Abstract
Interactions of human immunodeficiency virus type 1 (HIV-1) with immature dendritic cells (DC) are believed to be multifactorial and involve binding to the CD4 antigen, DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), mannose binding C-type lectin receptors (MCLR), and heparan sulfate proteoglycans (HSPG). In this study we assessed the relative contributions of these previously defined virus attachment factors to HIV binding and accumulation in DC and the subsequent transfer of the bound virus particle to CD4+ T cells. Using competitive inhibitors of HIV-1 attachment to DC, we have identified the existence of DC-SIGN-, MCLR-, and HSPG-independent mechanism(s) of HIV attachment and internalization. Furthermore, virus particles bound by DC independently of CD4, DC-SIGN, MCLR, and HSPG are efficiently transmitted to T cells. Treatment of virus particles with the protease subtilisin or treatment of immature DC with trypsin significantly reduced virus binding, thus demonstrating the role of HIV envelope glycoprotein interactions with unidentified DC-surface factor(s). Finally, this DC-mediated virus binding and internalization are dependent on lipid rafts. We propose that pathways to HIV-1 attachment and uptake in DC exhibit functional redundancy; that is, they are made up of multiple independent activities that can, at least in part, compensate for one another.
Human immunodeficiency type 1 (HIV-1) envelope glycoprotein binding to CD4 and an appropriate coreceptor results in the fusion of virus with host cell membranes and is the pathway leading to productive infection (11). In contrast, other molecules can also bind virus, but do not lead to fusion of the viral and cellular membranes. These molecules, called HIV-1 attachment factors, may serve to concentrate virus particles on the target cell surface or to transfer virus from the cell of initial virus contact to a CD4+ cell. Previous studies have identified members of the mannose binding C-type lectin receptor (MCLR) family, the dendritic cell (DC)-specific ICAM-3-grabbing nonintegrin (DC-SIGN), and liver/lymph node-specific ICAM-3 grabbing nonintegrin) (LSIGN or DCSIGNR) as HIV attachment factors (1, 4, 17, 18, 42, 54). More importantly, virus particles bound to these lectin receptors could be efficiently transmitted to CD4+ T cells expressing the appropriate receptor and coreceptor(s) complex (3, 40, 41, 62). Furthermore, it has also been suggested that different members of the MCLRs expressed on varied subsets of DC are largely responsible for all virus attachment to DC and that the virus binding could be competitively inhibited in the presence of excess carbohydrates such as mannan (49, 56, 57). In addition to these high-affinity ligand-receptor interactions, nonspecific interactions between the HIV env glycoprotein and the charged surfaces of the heparan sulfate proteoglycans (HSPG) could also contribute to virus attachment in certain cell types (7, 33, 51, 58). Interestingly, HSPG-bound virus particles could also be transmitted to target T cells (7).
Identification of the attachment factors on DC has important in vivo significance. It has been postulated that certain subsets of DC present in the peripheral mucosa are the first immune-competent cells to encounter lentiviruses (22, 53). Subsequent to virus capture by DC, infectious HIV particles are believed to be transported by DC to the draining lymph nodes where virus replication can be amplified (9, 30, 31, 48, 54). Although productive infection of DC themselves has been controversial, DC can capture virions and retain them in an infectious state for an extended period of time (9, 16, 19, 43, 44). These virus-bearing DC may then facilitate a more efficient spread of virus to the surrounding permissive CD4+ T cells (6, 9, 15, 20, 39, 43, 44, 63).
Previous studies by us and others have identified the existence of DC-SIGN-independent mechanisms of lentivirus attachment to DC (2, 20, 61). More importantly, these particles that were captured by DC in a DC-SIGN-independent fashion were transmitted to CD4+ T cells (20). Thus, we sought to characterize additional HIV capture mechanisms that are present on DC. Here, we report the existence of additional virus attachment mechanisms on DC in addition to the previously reported mechanisms, CD4, DC-SIGN, MCLR, and HSPG. The preincubation of DC with either saturating concentrations of mannan or neutralizing CD4 monoclonal antibodies, the enzymatic removal of HSPG, or a combination of these methods did not prevent virus binding to DC. Virus particles bound independently of mannose receptors were internalized by DC and were transferred to T cells. Interestingly, HIV-1 attachment to DC was significantly inhibited by pretreatment of the cells with cholesterol-sequestering reagents, such as methyl-β-cyclodextrin (MβCD) and fillipin III. These studies suggest that virus binding to DC is dependent on receptors concentrated in lipid rafts and that membrane fluid dynamics significantly impact initial virus interactions with DC. More importantly, binding of HIV-1 by DC is multifactorial, and all these virus-attachment mechanisms can potentially contribute to the virus reservoir in vivo.
MATERIALS AND METHODS
Cells and viruses.
The isolation and characterization of immature DC have been previously described (20, 50). Briefly, peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of healthy human donors by Ficoll gradients. Primary CD14+ monocytes were isolated from PBMC by CD14-coated magnetic beads and the auto-MACS protocol (Miltenyi Biotech, Auburn, Calif.), and cell purity of the isolated cell populations was routinely assessed to be >95% CD14+ by analysis with a fluorescence-activated cell sorter. CD14+ monocytes (3 × 106 cells/ml) were cultured in RPMI medium-10% fetal bovine serum (FBS) in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4 (1,000 U/ml each; Peprotech, Rocky Hill, N.J.) for a period of 6 to 8 days, at which point the cells attained a veiled cell (DC) phenotype (CD209+ CD14− HLA-DR+ CD80+ CD83−). The cell lines Jurkat, Jurkat-CCR5 (constitutively expressing the CCR5 coreceptor), THP1, and THP1/DC-SIGN have been described previously (20). The cell lines were cultured in RPMI medium-10% FBS (Invitrogen, Carlsbad, Calif.) supplemented with penicillin and streptomycin (complete RPMI medium). The HIV-1 molecular clones Lai (CXCR4-tropic), SF162 (CCR5-tropic), and NL4-3/Ba-L (CCR5-tropic) have been described previously (20, 60). Virus stocks for all molecular clones were generated by calcium phosphate-mediated transfections of HEK293T cells. Two days posttransfection, cell-free virus supernatants were harvested by centrifugation at 1,000 × g for 10 min and stored at −80°C until use. The primary virus strain 93BR019 (subtype B/F, CXCR4-tropic) was obtained from the AIDS repository and expanded in primary human PBMC by defined protocols. Cell-free virus supernatants were harvested on day 7 at peak p24gag content. All virus titers were determined on MAGI cells (23), and the p24gag content of the viral supernatants was determined by an enzyme-linked immunosorbent assay (ELISA; Beckman Coulter, Hialeah, Fla.).
Virus binding and transfer.
DC (4 × 104 cells/well) were infected with virus stocks (30 ng of p24gag) in 50 μl of total volume in a 96-well V-bottom plate for 2 h in triplicate. To potentially inhibit virus binding to DC, cells were treated with increasing amounts of mannan (Sigma, St. Louis, Mo.) for 30 min at 4°C, exposed to neutralizing CD4 monoclonal antibodies (Sim.4; 1 μg/ml), or treated with heparinase I (10 U/ml; Sigma) for 1 h at 37°C (33) prior to virus exposure at either 37 or 4°C. In addition, in some experiments, DC were treated with 500 μM amantadine (Sigma), 5 mM methyl-β-cyclodextrin (Sigma), or 2.5 μg of fillipin III (Calbiochem, San Diego, Calif.) per ml in a final volume of 150 μl for 30 min at 37°C and then exposed to virus for 2 h at 37°C. Cell viability was routinely assayed by trypan blue exclusion and was determined to be >95%. Virus binding assays were also performed with THP1 and THP1/DC-SIGN cells under identical assay conditions. Briefly, 4 × 104 THP1 or THP1/DC-SIGN cells were incubated in the presence or absence of mannan for 30 min at 4°C prior to virus exposure for 2 h at 4°C. All infections were performed in triplicate. Cells were vigorously washed five times with cold phosphate-buffered saline (PBS) interspersed with centrifugations to remove unadsorbed virus and finally lysed with 100 μl of PBS containing 1% Triton X-100. The lysates were cleared of cell debris by centrifugation (10,000 × g for 5 min at 4°C), and the p24gag content of the lysate was determined by ELISA. The percentage of virus particles bound by DC was calculated by the following formula: [(ng of p24gag associated with DC)/(ng of input p24gag) × 100]. The ability of DC, THP1, and THP1/DC-SIGN cells to transfer virus particles to T cells was determined by coculturing HIV-exposed DC, THP1, or THP1/DC-SIGN cells with Jurkat-CCR5 T cells (1:10 ratio) in a round-bottom 96-well plate (final volume, 200 μl). Briefly, virus-exposed cells were washed extensively, as described above, to remove unadsorbed virus, resuspended in 50 μl of complete medium, and transferred to round-bottom 96-well plates containing 2 × 105 Jurkat (or Jurkat-CCR5) T cells in a total volume of 200 μl. Cell-free virus supernatants were harvested 3 days after coculture, and the level of virus replication was determined by measuring the p24gag content of the cell-free supernatants by ELISA.
HIV-1 env gp120 binding.
DC (5 ×104 cells/well) were incubated with 250 ng of HIV-1SF162 (CCR5-tropic) gp120 in 50 μl of buffer (RPMI medium-10 mM HEPES-1% bovine serum albumin) for 1 h at 4°C in a 96-well V-bottom plate in triplicate. To potentially inhibit the binding of gp120 to DC, cells were pretreated with increasing concentrations of mannan for 30 min at 4°C prior to exposure to the monomeric env glycoprotein. Cells were washed three times with cold PBS, pelleted by centrifugation (500 × g for 5 min) at 4°C, and lysed with 200 μl of PBS containing 1% Triton X-100. Lysates were cleared of cell debris by centrifugation (10,000 × g for 5 min at 4°C), and processed for an ELISA against gp120. The percentage of gp120 binding by DC was determined by the following formula: [(ng of gp120 associated with DC)/(ng of input gp120) × 100].
Protease treatments.
Viral (Lai) supernatants from HEK293T transfections harvested 2 days posttransfection were clarified by centrifugation (500 × g for 5 min) and spun over a 20% sucrose cushion for 1 h at 4°C (64,000 × g). The viral pellet was resuspended in a solution of 20 mM Tris-HCl (pH 8.0)-1 mM CaCl2 in the presence or absence of 2 mg of subtilisin/ml (Sigma) and incubated overnight at 37°C (36). The protease reaction was stopped by the addition of serum-containing medium (Dulbecco's modified Eagle's medium-10% FBS), followed by another centrifugation over 20% sucrose at 64,000 × g for 1 h. The viral pellet was resuspended in medium, and the p24gag content was quantitated by ELISA. Subtilisin-treated viral supernatants were used for DC infections, as described above. The efficiency of subtilisin treatment was determined by Western blotting. Briefly, equal amounts (30 ng of p24gag) of virus supernatants were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel, transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, Mass.), probed sequentially with an anti-HIV-1 gp120 monoclonal antibody (clone 902; AIDS Repository) and a horseradish peroxidase-conjugated donkey anti-mouse antibody (Jackson Laboratory, Bar Harbor, Me.); the proteins were detected by enhanced chemiluminescence (Amersham BioSciences, Piscataway, N.J.). Alternatively, DC (4 ×104 cells/well) were treated with 0.25% trypsin at 37°C for 30 min and then washed with cold RPMI medium-10% FBS to stop the protease digestion. The cell pellet was then incubated with mannan (100 μg/ml) for 30 min at 4°C prior to virus exposure (30 ng of p24gag) for 2 h at 4°C in a 96-well V-bottom plate. Cell viability was monitored by trypan blue exclusion, and there was typically less than 5% cell death. Cells were washed four times with cold PBS, lysed in 100 μl of PBS-1% Triton X-100, and processed for ELISA against p24gag, as described above.
Confocal microscopy.
As controls for the effective functioning of amantadine and MβCD, drug-treated DC (as described above) were incubated with fluorescein isothiocyanate (FITC)-conjugated transferrin substrate (50 μg/ml) (Sigma) for 1 h at 4°C and then shifted to 37°C for 1 h, cytospun onto coverslips, and then fixed in 4% paraformaldehyde. Coverslips were rinsed extensively with PBS, stained with DAPI (4′,6′-diamidino-2-phenylindole; 0.2 μg/ml in distilled H2O) for 5 min, washed once with distilled H2O, dehydrated in 100% methanol for 15 min, air dried, mounted in Vectashield (Vector Laboratories, Burlingame, Calif.), and sealed with nail polish. Images of the stained cells were observed and collected with the Delta Vision SA3.1 microscope (Applied Precision Inc., Issaquah, Wash.). By using appropriate filters and excitation wavelengths for each fluorophore, a Z-series of 0.2-μm sections was collected for each sample. Images were deconvoluted with the DeltaVision software.
RESULTS
Virus capture by DC.
Some recent studies have suggested that MCLR are the predominant HIV-1 attachment factors on DC and that CD4 and MCLR could account for all virus binding to these cell types (21, 26, 56, 57). These studies utilized a biotin-conjugated or fluorescently conjugated monomeric HIV-1 gp120 envelope glycoprotein as the source of the virus envelope protein. However, because monomeric gp120 does not present all of the conformational epitopes that are present on the envelope as found on the virions (13, 34, 52), we performed virus binding assays with infectious virus particles expressing the native oligomeric envelope on its surface. DC, differentiated from monocytes in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4, were preincubated with increasing concentrations of mannan (ranging from 0.05 to 5 mg/ml) prior to infection with two different isolates of HIV-1 that differ in their coreceptor usage (Lai and NL4-3/Ba-L) (Fig. 1A and B). To prevent nonspecific macropinocytic or phagocytic uptake of virus particles by DC, the binding studies were performed at 4°C to inhibit all receptor-independent, energy-dependent membrane uptake processes. The determination of virus binding was achieved by measuring the amount of p24gag found associated with DC after extensive washing of the cells to remove unbound virus particles, and this amount was expressed as a percentage of the input virus amount (nanograms of p24gag).
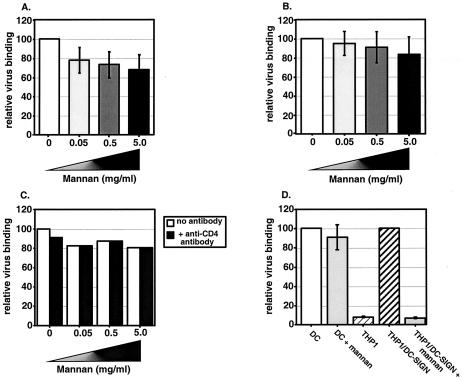
FIG. 1.
HIV-1 binding to DC can occur in the presence of competitive inhibitors of MCLR and CD4. Cells (4 × 104 cells/well) were incubated with increasing concentrations of mannan at 4°C and then exposed to either Lai (A) or NL4-3/Ba-L env (B) virus particles (30 ng of p24gag) for 2 h at 4°C. See Materials and Methods for experimental details. Cell-associated virus binding was determined by a p24gag ELISA. The mean level of Lai or NL4-3/Ba-L env binding to DC in the presence of increasing amounts of mannan was normalized to the level of virus binding to DC in the absence of mannan (set at 100%) and reported as relative virus binding. Infections with HIV/Lai (A) and NL4-3/Ba-L (B) were performed at least five independent times with DC derived from five independent donors. The results shown here are the averages of the mean percent binding observed for all the experiments. (C) A representative experiment of Lai virus binding to DC in the presence of a constant amount of anti-CD4-neutralizing monoclonal antibodies (Sim.4; 1 μg/ml) and increasing amounts of mannan. The mean level of virus binding to DC in the presence of either CD4-neutralizing antibodies or mannan was normalized to the level of virus binding to DC in the absence of mannan and CD4-neutralizing antibody (set at 100%) and reported as relative virus binding. Note that presence of anti-CD4 monoclonal antibodies had no synergistic effect with mannan in inhibiting Lai binding to DC. One representative experiment out of two is shown. (D) DC, THP1, or THP1/DC-SIGN cells (4 ×104) were incubated with Lai virus particles in the presence or absence of mannan (50 μg/ml) for 2 h at 4°C, and the cell-associated virus fraction was determined by p24gag ELISA. The experiment was performed at least three independent times, and each experiment was performed in triplicate. The mean level of virus binding to THP1 cells and THP1/DC-SIGN cells in the presence of mannan (50 μg/ml) was normalized to the level of virus binding to THP1/DC-SIGN cells in the absence of mannan (set at 100%) and reported as relative virus binding. Also, in each experiment, the mean level of virus binding to DC in the presence of mannan (50 μg/ml) was normalized to the level of virus binding to DC in the absence of mannan.
In contrast to previously published results obtained with monomeric gp120 (21, 26, 56, 57), the preincubation of DC with mannan had a limited effect on the ability of these cells to bind either of the two viral isolates. We observed only a 30% inhibition in HIV/Lai binding to DC even when the cells were preincubated with 5 mg of mannan/ml (Fig. 1A). Furthermore, mannan competition had minimal impact (<20%) on the ability of DC to bind the CCR5-tropic isolate NL4-3/Bal env (Fig. 1B). We have repeated this assay with both primary and lab-adapted HIV-1 isolates (CXCR4-tropic strains, MN and 93BR019, and the R5-tropic strain, NL4-3/JR-CSF env) with similar results (data not shown). To ascertain whether under our assay conditions, mannan was functioning properly, we incubated THP1/DC-SIGN cells with mannan prior to virus exposure. THP1/DC-SIGN cells, selected to constitutively express DC-SIGN, can bind virus particles with high efficiency, while the parental THP1 cells display negligible levels of virus binding (Fig. 1C). As expected, virus binding to THP1/DC-SIGN cells is completely inhibited by preincubation with mannan (50 μg/ml), while competition with mannan has limited effect on virus particle binding to DC (Fig. 1C).
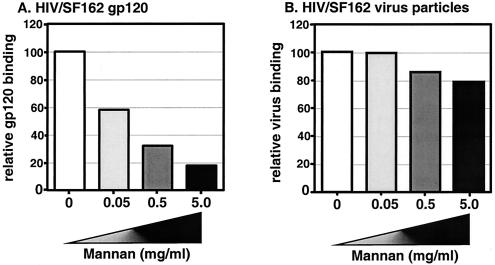
As previously suggested, it is possible that the residual virus attachment to DC in the presence of an excess amount of mannan could be attributed to the CD4 receptor (56, 57). To address this possibility, we exposed DC to a combination of neutralizing CD4 monoclonal antibody (Sim.4) and increasing concentrations of mannan prior to incubation with HIV/Lai (Fig. 1C). The prevention of virus binding to the CD4 protein and MCLR again had a negligible impact on the ability of DC to bind virus particles. In fact, pretreatment of DC with 5 mg of mannan/ml and neutralizing CD4 monoclonal antibodies inhibited Lai attachment by only 20% (Fig. 1D). In contrast to the results obtained with virus particles, there was a dose-dependent decrease in SF162 gp120 (monomeric) binding to DC with increasing concentrations of mannan (Fig. 2A), a finding that is in complete agreement with previously published results that have suggested that monomeric gp120 binding to DC is largely dependent on MCLR (21, 26, 56, 57). Since the monomeric gp120 was derived from the HIV/SF162 strain, we performed a binding assay with DC and SF162 virus particles. We performed an ELISA to determine the amount of gp120 present on intact HIV/SF162 virus particles and then subsequently used this virus stock for virus binding assays with primary DC (Fig. 2B). The input amount of 30 ng of virus particles (p24gag) per well translated to 0.7 ng of virus-associated gp120, as opposed to the initial input of 250 ng of monomeric gp120 per well (Fig. 2B). Even though the amounts of monomeric gp120 used in the binding experiments were much greater than that present on intact HIV/SF162 virus particles, monomeric gp120 binding to DC was completely inhibited by saturating concentrations of mannan while binding of virus particles to DC was not (Fig. 2).
FIG. 2.
Monomeric gp120 binding to DC is dependent on MCLR. DC were untreated or incubated with mannan prior to incubation with either (A) 250 ng of SF162 monomeric gp120 or (B) HIV/SF162 virus particles (30 ng of p24gag) at 4°C. The mean level of gp120 binding to DC in the presence of increasing amounts of mannan was normalized to the level of gp120 binding to DC in the absence of mannan (set at 100%) and reported as relative gp120 binding. The mean level of HIV/SF162 virus particle binding to DC in the presence of increasing amounts of mannan was normalized to the level of virus binding to DC in the absence of mannan (set at 100%) and reported as relative virus binding. The results from one representative experiment out of two are shown, and each experiment was performed in triplicate.
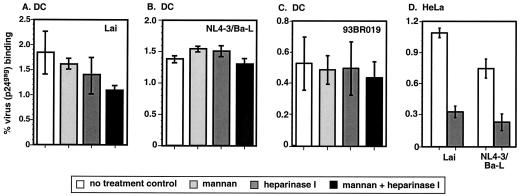
Besides CD4 and mannose receptors, HSPG have also been suggested as putative HIV attachment factors (7, 33, 51, 58). Previous studies have demonstrated that expression of HSPG (syndecans) on primary human macrophages and in some cell lines rendered the cells competent for HIV-1 capture (7, 33, 51, 58). It is believed that the surface proteoglycans are responsible for weak charge-based interactions with the HIV-1 envelope. Complete digestion of these proteoglycan side chains on macrophages and HeLa cells by heparinases abolished virus binding to these cells (7, 33, 51, 58). Therefore, we wanted to determine if HSPG expressed on DC surfaces could account for the virus attachment observed in the presence of MCLR inhibitors (Fig. 1A and B). To address this issue, DC were treated with a heparinase I enzyme that removes all HSPG side chains prior to virus exposure. Additionally, we used HeLa cells as controls for heparinase I treatment. Furthermore, in some cultures, treatment of the cells with heparinase I was followed by incubation with mannan prior to virus exposure. Data shown here are from binding assays performed with the HIV-1 isolates Lai, NL4-3/Bal and the primary isolate 93BR019 (Fig. 3A, B, and C, respectively). The results shown in Fig. 3 suggest that the removal of HSPG side chains has no effect on virus attachment to DC, while treatment of HeLa cells with heparinase I inhibits their ability to bind virus particles (Fig. 3D). Though the inhibition of binding to MCLR in addition to the removal of HSPG side chains had a limited effect on Lai binding to DC (reduced by 40% compared to virus binding by untreated DC) (Fig. 3A), it had no impact on the ability of DC to capture NL4-3/Ba-L (Fig. 3B) or 93BR019 (Fig. 3C) virus particles. These binding studies suggest the presence of additional attachment factors on DC that can mediate HIV-1 binding.
FIG. 3.
HSPG do not play a role in HIV-1 binding to DC. DC (4 ×104 cells/well) were incubated with mannan (50 μg/ml), treated with heparinase I (10 U/ml), or exposed to a combination of both reagents and then exposed to Lai (A), NL4-3/Ba-L (B), or 93BR019 (C) virus particles (30 ng of p24gag) for 2 h at 4°C. (D) HeLa cells (4 × 104 cells/well) were left untreated or treated with heparinase I (10 U/ml) and then exposed to either Lai or NL4-3/Ba-L. The percent virus (p24gag) binding represents the mean ± standard deviation of triplicate cultures. The results from one representative experiment out of three with DC derived from three independent donors are shown.
Virus transfer.
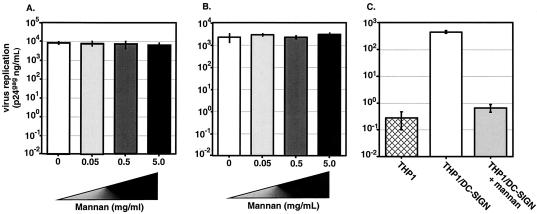
Finally, to determine if the virus particles captured by DC in the presence of an excess amount of mannan are competent to be transferred to T cells, we cocultured virus-exposed DC with Jurkat-CCR5 T cells. The level of virus replication was determined by measuring the p24gag content of the cell-free supernatants 3 days postinitiation of coculture. As shown in Fig. 4A and B, all cocultures demonstrated equivalent levels of viral replication. Interestingly, virus particles (both HIV/Lai and NL4-3/Ba-L env) captured by DC even in the presence of 5 mg of mannan/ml were efficiently transferred to T cells. As controls for the effective functioning of mannan in our virus transfer assays, THP1 and THP1/DC-SIGN cells were exposed to HIV/Lai particles and then cocultured with Jurkat T cells. Since THP1 cells display negligible levels of virus binding (Fig. 1D), they do not transmit virus to T cells (Fig. 4C), while THP1/DC-SIGN cells can bind virus efficiently (Fig. 1D) and can transmit these bound virus particles to T cells (Fig. 4C). Virus binding and transmission are completely dependent on DC-SIGN since preincubation with mannan completely inhibits virus binding (Fig. 1D) and, hence, prevents virus transfer to T cells (Fig. 4C). These results demonstrate that virus particles captured by DC independently of MCLR are infectious and are competent for transfer to T cells, where they can initiate robust virus replication.
FIG. 4.
DC can mediate HIV-1 transmission in a MCLR-independent manner. DC (2 × 104 cells/well) were preincubated with increasing concentrations of mannan and then incubated with Lai (A) or NL4-3/Ba-L env (B) at 4°C, extensively washed, and then cocultured with Jurkat-CCR5 T cells (2 × 105 cells/well). (C) THP1 or THP1/DC-SIGN cells (2 × 104 cells/well) in the presence or absence of mannan were incubated with Lai, extensively washed, and then cocultured with Jurkat-CCR5 T cells. Culture supernatants were assayed for p24gag content 3 days after the start of the coculture. Shown here are the results from one representative experiment out of three; each infection was performed in triplicate, and the mean amounts of p24gag content with standard deviations are shown here. The three independent experiments were performed with DC from three independent donors.
Requirement of protein interactions for HIV attachment and uptake.
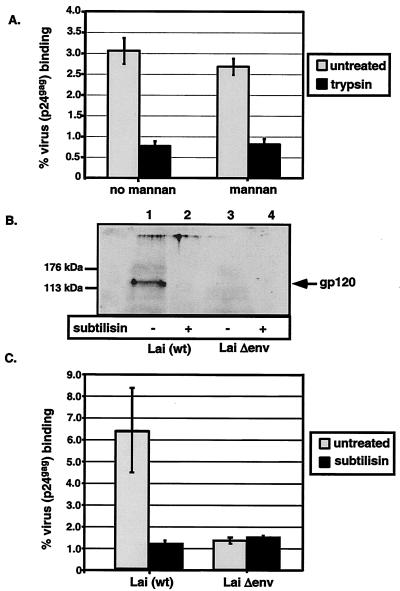
The data presented so far suggest the presence of additional HIV-1 attachment factors on DC. It is also possible that virus uptake by DC can occur by receptor-independent mechanisms. Antigen-presenting cells, such as DC, can sample their immediate environment by fluid uptake via membrane-ruffling processes such as macropinocytosis that allow uptake of various antigens without the requirement for a specific receptor (32). To determine if cell surface proteins present on the plasma membranes of DC are required for MCLR-independent binding, we treated DC with trypsin for a period of 30 min to remove cell surface-exposed proteins. To control for protease digestion, cells were stained for DC-SIGN expression and analyzed with a fluorescence-activated cell sorter. There was a 50-fold decrease in DC-SIGN expression on DC after trypsin treatment (data not shown). Subsequent to the protease treatment, cells were incubated with virus (Lai)- containing supernatants, and binding assays were performed as described previously. Trypsin treatment reduced binding of HIV/Lai to DC by >85% (Fig. 5A). Furthermore, incubation of trypsin-treated DC with mannan prior to virus exposure did not reduce the binding of virus particles any further, suggesting that trypsin-resistant MCLR are not responsible for the residual virus particle binding (Fig. 5A). These results suggest a requirement for a surface-exposed, trypsin-sensitive DC-specific factor(s) for HIV-attachment.
FIG. 5.
Protease treatment of immature DC or virus particles reduces virus particle binding to DC surface. (A) DC (4 × 104/well) were treated with 0.25% trypsin at 37°C for 30 min and then washed with cold complete RPMI medium to stop the protease digestion. The cell pellet was then incubated with mannan (100 μg/ml) for 30 min prior to virus exposure (30 ng of p24gag) for 2 h at 4°C. Virus binding to trypsin-treated and untreated DC was assessed as described in Materials and Methods. (B) Immunoblot analysis of Lai (lanes 1 and 2) and LaiΔenv (lanes 3 and 4) virus preparations isolated and purified from HEK293T cell supernatants that were either left untreated (lanes 1 and 3) or treated with the protease subtilisin (lanes 2 and 4). The blots were reacted with an antibody specific for the Lai gp120 glycoprotein. (C) DC (4 × 104 cells) were incubated with untreated or subtilisin-treated virus preparations (30 ng of p24gag) at 4°C for 2 h. The percent virus (p24gag) binding in panels A and C represents means ± standard deviations of triplicate cultures. The results from one representative experiment out of three are shown.
Some studies have suggested a role for host proteins, such as LFA-1 and VLA-4 that are incorporated into the virus particles during budding, in virus attachment to target cells (8, 10, 12, 25, 35). To test for the putative contribution of host-derived, virus membrane-incorporated proteins, we produced virus stocks that were envelope deficient (Lai Δenv) along with wild-type Lai in HEK293T cells by using similar transfection protocols and tested the ability of the viruses to bind DC. In addition, we also subjected both wild-type Lai and Lai Δenv virus stocks to subtilisin treatment. Treatment with the protease subtilisin removes all surface-exposed viral membrane proteins (36). Virus particles of Lai and Lai Δenv incubated in the presence or absence of subtilisin were pelleted through sucrose cushions and tested for HIV-1 envelope glycoprotein expression via Western blot analysis with gp120-specific monoclonal antibodies (Fig. 5B). The expression of gp120 was evident in the wild-type virus (Lai) preparations (lane 1) and absent in virus preparations when they were treated with subtilisin (lane 2). As expected, Lai Δenv virus particles did not express the envelope glycoproteins (Fig. 5B, lanes 3 and 4). Additionally, we performed an ELISA to determine the concentrations of the p24gag capsid protein in the virus preparations. As expected, subtilisin treatment had no effect on p24gag amounts in the virus preparations (data not shown). Binding of Lai to DC was inhibited by subtilisin pretreatment (Fig. 5C). Furthermore, Lai Δenv, compared to wild-type Lai, demonstrated minimal binding to DC, and subtilisin treatment of Lai Δenv particles had no impact on this process (Fig. 5C). These results demonstrate that much of the virus particle binding to DC is dependent on the HIV-1 envelope glycoproteins and that host proteins incorporated into virus membranes presumably make minimal contributions to this process.
Role of lipid rafts in virus binding and uptake.
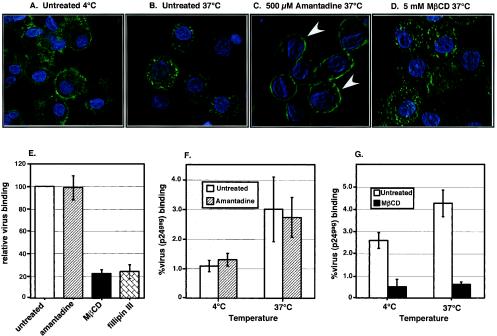
Regardless of the mechanism of virus attachment to DC, the fate of the virus particles is ultimately determined by targeting to a membrane-trafficking pathway. Hence, we next wanted to ascertain the mechanism of virus internalization by pharmacological inhibition of selective endocytosis pathways. We first examined the role of clathrin-coated pits in virus uptake by treating DC with amantadine, an inhibitor of clathrin-coated pit assembly. Since endocytosis of the transferrin receptor is mediated specifically via clathrin-coated pits, we used transferrin-FITC as a substrate in confocal microscopic analysis to verify that inhibitors used in this assay were functioning effectively. At 4°C, transferrin-FITC is localized predominantly at the plasma membrane, indicative of substrate binding but with no internalization (Fig. 6A). When the cells were shifted to 37°C, there was a rapid internalization of transferrin-FITC (Fig. 6B) that was completely inhibited by preincubation of the cells with amantadine, an inhibitor of clathrin-coated pit mediated endocytosis (Fig. 6C). In contrast, treatment with MβCD, which removes cholesterol from the plasma membrane, did not prevent intracellular accumulation of transferrin-FITC (Fig. 6D).
FIG. 6.
Binding of HIV-1 to DC is cholesterol dependent. DC were exposed to the endocytosis inhibitors and then incubated with FITC-labeled transferrin at 4°C. Cells were also stained with DAPI to help in the visualization of the cellular architecture. The images are the composite of a series of Z sections collected through the entire thickness of the cell monolayer and projected onto a two-dimensional plane. (A) At 4°C, transferrin-FITC can bind to its receptor but is not internalized, as evident from the staining observed predominantly at the plasma membrane. (B) Cells were shifted to 37°C for a period of 15 min and then monitored again for transferrin localization. Note the prominent intracellular green fluorescence indicative of uptake of transferrin-FITC. (C) Treatment with 500 μM amantadine prior to incubation with transferrin-FITC for 1 h at 37°C resulted in transferrin-FITC staining at the plasma membrane (indicated by white arrowheads) and completely abolished the intracellular staining, while treatment with 5 mM MβCD (D) had no effect on the intracellular accumulation of transferrin. (E) DC (4 × 104 cells) treated with the endocytosis inhibitors were exposed to Lai virus particles (30 ng of p24gag) for 2 h at 37°C. The mean level of virus (Lai) binding in the presence of endocytosis inhibitors was normalized to the level of virus binding in the absence of inhibitors (set at 100%). This experiment was performed at least five independent times with DC derived from five independent donors, and each experiment was performed in triplicate. The results shown are the averages of the mean percent binding (± standard deviations) observed for all the experiments. (F) DC (4 × 104 cells) were treated with amantadine (500 μM) and then exposed to Lai virus particles (30 ng of p24gag) for 2 h either at 4 or 37°C. (G) DC (4 × 104 cells) were treated with MβCD (5 mM) and then exposed to Lai virus particles (30 ng of p24gag) for 2 h either at 4 or 37°C. The percent virus (p24gag) binding values shown in panels F and G represent the means ± standard deviations of triplicate cultures. The results from one representative experiment out of three with DC derived from three independent donors are shown in panels F and G.
To further ascertain the mode of virus internalization in DC, we treated DC with endocytosis inhibitors prior to virus (HIV/Lai) exposure at 37°C. The level of virus accumulation was again determined by measurement of the cell-associated p24gag protein, as described previously. This experiment was performed multiple times with DC from multiple donors, and the data shown are the mean results of at least five independent experiments (Fig. 6E). Surprisingly, treatment of DC with amantadine had no effect on the endocytosis of virus particles (Fig. 6E), suggesting that virus internalization can occur via a clathrin-independent mechanism. Interestingly, virus internalization was dramatically inhibited (>80% inhibition) by the sterol binding agent MβCD and fillipin III, an agent that extracts cholesterol from caveolae (37, 38) (Fig. 6E), supporting the role for lipid rafts in the endocytosis of virus particles. To determine whether this inhibition was at the level of virus particle binding to the cell surface or whether virus particle accumulation was affected by the depletion of cholesterol from the plasma membranes of DC, we performed virus binding assays at 4 and 37°C in the presence or absence of drugs. At 4°C, where there is no virus particle internalization, amantadine has no effect on virus particle binding (Fig. 6F). Furthermore, when the assay is performed at 37°C, amantadine has no effect on the accumulation of virus particles within the cells (Fig. 6F). In contrast, treatment of DC with MβCD abolishes virus binding at 4°C and, hence, also prevents the accumulation of virus particles within the cells (Fig. 6G). These data provide evidence for the presence of DC-specific HIV-receptors in the cholesterol-enriched regions of the lipid bilayer.
DISCUSSION
Although the establishment of productive infection by HIV-1 in target cells requires the binding of the HIV envelope glycoprotein to the CD4 molecule and subsequent interaction with the chemokine coreceptor, CXCR4 or CCR5, the initial virus attachment can be mediated by a number of different molecules. These attachment molecules need not facilitate virus fusion with cells expressing these attachment factors per se but, rather, could mediate efficient trans infection of CD4+ target cells. Our data suggest the existence of CD4-, DC-SIGN-, MCLR-, and HSPG-independent mechanism(s) of HIV-1 attachment to DC and demonstrate that virus particles captured by DC independently of all previously known attachment mechanisms are still capable of being transmitted to T cells. However, attachment of infectious virus particles to DC is still largely mediated by the virus envelope glycoprotein and its interactions with DC-specific factors. Finally, we provide evidence that the attachment factors are concentrated in lipid rafts and that cholesterol-enriched lipid rafts play a vital role in virus uptake by DC.
In contrast to the results presented here, previous reports showed that HIV gp120 binding to different subsets of DC including peripheral blood monocyte-derived DC was completely dependent on CD4 and MCLR (56, 57). However, those studies used monomeric gp120 to measure binding, while we measured binding of virions. It is possible that trimeric gp120 on virion surfaces presents structural or conformational epitopes not present on monomeric gp120 that are necessary for MCLR (or DC-SIGN)-independent binding. It is also formally plausible that the affinity of the oligomeric env glycoprotein for MCLR is much higher than that exhibited by its monomeric gp120 counterpart, such that it cannot be completely inhibited by saturating concentrations of mannan. Alternatively, there might be posttranslational modifications of gp120 in virions that are not present in the recombinant gp120 preparations used. Furthermore, in a previous study, virus entry into DC was quantified by the integration of the HIV cDNA into the host genome, which was inhibited to a large extent by the preincubation of DC with 5 mg of mannan/ml (57). In contrast, our binding assay measures the ability of DC to capture HIV particles and does not make any conclusions about the ability of these captured virus particles to establish productive infections in DC. Rather, the captured virus particles are most probably targeted for trans infection of target T cells. There also might be a hierarchical aspect to HIV particle binding to DC, and the relative binding affinity of virus to MCLR (and DC-SIGN) may be lower than its binding to a DC-specific attachment factor(s) that is as yet unidentified. While DC-specific MCLR may play a role in virus binding, this role cannot explain the complete binding profile of HIV to DC (Fig. 1 and 2). Hence, the data presented in this study argue for an MCLR-independent binding of HIV to DC.
Host proteins, such as LFA-1 and VLA-4, incorporated into the budding virus particle membranes have also been postulated as potential attachment factors for virus binding through interaction with their ligands, ICAM-1 and VCAM-1, respectively, (8, 10, 25, 35). Viral replication in human PBMC could potentially result in the acquisition of host integrins in the budding virus particles. Though treatment of virus particles with subtilisin prevented virus attachment to DC (Fig. 5B), this protease treatment would also remove any host integrins from the virus membranes, thus obscuring any potential contribution of the host factors to virus attachment. We were able to demonstrate the involvement of the HIV env glycoprotein with virus attachment by two independent experimental strategies. First, we utilized infectious viral supernatants generated from transfections of HEK293T cells that are deficient for expression for most adhesion molecules, thus obviating the contributions, if any, of host integrins in virus attachment. Second, the binding of env-deficient virions to DC was significantly reduced relative to the binding of env-containing virions (Fig. 5A), thus demonstrating a role for HIV env glycoprotein-specific interactions with a DC-specific factor(s).
Trypsin treatment of DC inhibited virus binding, suggesting a requirement for a plasma membrane-expressed receptor(s). The disruption of lipid rafts also resulted in a reduction in virus attachment to cells, suggesting that the HIV attachment factor(s) is preferentially concentrated in the cholesterol-rich microdomains of the DC membranes. The removal of cholesterol from the DC plasma membranes, either by MβCD or fillipin III, could result in the dispersion of a HIV attachment factor(s). This possibility suggests the potential requirement for multiple oligomeric env glycoprotein-mediated interactions with spatially restricted concentrations of DC-specific factor(s) for efficient virus attachment. The functional involvement of lipid rafts and the concentration of receptors in plasma membrane microdomains have been invoked for the entry of diverse viral pathogens, such as Semliki Forest virus (28), ecotropic murine leukemia virus (27), simian virus 40 (38), and the filoviruses, Ebola and Marburg (5). Previous studies have also speculated on the role of lipid rafts in HIV env-mediated membrane fusion for the productive infection of human cell lines (24, 29, 45, 59). The data detailed in this report, to our knowledge, are the first to document the involvement of lipid rafts in HIV attachment to DC. Furthermore, we demonstrate that HIV internalization in DC seems to depend on specific cholesterol-rich microdomains. Though previous studies have demonstrated a requirement for clathrin-mediated endocytosis for HIV particles (14), our data provide evidence for an alternative entry pathway for HIV, such as caveola-mediated internalization. It is important to note that the depletion of cholesterol from the plasma membrane can lead to an inhibition of clathrin-coated pit-mediated endocytosis, as well as to a reduction in uptake through lipid rafts or caveolae (47, 55). Hence, treatment of DC with MβCD could potentially inhibit both clathrin- and nonclathrin-mediated endocytosis, while treatment with amantadine does not prevent the alternative pathway for HIV endocytosis.
These results have potential repercussions for the design of effective microbicides against HIV. It is believed that in vivo circulating CD14+ blood monocytes are precursors to antigen-capturing tissue DC, including the epidermal Langerhans cells and the dermal DC subsets (46). These immature DC subsets can potentially capture infectious particles in the plurostratified genital mucosa and transport HIV to secondary lymphoid tissues, where virus replication can be amplified (54). If DC are indeed the initial cell types that encounter virus in the peripheral mucosal tissue, prevention of HIV attachment to DC by lipid-raft-disrupting agents could inhibit the initial virus transmission event. A corollary of these studies is that the use of lectins or reagents that inhibit virus binding to MCLR will be of limited potential in the prevention of HIV-1 transmission in the genital mucosa.
Acknowledgments
We acknowledge members of the Fred Hutchinson Cancer Research Center flow cytometry, electron microscopy, and confocal microscopy core facilities for help with cell sorting, electron microscopy, and immunofluorescence, respectively. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): the CD4 monoclonal antibody (Sim.4) from James Hildreth and the gp120 monoclonal antibody (902) from Bruce Chesebro. We are grateful to Wei Chun Goh for her critical reading of the text.
This work was supported by NIH grants RO1 AI47708 to L. Stamatatos and R37 AI30827 and R01 AI51153 to M. Emerman.
REFERENCES
- 1.Baribaud, F., S. Pohlmann, and R. W. Doms. 2001. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286:1-6. [DOI] [PubMed] [Google Scholar]
- 2.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 8.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 10.Cantin, R., G. Martin, and M. J. Tremblay. 2001. A novel virus capture assay reveals a differential acquisition of host HLA-DR by clinical isolates of human immunodeficiency virus type 1 expanded in primary human cells depending on the nature of producing cells and the donor source. J. Gen. Virol. 82:2979-2987. [DOI] [PubMed] [Google Scholar]
- 11.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 12.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72:9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., V. Finkel, E. Delgado, and R. M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9:21-29. [DOI] [PubMed] [Google Scholar]
- 20.Gummuluru, S., V. N. KewalRamani, and M. Emerman. 2002. Dendritic cell-mediated viral transfer to T cells is required for human immunodeficiency virus type 1 persistence in the face of rapid cell turnover. J. Virol. 76:10692-10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, P. W., K. B. Flummerfelt, A. de Parseval, K. Gurney, J. H. Elder, and B. Lee. 2002. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J. Virol. 76:12855-12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 25.Liao, Z., J. W. Roos, and J. E. Hildreth. 2000. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retrovir. 16:355-366. [DOI] [PubMed] [Google Scholar]
- 26.Lin, G., G. Simmons, S. Pohlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, X., and J. Silver. 2000. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology 276:251-258. [DOI] [PubMed] [Google Scholar]
- 28.Lu, Y. E., T. Cassese, and M. Kielian. 1999. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 73:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masurier, C., N. Guettari, C. Pioche, R. Lacave, B. Salomon, F. Lachapelle, D. Klatzmann, and M. Guigon. 1997. The role of dendritic cells in the transport of HIV to lymph nodes analysed in mouse. Adv. Exp. Med. Biol. 417:411-414. [DOI] [PubMed] [Google Scholar]
- 31.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 33.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas, 3rd, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 36.Ott, D. E., L. V. Coren, B. P. Kane, L. K. Busch, D. G. Johnson, R. C. Sowder, 2nd, E. N. Chertova, L. O. Arthur, and L. E. Henderson. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 38.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 39.Pinchuk, L. M., P. S. Polacino, M. B. Agy, S. J. Klaus, and E. A. Clark. 1994. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity 1:317-325. [DOI] [PubMed] [Google Scholar]
- 40.Pohlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohlmann, S., G. J. Leslie, T. G. Edwards, T. Macfarlan, J. D. Reeves, K. Hiebenthal-Millow, F. Kirchhoff, F. Baribaud, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75:10523-10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 44.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randolph, G. J., S. Beaulieu, S. Lebecque, R. M. Steinman, and W. A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282:480-483. [DOI] [PubMed] [Google Scholar]
- 47.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell. 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowland-Jones, S. L. 1999. HIV: the deadly passenger in dendritic cells. Curr. Biol. 9:R248-R250. [DOI] [PubMed] [Google Scholar]
- 49.Saifuddin, M., M. L. Hart, H. Gewurz, Y. Zhang, and G. T. Spear. 2000. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. J. Gen. Virol. 81:949-955. [DOI] [PubMed] [Google Scholar]
- 50.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinman, R. M. 2000. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100:491-494. [DOI] [PubMed] [Google Scholar]
- 55.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turville, S. G., J. Arthos, K. MacDonald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 57.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 58.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 59.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. W. Ruscetti, J. M. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoeteweij, J. P., and A. Blauvelt. 1998. HIV-dendritic cell interactions promote efficient viral infection of T cells. J. Biomed. Sci. 5:253-259. [DOI] [PubMed] [Google Scholar]