Abstract
Rabies virus (RV) vaccine strain-based vectors show significant promise as potential live-attenuated vaccines against human immunodeficiency virus type 1 (HIV-1). Here we describe a new RV construct that will also likely have applications as a live-attenuated or killed-particle immunogen. We have created a RV containing a chimeric HIV-1 Env protein, which contains introduced cysteine residues that give rise to an intermolecular disulfide bridge between gp120 and the ectodomain of gp41. This covalently linked gp140 (gp140 SOS) is fused in frame to the cytoplasmic domain of RV G glycoprotein and is efficiently incorporated into the RV virion. On the HIV-1 virion, the gp120 and gp41 moieties are noncovalently associated, which leads to extensive shedding of gp120 from virions and virus-infected cells. The ability to use HIV-1 particles as purified, inactivated immunogens has been confounded by the loss of gp120 during preparation. Additionally, monomeric gp120 and uncleaved gp160 molecules have been shown to be poor antigenic representations of virion-associated gp160. Because the gp120 and gp41 portions are covalently attached in the gp140 SOS molecule, the protein is maintained on the surface of the RV virion throughout purification. Surface immunostaining and fluorescence-activated cell sorting analysis with anti-envelope antibodies show that the gp140 SOS protein is stably expressed on the surface of infected cells and maintains CD4 binding capabilities. Furthermore, Western blot and immunoprecipitation experiments with infected-cell lysates and purified virions show that a panel of neutralizing anti-envelope antibodies efficiently recognize the gp140 SOS protein. The antigenic properties of this recombinant RV particle containing covalently attached Env, as well as the ability to present Env in a membrane-bound form, suggest that this approach could be a useful component of a HIV-1 vaccine strategy.
Evidence suggests that an immunogen capable of eliciting virus-neutralizing antibodies may be an important component of an effective human immunodeficiency virus type 1 (HIV-1) vaccine (19, 32, 42, 69, 87, 99). Such an immunogen should faithfully represent the antigenic structure of the virion-associated envelope complex, since neutralizing capacity has been observed with antibodies directed against epitopes contained on the native Env trimer (10, 12, 68, 73, 82). However, formulating vaccines capable of eliciting neutralizing antibodies has been quite difficult because of the labile nature of gp120-gp41 interactions and the antigenic differences between virion-associated gp160 and monomeric or dissociated subunits (11, 15, 53, 54, 67).
Following oligomerization in the endoplasmic reticulum, the gp160 precursor protein is cleaved by cellular proteases and is transported to the cell surface (28, 49). The mature, virion-associated form of the HIV-1 Env glycoprotein is a trimeric molecule composed of three gp120 and three gp41 subunits held together by weak noncovalent interactions (30, 73, 103). This structure is highly flexible and undergoes substantial conformational changes upon gp120 binding with CD4 and chemokine coreceptors, which leads to exposure of the fusion peptides of gp41 that insert into the target cell membrane and mediate viral entry (16, 44, 46, 47, 58, 93, 103). During the course of HIV-1 infection, multiple forms of gp120 and gp41 subunits are shed from virions and virus-infected cells due to the noncovalent interactions between gp120 and gp41 and between gp41 subunits (38, 54, 62, 71). This “viral debris” is an attractive target for the humoral immune response; however, these dissociated, largely monomeric subunits are immunologically distinct from virion-associated gp160 multimers. It is widely held that this antigen shedding is an immune avoidance mechanism evolved by HIV-1 to elicit an inappropriate antibody response and thus draw the hosts' immune defenses away from more conserved and possibly neutralizing Env determinants (45, 71, 103).
While highly variable, HIV-1 Env does contain conserved functional domains associated with receptor interactions and membrane fusion. On intact trimers, these conserved regions are shielded by carbohydrates moieties (66, 76) and the gp120 variable loop domains overlap and mask the coreceptor binding site until after gp120 has bound to CD4 (46, 59, 78, 91, 102). The end result is that the virus effectively diminishes the exposure of conserved epitopes related to the vital function of membrane fusion. Virus-neutralizing antibodies are known to interfere with receptor binding and membrane fusion (64, 68, 71). Indeed, broadly reactive sera and several neutralizing monoclonal antibodies (MAbs) are predominantly directed against discontinuous, conformational epitopes on mature Env (60, 90, 102, 103). These well-described epitopes include the N-linked glycan-dependent epitope recognized by MAb 2G12 (97), those associated with the CD4 binding site of gp120 (74, 75, 94, 96), and epitopes induced by gp120 binding to CD4 (CD4-inducible epitopes, CD4i) (95).
A large body of evidence indicates that oligomeric Env and monomeric Env differ substantially in their exposed epitopes. Antibodies that recognize monomeric gp120 do not react well with the multimeric form and tend to be nonneutralizing (29, 63, 83, 101, 106). Conversely, a correlation has been seen between antibodies directed against multimeric Env and neutralization (29, 36, 70, 79, 82, 106). The limitations of soluble, monomeric gp120-based vaccines is that vaccine-elicited antibodies have narrow specificities, react strongly to epitopes on denatured gp120, and do not neutralize primary viral isolates (2, 9, 29, 48, 71, 106; R. B. Belshe, D. P. Bolognesi, M. L. Clements, L. Corey, R. Dolin, J. Mestecky, M. Mulligan, D. Stablein, and P. Wright, Letter, JAMA 272:431, 1994). In contrast, sera from HIV-1-infected individuals, while containing antibodies to gp160 precursors and dissociated subunits (61, 68, 83), can also contain neutralizing antibodies directed against conformational epitopes and oligomeric Env (10, 60, 71, 90). To neutralize infectious virus, antibodies must recognize the mature form of Env, which is responsible for receptor binding and membrane fusion.
The recent disappointment of a phase III human trial (P. W. Berman, Keystone Symp. HIV Vacc. Dev. Immunol. Biol. Challenges, 2003) with a gp120-based vaccine has again confirmed the need to improve Env-based immunogens. Whatever the vaccine formulation, it must be capable of eliciting antibodies directed against native Env if it is to generate neutralizing antibodies to HIV-1 infection. Numerous groups have approached the issue of improving gp120-based immunogens by devising methods that create mutant or soluble envelope proteins that maintain many of the oligomeric and conformational properties of virion-associated gp160. One approach involves mutating or removing the cleavage site between gp120 and gp41 and truncating gp41 N-terminal domain to the transmembrane (TM). The resulting uncleaved Env protein (gp140unc) is soluble, is linked via a peptide bond, and is oligomerized by noncovalent interactions between gp41 subunits (3, 16, 27, 88). However, immunogenicity studies with gp140unc proteins have failed to generate primate isolate-neutralizing antibodies (26, 77, 98). Data indicate that the retained peptide bond causes a structural perturbation in gp120 that exposes epitopes not accessible on virions and leads to inefficient coreceptor binding (26, 27, 31). Recent efforts to improve the multimerization of these soluble molecules have involved the addition of trimerization motifs at the C terminus of uncleaved gp140 glycoproteins (104, 105).
A different tactic has been applied in using glycosylation mutants and viruses with variable loop deletions and soluble molecules. Since these structures overlap conserved regions on Env trimers, removing them may yield greater exposure of neutralizing epitopes (14, 76, 80). gp120 proteins with variable loop deletions are properly folded (7) and maintain CD4 binding (46, 102). Still another approach is to maintain the gp120/gp41 cleavage site but to stabilize the mature protein by introduction of intermolecular disulfide bonds between gp120 and the gp41 ectodomain (gp41ecto) (5, 80). These so-called gp140 SOS molecules are soluble and fully processed, maintain many critical neutralizing epitopes in gp120 and gp41, bind CD4, and can expose CD4i epitopes (5, 6, 80, 81, 86). Furthermore, Binley et al. recently demonstrated that a gp140 SOS protein could be incorporated into pseudotyped virions and that these particles could be useful tools in vaccine design and studies of HIV-1 fusion and entry mechanisms (4).
This report describes a new application for a gp140 SOS protein that seeks to create a true HIV-1 envelope mimic by anchoring gp140 on the surface of a replication-competent recombinant rabies virus (RV) particle, thereby presenting HIV-1 Env as a membrane-bound antigen as it is in the natural infection. RV has proven to be a highly immunogenic vaccine vector capable of stably expressing a variety of foreign proteins. Recombinant RV vectors have applications as both live-attenuated and killed-particle vaccines (55). Here, a covalently linked gp140 SOS protein expressing gp120 and the gp41 ecto- and TM domains of pathogenic SHIV Env 89.6P is fused in frame to the cytoplasmic tail domain (CD) of RV G glycoprotein, which allows for efficient incorporation of this chimeric Env into the RV virion (34, 35). Our characterization of this virus indicates that the gp140 SOS protein is stably expressed and maintains many of the desirable features of an envelope-based component of an HIV-1 vaccine. Thus, this RV construct could potentially be used as either a live-attenuated or killed-particle immunogen.
MATERIALS AND METHODS
Plasmid construction and virus recovery.
The plasmid encoding the recombinant RV vaccine vector BNSP was described previously (51). The introduction of the arginine-to-glutamic acid substitution at position 333 of RV-G protein was performed as described previously for RV vector pSPBN-333, resulting in pBNSP-333 (51). To construct the recombinant RV vector pBNSP expressing SHIV89.6P Env containing gp120 and gp41 ecto- and TM domains of SHIV89.6P Env fused to RV G CD, the SHIV89.6P Env was amplified by PCR from pKB9SHIV(89.6P) (National Institutes of Health AIDS Research and Reference Reagent Program [ARRRP]) by using Vent polymerase (New England Biolabs Inc.) and the primers RP27 5′-GGG CTG CAG CTC GAG CGT ACG AAA ATG AGA GTG AAG GAG ATC AGG-3′ and RP32 5′-GCC CCG TTA ACT ATA GAA AGT ACA GCA AAA-3′. The PCR product was digested with BsiWI/HpaI and was cloned to pBS2H-NL4-3-G (35). The resulting plasmid was designated pBS289.6P-RVG. To introduce the chimeric HIV-1/RV G Env gene into the pBNSP vector, pBS289.6P-RVG was digested with BsiWI/XbaI and the 2.2-kb fragment was gel eluted and cloned into the previously BsiWI/NheI-digested pBNSP. The plasmid was entitled pBNSP-89.6P-RVG.
For construction of RV vector expressing covalently associated gp140 SOS, we introduced two cysteine residues into the 89.6P-RVG Env expressed from pBS289.6P-RVG by using site-directed mutagenesis (QuikChange Multi; Stratagene). The cysteine mutations are in positions analogous to those described by Binley et al. for HIV-1JRFL gp140 SOS (5). At position 499 of 89.6P gp120, an Ala→Cys mutation was introduced with 5′ phosphorylated primer RP185 (5′-GGAGTAGCACCCACCAGGTGCAAGAGAAGAACAGTGC-3′). At position 603 of 89.6P gp41 a Thr→Cys mutation was introduced with 5′ phosphorylated primer RP186 (5′-GCTCTGGAAAACTCATTTGCTGCACTTCTGTGCCTTGG-3′). The resulting plasmid was entitled pBS289.6P-RVG-SOS. pBS289.6P-RVG-SOS was digested with BsiWI/XbaI, and the 2.2-kb fragment was gel eluted and was cloned into BsiWI/NheI-digested pBNSP-333, creating pBNSP-333-89.6P-RVG-SOS. Of note, replacement of 89.6P CD (165 residues) with RV G CD (44 residues) yields a gp41 protein that is approximately 32 kDa. To be consistent with the published literature, however, the Env proteins will be referred to as gp140, gp120, and gp41 throughout. Infectious virus was recovered from plasmid DNA by using the T7-based RV recovery system described previously (33, 85).
Immunostaining for envelope expression.
BSR cells (a BHK-21 clone) were infected with BNSP-333, BNSP-89.6P-RVG, or BNSP-333-89.6P-RVG-SOS at a multiplicity of infection (MOI) of 0.1 for 48 h. Cells were fixed with 3% paraformaldehyde (PFA) for 30 min at room temperature and were then washed three times in 10 mM PBS-glycine. Cells were permeabilized with 1% Triton X-100 for 5 min at room temperature and were then washed twice with PBS-glycine. Cells were stained with either fluorescein isothiocyanate (FITC)-labeled anti RV-N (Centacor) or anti-envelope MAb 2G12 (ARRRP), which is directed against a conformational C3-V4 epitope (97). 2G12 staining was detected with a goat anti-human-FITC secondary antibody (Jackson ImmunoResearch).
Multicycle growth curve.
BSR cells were plated in 60-mm dishes and 16 h later were infected with an MOI of 0.01 with BNSP-333, BNSP-89.6P-RVG, or BNSP-333-89.6P-RVG-SOS. After incubation at 37°C for 1 h, inocula were removed and cells were washed three times with PBS to remove any unabsorbed virus. Three milliliters of complete medium was added back, and 100 μl of culture supernatants were removed at the indicated time points after infection. Virus aliquots were titered in duplicate on BSR cells.
FACS analysis: cell surface expression of 89.6P Env.
In 60-mm dishes, BSR cells were infected with RV constructs at an MOI of 1 for 48 h. Culture supernatants were removed and monolayers were washed once with PBS. Cells were released from the dish by treatment with 50 mM EDTA in phosphate-buffered saline (PBS), 3 ml/dish. Intact cells were washed three times with fluorescence-activated cell sorter (FACS) buffer (2% fetal bovine serum in PBS) and were then incubated with 2G12 (ARRRP) 1:500 in FACS buffer for 1 h at room temperature. Cells were washed twice in FACS buffer and were then incubated with goat anti-human-FITC antibody (Jackson ImmunoResearch) for 30 min at room temperature. Samples were washed twice and were then fixed with 3% PFA prior to FACS analysis.
FACS analysis: soluble CD4 binding and 17b reactivity.
In 60-mm dishes, BSR cells were infected with RV constructs at an MOI of 2 for 48 h. Cell removal by EDTA treatment was done as described above. Samples were incubated for 1 h at 37°C in the presence or absence of soluble CD4 (sCD4) (ARRRP), 20 μg/sample, and were then washed twice in FACS buffer. Antibody 17b (ARRRP) was added to each at 1:200 for 1 h at room temperature. Cells were washed twice in FACS buffer and were then incubated with goat anti-human-FITC antibody (Jackson ImmunoResearch) for 30 min at room temperature. Samples were washed twice and were then fixed with 3% PFA prior to FACS analysis. The increase (n-fold) in 17b binding was calculated by comparing the mean intensity of FITC signal in the presence or absence of sCD4.
Cell lysates and immunoprecipitation (IP).
In T25 flasks, 107 BSR cells were infected with BNSP-333, BNSP-89.6P-RVG, or BNSP-333-89.6P-RVG-SOS at an MOI of 2 for 2 h. Cells were washed twice in Met-Cys-free medium (Cellgro) and were incubated for 48 h with 200 μCi of [35S]Met-Cys (Redivue Promix; Amersham) per flask. After 48 h, supernatant was removed and monolayer was washed briefly with PBS. Radiolabeled cell lysates were made in detergent solution (1% NP-40, 0.4% deoxycholate, no sodium dodecyl sulfate [SDS]) with protease inhibitors (Sigma), then cellular debris was cleared via centrifugation for 5 min at 14,000 rpm.
IP reactions were performed at 4°C, overnight, on a rotating wheel. One hundred microliters of lysate was used per reaction. Neutralizing antibodies 2G12 (glycan-dependent C3-V4 epitope), 447 (V3 loop), IgG1b12 (CD4 binding domain), and 2F5 (gp41) were obtained from ARRRP. Nonneutralizing antibody C11 was a kind gift from J. Robinson. Antibodies were used at 1:100/reaction. After overnight incubation, 20 μl of r Protein G agarose (Invitrogen) was added to each and samples were returned to 4°C for 2 h. Samples were washed three times in radio-IP assay buffer and were resuspended in 1× Laemmli buffer plus 2-mercaptoethanol (2-ME). Similar 35S counts were loaded for each. Samples were boiled 4 min prior to loading on a 10% Tris-glycine gel (Bio-Rad). Following SDS-polyacrylamide gel electrophoresis (PAGE), gels were dried onto chromatography paper and were exposed on a phosphor screen for 48 h. Screen was read by using QuantityOne software (Bio-Rad).
Virus purification: Western blot.
In T150 flasks, 107 BSR cells were infected with RV constructs at an MOI of 1 for 4 days. Culture supernatants were cleared of cellular debris by spinning for 5 min at 1,400 rpm (Eppendorf) and were then layered over 20% buffered sucrose. Samples were loaded into an SW28 swinging bucket rotor (Beckman) and were spun at 25,000 rpm for 1 h at 4°C. Liquid was decanted, and virus pellets were resuspended in 1× Laemmli buffer without 2-ME. Prior to boiling, samples tested under reduced conditions had dithiothreitol (DTT) added to a final concentration of 100 mM. SDS-PAGE was performed on a 10% Tris-glycine gel (Bio-Rad); proteins were transferred to a polyvinyl difluoride membrane (Osmonics) and were then blocked for 1 h at room temperature with 5% milk powder in PBS. Blots were washed three times with 0.1% PBS-Tween 20 and were then probed with sheep anti-gp120 polyclonal antibody (ARRRP) at 1:1,000 or MAb 2F5 (ARRRR) at 1:5,000 overnight at 4°C. Blots were washed three times with 0.1% PBS-Tween 20, and envelope-specific signal was detected with donkey anti-sheep-horseradish peroxidase (HRP) (Jackson ImmunoResearch) at 1:5,000 or goat anti-human-HRP (Jackson ImmunoResearch) at 1:10,000. Blots were washed two additional times in 0.1% PBS-Tween 20 and were then placed in PBS and developed by chemiluminescence as per the manufacturer's instructions (NEN). Blots were read on X-Omat AR film (Kodak).
Virus purification: envelope incorporation.
In T75 flasks, 107 BSR cells were infected with RV constructs at an MOI of 5 for 2 h, washed three times with PBS, and incubated overnight in complete medium (Dulbecco's modified Eagle's medium-10% fetal bovine serum). At 16 h postinfection, cells were washed twice in Met-Cys-free medium and were labeled for 24 h with 500 μCi of [35S]Met-Cys per flask. Culture supernatants were cleared of cellular debris by centrifugation at 1,400 rpm (Eppendorf) for 5 min. Supernatants were then layered over 20% buffered sucrose, loaded into an SW28 rotor (Beckman), and spun for 1 h at 25,000 rpm at 4°C. Liquid was decanted and pelleted virus was resuspended in 1× Laemmli buffer plus 2-ME. Similar 35S counts were loaded for each. SDS-PAGE and phosphor screen exposure were done as described above. Quantitation of proteins incorporated into RV virion was done with QuantityOne software (Bio-Rad). Percent incorporation of 89.6P-G envelopes was determined by comparison of the gp120 band with RV-N protein band for each isolate.
Virus purification: killed-particle enzyme-linked immunosorbent assay.
Liter quantities of serum-free BNSP-333-89.6P-RVG-SOS virus were grown on BSR cells in a Celligen Plus Bioreactor (New Brunswick Scientific). Harvested virus supernatant was concentrated approximately 10-fold (Millitan system; Millipore), and purified virus was isolated from a continuous sucrose gradient (70 to 20%). Virus was inactivated by overnight treatment with β-propiolactone (Sigma) at 4°C, followed by 0.5 h of incubation at 37°C. Purified-killed virus was coated onto a 96-well plate (Nunc; Maxisorp) at 100 ng/well in coating buffer (50 mM Na2CO3, pH 9.6). After overnight incubation at 4°C, the plate was washed four times with wash buffer (0.1% Tween 20 in PBS) and was blocked for 1 h at room temperature with 5% milk powder in PBS. Anti-Env MAbs 2G12, b12, and 2F5 were diluted in PBS and were added to the plate in a fivefold dilution scheme ranging from 5 μg/ml to 1.6 ng/ml. A mouse anti-hepatitis C E2 MAb (catalog no. 4101; Immunodiagnostics) was similarly diluted and served as a negative control. The plate contents were incubated at 37°C for 1 h and was then washed six times. Peroxidase-conjugated secondary antibody, either goat anti-human-HRP or goat anti-mouse-HRP (Jackson ImmunoResearch) were added at 1:1,000 and the plate contents were incubated for 30 min at room temperature. Following four wash cycles, OPD substrate (Sigma) was added and the plate contents were incubated for 15 min at room temperature protected from light. The reaction was stopped with the addition of 2 M H2SO4, and the plate was read at 490 nm.
RESULTS
Construction of recombinant RVs expressing SHIV 89.6P envelope proteins.
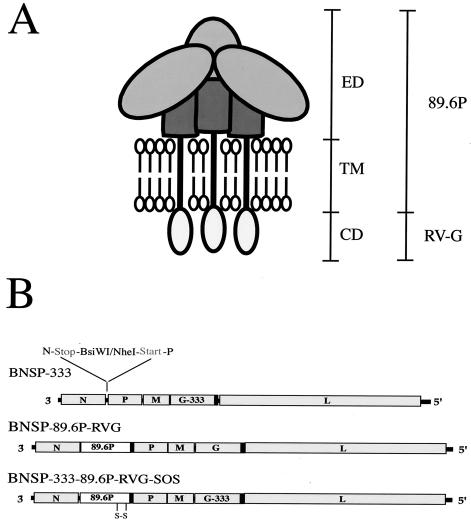
BNSP is a previously described RV-based vector containing an additional transcription unit between N and P genes for expression of foreign proteins (50). Because a potential use for these vectors is as live-virus vaccines, we introduced an amino acid change in the RV G protein at position 333 from arginine (R) to glutamic acid (E) by site-directed mutagenesis and PCR to create BNSP-333 (Fig. 1B). The position 333 mutation has been shown to result in slower uptake of the virus by cells (21), to interfere with certain RV strains' ability to infect motor neurons, and to diminish efficient spread of the virus in neuroblastoma cells (18). BNSP-333 was the target for the introduction of the 89.6P-RVG-SOS envelope protein (89.6P-G-SOS). BNSP without the 333 mutation was the target for the 89.6P RVG envelope (89.6P-G). The presence of the 333 mutation has previously been shown to have no effect on the growth characteristics or immunogenicity of RV vectors (51). BNSP 89.6P-G expresses a fully functional HIV-1 envelope and therefore will serve as a good comparator for the antigenic properties of our 89.6P-G-SOS protein. Previous results obtained by our laboratory and others indicated the requirement of the RV G cytoplasmic domain for efficient incorporation of HIV-1 gp140 into rhabdovirus virions (35, 43, 56, 57). Therefore, both Env-expressing viruses described here had their cytoplasmic domains replaced with that of RV G (Fig. 1B, black boxes).
FIG. 1.
Construction of recombinant RV-expressing SHIV 89.6P envelope proteins. (A) Schematic representation of membrane-associated trimeric gp160. The gp120 and gp41 ecto- and TM domains (ED and TM) are fused in frame to the CD of RV G protein (RVG). The envelope protein described herein is derived from the pathogenic SHIV-89.6P strain. The schematic was adapted from Binley and Moore (5). (B) BNSP-333 is an RV vaccine strain-based vector containing a minimal RV transcription unit between the N and P genes and an arginine-to-glutamic acid substitution at position 333 of RV G protein. A BNSP vector without the 333 mutation was the target to introduce the cDNA sequence encoding SHIV-89.6P-RVG envelope by using the BsiWI and NheI sites, resulting in BNSP-89.6P-RVG. BNSP-333 was the target for introduction of 89.6P-RVG SOS envelope to create BNSP-333-89.6P-RVG-SOS. In the SOS construct, site-directed mutagenesis was used to introduce cysteine residues at positions 499 (Ala→Cys) and 603 (Thr→Cys) of 89.6P gp160, which allows an intermolecular disulfide bond to form between gp120 and the gp41 ectodomain. All viruses were recovered by standard methods. The presence of the 333 mutation has been shown to have no effect on virus growth or immunogenicity (51).
The cDNAs encoding the plus strand RNA sequence of the respective recombinant RVs were cotransfected into BSR cells stably expressing T7 RNA polymerase (8) along with support plasmids expressing RV N, P, L, and G proteins, all under control of the T7 promoter. Through standard methods (50, 52), infectious virus was recovered for all three constructs. The sequences of the modified G genes and Env inserts were verified by sequencing of reverse transcriptase PCR products of virus RNA (data not shown).
Replication and 89.6P envelope expression of recombinant RVs.
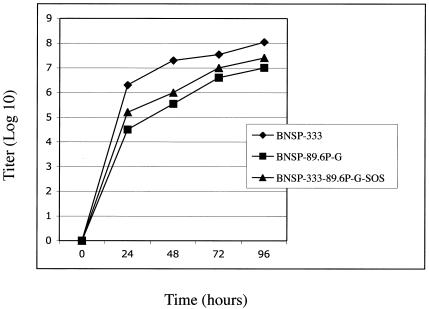
To ensure that both Env-containing viruses grew with similar kinetics and to similar titers, a mutlicyle growth curve was generated by infecting BSR cells at an MOI of 0.01 and sampling the cultures at 24, 48, 72, and 96 h postinfection. As illustrated in Fig. 2, both Env viruses grew in a similar fashion and to similar titers. This result is to be expected because the 89.6P-G Env proteins described here are identical except for the two introduced cysteine residues in the 89.6P-G-SOS protein. The titers for both, however, were approximately 10-fold lower than that of the RV vector control BNSP-333. These data are consistent with previous observations that expression of HIV-1 glycoproteins from recombinant rhabdovirus genomes results in a 3- to 10-fold reduction in titer compared to that for vector not expressing Env (39, 43, 85).
FIG. 2.
Multicycle replication growth curve of RV vectors. BSR cells were infected with BNSP-333, BNSP-89.6P-RVG, or BNSP-333-89.6P-RVG-SOS at an MOI of 0.01. Aliquots of culture supernatants were collected at indicated time points postinfection, and viral titers were determined in duplicate on BSR cells.
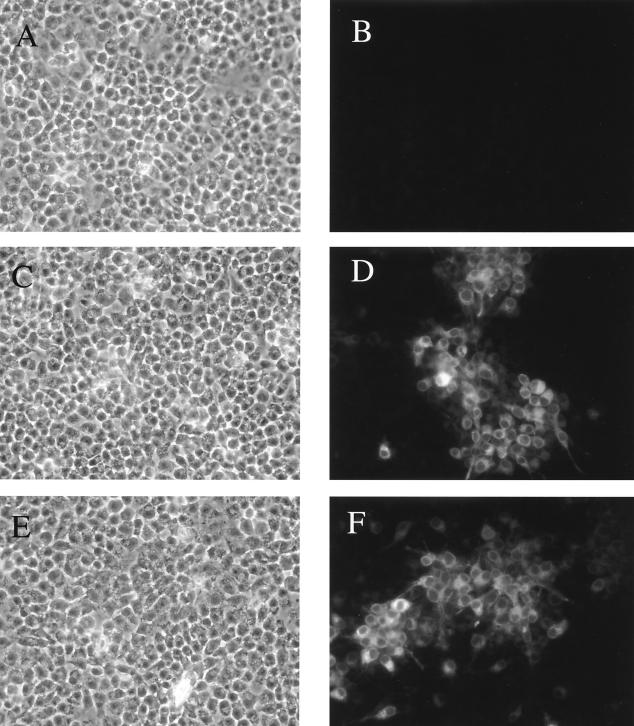
To ensure the introduced cysteine residues and the potential disulfide bond that forms between them had no deleterious effects on envelope expression, BSR cells (a BHK-21 clone) were infected with RVs for 48 h and were stained with anti-Env MAb 2G12 directed against a conformational C3-V4 epitope (97). As shown in Fig. 3, no specific binding to BNSP-333-infected cells was detected (Fig. 3B), while 2G12 recognizes 89.6P-G (Fig. 3D) and 89.6P-G-SOS (Fig. 3F) envelope proteins in a similar manner. These results clearly indicate that the envelope proteins are expressed with similar efficiency and that both are in a form recognized by an antibody that binds an epitope on the native Env trimer.
FIG. 3.
HIV-1 envelope immunostaining with antibody 2G12 confirms 89.6P Env expression from RV vectors. BSR cells were infected with RV constructs at an MOI of 0.1 for 48 h. Cells were fixed with 3% PFA and were permeabilized with 1% Triton X-100. Human MAb 2G12, directed against a conformational C3-V4 epitope, was used to detect 89.6P envelope expression. Panels A, C, and E are light-field images of the 2G12-stained cells shown in panels B, D, and F, respectively.
Previously, we demonstrated that HIV-1 envelope glycoproteins containing the CD of RV G are fully functional and capable of fusion on and infection of cell lines expressing CD4 and coreceptor molecules (35). However, if the intermolecular disulfide bond in the SOS protein forms with good efficiency, we would expect the gp140 SOS virus to be nonfusogenic, as its envelope is covalently associated and is therefore incapable of releasing gp120 and exposing the fusion peptides after receptor binding (17, 23, 44, 103). To determine fusogenic capabilities of the 89.6P-G-SOS Env, SupT1 cells (a human T cell line) were infected and cultures were examined 72 h later for syncytia induced by envelope fusion. As illustrated in Fig. 4, BNSP-333 does not induce fusion on SupT1 cells (BNSP-333-infected cells [Fig. 4B]), but the HIV 89.6P-G envelope expressed by BNSP 89.6P-RVG is completely functional and causes extensive fusion and syncytia (Fig. 4C). The 89.6P-G-SOS protein, however, does not yield visible syncytia (Fig. 4D). If the 89.6P-G-SOS culture is incubated a further 48 h some smaller syncytia were observed (not shown). This fusion could be caused by an accumulation of envelope protein on the surface of virus-infected cells, or likelier, due to the imperfect formation of the disulfide bond in the 89.6P-G-SOS protein. Previously, the bond formation in a soluble gp140 SOS protein based on HIV-1NL4-3 envelope was shown to be ∼50% when expressed from 293T cells (5) and ∼91% when expressed from CHO cells (86). Equivalent infection of all cells with RV was confirmed by staining with an antibody specific for RV N protein (data not shown).
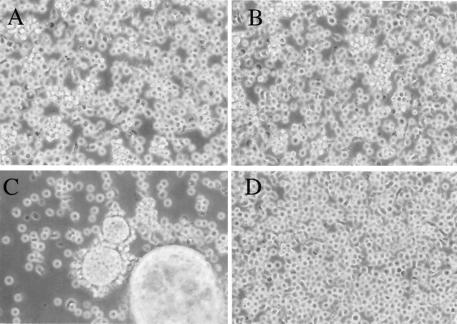
FIG. 4.
Expressed 89.6P-G-SOS glycoprotein is not fusogenic. SupT1 cells (a human T-cell line) were infected with BNSP-333 (B), BNSP-89.6P-RVG (C), or BNSP-333-89.6P-RVG-SOS (D) at an MOI of 0.1, and cultures were examined 72 h later for syncytia induced by envelope fusion. Uninfected SupT1 cells are shown in panel A.
Cell surface expression of properly folded 89.6P RVG SOS envelope.
A possible explanation for the lack of syncytia observed with the SOS virus is that, although expressed from the RV genome, the SOS protein is retained intracellularly and therefore does not go to the cell surface to be available for virion incorporation. Since it is our goal to create a RV that mimics virion-associated HIV-1 gp160, it is essential to determine if our gp140 SOS protein is (i) expressed at the cell surface of infected cells, thus available for virion incorporation, and (ii) properly folded and fully processed. The primary gp120/gp41cleavage site is maintained in the gp140 SOS protein; therefore, the protein should be available for complete processing into mature envelope. Generally, unprocessed Env is sorted into the lysosomal pathway and very little is incorporated into HIV-1 virions (24, 25, 49).
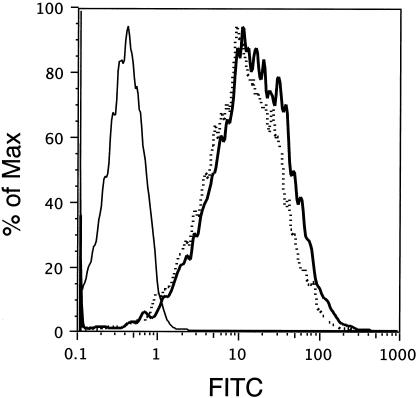
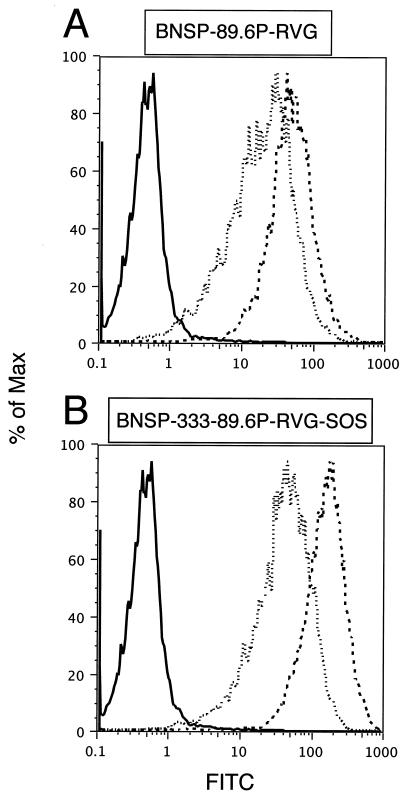
To assess folding and surface expression of the SOS protein, BSR cells were infected with RV constructs and 48 h later were released from the culture dish by treatment with 50 mM EDTA in PBS. Cells were stained with 2G12 and were subjected to FACS analysis. As Fig. 5 indicates, surface expression of the wild type (89.6P-G) and that of covalently linked 89.6P-G-SOS Env are identical. No reactivity was observed on BNSP-333-infected control cells. Therefore, both Env proteins traffic to the cell surface in a similar manner and 2G12 recognizes 89.6P-G SOS Env to the same extent as the functional Env 89.6P-G.
FIG. 5.
Cell surface expression of 89.6P-RVG-SOS envelope. BSR cells were infected with RV constructs at an MOI of 1 for 48 h. Cells were released from a culture dish by treatment with 50 mM EDTA in PBS and were stained with MAb 2G12 and FITC-labeled secondary antibody. Cells were fixed with 3% PFA prior to FACS analysis. Depicted are BNSP-333-infected cells (solid line), BNSP-89.6P-RVG (dotted line), and BNSP-333-89.6P-RVG-SOS (bold line).
gp140 SOS retains CD4 binding and reveals CD4i epitopes.
An additional desirable property of an envelope-based immunogen is that it retains receptor binding capabilities. The sequential binding of CD4 and chemokine coreceptors introduces structural changes into gp120 and gp41 that reveal conserved regions associated with membrane fusion (45, 46, 59, 78, 91, 102). Neutralizing antibodies directed against the CD4 binding domain (13, 41, 75) or CD4i epitopes (46, 78, 95) are among the most potent yet described. For these reasons, we analyzed if the modified Env retained CD4 binding by using soluble CD4 (sCD4) and neutralizing antibody 17b (46, 95). BSR cells were infected for 48 h and were then harvested by treatment with 50 mM EDTA in PBS. Cells were first incubated in the presence or absence of sCD4, followed by MAb 17b. As shown in Fig. 6, 17b binds both envelope proteins in the absence of sCD4, but in each case sCD4 significantly increases the antibody affinity for Env proteins. For 89.6P-G, there is a 2.5-fold increase in binding in the presence of sCD4 (top panel), and for 89.6P-G-SOS there is a fourfold increase (bottom panel). These results are consistent with previous observations that 17b displays a certain level of gp120 binding in the absence of sCD4 but a significantly higher affinity when gp120 is complexed with CD4 (91, 95). In addition, soluble gp140 SOS proteins showed strong induction of the 17b epitope in the presence of sCD4 (5, 80, 81, 86). The above FACS analysis reveals that not only is the gp140 SOS protein properly expressed and processed to the cell surface but also that it retains CD4 binding and is capable of the conformational changes required to reveal CD4i epitopes.
FIG. 6.
89.6P-RVG-SOS envelope maintains CD4 binding and can reveal a CD4i epitope. BSR cells were infected with RV constructs at an MOI of 2 for 48 h. Cell removal was conducted by EDTA-PBS treatment; cells were incubated for 1 h at 37°C in the presence or absence of 20 μg of soluble CD4 (sCD4)/sample. Antibody 17b, which recognizes a CD4i epitope on gp160, was added to each at 1:200 for 1 h at room temperature. Cells were incubated with FITC-labeled secondary antibody and were fixed with 3% PFA prior to FACS analysis. The top panel shows that for 89.6P-G Env there is a 2.5-fold increase in 17b binding in the presence of sCD4. The bottom panel shows a fourfold increase in 17b binding in the presence of sCD4 for 89.6P-G-SOS Env. The figure shows representative data from two independent experiments.
IP of radiolabeled gp140 proteins with a panel of anti-envelope antibodies.
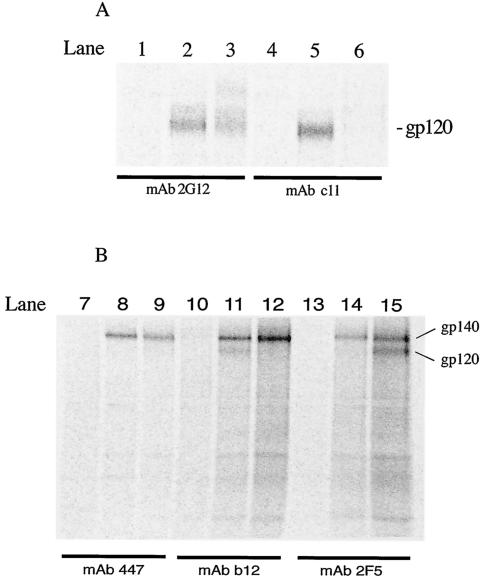
After confirming that the gp140 SOS protein retained CD4 binding and 17b reactivity at the cell surface, we next characterized the protein interaction with a panel of neutralizing antibodies to determine if important antibody epitopes were represented throughout the envelope protein. For this analysis we performed IP reactions on infected-cell lysates under native conditions. 35S-radiolabeled lysates were incubated with anti-Env antibodies, and antibody-envelope complexes were immunoprecipitated and were analyzed on a phosphorimager. Similar 35S counts were loaded for each, and proteins were separated by SDS-PAGE. As shown in Fig. 7, all of the neutralizing antibodies tested, 2G12 (directed against a glycan-dependent C3-V4 epitope), 447 (specific for V3 loop), IgG1b12 (specific for CD4 binding domain), and 2F5 (which recognizes a linear gp41 epitope), readily recognize the envelope glycoproteins from both 89.6P-G- and 89.6P-G-SOS expressing viruses. Interestingly, nonneutralizing antibody C11, whose epitope is occluded in the context of mature gp120-gp41 complexes (63, 101), recognizes the 89.6P-G gp140 but not the 89.6P-G SOS protein (compare Fig. 7A, lanes 5 and 6). Since cell lysates and native conditions were used for the IP reactions, all forms of the 89.6P-G proteins, in all stages of processing, are available for antibody binding. It could be hypothesized that the presence of the introduced cysteine residues in gp140 SOS promotes more immediate and stable assembly of the mature protein during processing and therefore makes the C11 epitope inaccessible. The introduced cysteine residue at position 499 of gp140 SOS is near the C11 epitope but does not destroy it, as seen by C11 reactivity with gp120 molecules derived from other soluble gp140 SOS proteins (5). The IP results further suggest that the 89.6P-G-SOS protein is processed in a manner that yields a mature protein that faithfully represents many important neutralizing epitopes present on intact Env trimers. Moreover, an epitope occluded in mature Env complexes is not presented in the SOS protein.
FIG. 7.
Expressed 89.6P envelope proteins are recognized by a panel of anti-envelope antibodies in IP reactions. The 35S-labeled envelope glycoproteins from infected-cell lysates were immunoprecipitated with anti-envelope MAbs, boiled with SDS and 2-ME, and analyzed by SDS-PAGE. BNSP-333 lysates were loaded into lanes 1, 4, 7, 10, and 13; BNSP-89.6P-RVG samples are in lanes 2, 5, 8, 11, and 14; and BNSP-333-89.6P-RVG-SOS samples are in lanes 3, 6, 9, 12, and 15. The MAbs tested in all lanes are given under the black bars. Description of specific bands is given at right. Similar 35S counts were loaded for each sample.
Analysis of gp140 SOS in purified virions.
One of our goals is to create an RV particle to serve as an HIV-1 Env carrier for immunizations. If such an 89.6P-G-SOS containing virus is to be used as a killed-particle immunogen, the most critical question that must be addressed is, what is the cleavage state of the 89.6P-G SOS in RV virions? It is possible that some folded, uncleaved gp140 is transported to the cell surface and therefore is available for virion incorporation. The analysis thus far has been on infected cells and whole-cell lysates. To analyze the processing of virion-incorporated gp140, we sucrose purified recombinant RV virions and analyzed their reactivity with anti-Env antibodies. All samples were resuspended in 1× Laemmli buffer with 1% SDS (denatured). For samples run under reduced conditions, 100 mM DTT was added prior to boiling and SDS-PAGE.
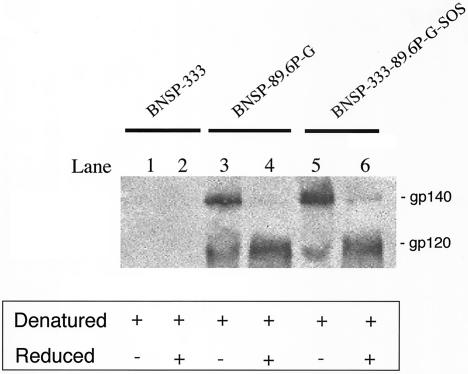
As shown in Fig. 8, under denatured conditions both HIV Env-expressing viruses contain 120- and 140-kDa bands (Fig. 8, lanes 3 and 5). The gp140 band is expected in the SOS virus because of the introduced disulfide bond. The presence of SDS-resistant gp140 in 89.6P-G virus is not unexpected because any intra- or intermolecular disulfide bonds between Env subunits would be maintained under these conditions. When the samples were electrophoresed under denatured and reduced conditions, the aggregates separated and the predominant envelope species was gp120 (Fig. 8, lanes 4 and 6). This indicates that the major Env species in both viruses is fully cleaved. This is expected for 89.6P-G because the envelope in this virus is functional and fusogenic (Fig. 4). If the peptide bond between gp120 and gp41 was maintained in the SOS protein, as it is in uncleaved gp140 proteins, the 140-kDa band would be unaffected by the addition of DTT. This is a potentially important finding, since it is our goal to have the gp140 SOS protein fully processed yet covalently associated at the virion surface. Similar results were obtained with cell lysates, and purified virion samples were probed with anti-gp41 MAb 2F5 (not shown).
FIG. 8.
Western blot analysis of Env proteins in purified RV virions. BSR cells were infected with RV constructs at an MOI of 1 for 4 days. Clarified culture supernatants were layered over 20% buffered sucrose, and virions were pelleted via ultracentrifugation. Isolated virions were resuspended in 1× Laemmli buffer with SDS (denatured). Samples run under reduced conditions had 100 mM DTT added prior to boiling and SDS-PAGE. Membrane was probed with anti-gp120 polyclonal antibody and was developed by chemiluminescence. The relative size of each band is given at right. All samples are denatured, and samples in lanes 2, 4, and 6 are also reduced. Under reduced conditions the gp140 species dissociate and the predominant Env species in both viruses is cleaved gp120 (compare lanes 4 and 6).
Incorporation and retention of envelope glycoproteins in RV virions.
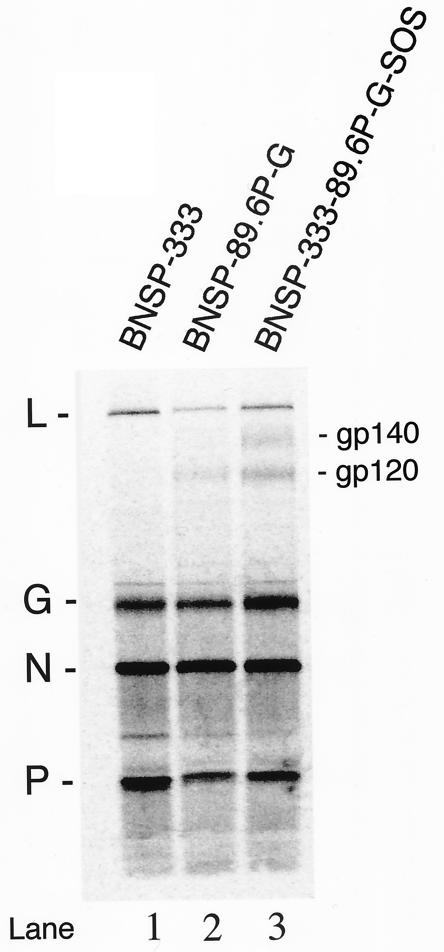
To determine the Env composition of our RV constructs, purified virions from the supernatant of 35S-radiolabeled infected cells were analyzed by SDS-PAGE and the proteins were quantitated by phosphorimaging. Similar 35S counts were loaded for each sample. As shown in Fig. 9, similar amounts of RV N protein are present in all three virion preparations (12,747 ± 809 counts); however, differing amounts of the G protein are present, with BNSP-333 89.6P-G-SOS seeming to have a greater amount than do the other two viruses (Fig. 9; compare lane 3, G band, with lanes 1 and 2). This apparent difference in G protein content between recombinants was reproducible in an independent experiment (not shown) and has been observed for VSV (38). It was previously demonstrated that foreign glycoproteins are incorporated into rhabdovirus virions in addition to the G protein and not as a replacement for any G proteins (84). Due to the variability of G, quantitation of 89.6P-G Env incorporation was calculated by comparison to RV N protein. The N protein is 3′ (upstream) of the cloning site for 89.6P-G Env; therefore, its expression is unaffected by the presence of the additional coding sequence. Therefore, N protein expression levels are the same between the control (BNSP-333) and the Env-expressing viruses. A clear band representing gp120 is seen in both 89.6P-G envelope viruses. Quantitation of gp120 bands indicates that ∼31% more 89.6P-G-SOS is retained in the virion than 89.6P-G. This is encouraging, since it is our hope that the covalent linkage of the gp140 would prevent undue shedding of gp120 during purification.
FIG. 9.
Incorporation and retention of Env glycoproteins in RV virions. The 35S-labeled virions from infected BSR cell cultures were purified over 20% buffered sucrose and were resuspended in 1× Laemmli buffer with SDS and 2-ME and were analyzed by SDS-PAGE. The positions of RV L, G, N, and P proteins are provided at the left. The positions of the gp120 and gp140 bands in the Env-expressing viruses are shown at right. A comparison was performed between RV-N protein and gp120 bands for each isolate.
The higher-molecular-weight species observed above gp120 in the 89.6P-G-SOS virus may be incompletely processed gp140. It is possible that the amount of envelope protein produced by RV at this MOI (3) saturates cellular protease pathways responsible for cleavage. A similar observation of uncleaved gp160 in virions has been reported for VSV recombinants expressing Env proteins (39). Further, overexpression of soluble gp140 proteins in transiently transfected 293T cells leads to a significant release of uncleaved molecules (5). In some instances, the problem of incomplete cleavage of envelope glycoproteins in culture can be overcome by the addition of exogenous furin (5, 80, 87, 100). In HIV-1 infection, gp160 precursor molecules are cleaved by cellular proteases of the furin family. We are currently analyzing the possible need and effectiveness of exogenous furin in our system. Of note, Western blot analysis of virions prepared under similar conditions, but with an input MOI of 1, showed only minimal reactivity with any higher-molecular-weight species of Env (Fig. 8). Taken together these results indicate that the majority of the 89.6P-G SOS protein retained within the RV virion is properly processed.
Purified 89.6P-SOS virus retains HIV-1-neutralizing antibody binding.
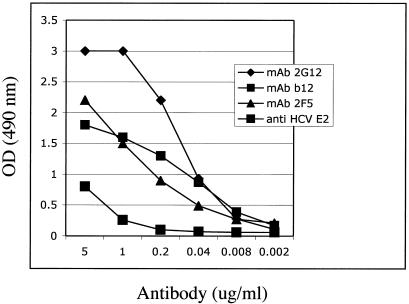
After demonstrating that gp140 SOS is retained in purified virions, we sought to determine a quantitative measure of Env epitope exposure and neutralizing antibody binding capacities. To address this, we adopted an enzyme-linked immunosorbent assay format where purified, inactivated BNSP-89.6P-RVG-SOS was coated onto a 96-well microtiter plate at 100 ng/well. The plate contents were incubated with serial dilutions of MAbs 2G12, b12 and 2F5, and specific antibody binding was determined. As shown in Fig. 10, all three anti-Env antibodies recognize the gp140 SOS displayed on the particle in a concentration-dependent manner. As may be expected, the conformational C3-V4 epitope of 2G12 appears to be presented to a greater extent than the CD4 binding site epitope of b12 or the gp41 epitope of 2F5. All three antibodies maintain specific binding to 89.6P-G-SOS, down to a concentration of approximately 40 ng/ml. These findings are significant because they demonstrate that milligram quantities of highly purified gp140 SOS virus can be prepared in a manner that maintains critical HIV-1-neutralizing epitopes.
FIG. 10.
Purified and inactivated 89.6P-SOS virus retains HIV-1 antibody binding. Sucrose gradient-purified BNSP-89.6P-RVG-SOS was inactivated by treatment with β-propiolactone and was coated onto a microtiter plate at 100 ng/well. Serial fivefold dilutions of MAbs were added to the plate at concentrations from 5 μg/ml to 1.6 ng/ml. The anti-HCV E2 antibody served as a negative control. OD, optical density.
DISCUSSION
This report describes a new application for a gp140 SOS protein where the molecule is expressed as a membrane-bound Env from a recombinant RV. Our results indicate that the 89.6P-G-SOS glycoprotein maintains many of the neutralizing epitopes present in HIV-1 gp160 and is very similar antigenically to the functional envelope expressed from BNSP-89.6P-RVG. The aim of this study was to create an envelope-based immunogen that mimics mature gp160, and the similarities between the 89.6P-G and 89.6P-G-SOS proteins are very encouraging and suggest that this approach may be an improvement on molecules not based on processed, multimeric Env. The antibody reactivity pattern of 89.6P-G-SOS is consistent with previous studies that described the relationship between antibody recognition of the native trimer and virus neutralization (36, 63, 64, 83, 92). The disulfide bridge in the SOS protein stabilizes Env so that a greater fraction is retained on the RV virion throughout purification. Although it is covalently associated, the SOS protein is capable of the conformational changes associated with receptor binding, as is seen by the induction of the 17b epitope in the presence of sCD4 (Fig. 6).
A recombinant viral particle displaying a significant portion of uncleaved gp140 would not be an optimal surrogate of an HIV-1 virion (26, 27). Given the results of the Western blot and viral protein analysis, we are cautiously optimistic that the majority of the 89.6P-G-SOS Env incorporated into and retained on our RV virion is completely processed and properly folded. The presence of a small fraction of uncleaved protein in our virions suggests that some improvements in our system may yet be needed to achieve complete Env processing. We are currently evaluating different cell lines in which to grow these recombinant viruses, studying the effect of input MOI on incorporation and processing efficiency, as well as addressing the need for exogenous furin to achieve complete cleavage.
Several reports have provided detailed analysis of the processing and antigenic features of soluble gp140 SOS proteins (5, 7, 80, 81, 86). Binley et al. showed that the addition of furin resulted in complete processing of the protein and that the secreted gp140 SOS retained antigenic characteristics of virion-associated gp160 (5). It was later demonstrated that gp140 SOS proteins with variable loop deletions also displayed neutralizing epitopes; however, it was found that upon purification the gp140 SOS proteins became monomeric. This was attributed to the lack of TM and CD of gp41 in the soluble molecules (80, 86). Recently, successful stabilization of soluble SOS trimers has been achieved by the introduction of an isoleucine-to-proline mutation at position 559 of gp41. This SOSIP protein has been purified in trimeric form, and initial immunogenicity studies are under way in small animal models (81; N. Schulke, M. Vesanen, R. W. Sanders, S. Beddows, J. P. Gardner, V. A. R., M. Lu, P. J. Maddon, W. Olson, and J. P. Moore, Keystone Symp. HIV Vacc. Dev. Immunol. Biol. Challenges, abstr. 448, 2003). In agreement with a recent report where a gp140 SOS protein was incorporated into pseudotyped virions (4), our virion-associated 89.6P-G-SOS maintains several desirable attributes of an Env-based immunogen and may have advantages over uncleaved or monomeric proteins. By using a replication-competent virus to express the Env protein, we have the ability to easily produce and purify milligram quantities of our potential vaccine antigen. Immunogenicity studies will determine if gp140 SOS glycoproteins proved to be more successful than previous envelope-based immunogens in eliciting broadly reactive virus-neutralizing antibodies.
As suggested by other reports (107), we believe the context in which an antigen is presented to the immune system is paramount for the response that it will generate. Generally, only a few antigenic determinants are present on infectious agents that are both important for infection and are accessible to neutralizing antibodies (37, 107). The context of antigen presentation is not only important for HIV-1 vaccination, where soluble gp120 vaccines have failed (Berman, Keystone Symp., •, 2003), but for numerous other viruses as well. For RV, although all neutralizing determinants are contained on the G protein, soluble, purified G protein does not confer protection (20). In contrast, the anti-G response to the G protein contained in the killed RV vaccine is the immune correlate of protection in humans. The same is true for vesicular stomatitis virus, where neutralizing antibodies are only directed against the tip of the G protein exposed on the virion surface (107). Respiratory syncytial virus offers another example, where vaccines based on purified F glycoprotein failed because antibodies that recognized the purified protein bound poorly to virion-associated F protein and therefore were nonneutralizing (65, 72). Our method allows the presentation of a membrane-bound HIV-1 envelope in a similar context to a natural HIV-1 infection. Further, dissociation of gp120 monomers from our RV particle would be minimal due to the covalent association of the Env subunits.
In conclusion, the flexibility of the RV-based vector system will permit evaluation of gp140 SOS constructs as both live-attenuated and killed-particle vaccines. An effective HIV-1 vaccine will likely need to stimulate both humoral and cellular effector molecules (1, 11, 22, 40). As live-attenuated vaccines, rhabdovirus-based vectors have proven safe and capable of generating robust cellular and humoral responses to HIV-1 and simian immunodeficiency virus proteins in a variety of priming-boosting regimens (51; reviewed in reference 55). Also, as a killed-particle boost following live vector priming, RV expressing the HCV E2 glycoprotein elicited high levels of E2-specific antibodies in mice (89). A recombinant RV particle with a covalently attached HIV-1 envelope on its surface may likely prove to be a useful tool in developing an effective HIV-1 vaccine strategy.
Acknowledgments
We gratefully thank John Moore and Simon Beddows for their insights and advice on characterizing gp140 SOS proteins. Thanks go to Paul Hallberg at KCC Flow Cytometry facility/TJU. We thank Milosz Faber for his assistance with phosphorimaging and protein quantitation, Marie-Louise Dietzschold for her help with the Bioreactor, and Patrick Starfish for his excellent technical assistance. Numerous reagents were obtained through ARRRP, Division of AIDS, NIAID, NIH as listed in Materials and Methods.
This study was supported by NIH grant AI49153 to M.J.S. P.M.M. was supported in part by training grant 5T32AI07523 from the NIH Training Program in AIDS Research.
REFERENCES
- 1.Barouch, D. H., and N. L. Letvin. 2002. Viral evolution and challenges in the development of HIV vaccines. Vaccine 20:A66-A68. [DOI] [PubMed] [Google Scholar]
- 2.Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, et al. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. [DOI] [PubMed] [Google Scholar]
- 3.Berman, P. W., W. M. Nunes, and O. K. Haffar. 1988. Expression of membrane-associated and secreted variants of gp160 of human immunodeficiency virus type 1 in vitro and in continuous cell lines. J. Virol. 62:3135-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J. M., C. S. Cayanan, C. Wiley, N. Schulke, W. C. Olson, and D. R. Burton. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J. Virol. 77:5678-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley, J. M., R. W. Sanders, A. Master, C. S. Cayanan, C. L. Wiley, L. Schiffner, B. Travis, S. Kuhmann, D. R. Burton, S. L. Hu, W. C. Olson, and J. P. Moore. 2002. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 76:2606-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binley, J. M., R. Wyatt, E. Desjardins, P. D. Kwong, W. Hendrickson, J. P. Moore, and J. Sodroski. 1998. Analysis of the interaction of antibodies with a conserved enzymatically deglycosylated core of the HIV type 1 envelope glycoprotein 120. AIDS Res. Hum. Retrovir. 14:191-198. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 10.Burton, D. R., and D. C. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11:S87-S98. [PubMed] [Google Scholar]
- 11.Burton, D. R., and J. P. Moore. 1998. Why do we not have an HIV vaccine and how can we make one? Nat. Med. 4:495-498. [DOI] [PubMed] [Google Scholar]
- 12.Burton, D. R., and P. W. Parren. 2000. Vaccines and the induction of functional antibodies: time to look beyond the molecules of natural infection? Nat. Med. 6:123-125. [DOI] [PubMed] [Google Scholar]
- 13.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 14.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavacini, L. A., M. Duval, J. Robinson, and M. R. Posner. 2002. Interactions of human antibodies, epitope exposure, antibody binding and neutralization of primary isolate HIV-1 virions. AIDS 16:2409-2417. [DOI] [PubMed] [Google Scholar]
- 16.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 17.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 18.Coulon, P., J. P. Ternaux, A. Flamand, and C. Tuffereau. 1998. An avirulent mutant of rabies virus is unable to infect motoneurons in vivo and in vitro. J. Virol. 72:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 20.Dietzschold, B., T. J. Wiktor, W. H. Wunner, and A. Varrichio. 1983. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology 124:330-337. [DOI] [PubMed] [Google Scholar]
- 21.Dietzschold, B., W. H. Wunner, T. J. Wiktor, A. D. Lopes, M. Lafon, C. L. Smith, and H. Koprowski. 1983. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc. Natl. Acad. Sci. USA 80:70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 23.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 24.Doms, R. W., R. A. Lamb, J. K. Rose, and A. Helenius. 1993. Folding and assembly of viral membrane proteins. Virology 193:545-562. [DOI] [PubMed] [Google Scholar]
- 25.Dubay, J. W., S. R. Dubay, H. J. Shin, and E. Hunter. 1995. Analysis of the cleavage site of the human immunodeficiency virus type 1 glycoprotein: requirement of precursor cleavage for glycoprotein incorporation. J. Virol. 69:4675-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earl, P. L., B. Moss, and R. W. Doms. 1991. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 65:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 31.Edinger, A. L., C. Blanpain, K. J. Kunstman, S. M. Wolinsky, M. Parmentier, and R. W. Doms. 1999. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J. Virol. 73:4062-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emini, E. A., W. A. Schleif, J. H. Nunberg, A. J. Conley, Y. Eda, S. Tokiyoshi, S. D. Putney, S. Matsushita, K. E. Cobb, C. M. Jett, et al. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728-730. [DOI] [PubMed] [Google Scholar]
- 33.Finke, S., and K. K. Conzelmann. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J. Virol. 73:3818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley, H. D., J. P. McGettigan, C. A. Siler, B. Dietzschold, and M. J. Schnell. 2000. A recombinant rabies virus expressing vesicular stomatitis virus glycoprotein fails to protect against rabies virus infection. Proc. Natl. Acad. Sci. USA 97:14680-14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foley, H. D., M. Otero, J. M. Orenstein, R. J. Pomerantz, and M. J. Schnell. 2002. Rhabdovirus-based vectors with human immunodeficiency virus type 1 (HIV-1) envelopes display HIV-1-like tropism and target human dendritic cells. J. Virol. 76:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frankel, M. E., and W. Gerhard. 1979. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol. Immunol. 16:101-106. [DOI] [PubMed] [Google Scholar]
- 38.Gelderblom, H. R., H. Reupke, and G. Pauli. 1985. Loss of envelope antigens of HTLV-III/LAV, a factor in AIDS pathogenesis? Lancet ii:1016-1017. [DOI] [PubMed] [Google Scholar]
- 39.Haglund, K., J. Forman, H. G. Krausslich, and J. K. Rose. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112-121. [DOI] [PubMed] [Google Scholar]
- 40.Haynes, B. F., G. Pantaleo, and A. S. Fauci. 1996. Toward an understanding of the correlates of protective immunity to HIV infection. Science 271:324-328. [DOI] [PubMed] [Google Scholar]
- 41.Ho, D. D., J. A. McKeating, X. L. Li, T. Moudgil, E. S. Daar, N. C. Sun, and J. E. Robinson. 1991. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J. Virol. 65:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu, S. L., J. Klaniecki, T. Dykers, P. Sridhar, and B. M. Travis. 1991. Neutralizing antibodies against HIV-1 BRU and SF2 isolates generated in mice immunized with recombinant vaccinia virus expressing HIV-1 (BRU) envelope glycoproteins and boosted with homologous gp160. AIDS Res. Hum. Retrovir. 7:615-620. [DOI] [PubMed] [Google Scholar]
- 43.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 45.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 46.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwong, P. D., R. Wyatt, Q. J. Sattentau, J. Sodroski, and W. A. Hendrickson. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 74:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 49.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53:55-67. [DOI] [PubMed] [Google Scholar]
- 50.McGettigan, J. P., H. D. Foley, I. M. Belyakov, J. A. Berzofsky, R. J. Pomerantz, and M. J. Schnell. 2001. Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelope protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelope proteins from different HIV-1 isolates. J. Virol. 75:4430-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGettigan, J. P., R. J. Pomerantz, C. Siler, P. M. McKenna, H. D. Foley, B. Dietzschold, and M. J. Schnell. 2003. Second generation rabies-based vaccine vectors expressing human immunodeficiency virus type 1 Gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 77:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGettigan, J. P., S. Sarma, J. M. Orenstein, R. J. Pomerantz, and M. J. Schnell. 2001. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 75:8724-8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKeating, J. A., and P. Balfe. 1999. The role of the viral glycoprotein in HIV-1 persistence. Immunol. Lett. 65:63-70. [DOI] [PubMed] [Google Scholar]
- 54.McKeating, J. A., A. McKnight, and J. P. Moore. 1991. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J. Virol. 65:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKenna, P. M., J. P. McGettigan, and M. J. Schnell. 2003. Recombinant rhabdoviruses as potential vaccines for HIV-1 and other diseases. Curr. HIV Res. I:229-237. [DOI] [PubMed] [Google Scholar]
- 56.Mebatsion, T., and K. K. Conzelmann. 1996. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc. Natl. Acad. Sci. USA 93:11366-11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mebatsion, T., S. Finke, F. Weiland, and K.-K. Conzelmann. 1997. A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell 90:841-847. [DOI] [PubMed] [Google Scholar]
- 58.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore, J. P., and J. Binley. 1998. HIV. Envelope's letters boxed into shape. Nature 393:630-631. [DOI] [PubMed] [Google Scholar]
- 60.Moore, J. P., and D. D. Ho. 1993. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J. Virol. 67:863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9:S117-S136. [PubMed] [Google Scholar]
- 62.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 63.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murphy, B. R., G. A. Prince, E. E. Walsh, H. W. Kim, R. H. Parrott, V. G. Hemming, W. J. Rodriguez, and R. M. Chanock. 1986. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J. Clin. Microbiol. 24:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olofsson, S., and J. E. Hansen. 1998. Host cell glycosylation of viral glycoproteins—a battlefield for host defence and viral resistance. Scand. J. Infect. Dis. 30:435-440. [DOI] [PubMed] [Google Scholar]
- 67.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody—debris or virion? Nat. Med. 3:366-367. (Erratum, 3: 591.) [DOI] [PubMed] [Google Scholar]
- 69.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parren, P. W., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 72.Piedra, P. A., S. Grace, A. Jewell, S. Spinelli, D. Bunting, D. A. Hogerman, F. Malinoski, and P. W. Hiatt. 1996. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr. Infect. Dis. J. 15:23-31. [DOI] [PubMed] [Google Scholar]
- 73.Poignard, P., E. O. Saphire, P. W. Parren, and D. R. Burton. 2001. gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19:253-274. [DOI] [PubMed] [Google Scholar]
- 74.Posner, M. R., L. A. Cavacini, C. L. Emes, J. Power, and R. Byrn. 1993. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J. Acquir. Immune Defic. Syndr. 6:7-14. [PubMed] [Google Scholar]
- 75.Posner, M. R., T. Hideshima, T. Cannon, M. Mukherjee, K. H. Mayer, and R. A. Byrn. 1991. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J. Immunol. 146:4325-4332. [PubMed] [Google Scholar]
- 76.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 77.Richardson, T. M., Jr., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 79.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanders, R. W., L. Schiffner, A. Master, F. Kajumo, Y. Guo, T. Dragic, J. P. Moore, and J. M. Binley. 2000. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 74:5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanders, R. W., M. Vesanen, N. Schuelke, A. Master, L. Schiffner, R. Kalyanaraman, M. Paluch, B. Berkhout, P. J. Maddon, W. C. Olson, M. Lu, and J. P. Moore. 2002. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 76:8875-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 84.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schnell, M. J., H. D. Foley, C. A. Siler, J. P. McGettigan, B. Dietzschold, and R. J. Pomerantz. 2000. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc. Natl. Acad. Sci. USA 97:3544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schulke, N., M. S. Vesanen, R. W. Sanders, P. Zhu, M. Lu, D. J. Anselma, A. R. Villa, P. W. Parren, J. M. Binley, K. H. Roux, P. J. Maddon, J. P. Moore, and W. C. Olson. 2002. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J. Virol. 76:7760-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 88.Shu, W., H. Ji, and M. Lu. 1999. Trimerization specificity in HIV-1 gp41: analysis with a GCN4 leucine zipper model. Biochemistry 38:5378-5385. [DOI] [PubMed] [Google Scholar]
- 89.Siler, C. A., J. P. McGettigan, B. Dietzschold, S. K. Herrine, J. Dubuisson, R. J. Pomerantz, and M. J. Schnell. 2002. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology 292:24-34. [DOI] [PubMed] [Google Scholar]
- 90.Steimer, K. S., C. J. Scandella, P. V. Skiles, and N. L. Haigwood. 1991. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science 254:105-108. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan, N., Y. Sun, J. Binley, J. Lee, C. F. Barbas, 3rd, P. W. Parren, D. R. Burton, and J. Sodroski. 1998. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J. Virol. 72:6332-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thali, M., C. Furman, D. D. Ho, J. Robinson, S. Tilley, A. Pinter, and J. Sodroski. 1992. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 66:5635-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thali, M., U. Olshevsky, C. Furman, D. Gabuzda, M. Posner, and J. Sodroski. 1991. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J. Virol. 65:6188-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.VanCott, T. C., J. R. Mascola, R. W. Kaminski, V. Kalyanaraman, P. L. Hallberg, P. R. Burnett, J. T. Ulrich, D. J. Rechtman, and D. L. Birx. 1997. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J. Virol. 71:4319-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 100.Volchkov, V. E., H. Feldmann, V. A. Volchkova, and H. D. Klenk. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 95:5762-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wyatt, R., E. Desjardin, U. Olshevsky, C. Nixon, J. Binley, V. Olshevsky, and J. Sodroski. 1997. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J. Virol. 71:9722-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 103.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 104.Yang, X., M. Farzan, R. Wyatt, and J. Sodroski. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 74:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]