Abstract
Herpes simplex virus type 1 (HSV-1) ICP0 directs the degradation of cellular proteins associated with nuclear structures called ND10, a function thought to be closely associated with its broad transactivating activity. Roscovitine (Rosco), an inhibitor of cyclin-dependent kinases (cdks), inhibits the replication of HSV-1, HSV-2, human cytomegalovirus, varicella-zoster virus, and human immunodeficiency virus type 1 by inhibiting specific steps or activities of viral regulatory proteins, indicating the broad and pleiotropic effects that cdks have on the replication of these viruses. We previously demonstrated that Rosco inhibits the transactivating activity of ICP0. In the present study, we asked whether Rosco also affects the ability of ICP0 to direct the degradation of ND10-associated proteins. For this purpose, WI-38 cells treated with cycloheximide (CHX) were mock infected or infected with wild-type HSV-1 or an ICP0− mutant (7134). After release from the CHX block, the infections were allowed to proceed for 2 h in the presence or absence of Rosco at a concentration known to inhibit ICP0's transactivating activity. The cells were then examined for the presence of ICP0 and selected ND10-associated antigens (promyelocytic leukemia antigen [PML], sp100, hDaxx, and NDP55) by immunofluorescence. Staining for the ND10-associated antigens was detected in ≤20% of KOS-infected cells in the presence or absence of Rosco, demonstrating that Rosco-sensitive kinases are not required for ICP0's ability to direct the dispersal or degradation of these antigens. In contrast, >90% of 7134- and mock-infected cells stained positive for all ND10-associated antigens in the presence or absence of Rosco. Similar results were obtained with a non-ND10-associated antigen, DNA-PKcs, a known target of ICP0-directed degradation. The results of the PML and DNA-PKcs immunofluorescence studies correlated with a decrease in the levels of these proteins as determined by Western blotting. Thus, the differential requirement for Rosco-sensitive cdk activities distinguishes ICP0's ability to direct the dispersal or degradation of cellular proteins from its transactivating activity.
The molecular mechanisms responsible for the activation of herpes simplex virus (HSV) gene expression during productive infection and reactivation from latency are not well understood. Available evidence indicates, however, that the functions of viral regulatory proteins that activate viral-gene expression are themselves activated by cell cycle regulatory proteins. For example, two cellular proteins, HCF (36) and TAF250 (84), whose expression and activities are cell cycle associated, activate the functions of two viral regulatory proteins, VP16 (36) and ICP4 (12), respectively, and cyclin-dependent kinases (cdks), which drive the cell cycle, are required for the synthesis and activities of HSV immediate-early (IE) regulatory proteins (75-77). Notably, inhibitors of cdks have been shown to inhibit the replication of HSV type 1 (HSV-1) (74), HSV-2 (74), human cytomegalovirus (9), varicella-zoster virus (S. L. Taylor, P. R. Kinchington, A. Brooks, and J. F. Moffat, personal communication), and human immunodeficiency virus type 1 (74) at several steps in their life cycles by inhibiting, either directly or indirectly, the functions of key viral regulatory proteins. These observations suggest that cdks may regulate common cellular proteins in viral pathways or processes (e.g., viral transcription) necessary for their replication.
Among the HSV IE proteins whose regulatory functions require the activities of cdks is ICP0 (2, 16), a strong activator of any viral or cellular gene that exhibits a basal level of transcription (11, 22, 26, 31, 32, 64, 69, 86). Although the mechanism responsible for ICP0's potent transactivating activity is unknown, transactivation occurs at a transcriptional or pretranscriptional level that does not involve the binding of ICP0 to a specific DNA sequence (29, 43), and one or more cdks that are sensitive to the drug roscovitine (Rosco) (i.e., cdk-1, -2, -5, -7, and -9) are able to inhibit this activity (16). Whatever the mechanism of transactivation, ICP0 mutants impaired in transactivating activity are also impaired in the ability to direct the dispersal or degradation of selected cellular proteins associated with nuclear structures called ND10 (23, 28, 58, 59, 67). ND10 structures were first observed by electron microscopy >40 years ago and have since been linked to cancer, autoimmune diseases, and the replication of DNA-containing viruses (reviewed in reference 66). Among the many cellular proteins associated with ND10 are the promyelocytic leukemia antigen (PML) (58), sp100 (59), hDaxx (41), NDP55 (6), CREB-binding protein (19), ISG20 (35), and Rb (4). These proteins play roles in cellular transcription, proliferation, survival, and/or resistance to viral infection. ICP0 has been shown to direct the degradation of PML, sp100, and their sumoylated isoforms (14, 28, 67), as well as several non-ND10-associated proteins (27, 48, 55, 68), via the ubiquitin-proteasome pathway. Consistent with this activity, ICP0 possesses E3 ubiquitin-ligase activity in vitro (8, 85). E3 ubiquitin-ligases attach ubiquitin to cellular proteins, targeting them for degradation via the proteasome pathway (33). Notably, the ubiquitin-proteasome pathway is also required for ICP0's transactivating activity, as inhibitors of the pathway block transactivation (30). These findings, therefore, link ICP0's E3 ubiquitin ligase activity and its ability to direct the degradation of ND10-associated proteins directly to its transactivating activity. Although the precise roles of ND10, the cellular proteins associated with them, and the degradation of these proteins in HSV replication are not clear, the genomes of several DNA-containing viruses other than HSV, including human cytomegalovirus, Epstein-Barr virus, adenovirus, and papillomavirus, localize to sites immediately adjacent to ND10 upon infection, and each of these viruses encodes a protein able to alter or direct the degradation of ND10-associated proteins (reviewed in reference 24). During HSV replication, the ICP0-directed dispersal or degradation of ND10-associated proteins appears to provide a unique cellular environment that promotes viral transcription and DNA replication.
In addition to its transactivating activity, ICP0 has the ability to reset the cell cycle clock to late G1/S and G2/M checkpoints (20, 37, 54, 79), so that HSV-1 replication is independent of the stage of the cell cycle (10). As for ICP0's transactivating activity, ICP0 mutants unable to direct the dispersal or degradation of ND10-associated proteins are also impaired in the ability to reset the cell cycle clock (28, 37, 38, 54, 67). Consequently, ICP0-directed dispersal or degradation of ND10-associated proteins is likely a central event in both ICP0's transactivating and cell cycle-manipulating activities.
Having established that Rosco-sensitive cdks are required for the transactivating activity of ICP0, we asked whether Rosco-sensitive cdks are also required for ICP0's ability to promote the dispersal or degradation of ND10-associated proteins. Our results demonstrate that Rosco-sensitive cdks are not required for this activity. Based on these and other findings, we propose a model in which the ICP0-directed dispersal or degradation of ND10-associated proteins, which does not require Rosco-sensitive cdks, precedes its transactivating activity, which does require Rosco-sensitive cdks.
MATERIALS AND METHODS
Cells and viruses.
An African green monkey kidney cell line (Vero) and human embryonic lung cells (WI-38) were obtained from the American Type Culture Collection (Manassas, Va.) and propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum as described previously (73). L7 cells, a Vero cell line stably transformed with the gene encoding ICP0 and able to complement the replication of ICP0 null mutants, were propagated as described by Samaniego et al. (72). Low-passage (p11) HSV-1, strain KOS, was used as the wild-type virus and was propagated and assayed as described by Schaffer et al. (73). The HSV-1 recombinant KOS6β was grown and assayed as described previously (16). The KOS-derived ICP0 null mutants, 7134 and dlx3.1, were grown as previously described (10, 71), and titers of mutant stocks were determined on L7 cell monolayers. 7134 contains the lacZ gene in place of the open reading frame of ICP0, and dlx3.1 contains a large deletion (3.1 kb) that eliminates the promoter and most of the open reading frame of ICP0 (Fig. 1).
FIG. 1.
HSV-1 recombinant viruses used in this study. Diagram of the wild-type HSV genome (KOS; not drawn to scale) showing the unique long (UL) and unique short (US) regions of the genome, each flanked by inverted repeat sequences (ab UL b′a′ and a′c′ US ca) shown as open boxes. Beneath the diagram of the HSV-1 genome are shown short arrows indicating transcripts of relevant genes and the genomes of recombinant viruses with their respective inserted lacZ cassettes and deletions (parentheses). The bent arrows indicate the transcription start sites of the Escherichia coli lacZ cassettes (open boxes). KOS6β contains the ICP6 promoter-E. coli lacZ reporter cassette cloned into the BglII site between the UL49 and UL50 genes of HSV (16). dlx3.1-6β contains the ICP6 promoter-E. coli lacZ reporter cassette cloned into the same site in the background of the ICP0 null mutant, dlx3.1 (71). dlx3.1 contains a 3.1-kb deletion in both copies of the ICP0 gene (71), and 7134 contains the E. coli lacZ cassette cloned in place of the complete open reading frames of both copies of ICP0 (11). The lacZ cassette is under the control of the ICP0 promoter in 7134.
Construction and characterization of dlx3.1-6β.
L7 cells were cotransfected with 1 μg of infectious dlx3.1 DNA, 2.5 μg of pUIClacZ (16), and 100 ng of the ICP0 expression plasmid, pSH (as an inducer of recombination), as described by Jordan and Schaffer (43). pUIClacZ contains the ICP6 promoter::lacZ cassette cloned into the unique BglII site between the divergent promoters of the UL49 and UL50 genes of HSV-1 (16). Viral progeny of the cotransfection were plated on L7 cells in medium containing 2% methylcellulose and the β-galactosidose (β-Gal) chromogenic substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Recombinants containing the ICP6 promoter::lacZ cassette produced blue plaques on L7 cells. One blue plaque (dlx3.1-6β) was isolated and plaque purified three times. KOS6β, dlx3.1, and dlx3.1-6β viral DNAs were isolated from infected Vero cells, digested with KpnI or SacI and PstI, and subjected to Southern blot analyses as previously described for KOS6β (16) and dlx3.1 (71). The KpnI digestion was used to test for the correct insertion of the ICP6 promoter::lacZ cassette, and the SacI and PstI digestions were used to determine whether deletion of the ICP0 locus characteristic of dlx3.1 was maintained. To compare the replication efficiencies of the two pairs of viruses, one-step growth curves were performed with 5 PFU (each) of KOS, KOS6β, dlx3.1, and dlx3.1-6β/cell in Vero cells as described by Cai and Schaffer (10).
Drugs.
Rosco (CalBiochem, San Diego, Calif.) was prepared in dimethyl sulfoxide (DMSO) as a 50 mM stock and used at various concentrations ranging from 1 to 100 μM, or as indicated in the figure legends. Cycloheximide (CHX; Sigma Chemical, St. Louis, Mo.) was prepared in serum-free Dulbecco's modified Eagle's medium as a stock solution of 20 mg/ml and used in WI-38 cell medium at a concentration of 50 μg/ml.
Transactivation of the ICP6 promoter by ICP0.
The design of the transactivation experiments is illustrated in Fig. 2A. Briefly, WI-38 cells were plated in 24-well plates (5 × 104 cells per well). Twenty-three hours later, the cells were treated with CHX for 1 h and either mock infected or infected with 5 PFU of KOS6β or dlx3.1-6β/cell for 1 h at 37°C in the presence of CHX. After virus adsorption (t = 1 h postinfection [p.i.]), the inoculum was removed, the monolayers were washed three times with phosphate-buffered saline (PBS) containing CHX, 1 ml of WI-38 cell growth medium containing CHX was added to each well, and the monolayers were incubated for an additional 4.5 h (t = 5.5 h p.i.). At 5.5 h p.i., the cells were preincubated for 0.5 h in medium containing CHX in the presence or absence of Rosco. At 6 h p.i., duplicate monolayers were either harvested directly (t = 0 h postrelease [p.r.] of the CHX block) or washed with PBS with or without Rosco and incubated in medium with or without Rosco for an additional 6 h. At the end of the second 6-h treatment period (t = 12 h p.i.), samples were harvested. For studies of the kinetics of ICP6 promoter activation, KOS6β-infected cells were released into WI-38 cell growth medium, and samples were harvested at 1-h intervals from 1 to 6 h p.r. of the CHX block. At the end of the experiment, cell extracts were prepared and β-Gal assays were performed as described below.
FIG. 2.
Effect of Rosco on ICP0-mediated induction of β-Gal from the ICP6 promoter in KOS6β and dlx3.1-6β. (A) Experimental design. WI-38 cells were plated, pretreated with CHX for 1 h (t = −1 h p.i.), and then either mock infected or infected with KOS6β or dlx3.1-6β (t = 0 h p.i.) in the presence of CHX for 6 h (t = 6 h p.i.). The cells were then harvested or released into non-Rosco-containing (− Rosco; normal) medium and harvested at 1-h intervals thereafter or released into medium in the presence of the indicated concentrations of Rosco (+ Rosco) for an additional 6 h. At the end of the 6-h treatment period (t = 12 h p.i.), cell extracts were prepared. β-Gal and total-protein (Bradford) assays were performed on all samples. A+, polyadenylated transcripts. (B) β-Gal activity in KOS6β-infected WI-38 cells. Negative controls (Controls), kinetics of ICP6 promoter activity in the absence of Rosco (Kinetics) from 0 to 6 h p.r. of the CHX block, and ICP6 promoter activity in the presence of Rosco (Rosco) at 6 h p.r. of the CHX block are indicated by the brackets above and below the graph. All samples were compared to mock-infected cells (t = −1 h p.i.) as a control (far-left sample), which was given the arbitrary value of 1. +, present; −, absent. (C) β-Gal activity in KOS6β- or dlx3.1-6β-infected WI-38 cells. Infection conditions were as described for panel B; however, replicate cultures were infected with the HSV-1 recombinant, dlx3.1-6β, and β-Gal activity was measured at 0 and 6 h p.r. of the CHX block. Negative controls (Controls), activation of the ICP6 promoter in the absence of Rosco (− R), and the presence of 50 μM Rosco (+ R) 6 h p.r. of the CHX block are indicated by the brackets above and below the graph. All samples were compared to mock-infected cells (t = −1 h p.i.) as a control (far-left sample), which was given the arbitrary value of 1. In all cases, the data represent the means of duplicate samples for each graph, and the error bars indicate the standard deviation of the mean.
β-Gal and protein assays.
Cell extracts were prepared by lysing cells in 50 μl of cold Triton-Tris lysis buffer (1% Triton X-100, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, and fresh 1 mM dithiothreitol) (89). β-Gal activity was assayed as described previously (70). Briefly, 10 μl of cell extract was added to 90 μl of a solution containing 11 mM KCl, 88 mM sodium phosphate buffer (pH 7.3), 1 mM MgCl2, 5 mM 2-mercaptoethanol, and freshly added 4.4 mM chlorophenol red-β-d-galactopyranoside (Calbiochem). Reaction mixtures in 96-well plates were incubated for 45 min at 37°C and for 14 to 18 h at room temperature. Optical densities were read at 595 nm on a plate reader (Molecular Dynamics, Sunnyvale, Calif.), and the amount of β-Gal activity was determined by a linear standard curve. β-Gal assays were standardized relative to the protein concentration (Bio-Rad [Hercules, Calif.] protein assay) according to the manufacturer's protocol.
Immunofluorescence.
Infections performed to examine the expression of ICP0 and individual cellular antigens were conducted essentially as described above (see “Transactivation of the ICP6 promoter by ICP0”), except the cells were plated on collagen-coated coverslips and mock infected or infected with wild-type HSV-1 or 7134. At the time of harvest, the medium was removed, and the coverslips were washed twice with PBS and either fixed and permeabilized as described by Ascoli and Maul (6) for the staining of ICP0 and PML, sp100, or NDP55 or fixed and permeabilized as described by Parkinson et al. (68) for the staining of ICP0 and either hDaxx or DNA-PKcs. The following primary antibodies (dilutions indicated in parentheses) were used for the dual staining of each cellular antigen and ICP0: PML (1:500) (PML-14; Gerd Maul, Wistar Institute, Philadelphia, Pa.) and ICP0 (1:500) (H1112; Rumbaugh-Goodwin Institute for Cancer Research, Plantation, Fla.), hDaxx (1:10) (monoclonal antibody [MAb 514] [41]) and ICP0 (1:100) (J17 [89]), sp100 (1:10) (MAb 1150 [21]) and ICP0 (1:100) (J17), NDP55 (1:10) (MAb 138 [6]) and ICP0 (1:100) (J17), and DNA-PKcs (1:10) (sc-5282; Santa Cruz Biotechnology, Santa Cruz, Calif.) and ICP0 (1:100) (J17). The ICP0 rabbit polyclonal antibody, J17, used in dual staining with all cellular antigens (except PML) was preadsorbed overnight at 4°C on 3.7% formaldehyde-fixed WI-38 cells prior to its use in immunofluorescence tests to reduce background staining of uninfected WI-38 cells. Primary antibodies against each cellular antigen and ICP0 were diluted together in PBS containing 1% bovine serum albumin (BSA), and 75 μl was placed on each coverslip. The coverslips were incubated for 1 h at room temperature in a humidified chamber. They were washed twice with PBS containing 1% BSA at room temperature, and 75 μl of secondary antibodies was placed on each coverslip. All secondary antibodies described in this study were purchased from Jackson ImmunoResearch, West Grove, Pa. The following secondary antibodies (dilutions indicated in parentheses) were used for each primary antibody: PML and ICP0 (J17) (1:100) (goat anti-rabbit immunoglobulin G [IgG] conjugated with rhodamine red-X), hDaxx, sp100, DNA-PKCS, and ICP0 (H1112) (1:100) (goat anti-mouse IgG conjugated with fluorescein isothiocyanate), and NDP55 (1:100) (goat anti-mouse IgM conjugated with fluorescein isothiocyanate). All secondary antibodies were diluted individually in PBS containing 1% BSA, and 75 μl was added to each coverslip in the following order. First, the appropriate secondary antibody which recognized each primary antibody against ICP0 was added to the coverslips, and the coverslips were incubated for 40 min at room temperature in a humidified chamber and washed two times with PBS containing 1% BSA. Second, the appropriate secondary antibody which recognized each primary antibody against a given cellular antigen was added to the coverslips, and the coverslips were incubated for 40 min at room temperature in a humidified chamber, washed two times with PBS containing 1% BSA and two times with distilled water, and mounted with 7 μl of Prolong Antifade Solution (Molecular Probes, Eugene, Oreg.) per coverslip. The cells were viewed by fluorescence microscopy with a Nikon Eclipse TE300 fluorescence microscope at ×400 magnification and photographed with an RT Slider digital camera (Diagnostic Instruments, Sterling Heights, Mich.) with Photoshop software (Adobe Systems Inc., Mountain View, Calif.). Images were assembled and labeled with Canvas 8 software (Deneba Systems, Miami, Fla.) and printed using a Fuji (Edison, N.J.) Fujix Pictrography 3500 printer.
At least 200 cells from random fields were analyzed for each preparation, and staining for a given cellular antigen and for ICP0 was noted. Each cell was categorized into one of four groups: cells that stained positive (i) only for a given cellular antigen, (ii) only for ICP0, (iii) for both, or (iv) for neither. The percentage of cells in each category was determined by dividing the number of cells in a given category by the total number of cells counted in all four categories.
Preparation of infected cell lysates for PML and DNA-PKcs protein determination.
WI-38 cells were plated at 2.0 × 106 per 100-mm-diameter plate in 10 ml of WI-38 culture medium. Twenty-three hours later, the cells were treated with 10 ml of WI-38 medium containing CHX. One hour later, the cells were washed with 5 ml of PBS containing CHX and mock infected or infected with 1 ml of KOS or 7134 at a multiplicity of infection of 5 PFU/cell in WI-38 medium containing CHX. After the 1-h adsorption period, the inoculum was removed, the cultures were washed twice with 5 ml of PBS containing CHX, and 10 ml of WI-38 medium containing CHX was added to each plate. After an additional 4.5-h incubation, the culture medium was aspirated and fresh medium containing CHX and either DMSO (vehicle) or Rosco in DMSO was added to the cultures. The monolayers were incubated for an additional 30 min, the medium was removed, and the cultures were washed twice with PBS containing DMSO or Rosco in DMSO. At this time, 0 h p.r. of the CHX block, and at selected intervals (2, 4, or 8 h) thereafter, samples were washed with PBS, scraped into 5 ml of PBS, and centrifuged at 4°C and 1,000 × g for 10 min. The supernatant was aspirated, and the resulting pellet was flash-frozen in liquid N2 and thawed. To detect PML, the cells were lysed in 30 μl of RIPA buffer (25 mM NaCl, 50 mM Tris [pH 7.5], 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, and 0.5% deoxycholic acid) containing 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml, and 1 μg of leupeptin/ml. To detect DNA-PKCS, the cell pellets were lysed in 1 ml of RIPA buffer plus the protease inhibitors previously mentioned. The lysed extracts were sonicated by two 30-s pulses in a Misonix (Farmingdale, N.Y.) Sonicator 3000 at ∼105 W and held on ice.
Immunoprecipitations.
To detect DNA-PKCS in infected cell cultures, 1 μg of undiluted anti-DNA-PKCS monoclonal mouse IgG antibody (no. sc-5282; Santa Cruz Biotechnology) was added to each 1 ml of lysate prepared as described above and incubated overnight at 4°C with gentle agitation. The next day, samples were incubated with 20 μl (∼10 mg) of protein A-agarose (Invitrogen Life Technologies, Carlsbad, Calif.) for 2 h at 4°C with gentle agitation and then centrifuged for 2 min at 3,300 × g at 4°C. The protein A-agarose pellet was washed twice in RIPA buffer containing protease inhibitors. To elute DNA-PKCS, the final pellet was resuspended in 20 μl of 1× Laemmli buffer (46), heated for 5 min at 95°C, and centrifuged for 10 min at 16,100 × g at room temperature. The supernatant from each sample was then subjected to Western blot analysis.
Western blotting.
To detect PML in cell lysates, 5 μl of 4× Laemmli buffer was added to 15 μl of each cell lysate and heated at 95°C for 5 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (8 and 6% acrylamide for PML and DNA-PKCS, respectively; 29:1 bisacrylamide-acrylamide) at 30 V for 16 h. The proteins were transferred onto nitrocellulose membranes (Osmonics, Westborough, Mass.) by using a Mini Trans-Blot cell (Bio-Rad Laboratories, Hercules, Calif.) at 4°C and 70 V. The membranes were blocked for 2 h at room temperature with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T). Primary-antibody incubations were performed in 5% nonfat dry milk in TBS-T for 2 h at room temperature with antibodies directed either against PML (no. sc-5621 [rabbit polyclonal IgG diluted 1:500]; Santa Cruz Biotechnology) or DNA-PKCS (no. sc-5282 [mouse monoclonal IgG diluted 1:500]; Santa Cruz Biotechnology). The membranes were washed six times in 1 h in TBS-T (20 ml per wash). For the secondary antibody, goat anti-rabbit IgG- or goat anti-mouse IgG-horseradish peroxidase (Pierce Biochemicals, Rockford, Ill.) was diluted 1:20,000 in 5% nonfat dry milk in TBS-T for PML and DNA-PKCS, respectively, and the membranes were incubated for 30 min at room temperature. The membranes were then washed six times in 1 h in TBS-T (20 ml per wash) and exposed to film (Hyperfilm ECL; Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) by using Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences, Boston, Mass.). The film was developed in a Kodak M35A X-OMAT processor. Exposures were scanned (Arcus II scanner; AGFA, Mortsel, Belgium), assembled, and printed by using Adobe Photoshop. To quantitate the relative levels of PML or DNA-PKCS, autoradiographs were scanned by using a Bio-Rad Fluor-S MultiImager, and band intensities were calculated with Multi-Analyst software.
RESULTS
Kinetics of ICP0-mediated transactivation of the ICP6 promoter in WI-38 cells.
It was shown previously in Vero cells that Rosco-sensitive cdks are required for the transactivating activity of ICP0 and for its posttranslational modification state (16, 77). Because ICP0's transactivating activity is thought to be linked to its ability to target ND10-associated proteins for dispersal or degradation, we wanted to determine whether Rosco-sensitive cdks are also required for this activity by using immunofluorescence tests and Western blotting assays. Because most antibodies directed against ND10-associated proteins recognize only human forms of these proteins, it was necessary to perform the proposed studies with a human cell line. WI-38 cells were chosen for the tests. Prior to examining the effects of Rosco on ICP0's ability to target ND10-associated proteins for dispersal or degradation, we determined the kinetics and levels of ICP0-mediated transactivation and the concentration of Rosco needed to inhibit this activity in WI-38 cells.
As in our previous studies with Vero cells, we utilized ICP6 promoter activity as a measure of the transactivating activity of ICP0, as ICP0 has been shown to be a unique and potent transactivator of this promoter (15, 17, 34, 72, 81-83). These tests were performed using the HSV-1 recombinant KOS6β, which contains an ICP6 promoter::lacZ reporter cassette inserted into the HSV-1 genome between the divergently transcribed genes UL49 and UL50 (Fig. 1). A protocol designed to measure the kinetics of ICP0-mediated induction of β-Gal activity from the ICP6 promoter in KOS6β-infected cells after the release of a CHX block was used in these tests (Fig. 2A) (16). Briefly, the presence of CHX in infected cultures results in the accumulation of IE viral transcripts, which are subsequently translated into IE proteins when CHX is removed. When CHX is removed in the absence of Rosco, fully functional ICP0 is synthesized and induces transcription of the ICP6 promoter::lacZ cassette. When CHX is removed in the presence of Rosco, the effect of Rosco on ICP0's transactivating activity can be assessed. This protocol was developed specifically because Rosco inhibits the synthesis of IE transcripts, including those specific for ICP0 (76, 77). As shown in Fig. 2B (Kinetics), in the absence of Rosco, the levels of ICP0-induced β-Gal activity controlled by the ICP6 promoter increased as a function of time postrelease of the CHX block. Measurable increases in β-Gal activity were observed from 2 (≥5-fold) to 6 (≥380-fold) h p.r. of the CHX block compared to mock-infected cells or KOS6β-infected cells treated only with CHX (Fig. 2B, Controls). In previous studies, a similar increase in ICP6 promoter activity (110-fold) was observed in KOS6β-infected Vero cells from 0 to 6 h p.r. of the CHX block (16). Because the maximal level of β-Gal activity was observed 6 h p.r. of the CHX block, this time point was chosen to examine the effect of Rosco on the transactivating activity of ICP0 in WI-38 cells.
Effect of Rosco on ICP0-mediated transactivation of the ICP6 promoter in WI-38 cells.
Although 100 μM Rosco inhibits ICP0's transactivating activity 50-fold in Vero cells (16), the concentration of Rosco able to inhibit ICP0's transactivating activity in WI-38 cells was unknown. To address this question, concentrations of Rosco ranging from 1 to 100 μM were added to WI-38 cell cultures after release of the CHX block, and β-Gal assays were performed 6 h later. Concentrations ranging from 1 to 100 μM inhibited ICP0's ability to induce β-Gal activity from the ICP6 promoter (Fig. 2B, Rosco) ≥2-, ≥13-, ≥21-, and ≥28-fold (Fig. 2B, Kinetics). Thus, in WI-38 cells, Rosco inhibited ICP0-mediated transactivation of the ICP6 promoter at all concentrations tested. Because 50 μM Rosco was as effective as 100 μM Rosco, 50 μM was utilized in subsequent tests.
Transactivation of the ICP6 promoter by ICP0.
Although others have reported that ICP0 is a specific and potent transactivator of the ICP6 promoter, we wanted to test the validity of these observations in WI-38 cells. For this purpose, we compared the β-Gal activity of KOS6β with that of the recombinant virus dlx3.1-6β (Fig. 1). dlx3.1-6β was constructed in the same manner as KOS6β except that the ICP6 promoter::lacZ cassette was inserted in the genetic background of the ICP0 null mutant, dlx3.1 (71) (Fig. 1). The site of insertion of the ICP6::lacZ cassette and retention of the ICP0 deletion were verified by Southern blot analysis (data not shown). In one-step growth curves in Vero cells, the replication efficiencies of dlx3.1-6β and dlx3.1 were comparable (data not shown), indicating that insertion of the ICP6 promoter::lacZ cassette had no major effect on the replication of dlx3.1-6β relative to dlx3.1 in cell culture.
Using KOS6β, dlx3.1-6β, and the CHX block protocol (Fig. 2A), we asked whether ICP0 is a specific transactivator of the ICP6 promoter in WI-38 cells. As shown in Fig. 2C, in the absence of Rosco, a 20-fold reduction in β-Gal activity was observed 6 h p.r. of the CHX block in extracts of cells infected with dlx3.1-6β compared to cells infected with KOS6β. The presence of 50 μM Rosco also produced a 20-fold reduction in β-Gal activity in KOS6β-infected cells relative to the “no-drug” control 6 h p.r. of the block. In contrast, the basal β-Gal activity in dlx3.1-6β-infected cells was unaffected by Rosco. These results demonstrate that ICP0 is the primary transactivator of the ICP6 promoter and that Rosco effectively inhibits this activity in WI-38 cells, confirming previous reports that ICP0 is a specific and potent transactivator of the ICP6 promoter (15, 17, 34, 72, 81-83) and that Rosco did not affect the basal activity of the ICP6 promoter (16).
Effects of Rosco on ICP0-directed degradation of the ND10-associated antigen PML.
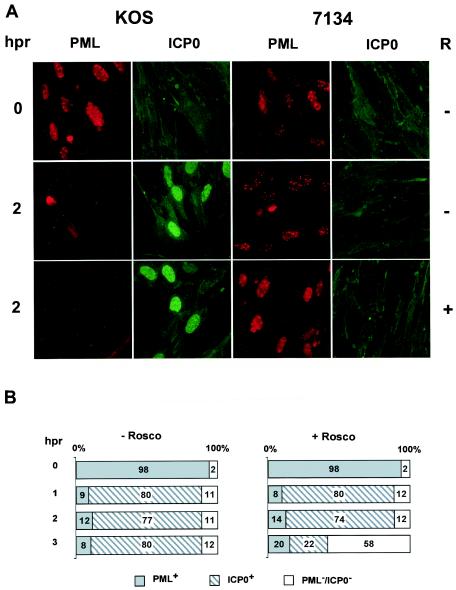
ICP0 alters nuclear structures called ND10, in part by targeting cellular proteins associated with these structures for degradation (14, 28, 58, 59, 67). It has been shown that the proteasome pathway is required for the targeted degradation of these proteins by ICP0, which is in turn required for ICP0's transactivating activity (28). Since Rosco inhibits ICP0's transactivating activity, we asked whether Rosco also affects the ability of ICP0 to degrade the prototypic ND10-associated antigen, PML, during viral infection. For this purpose, WI-38 cells were mock infected or infected with wild-type KOS or the ICP0 null mutant 7134 (Fig. 1) using the CHX block protocol. The infected cells were fixed and permeabilized at the time of release of the CHX block or 2 h p.r. into medium with or without Rosco. The 2-h time point was chosen because ICP0 has been shown to induce the loss of ND10-associated antigen-specific staining by ≥90% within 2 h p.r. of the CHX block in HSV-1-infected cells (59). In the present tests, PML staining was nuclear and punctate at the time of release of the CHX block, as expected of an ND10-associated antigen in cells that do not express ICP0 (Fig. 3A, 0 h p.r.). By 2 h p.r., however, PML-specific staining in KOS-infected cells (cells staining positive for ICP0) was either undetectable or greatly reduced in both the presence and absence of Rosco. In contrast, 7134-infected (Fig. 3A, 2 h p.r.) and mock-infected (data not shown) cells exhibited abundant PML-specific staining but no ICP0-specific staining in the absence or presence of Rosco.
FIG. 3.
PML- and ICP0-specific immunofluorescence in WI-38 cells infected with wild-type KOS (ICP0+) or 7134 (ICP0−). (A) At 0 and 2 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco (R), infected cells were washed, fixed, permeabilized, and probed with primary and secondary antibodies to detect PML and ICP0 by immunofluorescence. (B) Percentages of KOS-infected cells staining positive for PML, ICP0, or neither protein at 0, 1, 2, and 3 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco. At least 200 cells were counted per time point per treatment group. The results obtained for mock- and 7134-infected cells at 0, 1, 2, and 3 h p.r. (data not shown) were indistinguishable from those for KOS-infected cells at 0 h p.r. of the CHX block.
To quantify this effect, the kinetics of the disappearance of PML staining relative to ICP0 staining was measured by counting individual cells that stained positive for PML, ICP0, both, or neither antigen at 1-h intervals from 0 to 3 h p.r. of the CHX block in KOS- (Fig. 3B), mock-, or 7134-infected cells (data not shown). At 0 h p.r., PML staining was observed in 98% of KOS-infected cells in untreated or Rosco-treated cells. After 1 and 2 h p.r., however, only 8 to 14% of the cells stained positive for PML, whereas 74 to 80% of the cells stained positive for ICP0, in both the absence and presence of Rosco. Thus, ICP0's ability to reduce PML staining was not affected by Rosco. By 3 h p.r. in the absence of Rosco, only 8% of cells stained positive for PML, 80% stained positive for ICP0, and 12% did not stain for either antigen. In contrast, by 3 h p.r. in the presence of Rosco, 20% of cells were PML positive, only 22% were ICP0 positive, and 58% did not stain for either antigen. Thus, in the presence of Rosco, the percentage of cells exhibiting PML staining actually increased over time, the percentage of cells staining positive for ICP0 decreased, and the percentage of cells staining negative for both antigens increased markedly. These observations suggest that Rosco inhibited ICP0 synthesis by 3 h p.r., an effect that correlates with a previously observed decrease in IE transcript accumulation after release from the CHX block in the presence of Rosco (77). Alternatively or simultaneously, the absence of the activities of Rosco-sensitive cdks may affect the stability of ICP0. While others have observed transient or limited colocalization between ICP0 and PML during HSV-1 infection (58, 59), colocalization was not observed in this study. Our inability to detect the colocalization of ICP0 and PML is likely due to the time points and/or cell type used in this experiment. As expected, at all time points tested, the percentages of cells staining positive for PML in mock- and 7134-infected cells (∼95%) (data not shown) were similar to that in KOS-infected cells at 0 h p.r. Individual cells that stained positive for both ICP0 and PML were not observed.
ICP0-directed degradation of PML: Western blot analysis.
Previous studies have demonstrated that the ICP0-directed reduction in PML staining of ND10 during HSV-1 infection correlates with the degradation of the PML protein via the ubiquitin-proteasome pathway and not simply the dispersal and antigenic alteration of PML (28). To measure levels of PML protein, we performed Western blot analysis. WI-38 cells were mock infected or infected with KOS or 7134 and harvested 0 and 2 h p.r. of the CHX block. Western blots of these samples showed a marked decrease in the level of an ∼127-kDa isoform of PML in KOS-infected cells 2 h p.r., both in the absence and presence of Rosco (Fig. 4), compared to mock- and 7134-infected cells. In two independent tests, PML levels in KOS-infected cells 2 h p.r. decreased to an average of 10% in the absence of Rosco and 17% in the presence of Rosco compared to KOS-infected cells at 0 h p.r., set at 100%. No major reduction in the levels of this PML isoform was observed in mock- or 7134-infected cells at 2 h p.r. Minor variations in the levels of PML in some mock- and 7134-infected samples (e.g., Fig. 4, lane Mock, at 0 and 2 h p.r. in the absence of Rosco) were not reproducible in repeat experiments. Thus, Rosco did not affect the ability of ICP0 to direct the degradation of at least one PML isoform. Notably, although different antibodies to PML were used in the immunofluorescence and Western blot assays, both antibodies recognize the ∼127-kDa isoform of PML that is degraded in KOS-infected cells, as determined by Western blotting (data not shown), supporting the results of both immunofluorescence and Western blot assays.
FIG. 4.
Western blot analysis of PML from mock-, KOS-, and 7134-infected WI-38 cells. WI-38 cells were pretreated with CHX (50 μg/ml) for 1 h, mock infected or infected with 5 PFU of KOS or 7134/cell in CHX-containing medium for 6 h, and harvested at 0 (top) and 2 (bottom) h p.r. of the CHX block in the absence (−) or presence (+) of Rosco. Cell extracts were prepared and analyzed by SDS-PAGE and Western blotting. The numbers on the right indicate the molecular mass marker (in kilodaltons), and the arrows on the left indicate the PML isoform (∼127 kDa) of interest.
Effect of Rosco on ICP0-directed dispersal and/or degradation of ND10-associated hDaxx-, sp100-, and NDP55-specific staining.
Because the ICP0-directed degradation of PML was unaffected by Rosco, we next asked whether Rosco affects ICP0's ability to affect the staining of three other ND10-associated antigens, hDaxx, sp100, and NDP55, using the same experimental approach used for PML. ICP0 reduced hDaxx-specific (Fig. 5A), sp100-specific (Fig. 5B), and NDP55-specific (Fig. 5C) staining in KOS-infected cells in the presence and absence of Rosco at 2 h p.r., relative to 7134-infected (Fig. 5A to C) and mock-infected (data not shown) cells. Similar to PML, the staining patterns of these three antigens were nuclear and punctate in cells that did not express ICP0.
FIG. 5.
hDaxx-, sp100-, NDP55-, and ICP0-specific immunofluorescence in WI-38 cells infected with KOS or 7134. (A to C) At 2 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco (R), infected cells were washed, fixed, permeabilized, and probed with primary and secondary antibodies to detect hDaxx and ICP0 (A), sp100 and ICP0 (B), or NDP55 and ICP0 (C) by immunofluorescence. (D to F) Percentages of KOS-infected cells staining positive for hDaxx and ICP0 (D), sp100 and ICP0 (E), or NDP55 and ICP0 (F) at 0 and 2 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco. At least 200 cells were counted per time point per treatment group. The results obtained for mock- and 7134-infected cells at 0 and 2 h p.r. (data not shown) were indistinguishable from those for KOS-infected cells 0 h p.r. of the CHX block.
The percentage of cells staining positive for hDaxx, sp100, and NDP55 relative to those staining positive for ICP0 was determined at 0 and 2 h p.r. in mock-, KOS-, or 7134-infected cells. hDaxx (Fig. 5D), sp100 (Fig. 5E), and NDP55 (Fig. 5F) staining was observed in 92, 95, and 92% of all KOS-infected cells, respectively, at 0 h p.r. in the absence of Rosco. Similar results were obtained in the presence of Rosco. No ICP0-specific staining was detected in these cells at 0 h p.r. At 2 h p.r., however, only 4 to 7% of KOS-infected cells stained positive for hDaxx (Fig. 5D), sp100 (Fig. 5E), or NDP55 (Fig. 5F), while 86 to 93% of these cells were positive for ICP0 in the absence or presence of Rosco. Of the three ND10-associated antigens examined, only NDP55 was detected in the same KOS-infected cells as ICP0, and these costained cells represented only 1% of all cells counted (Fig. 5F). The percentages of hDaxx, sp100, and NDP55 staining in mock- and 7134-infected cells at 0 and 2 h p.r. were similar to those observed in KOS-infected cells at 0 h p.r. in untreated and Rosco-treated cells (between 91 and 95% [data not shown]). The results observed for sp100 are similar to immunofluorescence and Western blotting results reported previously (14, 58, 65, 67). Efforts to measure levels of hDaxx, sp100, and NDP55 by Western blot analysis utilizing the same primary antibodies used in immunofluorescence tests were unsuccessful. Consequently, confirmatory Western blot tests were not performed. The results of tests for hDaxx-, sp100-, and NDP55-specific staining (Fig. 5) correlate with those of PML immunofluorescence tests (Fig. 3) and indicate that Rosco does not inhibit ICP0's ability to disperse and/or reduce the expression of at least four ND10-associated antigens in HSV-1-infected cells.
Effect of Rosco on ICP0-directed degradation of DNA-PKCS.
We next asked whether Rosco-sensitive cdks are required for ICP0 to direct the degradation of a cellular protein not associated with ND10, DNA-PKCS (48, 68). In immunofluorescence tests, cells were fixed and permeabilized at 0, 4, and 8 h p.r. of the CHX block. We chose these time points because ICP0-mediated degradation of DNA-PKCS has been reported to be delayed relative to the degradation of ND10-associated proteins (48, 68). The outcomes of these tests demonstrate that in the presence of ICP0, the number of KOS-infected cells staining positive for DNA-PKCS decreased gradually from 0 to 8 h p.r. (Fig. 6A), in both the absence and presence of Rosco, relative to 7134-infected (Fig. 6A) and mock-infected (data not shown) cells. In contrast to the staining of ND10-associated antigens, DNA-PKCS staining was diffuse throughout the nucleus, and many cells stained positive for both ICP0 and DNA-PKCS at 4 h p.r. of the CHX block in the absence or presence of Rosco (Fig. 6A). In some costained cells, a diminution in the intensity of DNA-PKCS staining was apparent relative to DNA-PKCS staining in 7134-infected cells at 4 h p.r. (Fig. 6A).
FIG. 6.
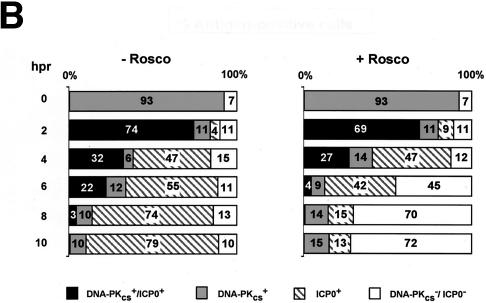
DNA-PKCS- and ICP0-specific immunofluorescence in WI-38 cells infected with KOS or 7134. (A) At 0, 4, and 8 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco (R), infected cells were washed, fixed, permeabilized, and probed with primary and secondary antibodies to detect DNA-PKCS and ICP0 by immunofluorescence. Individual KOS-infected cells which stained positive for both DNA-PKCS and ICP0 (t = 4 h p.r. of the CHX block) are indicated by the arrows. (B) Percentages of DNA-PKCS+/ICP0+, DNA-PKCS+, ICP0+, and DNA-PKCS−/ICP0− staining in KOS-infected cells at 0, 2, 4, 6, 8, and 10 h p.r. of the CHX block in the absence (−) and presence (+) of Rosco. At least 200 cells were counted per time point per treatment group. The results obtained for mock- and 7134-infected cells at 0, 2, 4, 6, 8, and 10 h p.r. (data not shown) were indistinguishable from those for KOS-infected cells at 0 h p.r. of the CHX block.
As shown in Fig. 6B, DNA-PKCS staining was observed in 93% of KOS-infected cells at 0 h p.r. in the absence or presence of Rosco. A gradual decrease in the percentage of cells staining positive for DNA-PKCS (the sum of DNA-PKCS+/ICP0+ and DNA-PKCS+) was observed between 2 and 10 h p.r. (85 to 10 and 80 to 15% in the absence and presence of Rosco, respectively). In contrast to results obtained with the ND10-associated antigens, DNA-PKCS and ICP0 immunoreactivities were detected simultaneously in a large number of cells at 2 and 4 h p.r. ICP0 staining (the sum of DNA-PKCS+/ICP0+ and ICP0+) in untreated cells remained constant (∼80%) from 2 to 10 h p.r.; in contrast, ICP0 staining in Rosco-treated samples gradually decreased from 78 to 13%, and the percentage of cells that did not stain for either antigen increased from 11 to 72%. The reduction in the number of ICP0-positive cells and the increase in cells that did not stain for either antigen in the presence of Rosco are similar to the findings made in the studies of PML in the presence of Rosco (Fig. 3B). It is clear from these studies that expression of ICP0 correlated with a reduction in the number of DNA-PKCS-positive cells but that relative to studies of the ND10-associated antigens, this reduction occurred over a longer period (0 to 8 h p.r.) and a greater percentage of cells exhibited both DNA-PKCS and ICP0 staining.
ICP0-directed degradation of DNA-PKCS: Western blot analysis.
To validate the observation that Rosco had no effect on the ability of ICP0 to direct the degradation of DNA-PKCS, extracts of mock-, KOS-, and 7134-infected cells were analyzed for DNA-PKCS protein levels by Western blotting (Fig. 7). Approximately equivalent levels of DNA-PKCS protein were detected at 0 h p.r. in the absence or presence of Rosco in mock-, KOS-, and 7134-infected cells. A decrease in the levels of the DNA-PKCS protein was detected only in KOS-infected cells at 4 and 8 h p.r., in both the absence and presence of Rosco. In two independent experiments, the levels of DNA-PKCS in KOS-infected cells at 4 h p.r. decreased to an average of 49% (without Rosco) and 14% (with Rosco) relative to KOS-infected cells at 0 h p.r. (100%). This decrease was further enhanced or maintained at 8 h p.r. to an average of 14% (without Rosco) and 16% (with Rosco) relative to KOS-infected cells at 0 h p.r. (100%). The levels of two polypeptides with increased electrophoretic mobilities relative to full-length DNA-PKCS also decreased gradually from 4 to 8 h p.r. in all KOS-infected samples. Previous reports suggested that these smaller polypeptides are proteolytic products of full-length DNA-PKCS (52, 60, 80).
FIG. 7.
Immunoprecipitation and Western blot analyses of DNA-PKCS from mock-, KOS-, and 7134-infected WI-38 cells. WI-38 cells were pretreated with CHX (50 μg/ml) for 1 h, mock infected or infected with 5 PFU of KOS or 7134/cell in CHX-containing medium for 6 h, and harvested at 0, 4, and 8 h p.r. of the CHX block in the absence (−) or presence (+) of Rosco. Extracts and immunoprecipitations of harvested cells were prepared and analyzed by SDS-PAGE and Western blotting. The molecular mass markers (in kilodaltons), bands specific for full-length DNA-PKCS (arrows), and bands specific for degradation products of DNA-PKCS (dots) are indicated on the left.
Taken together, the results of immunofluorescence tests and Western blot analysis indicate that, as for PML and sp100 (14, 58, 65, 67), Rosco-sensitive cdks are not required for the ICP0-directed degradation of DNA-PKCS. On the other hand, the ICP0-directed degradation of DNA-PKCS in the absence of Rosco proceeded more slowly than the degradation of ND10-associated antigens in the absence of Rosco, and cells staining for both ICP0 and DNA-PKCS were common.
DISCUSSION
Rosco-sensitive cdks are required for the transactivating activity of ICP0 but not for its ability to direct the dispersal or degradation of cellular proteins.
Because a strong link exists between the transactivating activity of ICP0, which requires Rosco-sensitive cdks, and its ability to disperse or degrade ND10-associated proteins (3, 28, 58, 59, 67), we asked whether the mechanism by which Rosco inhibits ICP0's transactivating activity is by blocking ICP0-directed degradation of ND10-associated proteins. Our results demonstrate that in WI-38 cells, Rosco-sensitive cdks do not play a role in ICP0-directed dispersal or degradation of several ND10-associated antigens or of a non-ND10-associated antigen. (Note that while the present study focused on the roles of Rosco-sensitive cdks [i.e., cdk-1, -2, -5, -7, and -9] in ICP0-targeted degradation of cellular proteins, it is possible that this activity may require cdks that are not sensitive to Rosco [i.e., cdk-4, -6,and -8]).
An additional point should be made prior to discussing the implications of ND10-associated protein degradation for the functions of ICP0. The two kinds of tests used in these studies assess different properties of the cellular proteins whose degradation was examined. Immunofluorescence analysis detects the presence and locations of protein or antigen isoforms by antibody-antigen interactions, whereas Western blot analysis detects the levels of these isoforms. Although we have shown by both immunofluorescence and Western blotting that ICP0 directs the degradation of one PML isoform, we were unable to demonstrate by Western blotting that ICP0 promotes the degradation of hDaxx, sp100, and NDP55. In this regard, it has been shown that the degradation of PML and sp100, and even more so, their sumoylated isoforms, is directed by ICP0 (as determined by Western blotting), correlating with the loss of PML and sp100 staining as determined by immunofluorescence analysis (14, 28, 67). For PML, because its isoforms and their stabilities vary with cell type and species in the presence of ICP0, it is unclear if the degradation of all or selected PML isoforms is important for the transactivating activity or other functions of ICP0 (24). The results of the sp100 immunofluorescence tests in the present study (Fig. 5B and E) are consistent with the likelihood that Rosco does not affect ICP0-directed degradation of sp100. The levels of hDaxx and NDP55 during HSV infection have not been examined by Western blot analysis, so it remains to be determined if ICP0 directs the degradation of these two proteins or simply alters the subcellular distribution of antigenically altered isoforms.
Western blot analysis of PML and DNA-PKCS in cells expressing ICP0 in the absence of Rosco revealed that ICP0 rapidly induced the degradation of one isoform of PML by 2 h p.r. of the CHX block (Fig. 4), whereas degradation of DNA-PKCS was not apparent until 8 h p.r. (Fig. 7). This difference in the rates of degradation of the two proteins suggests possible differences in the mechanisms of their degradation, even though ICP0 has been shown to induce the degradation of both antigens via the ubiquitin-proteasome pathway (28, 68). The pattern of nuclear staining and the time required for degradation of DNA-PKCS differ from that of PML, which suggests the possibility that ICP0 may preferentially direct the degradation of ND10-associated antigens. In support of this concept, available evidence indicates that components of the ubiquitin-proteasome pathway localize to ND10 in the absence or presence of ICP0 (5, 25, 47). Because ICP0 (i) localizes transiently to ND10 prior to its targeted degradation of ND10-associated proteins (59) and (ii) possesses E3 ubiquitin ligase activity (8, 85), a link may exist between the association of proteins with ND10 and their preferential degradation directed by ICP0.
Roles of ND10-associated proteins (hDaxx, sp100, NDP55, and PML) in ICP0 function.
The fact that the genomes of several DNA-containing viruses localize to sites adjacent to ND10 prior to the initiation of viral transcription and DNA replication is well established (reviewed in reference 24). Many of these viruses encode proteins with ND10-altering activities similar to that of ICP0, suggesting that they may utilize a common strategy to initiate replication. Despite these common features, the role(s) that dispersal or degradation of individual ND10-associated proteins plays at the molecular level in facilitating viral replication and, specifically, in ICP0's transactivating and cell cycle-altering activities, is poorly understood. Several studies suggest that hDaxx, sp100, and PML are linked with activities that suppress transcription (4, 39, 49, 50, 53, 78, 88), whereas the biological activity of NDP55 remains to be characterized. In the cases of hDaxx, sp100, and PML, ICP0 may relieve the transcription-repressive activities of these proteins through their dispersal or degradation to activate viral-gene expression. Of the four ND10-associated proteins examined in this study, PML is the best characterized. In addition to its altered nuclear localization as a fusion protein with retinoic acid receptor α in acute promyelocytic leukemia (18, 45), PML is apparently necessary for the formation of ND10 (41). In a recent report examining the significance of PML in HSV-1 infection, Lopez et al. hypothesized that overexpression of PML would lead to a block in the ability of ICP0 to alter ND10-associated proteins, resulting in the inhibition of HSV gene expression and reduced replication efficiency (56). Contrary to their prediction, overexpression of PML in the presence of ICP0 did not result in alteration of ND10, diminished HSV gene expression, or reduced replication. The authors concluded that the alteration of ND10 is not necessary for HSV replication. Two potential problems with the interpretation of these experiments are that (i) excessive amounts of PML may not alter the intrinsic structure of ND10 and (ii) ICP0 is likely able to induce the dispersal or degradation of selected ND10-associated proteins despite the overexpression of PML. While this report examined the effects of overexpression of PML on ICP0 function, the same group has recently reported that the absence of PML also has no apparent effect on HSV-1 replication but is essential for the antiviral effects of interferon on HSV-1 replication (13). This observation suggests that PML (and potentially other ND10-associated proteins) may not play a role in the transactivating activity of ICP0 in cell culture; however, these data do not exclude a role for other ND10-associated proteins or for PML in ICP0's transactivating activity in vivo.
Role of Rosco-sensitive cdks in regulating ICP0 function: a model.
To provide a possible explanation for the mechanism by which Rosco-sensitive cdks differentially affect ICP0's ND10-altering and transactivating functions, a model based on existing evidence is proposed (Fig. 8). Upon HSV-1 infection of cycling cells, ICP0 is expressed and cdks alter the posttranslational modification state of ICP0, either directly or indirectly, to yield multiple ICP0 isoforms which localize to ND10 (Fig. 8a and b) (40). The cdk-dependent, posttranslationally modified forms of ICP0 are essential for its transactivating activity. ICP0's E3 ubiquitin-ligase activity (8, 85) promotes the ubiquitination of selected ND10-associated proteins, targeting them for degradation via the proteasome pathway (14, 28, 67) (Fig. 8c). This process may play a role in ICP0's transactivating activity (30) (and possibly in its cell cycle-modulatory activity). Because ICP0 activates transcription in a DNA sequence-independent manner (29, 43), it is likely that the activation of and/or interactions between cdk-modified ICP0 isoforms and cellular proteins serve to induce transcription of viral and cellular genes (Fig. 8d) (40).
FIG. 8.
Model of the role of Rosco-sensitive cdks in regulating ICP0 function. Shown is a temporal model outlining the role of cdks in ICP0 function during an HSV infection either in the absence (b to e) or in the presence (f to i) of Rosco using the CHX block protocol shown in Fig. 2A.
Based on this model, how do Rosco-sensitive cdks affect the functions of ICP0? From the collective studies of ICP0 functions in the presence of Rosco, the activities of Rosco-sensitive cdks are not required for ICP0's nuclear localization and association with ND10 (Fig. 8e) (16, 77). They are, however, required for ICP0's posttranslational processing (Fig. 8e) (16, 77). Rosco-sensitive cdks are also dispensable for ICP0's E3 ubiquitin-ligase activity and proteasome-mediated degradation or dispersal of ND10-associated proteins (Fig. 8f; also see Fig. 3 to 5). Following the degradation or dispersal of ND10-associated proteins, isoforms of ICP0 not modified by cdks are unable to interact with and/or activate cellular factors involved in transcriptional transactivation (Fig. 8g). Notably, cdk-mediated posttranslational modification of ICP0 may occur either before or after the degradation of ND10-associated proteins, a modification that could protect ICP0 from self-mediated degradation. Whether cdks are required for ICP0's cell cycle-blocking activity is unclear, as both Rosco and ICP0, independently, are able to inhibit cell cycle progression (37, 54, 63). An alternative (but not mutually exclusive) model to that just presented is that Rosco-sensitive cdks alter the activities or levels of cellular proteins (Fig. 8d and g) that play pivotal roles in ICP0's transactivating activity, thus inhibiting ICP0-mediated transactivation through effects on cellular proteins. At present, the cellular proteins targeted by Rosco-sensitive cdks, as they relate to ICP0 function, have not been identified. Because Rosco also inhibits virion-induced activation of HSV-1 IE gene expression (44, 76, 77) and the ability of the HSV-1 major IE transactivator, ICP4, to activate gene expression (77), Rosco-sensitive cdks could, in theory, regulate a common set of cellular proteins required for HSV transcription.
Based on the model shown in Fig. 8, we hypothesize that Rosco-sensitive cdks are essential for the appropriate posttranslational modification of ICP0, which is in turn required for its full biological activity. Consistent with this hypothesis, the activities of many regulatory proteins of DNA-containing viruses are posttranslationally modified by cellular cdks. For example, cdk-mediated posttranslational modification of the papillomavirus replication initiation protein, E1, is important for viral DNA replication (51, 57, 61), and similar modifications of simian virus 40 large T antigen are important for its nuclear import and for viral DNA replication (42, 62). In a previous study, the posttranslational modification state of ICP0 mediated by Rosco-sensitive cdks appeared to occur by mechanisms other than, or in addition to, phosphorylation (16). Amino acid sequence analysis of ICP0 (MacVector; Genetics Computer Group, Madison, Wis.) suggests that it is potentially posttranslationally modified in multiple ways, including phosphorylation, nucleotidylylation, acetylation, glycosylation, and myristoylation. Phosphorylation and nucleotidylylation are the only modifications of ICP0 reported to date (1, 7, 87). Of these, only the phosphorylation state of ICP0 has been examined in the presence of Rosco or Rosco-sensitive cdks. In one report, cdk-1, a Rosco-sensitive cdk, was shown to phosphorylate ICP0 directly in vitro (3), and the phosphorylation state of ICP0 was shown to be sensitive to Rosco (2). In our hands, a 33% reduction in the level of phosphorylation of ICP0 synthesized in the presence of Rosco was observed (16). Structure-function studies have not yet established whether Rosco-sensitive cdks phosphorylate ICP0 directly or indirectly in vivo. Ultimately, determining how cdks modify ICP0 will be critical to understanding the roles of these modifications in the many functions of ICP0.
Acknowledgments
This work was supported by Public Health Service grants RO1CA20260 from the National Cancer Institute (P.A.S.) and RO1AI41136 from the National Institute of Allergy and Infectious Diseases (G.G.M.). D.J.D. was supported by American Cancer Society Fellowship PF-00-021-01-MBC.
We thank members of the Schaffer laboratory and the Boston area herpesvirus group for helpful comments and suggestions.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcalay, M., L. Tomassoni, E. Colombo, S. Stoldt, F. Grignani, M. Fagioli, L. Szekely, K. Helin, and P. G. Pelicci. 1998. The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein. Mol. Cell. Biol. 18:1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anton, L. C., U. Schubert, I. Bacik, M. F. Princiotta, P. A. Wearsch, J. Gibbs, P. M. Day, C. Realini, M. C. Rechsteiner, J. R. Bennink, and J. W. Yewdell. 1999. Intracellular localization of proteasomal degradation of a viral antigen. J. Cell Biol. 146:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascoli, C. A., and G. G. Maul. 1991. Identification of a novel nuclear domain. J. Cell Biol. 112:785-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaho, J. A., C. Mitchell, and B. Roizman. 1993. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J. Virol. 67:3891-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 10.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W. Z., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrozza, M. J., and N. A. DeLuca. 1996. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol. Cell. Biol. 16:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 15.Davido, D. J., and D. A. Leib. 1996. Role of cis-acting sequences of the ICPO promoter of herpes simplex virus type 1 in viral pathogenesis, latency and reactivation. J. Gen. Virol. 77:1853-1863. [DOI] [PubMed] [Google Scholar]
- 16.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai, P., R. Ramakrishnan, Z. W. Lin, B. Osak, J. C. Glorioso, and M. Levine. 1993. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J. Virol. 67:6125-6135. (Erratum, J. Virol. 68:1264, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de The, H., C. Lavau, A. Marchio, C. Chomienne, L. Degos, and A. Dejean. 1991. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66:675-684. [DOI] [PubMed] [Google Scholar]
- 19.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G(1)/S block in asynchronously growing cells and prevents G(1) entry in quiescent cells. Virology 267:335-349. [DOI] [PubMed] [Google Scholar]
- 21.Epstein, A. L. 1984. Immunobiochemical characterization with monoclonal antibodies of Epstein-Barr virus-associated early antigens in chemically induced cells. J. Virol. 50:372-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D. 1985. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 4:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 24.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 20:7266-7273. [DOI] [PubMed] [Google Scholar]
- 25.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, R. D. 1984. trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everett, R. D., A. Orr, and M. Elliott. 1991. High level expression and purification of herpes simplex virus type 1 immediate early polypeptide Vmw110. Nucleic Acids Res. 19:6155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelman, I. H., and S. Silverstein. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 82:5265-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gius, D., and L. A. Laimins. 1989. Activation of human papillomavirus type 18 gene expression by herpes simplex virus type 1 viral transactivators and a phorbol ester. J. Virol. 63:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glickman, M. H., and A. Ciechanover. 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82:373-428. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62:196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gongora, C., G. David, L. Pintard, C. Tissot, T. D. Hua, A. Dejean, and N. Mechti. 1997. Molecular cloning of a new interferon-induced PML nuclear body-associated protein. J. Biol. Chem. 272:19457-19463. [DOI] [PubMed] [Google Scholar]
- 36.Goto, H., S. Motomura, A. C. Wilson, R. N. Freiman, Y. Nakabeppu, K. Fukushima, M. Fujishima, W. Herr, and T. Nishimoto. 1997. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev. 11:726-737. [DOI] [PubMed] [Google Scholar]
- 37.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobbs, W. E., D. E. Brough, I. Kovesdi, and N. A. DeLuca. 2001. Efficient activation of viral genomes by levels of herpes simplex virus ICP0 insufficient to affect cellular gene expression or cell survival. J. Virol. 75:3391-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319-3330. [DOI] [PubMed] [Google Scholar]
- 40.Ishov, A. M., and G. G. Maul. 1996. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 134:815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jans, D. A., M. J. Ackermann, J. R. Bischoff, D. H. Beach, and R. Peters. 1991. p34cdc2-mediated phosphorylation at T124 inhibits nuclear import of SV-40 T antigen proteins. J. Cell Biol. 115:1203-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan, R., and P. A. Schaffer. 1997. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J. Virol. 71:6850-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan, R., L. Schang, and P. A. Schaffer. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J. Virol. 73:8843-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakizuka, A., W. H. Miller, Jr., K. Umesono, R. P. Warrell, Jr., S. R. Frankel, V. V. Murty, E. Dmitrovsky, and R. M. Evans. 1991. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66:663-674. [DOI] [PubMed] [Google Scholar]
- 46.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 47.Lallemand-Breitenbach, V., J. Zhu, F. Puvion, M. Koken, N. Honore, A. Doubeikovsky, E. Duprez, P. P. Pandolfi, E. Puvion, P. Freemont, and H. de The. 2001. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 193:1361-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lees-Miller, S. P., M. C. Long, M. A. Kilvert, V. Lam, S. A. Rice, and C. A. Spencer. 1996. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 70:7471-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehembre, F., S. Muller, P. P. Pandolfi, and A. Dejean. 2001. Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene 20:1-9. [DOI] [PubMed] [Google Scholar]
- 50.Lehming, N., A. Le Saux, J. Schuller, and M. Ptashne. 1998. Chromatin components as part of a putative transcriptional repressing complex. Proc. Natl. Acad. Sci. USA 95:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lentz, M. R., D. Pak, I. Mohr, and M. R. Botchan. 1993. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J. Virol. 67:1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Romancer, M., S. C. Cosulich, S. P. Jackson, and P. R. Clarke. 1996. Cleavage and inactivation of DNA-dependent protein kinase catalytic subunit during apoptosis in Xenopus egg extracts. J. Cell Sci. 109:3121-3127. [DOI] [PubMed] [Google Scholar]
- 53.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829-5835. [DOI] [PubMed] [Google Scholar]
- 56.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma, T., N. Zou, B. Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 59.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74:2679-2690. [DOI] [PubMed] [Google Scholar]
- 60.McConnell, K. R., W. S. Dynan, and J. A. Hardin. 1997. The DNA-dependent protein kinase catalytic subunit (p460) is cleaved during Fas-mediated apoptosis in Jurkat cells. J. Immunol. 158:2083-2089. [PubMed] [Google Scholar]
- 61.McShan, G. D., and V. G. Wilson. 2000. Contribution of bovine papillomavirus type 1 E1 protein residue 48 to replication function. J. Gen. Virol. 81:1995-2004. [DOI] [PubMed] [Google Scholar]
- 62.McVey, D., S. Ray, Y. Gluzman, L. Berger, A. G. Wildeman, D. R. Marshak, and P. Tegtmeyer. 1993. cdc2 phosphorylation of threonine 124 activates the origin-unwinding functions of simian virus 40 T antigen. J. Virol. 67:5206-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 64.Mosca, J. D., D. P. Bednarik, N. B. Raj, C. A. Rosen, J. G. Sodroski, W. A. Haseltine, G. S. Hayward, and P. M. Pitha. 1987. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 84:7408-7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Negorev, D., and G. G. Maul. 2001. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 20:7234-7242. [DOI] [PubMed] [Google Scholar]
- 67.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rouet, R., G. Raguenez, and J.-P. Salier. 1992. Optimized assays for quantifying transient expression of co-transfected β-galactosidase and CAT reporter genes. BioTechniques 13:700-702. [PubMed] [Google Scholar]
- 71.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samaniego, L. A., N. Wu, and N. A. DeLuca. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 71:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaffer, P. A., G. M. Aron, N. Biswal, and M. Benyesh-Melnick. 1973. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology 52:57-71. [DOI] [PubMed] [Google Scholar]
- 74.Schang, L. M., A. Bantly, M. Knockaert, F. Shaheen, L. Meijer, M. H. Malim, N. S. Gray, and P. A. Schaffer. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schang, L. M., A. Bantly, and P. A. Schaffer. 2002. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4. J. Virol. 76:7724-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seeler, J. S., A. Marchio, D. Sitterlin, C. Transy, and A. Dejean. 1998. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc. Natl. Acad. Sci. USA 95:7316-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song, B., J. J. Liu, K. C. Yeh, and D. M. Knipe. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326-334. [DOI] [PubMed] [Google Scholar]
- 80.Song, Q., S. P. Lees-Miller, S. Kumar, Z. Zhang, D. W. Chan, G. C. Smith, S. P. Jackson, E. S. Alnemri, G. Litwack, K. K. Khanna, and M. F. Lavin. 1996. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 15:3238-3246. [PMC free article] [PubMed] [Google Scholar]
- 81.Stabell, E. C., and P. D. Olivo. 1992. Isolation of a cell line for rapid and sensitive histochemical assay for the detection of herpes simplex virus. J. Virol. Methods 38:195-204. [DOI] [PubMed] [Google Scholar]
- 82.Sze, P., and R. C. Herman. 1992. The herpes simplex virus type 1 ICP6 gene is regulated by a ‘leaky’ early promoter. Virus Res. 26:141-152. [DOI] [PubMed] [Google Scholar]
- 83.Tebas, P., E. C. Stabell, and P. D. Olivo. 1995. Antiviral susceptibility testing with a cell line which expresses β-galactosidase after infection with herpes simplex virus. Antimicrob. Agents Chemother. 39:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Umene, K., and T. Nishimoto. 1996. Inhibition of generation of authentic genomic termini of herpes simplex virus type 1 DNA in temperature-sensitive mutant BHK-21 cells with a mutated CCG1/TAFII250 gene. J. Virol. 70:9008-9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber, P. C., and B. Wigdahl. 1992. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J. Virol. 66:2261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilcox, K. W., A. Kohn, E. Sklyanskaya, and B. Roizman. 1980. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol. 33:167-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu, W. S., S. Vallian, E. Seto, W. M. Yang, D. Edmondson, S. Roth, and K. S. Chang. 2001. The growth suppressor PML represses transcription by functionally and physically interacting with histone deacetylases. Mol. Cell. Biol. 21:2259-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu, Z., W. Cai, and P. A. Schaffer. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 68:3027-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]