Abstract
Rapid transmission of Borna disease virus occurred upon cohabitation of persistently infected and naive rats. Infectious virus, which was abundantly present in fresh urine samples of carrier rats, entered the brains of recipient rats via the olfactory route. Thus, susceptible farm animals possibly acquire the virus from persistently infected rats.
Borna disease virus (BDV) predominantly infects horses and sheep, where it can cause a fatal neurological disease called Borna disease (BD) (9, 20). Many fundamental questions regarding the epidemiology of BDV remain unsolved. Since transmission of BDV from diseased horses or sheep to uninfected animals does not appear to take place, a different natural reservoir of BDV which has not yet been identified must exist (22). Although indirect experimental evidence suggests transmission of BDV via the olfactory system (14), proof of this hypothesis is lacking. Thus, animal model systems which might help to answer some of these questions are urgently needed. Intracerebral or intranasal infection of adult Lewis rats with BDV leads to severe, often fatal neurological disease with symptoms resembling naturally occurring BD (4, 18). Histopathologically, a nonpurulent meningoencephalitis can be diagnosed. The disease is immune system mediated (23). Infection of newborn Lewis rats leads to lifelong virus persistence in the absence of gross inflammation and disease, likely a result of thymic clonal deletion of BDV-specific T cells (2, 21). In contrast to rats infected as adults, in which BDV is confined to the central and peripheral nervous systems, rats infected as newborns have detectable BDV in virtually all tissues (8, 13, 14). Infectious virus was also reported to be present in body secretions of these rats, such as urine and saliva (8, 14). Prompted by previous studies which suggested occasional BDV transmission from carrier rats to naive adult rats (14, 15), we examined whether some of the open questions in BDV epidemiology could be clarified with this animal model.
Ten persistently infected rats were available for this study. They had been infected as newborns with rat BDV (22, 25). They were between 3 and 14 months old when the cohabitation experiments were performed. In the first experiment, the 10 carrier rats were cohoused with one naive adult rat each. Seven recipient rats developed neurological symptoms between days 27 and 46 after the beginning of the cohabitation period (Table 1). They were usually sacrificed within 24 h after onset of disease. All diseased animals showed the typical signs of BD, such as apathy, ruffled fur, and bloody noses. Rats 69, 5, and 2, which were cohoused with carrier rats C, I, and J, respectively, were sacrificed in the absence of clinical symptoms at days 30 (rat 69), 77 (rat 2), and 95 (rat 5) after onset of cohabitation. The brains of all seven diseased rats as well as the brain of healthy rat 69 exhibited strong immunostaining for the N protein of BDV (Table 1), indicating successful transmission of BDV to these recipients. Perivascular and parenchymal immune cell infiltrates were further present in the brains of all diseased rats and in the brain of rat 69. Six of the seven rats with BD contained high levels of BDV-specific serum immunoglobulin G, whereas the serum antibody titers of the remaining animal (rat 52) and the symptom-less infected rat 69 were not clearly elevated (Table 1). No BDV-specific serum antibodies were present in recipient rats 2 and 5, which were cohoused with carrier rats I and J (Table 1). We found no viral antigen or signs of inflammation in the brains of these two animals, suggesting that carrier rats I and J were not able to transmit BDV. These two rats also failed to transmit the virus to naive rats 42 and 55 in a second attempt (Table 1), although postmortem analysis confirmed the carrier state of rats I and J (data not shown).
TABLE 1.
Transmission of BDV to naive Lewis rats by cohabitation with carrier rats
| Carrier rat (sex) | Recipient (sex) | Duration of contacta (days) | Day of first signs of disease | Infection statusb | Antibody titerc |
|---|---|---|---|---|---|
| A (f) | 12 (m) | 42 | 41 | + | 10,000 |
| B (f) | 6 (f) | 37 | 37 | + | 5,120 |
| C (f) | 69 (m) | 30 | None | + | <20 |
| D (m) | 52 (f) | 32 | 27 | + | 20 |
| E (m) | 65 (f) | 34 | 34 | + | 2,560 |
| F (f) | 71 (m) | 37 | 36 | + | 2,560 |
| G (m) | 74 (m) | 40 | 40 | + | 2,560 |
| H (m) | 80 (f) | 46 | 46 | + | 10,000 |
| I (m) | 5 (f) | 95 | None | − | <20 |
| 42 (f) | 66 | None | − | <20 | |
| J (f) | 2 (m) | 77 | None | − | <20 |
| 55 (m) | 88 | None | − | <20 |
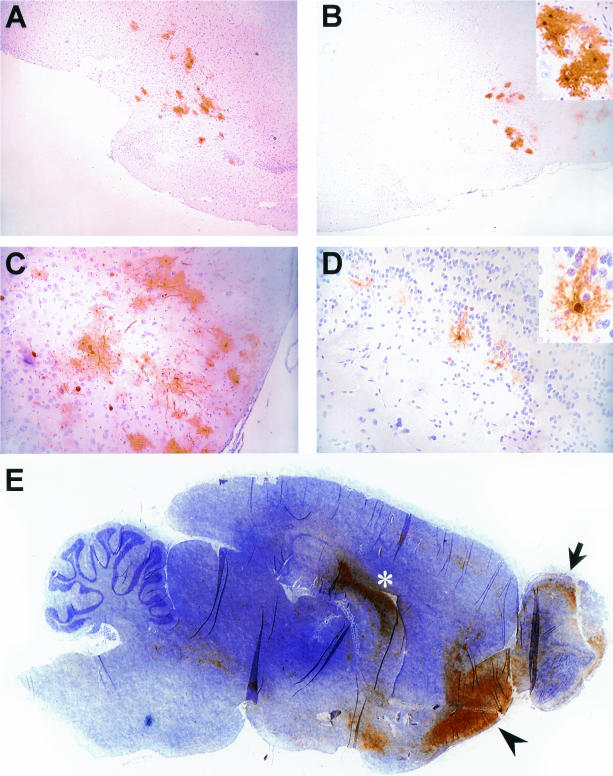
To determine the efficacy of BDV transmission, we cohoused naive rats for various times between 34 days and 1 h with carrier rat A or B (Table 2). All eight recipients that were cohoused with carrier rats A and B for 30 h or longer became infected, seroconverted, and exhibited neurological symptoms at the time of sacrifice. Of the eight recipients that were in contact with carrier rat A or B for only 21 to 28 h, three became infected and two of them developed neurological disease and BDV-specific serum antibodies. Infected recipient rat 28, which had been in contact with carrier rat B for 10 h, displayed transient mild symptoms but recovered subsequently, presumably due to a successful immune response. This rat had some BDV-specific antigen in the brain, confined to the hippocampus, and contained high levels of BDV-specific serum immunoglobulin G (Table 2). None of the six recipients that cohabitated with either of the two carrier rats for only 1 to 2 h became infected. However, one of them (rat 30) developed a serological response in the absence of detectable virus. It thus appeared that a brief contact was generally insufficient for virus transmission. Cohabitation for one or more days, on the other hand, frequently led to virus transmission and subsequent disease. To identify the sites of virus entry and initial replication in the brain, five naive rats were cohoused with carrier rat A and were sacrificed between 23 and 26 days after the onset of cohabitation. As expected, none of these rats displayed neurological symptoms during this short period. Immunohistochemical (IHC) analysis revealed that all but one rat became infected and that the degree of viral spread in the brains of the infected rats varied considerably. Whereas BDV had already spread to various brain regions in two rats (Fig. 1E and data not shown), few infected cells were found in the brains of two other rats (Fig. 1A to D). Intriguingly, in the latter rats, infected cells were almost exclusively located in the olfactory tubercle (Fig. 1A), the anterior olfactory nucleus (Fig. 1B and C), and the olfactory bulb (Fig. 1D). Additionally, some scattered BDV-positive cells were detectable in the septum, fornix, and thalamus (data not shown). The brain regions most strongest infected in the former two rats included the same brain regions as well as the ventral hippocampal commissure and the hippocampal CA3 region (Fig. 1E). Based on morphology, the majority of infected cells in these rats appeared to be neurons (Fig. 1, insets). Taken together, these data strongly suggest that BDV entered the brain via the olfactory route.
TABLE 2.
Efficacy of BDV transmission by carrier rats A and B
| Carrier rat | Recipient (sex) | Duration of contact | Day of first signs of disease | Time (days) between first contact and euthanization | Infection statusa | Antibody titerb |
|---|---|---|---|---|---|---|
| A | 13 (m) | 33 days | 33 | 33 | + | 2,560 |
| 1 (f) | 13 days | 34 | 34 | + | 20,000 | |
| 4 (m) | 13 days | 34 | 34 | + | 5,120 | |
| 3 (m) | 7 days | 33 | 33 | + | 2,560 | |
| 51 (m) | 5 days | 33 | 33 | + | 10,000 | |
| 24 (f) | 3 days | 33 | 33 | + | 10,000 | |
| 62 (f) | 30 h | 40 | 41 | + | 5,120 | |
| 76 (m) | 28 h | None | 45 | − | <10 | |
| 11 (m) | 24 h | 33 | 33 | + | 400 | |
| 38 (m) | 24 h | None | 41 | + | <10 | |
| 68 (f) | 24 h | None | 67 | − | <10 | |
| 40 (m) | 24 h | None | 44 | − | <10 | |
| 72 (m) | 21 h | None | 57 | − | <10 | |
| 79 (f) | 21 h | None | 102 | − | 40 | |
| 29 (m) | 1 h | None | 51 | − | <10 | |
| 35 (m) | 1 h | None | 44 | − | <10 | |
| B | 14 (m) | 34 days | 34 | 34 | + | 2,560 |
| 70 (m) | 21 h | 44 | 44 | + | 5,120 | |
| 28 (f) | 10 h | Transientc | 42 | + | 20,000 | |
| 34 (m) | 2 h | None | 56 | − | <10 | |
| 33 (m) | 2 h | None | 56 | − | <10 | |
| 30 (f) | 1 h | None | 51 | − | 800 | |
| 36 (m) | 1 h | None | 52 | − | <10 |
The infection status was assessed by IHC analysis of brain sections using monoclonal antibody Bo18.
Antibody titers were determined by IFA.
See text for details.
FIG. 1.
Identification of the route of BDV spread in brains of rats infected by cohabitation. Rats were exposed to carrier rat A for 7 days or less and sacrificed 23 to 25 days after onset of cohabitation. Sections of paraformaldehyde-fixed, paraffin-embedded brains were subjected to IHC to detect BDV-infected cells with BDV-N-specific monoclonal antibody Bo18 (3) (brown stain). Sections were counterstained with Mayer′s hematoxylin. (A and B) Two immediately adjacent brain regions of one section. BDV-positive cells in panel A are located in the area of the olfactory tubercle and the ventral pallidum. BDV-positive cells in panels B and C are located in the anterior olfactory nucleus. (D) Part of the olfactory bulb. (E) Ventral hippocampal commissure (asterisk), the region of the anterior olfactory nucleus (arrowhead), and the olfactory bulb (arrow). Brain regions were identified based on maps of Paxinos and Watson (16). (B and D insets) BDV-positive neurons at a higher magnification.
To determine the source of virus, various body secretions of several carrier rats, including urine, feces, saliva, and nasal secretions, were examined for the presence of infectious BDV by titration on Vero cells. Feces, saliva, and nasal secretions collected from carrier rats A and G did not contain measurable amounts of infectious virus (data not shown), whereas urine did. In a first experiment, large samples of urine (0.5 ml) collected from carrier rats A, B, D, E, G, and H were used as the inoculum. Immunofluorescence (IFA) analysis of the exposed Vero cells after one cell culture passage revealed that BDV was present in urine of all rats analyzed (data not shown). Urine samples of rats A and B contained markedly less infectious BDV than the others. New urine samples from four of these six animals were available for more-careful titration. The BDV titers of these urines ranged from 500 to 2 × 104 focus-forming units per ml. Multiple urine samples taken from individual animals over a period of 3 weeks revealed some titer fluctuation (Table 3).
TABLE 3.
Titers of infectious BDV in urine of four carrier rats
| Carrier rat | Titer for sample:a
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| B | 1.5 × 103 | 1.5 × 103 | 5 × 102 | 1.5 × 103 | ND |
| D | 2 × 104 | 2 × 103 | ND | 3 × 103 | 1.3 × 104 |
| E | NDb | 2 × 104 | 3 × 103 | ND | 2 × 104 |
| H | 2 × 104 | 1.5 × 104 | 8 × 103 | 1.5 × 104 | 1.5 × 104 |
Titers are expressed as focus-forming units per milliliter of urine on Vero cells.
ND, no urine available.
To further investigate the mode of transmission, four naive female adult rats were housed individually for 19 to 134 days in cages in which carrier rats A, B, and D had been kept before. We used only cages in which carrier rats had been housed for several days, and we brought the recipient rats into these cages as soon as the carrier rats had been removed. Used cages were replaced every 5 to 7 days. None of the four recipient rats that we used for this experiment became diseased, and none contained viral antigen in the brain. In addition, BDV-specific serum antibody titers of these rats remained below 1:20. Thus, the used cages were not infectious for rats. Rather, cohousing of infected and uninfected animals appeared to be required for virus transmission. If BDV was transmitted by urine, this way of transmission was efficient only when the urine was fresh. We therefore determined whether fresh urine of carrier rats was infectious for naive rats if administered experimentally into the noses of the recipient animals. Six Lewis rats were administered 10-μl samples of urine by the intranasal route. Based on the titration on Vero cells, this inoculum contained approximately 200 focus-forming units of BDV. Five of the six inoculated rats developed severe BD within 24 to 30 days postinoculation. Infection with BDV of these animals was confirmed by postmortem immunostaining of brain sections (data not shown). No BDV antigen could be detected in the brain of the remaining healthy rat, which was sacrificed on day 36 after intranasal inoculation.
Our results are in agreement with and largely extend original observations by Nitzschke (15), who reported in 1963 that four of nine adult rats which were housed together with carrier rats for 4 to 8 weeks acquired the infection. Our study revealed that much shorter cohousing periods are sufficient for efficient transmission of BDV. Animal-to-animal contact for at least 2 days guaranteed successful infection, although as little as 10 h of exposure was sometimes sufficient. The majority of infected animals in our study became sick at about 5 to 6 weeks after first contact with carrier rats. In another study in which carrier rats infected their mothers when kept in contact for 6 months (14), the first signs of neurological disease were observed in mother rats when the offspring were 3 to 5 months old. The much shorter incubation times in our experiments might be explained by assuming that carrier rats do not secrete infectious virus particles at a very young age. This notion is supported by our observation that none of the 16 mothers of rats infected as newborns in our facility ever acquired the infection (unpublished data). We always separated mother and offspring before day 40 postpartum.
BDV transmission via urine was discussed by Zwick and coworkers more than 70 years ago (26). For several reasons we assume that transmission of BDV by our carrier rats occurred via urine rather than via saliva or tears, which were previously reported to harbor infectious virus (14). We failed to detect infectious BDV in saliva or nasal secretions of both animals that we examined. In contrast, up to 20,000 infectious units of BDV per ml of urine could be detected. The high titers of BDV in urine readily explain why our carrier rats transmitted the virus so efficiently. The view that BDV primarily is transmitted via urine is supported by the observation that transmission of BDV by direct experimental application of small volumes of fresh infectious urine into the noses of recipient animals was very efficient. Consistent with the detection of BDV in the urine of newborn infected rats, Morales (13) demonstrated large amounts of BDV antigen in the nerve fibers and epithelium as well as in the tunica muscularis of the bladders of these rats. Considering these facts, we were surprised that transmission of BDV from carrier to recipient rats required cohousing of the animals. Since infectious urine is adsorbed fast by the cage bedding used in our animal facility, a simple explanation might be that BDV loses its infectivity very quickly under these conditions. It remains unclear why 2 of our 10 carrier rats failed to transmit BDV to cage mates. Most likely, the titer of infectious virus in the urine of these two rats was below a critical threshold level. It is conceivable that the tolerance in rats I and J was incomplete and that the remaining antiviral immune response prevented efficient spread of BDV to the peripheral sites. This view is supported by our observation that rat J displayed nontypical neuropathology (data not shown) that appeared to result from an immune attack.
Morales and coworkers (14) noticed that infectious virus was present in the nasal mucous membrane of mother rats that acquired the virus from their experimentally infected litter. Our analysis of brain sections from rats that were sacrificed at 23 to 25 days after the onset of cohousing with carrier rats supports Morales's findings and the hypothesis that BDV entered the brain via the olfactory route. BDV-infected cells were mainly found in the olfactory bulb and regions of the olfactory cortex, such as the anterior olfactory nucleus and olfactory tubercle. From these regions, BDV seems to spread to the hippocampus via the anatomical connections including the fornix, septum, and ventral hippocampal commissure. Notably, a similar spread of BDV following experimental infection of Lewis rats by the intranasal route has been described (14), strengthening the view that BDV entry into the brain was via the olfactory route in our experimental setting.
The natural reservoir of BDV and the mode of transmission remain speculative. The popular view that BDV is transmitted from diseased horses or sheep is not supported well by epidemiological data (for comprehensive reviews see references 9 and 22). Viral RNA was found in saliva of naturally infected horses (7, 11, 19), but infectious BDV was not detected except in a single saliva specimen from a diseased horse (7). We favor the alternative hypothesis that horses, sheep, and other natural hosts of BDV acquire the infection from a different, unidentified source. We previously argued (22) that several epidemiological parameters are compatible with a rodent reservoir of BDV in areas of endemicity. From experimental work with rats infected as newborns, it is likely that infection of wild rats might not result in serious disease. Notably, inoculation of adult black hooded rats with BDV has been shown to induce persistent infection in the absence of disease (6). If persistently infected wild rats secreted infectious virus with the urine like Lewis rats infected as newborns, it is easily conceivable that animal feed might get contaminated and that susceptible farm animals could acquire BDV infections while eating such feed. No published study addressed the important question of whether rats or other rodents from regions of endemicity in Europe are persistently infected with BDV. Negative reports are available only from areas in Asia where BDV is not endemic (5, 24). Note that some other viruses, such as the Seoul type hantavirus and Lassa virus are transmitted horizontally via infectious urine (10, 12).
Since the debate on a possible role of BDV in human neuropsychiatric disorders has not come to an end (17), animal keepers and laboratory personal should be aware of the fact that rats infected as newborns frequently secrete large amounts of infectious BDV in the urine. This report provides a rational basis for future debates on the biological safety requirements for work with persistently infected rats.
Acknowledgments
We thank Christel Hässler and Rosita Frank for excellent technical assistance and Steven Rubin, Mikhail Pletnikov, Mathias Rauer, and Otto Haller for helpful discussions and critical comments on the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Billich, C., C. Sauder, R. Frank, S. Herzog, K. Bechter, K. Takahashi, H. Peters, P. Staeheli, and M. Schwemmle. 2002. High-avidity human serum antibodies recognizing linear epitopes of Borna disease virus. Biol. Psychiatry 51:979-987. [DOI] [PubMed] [Google Scholar]
- 2.Carbone, K. M., S. W. Park, S. A. Rubin, I. I. Waltrip, and G. B. Vogelsang. 1991. Borna disease: association with a maturation defect in the cellular immune response. J. Virol. 65:6154-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geib, T., C. Sauder, S. Venturelli, C. Hässler, P. Staeheli, and M. Schwemmle. 2003. Selective virus resistance conferred by expression of Borna disease virus nucleocapsid components. J. Virol. 77:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 5.Hagiwara, K., M. Asakawa, L. Liao, W. Jiang, S. Yan, J.-J. Chai, Y. Oku, K. Ikuta, and M. Ito. 2001. Seroprevalence of Borna disease virus in domestic animals in Xinjiang, China. Vet. Microbiol. 80:383-389. [DOI] [PubMed] [Google Scholar]
- 6.Herzog, S., K. Frese, and R. Rott. 1991. Studies on the genetic control of resistance of black hooded rats to Borna disease. J. Gen. Virol. 72:535-540. [DOI] [PubMed] [Google Scholar]
- 7.Herzog, S., K. Frese, and R. Rott. 1994. Ein Beitrag zur Epizootiologie der Bornaschen Krankheit beim Pferd. Wien. Tierarztl. Monschr. 81:374-379. [Google Scholar]
- 8.Herzog, S., C. Kompter, K. Frese, and R. Rott. 1984. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med. Microbiol. Immunol. 173:171-177. [DOI] [PubMed] [Google Scholar]
- 9.Ikuta, K., K. Hagiwara, H. Taniyama, and N. Nowotny. 2002. Epidemiology and infection of natural animal hosts, p. 87-124. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 10.Kariwa, H., M. Fujiki, K. Yoshimatsu, J. Arikawa, I. Takashima, and N. Hashimoto. 1998. Urine-associated horizontal transmission of Seoul virus among rats. Arch. Virol. 143:15-24. [DOI] [PubMed] [Google Scholar]
- 11.Lebelt, J., and K. Hagenau. 1996. Die Verteilung des Bornavirus in natürlich infizierten Tieren mit klinischer Erkrankung. Berl. Muench. Tierarztl. Wochenschr. 109:178-183. [PubMed] [Google Scholar]
- 12.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 13.Morales, J. A. 1988. Immunhistologische Verlaufsuntersuchungen über die Ausbreitung des Virus der Bornaschen Krankheit bei der Lewis-Ratte nach perinataler intrazerebraler Infektion. Vet. Red. thesis. Universität Giessen, Giessen, Germany.
- 14.Morales, J. A., S. Herzog, C. Kompter, K. Frese, and R. Rott. 1988. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med. Microbiol. Immunol. 177:51-68. [DOI] [PubMed] [Google Scholar]
- 15.Nitzschke, E. 1963. Untersuchungen über die experimentelle Bornavirus-Infektion bei der Ratte. Zentbl. Vetmed. Reihe B 10:470-527. [Google Scholar]
- 16.Paxinos, G., and C. Watson. 1998. The rat brain in stereotaxic coordinates. Academic Press, San Diego, Calif.
- 17.Planz, O., K. Bechter, and M. Schwemmle. 2002. Human Borna disease virus infection, p. 179-226. In K. M. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 18.Pletnikov, M., D. Gonzalez-Dunia, and L. Stitz. 2002. Experimental infection: pathogenesis of neurobehavioral disease, p. 125-178. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 19.Richt, J. A., J. E. Clements, S. Herzog, J. Pyper, K. Wahn, and H. Becht. 1993. Analysis of virus-specific RNA species and proteins in Freon-113 preparations of the Borna disease virus. Med. Microbiol. Immunol. 182:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richt, J. A., and R. Rott. 2001. Borna disease virus: a mystery as an emerging zoonotic pathogen. Vet. J. 161:24-40. [DOI] [PubMed] [Google Scholar]
- 21.Rubin, S. A., A. M. Sierra-Honigmann, H. M. Lederman, I. I. Waltrip, J. J. Eiden, and K. M. Carbone. 1995. Hematologic consequences of Borna disease virus infection of rat bone marrow and thymus stromal cells. Blood 85:2762-2769. [PubMed] [Google Scholar]
- 22.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 23.Stitz, L., T. Bilzer, and O. Planz. 2002. The immunopathogenesis of Borna disease virus infection. Front. Biosci. 7:541-555. [DOI] [PubMed] [Google Scholar]
- 24.Tsujimura, K., T. Mizutani, H. Kariwa, K. Yoshimatsu, M. Ogino, Y. Morii, H. Inagaki, J. Arikawa, and I. Takashima. 1999. A serosurvey of Borna disease virus infection in wild rats by a capture ELISA. J. Vet. Med. Sci. 61:113-117. [DOI] [PubMed] [Google Scholar]
- 25.Zocher, M., S. Czub, J. Schulte-Mönting, J. C. de la Torre, and C. Sauder. 2000. Alterations in neurotrophin and neurotrophin receptor gene expression patterns in the rat central nervous system following perinatal Borna disease virus infection. J. Neurovirol. 6:462-477. [DOI] [PubMed] [Google Scholar]
- 26.Zwick, W., J. Witte, and F. Bert. 1932. Ueber das Vorkommen des Virus der Bornaschen Krankheit im Harn, im Blut und im Liquor cerebrospinalis. Berl. Tieraerztl. Wochenschr. 48:336-338. [Google Scholar]