Abstract
A recombinant replication-defective adenovirus vector that can overexpress the ectodomain of the envelope protein of dengue virus type 2 (NGC strain) has been constructed. This virus was immunogenic in mice and elicited dengue virus type 2 specific B- and T-cell responses. Sera from immunized mice contained neutralizing antibodies that could specifically recognize dengue virus type 2 and neutralize its infectivity in vitro, indicating that this approach has the potential to confer protective immunity. In vitro stimulation of splenocytes (from immunized mice) with dengue virus type 2 resulted in a significant proliferative response accompanied by the production of high levels of gamma interferon but did not show significant changes in interleukin-4 levels. This is suggestive of a Th1-like response (considered to be important in the maturation of cytotoxic T lymphocytes that are essential for the elimination of virus-infected cells). The data show that adenovirus vectors offer a promising alternative strategy for the development of dengue virus vaccines.
Dengue viruses, which are members of the Flaviviridae family, are mosquito-borne human pathogens with a worldwide prevalence (12). There are four antigenically distinct serotypes of dengue viruses (28). There is neither an effective antiviral therapy for the treatment of dengue virus infections nor a licensed vaccine for their prevention (12, 27). Infection with any one dengue virus serotype provides lifelong homologous immunity with only transient cross-protection against the remaining three serotypes (19). Sequential infection in areas of hyperendemicity (where multiple serotypes cocirculate) has the potential to trigger life-threatening disease widely believed to be mediated by an antibody-dependent enhancement mechanism (33). This has prompted the view that a dengue vaccine must be tetravalent; that is, it must afford solid and long-lasting protection against all four dengue virus serotypes.
Several laboratories worldwide are exploring multiple approaches towards developing dengue virus vaccines based on live attenuated viruses (1, 21, 36), inactivated viruses (35), infectious clone-derived intertypic (18, 26) and chimeric (5, 13, 14, 43) viruses, antigen-encoding plasmids (23, 24), recombinant proteins expressed in heterologous systems (2, 22, 38, 40), and live vaccinia virus vectors encoding antigen genes (9, 31, 32). However, the major focus is on the live, empirically attenuated (1, 21, 36), and infectious clone-derived ChimeriVax vaccines based on the attenuated YF17D yellow fever vaccine vector (13, 14). Alternative attenuated vector backbones based on dengue type 1 (DEN-1) (29, 45), DEN-2 (18), and DEN-4 (8) viruses are being developed in parallel. All these strategies rely on the creation of monovalent vaccine viruses, which are mixed together to generate tetravalent formulations.
Recent studies in which the tetravalent live attenuated (21) and ChimeriVax (13) vaccines were tested in humans and nonhuman primates, respectively, revealed that the tetravalent formulations elicited an unbalanced immune response, which was predominantly specific to a single serotype. This has been ascribed to viral interference that apparently comes into play when all four vaccine viruses are mixed together and coinjected (21). The observation that the tetravalent ChimeriVax vaccine formulation is also apparently associated with the phenomenon of viral interference (despite all four of its component viruses having identical YF17D backbones, unlike the live attenuated tetravalent vaccine) underscores the difficulties, and more importantly the risk, inherent in the current strategy of creating a tetravalent dengue vaccine. This warrants investigation of other recombinant viral vector systems that may permit the creation of a single tetravalent dengue virus vaccine vector. From such a perspective, the adenovirus (Ad) expression system appears worth investigating, as vectors are available that can accommodate inserts of up to ∼35 kb (16, 44), making it possible to envisage the creation of a single vaccine vector that encodes critical protective antigens of all four dengue virus serotypes to provide complete protection against dengue.
Ad vectors offer several important advantages from a vaccine perspective (reviewed in references 34 and 39). They have an exceptional safety record as live viral vaccines (10) and are not particularly pathogenic in humans (17). Two of the most promising recent reports pertaining to nonhuman primate models of the Ebola virus (41) and the human immunodeficiency virus (4, 37) emphasize the potential of Ad-based vaccination strategy. However, one concern regarding Ad vectors for human use is that preexisting immunity to Ad can compromise the efficacy of Ad-based vaccines. Recent work on Ad-based human immunodeficiency virus (4) and Ebola vaccines (46) has suggested that DNA priming followed by vector boosting can effectively overcome the effect of prior Ad immunity. Though poxvirus vectors can accommodate very large inserts, a comparison of attenuated poxvirus vectors such as NYVAC (11) and MVA (4, 37) with replication-defective Ad vectors have shown the latter to be safer and more efficacious for vaccine applications, particularly with reference to the primer-booster injection strategy.
To investigate the utility of the Ad vector in developing dengue virus vaccines, in this study we constructed a replication-defective recombinant Ad (rAd) vector by inserting the envelope (E) protein-encoding gene of DEN-2 virus into the early region 1 (E1) of the Ad genome. The E protein, which is exposed on the surface of the virus, is the dominant virus antigen (25) that interacts with host cell receptors (6) and represents the major target of neutralizing antibodies (7, 30). We used the ectodomain of the DEN-2E virus molecule obtained by deletion of the carboxy-terminal transmembrane domain. This truncation does not compromise the structural-antigenic integrity of the E protein. In fact, studies have shown that the truncated version is more immunogenic than the full-length molecule (32). We also included in our construct the putative secretory signal encoded by the carboxy terminus of the premembrane protein. It was necessary to include this signal sequence, as its deletion inhibited expression of the E protein (our unpublished data).
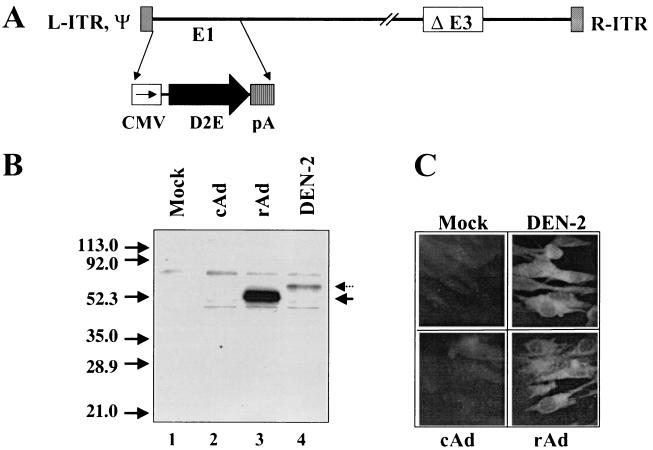
The E protein-encoding gene (DEN-2E virus gene) was isolated as a ∼1.3-kb cDNA fragment by reverse transcription-PCR using DEN-2 virus (NGC strain) genomic RNA as the template. The cloned gene was sequenced and verified by comparison with published data (20) to correspond to the carboxy-terminal 31-amino-acid-encoding portion (putative secretory signal sequence) of the premembrane gene followed by the amino-terminal 395-amino-acid-encoding portion (the ectodomain) of the E gene. The DEN-2E virus gene was placed under the transcriptional control of the cytomegalovirus (CMV) early promoter/enhancer in an appropriate shuttle vector, and the resultant expression cassette was then inserted into the E1 region of the Ad type 5 (Ad5) genome by using in vivo recombination in Escherichia coli and rescuing the resultant E1-lacking recombinant virus, rAdD2E, in the E1 trans-complementing human 293 cell line as described previously (15). The genome structure of rAdD2E (verified by PCR and extensive restriction analyses) is depicted in Fig. 1A.
FIG. 1.
rAd vector rAdD2E for the expression of DEN-2 virus E protein in mammalian cells. (A) Schematic representation of the linear genome of rAdDen2E. E1 denotes early region 1 of the Ad genome, which had been replaced by the E ectodomain expression cassette (CMV, CMV promoter/enhancer; D2E, cDNA encoding the E ectodomain of DEN-2 virus; pA, polyadenylation signal) in the recombinant virus. ΔE3 indicates a ∼2.7-kb deletion in the nonessential E3 region of the Ad genome. The gray-shaded boxes at either end of the viral genome represent the cis-acting elements critical for viral DNA replication (L-ITR and R-ITR, left and right inverted terminal repeats, respectively; ψ, packaging signal). (B) Immunoprecipitation (using DEN-2 virus-specific 3H5 mAb) of DEN-2E virus protein from infected BHK cells. Cells were either mock infected (lane 1) or infected with cAd (lane 2), rAdD2E (rAd; lane 3), or DEN-2 virus (lane 4). Infected cells were metabolically labeled with [35S]methionine, lysed, immunoprecipitated with 3H5 mAb, and analyzed on a denaturing 15% polyacrylamide gel. The positions and sizes (in kilodaltons) of prestained protein markers run on the same gel are indicated on the left of the panel. The arrows on the right indicate the positions of the full-length E protein expressed by DEN-2 virus (dotted arrow) and the C-terminally truncated version (E ectodomain) expressed by the rAd vector (solid arrow). (C) Indirect immunofluorescence micrograph of infected BHK cells. Cells were either mock infected or infected with the same viruses as described for panel B, fixed in cold acetone, and incubated with 3H5 mAb followed by fluorescein-labeled anti-mouse antibody to visualize E protein expression.
We examined the ability of this rAd to express DEN-2E virus protein in cultured mammalian cells. In the experiment shown in Fig. 1B, baby hamster kidney (BHK) cells were infected with rAdD2E, control Ad (cAd) (insertless Ad lacking E1), or DEN-2 virus and metabolically labeled with [35S]methionine. Lysates prepared from these cells were immunoprecipitated with 3H5 (42), the DEN-2E virus protein-specific monoclonal antibody (mAb), and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The 3H5 mAb specifically immunoprecipitated one major protein of the predicted size (∼53 kDa) from rAdD2E-infected cells (Fig. 1B, lane 3). The positive-control experiment carried out in parallel showed that DEN-2 virus-infected BHK cells expressed the full-length E protein (Fig. 1B, lane 4). We obtained similar results using COS cells in place of BHK cells. Interestingly, when we used 293 and HeLa cells, we detected the expression of rAd-encoded E ectodomain protein but not of DEN-2 virus-encoded full-length E protein (data not shown), presumably reflecting the inability of DEN-2 virus to successfully infect these cell types.
From a comparison of the expression levels attained (Fig. 1B; compare lanes 3 and 4), it is evident that rAdD2E can be used to achieve high levels of DEN-2E virus expression in infected cells. Using a purified preparation of recombinant E protein mutant containing the 3H5 epitope as a reference in a quantitative immunoblot assay, we estimated the levels of rAd-mediated E protein expression in infected BHK cells to be ∼2 μg/106 cells; in 293 cells, the levels were ∼8- to 10-fold higher (data not shown). Protein expression was also analyzed by immunofluorescence, as shown in Fig. 1C. Infected BHK cells were fixed in acetone and probed with 3H5 mAb. Antigen-antibody interactions were detected using fluorescein-tagged anti-mouse immunoglobulin G. Mock-infected as well as cAd-infected BHK cells failed to show any fluorescence in this assay. DEN-2 virus-infected BHK cells displayed intense fluorescence and served as the positive control. The rAdD2E-infected BHK cells manifested fluorescence comparable to that of the positive control. Interestingly, the difference in E protein expression levels evident in the immunoprecipitation experiment was not discernible in the immunofluorescence experiment. As the same antibody (3H5 mAb) was used in both techniques, the apparent discrepancy may be a reflection of differences in the detection methodologies.
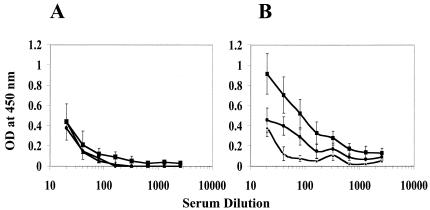
Next, we investigated the immunogenicity of the rAd vector. Groups of six BALB/c mice were immunized intraperitoneally with rAdD2E or cAd (107 PFU/mouse) at 0, 1, and 2 months. Sera (collected 1 week after each inoculation) were analyzed by enzyme-linked immunosorbent assays (ELISA) using live DEN-2 virus as the capture antigen. The data are depicted in Fig. 2. Sera collected after the initial injection had no significant antibody titers in either cAd- or rAdD2E-immunized animals. However, the presence of DEN-2 virus-specific antibodies could be discerned in the rAdD2E-immunized sera after the second injection; the levels detected increased quite significantly after the final injection (Fig. 2B). There was no change in the observed ELISA titers of cAd-immunized (Fig. 2A) or phosphate-buffered saline (PBS) (mock)-immunized (data not shown) mice after the second and third inoculations. The rAdD2E-induced antibodies specifically recognized the DEN-2 virus-expressed full-length E protein, as is evident from the data presented in Fig. 3. In this experiment, culture supernatants of DEN-2 virus-infected, [35S]methionine-labeled COS cells were immunoprecipitated with mouse immune sera collected after the first, second, and third immunizations. A control immunoprecipitation carried out in parallel using 3H5 mAb in place of mouse sera revealed the full-length E protein (Fig. 3, lane 2). The rAdD2E-immunized animals did not show any discernible anti-DEN-2 virus antibody response after the initial injection (Fig. 3, lane 5). However, after the booster immunizations an anti-DEN-2 antibody response was readily detectable in rAdD2E-immunized mouse sera (Fig. 3, lanes 8 and 11). The immunoprecipitated protein bands shown in lanes 8 and 11 comigrated with the 3H5 immunoprecipitated band shown in lane 2, indicating that the antibodies elicited by rAdD2E can efficiently recognize and immunoprecipitate the full-length E protein expressed by DEN-2 virus.
FIG. 2.
Anti-DEN-2 virus antibodies in rAd-immunized mouse sera. Groups of BALB/c mice (n = 6) were immunized intraperitoneally with either cAd (A) or rAdD2E (B) as described in the text. Anti-DEN-2 virus antibody titers in sera obtained after the first (triangles), second (circles), and third (squares) injections were determined with DEN-2 virus as the capture antigen and revealed with horseradish peroxidase-conjugated anti-mouse antibody. ELISA plates (96 well) coated overnight with DEN-2 virus (250 50% tissue culture infective doses [TCID50]) at 4°C were blocked (in 1× PBS-1% polyvinylpyrrolidone-0.2% horse serum for 2 to 4 h at 4°C), washed four times (using 1× PBS-0.1% Tween 20), and incubated for 45 min at 37°C with serial twofold dilutions of the individual mouse serum samples. The wells were washed (using 1× PBS-0.25% Tween 20) and incubated with anti-mouse immunoglobulin G-horseradish peroxidase conjugate (at a 1:5,000 dilution) for 45 min at 37°C, washed again, and incubated with 3,3′5,5′tetramethyl benzidine substrate for 25 min at 37°C. The color reaction was terminated by the addition of 1 M H2SO4, and the optical density (OD) was read at 450 nm. Each data point represents the average of six independent determinations (error bars represent standard deviations [SD]).
FIG. 3.
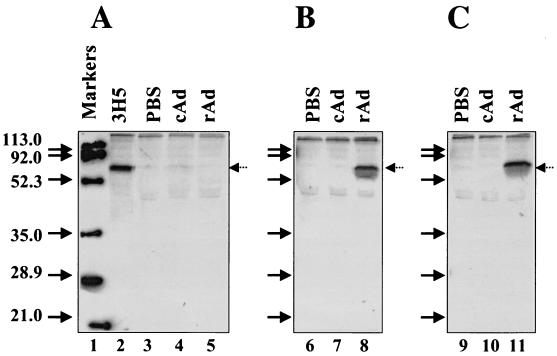
Analysis of rAdD2E-induced DEN-2 virus-specific antibodies in murine sera by radioimmunoprecipitation. Pooled sera (5 μl from each group) obtained from PBS (lanes 3, 6, and 9)-, cAd (lanes 4, 7, and 10)-, and rAd (lanes 5, 8, and 11)-immunized mice collected after the first (A), second (B), and third (C) vaccinations were incubated separately (overnight at 4°C) with 500 μl of DEN-2 virus-infected, [35S]methionine-radiolabeled COS cell culture supernatant. The resultant antigen-antibody complexes were captured using protein G Sepharose, washed, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A control immunoprecipitation using 3H5 mAb (1 μl) instead of murine antiserum (lane 2) was performed in parallel. Lane 1 shows prestained markers whose positions were traced with radioactive ink on the dried gel prior to autoradiography. The sizes of these markers are indicated (in kilodaltons) on the left side of the panel. The arrows on the right side of each panel indicate the positions of the DEN-2 virus-expressed full-length E protein.
To determine whether the antibodies induced by rAdD2E vaccination could neutralize DEN-2 virus infectivity, BHK cells in 96-well plates were infected with DEN-2 virus preincubated with twofold serial dilutions of heat (56°C for 30 min)-inactivated immune sera collected after each of the three vaccinations. Simultaneously, we also analyzed immune sera collected from animals that were immunized with a commercially available preparation of inactivated DEN-2 virus antigen. As the rAd vector used here is replication defective (i.e., lacking E1), we reasoned that it would be appropriate to compare its capacity to induce neutralizing antibodies with that of inactivated DEN-2 (iDEN-2) virus rather than with that of replication-competent live DEN-2 virus. The results are summarized in Table 1. It is evident from the data that at least one booster injection is necessary before virus-neutralizing antibodies can be detected in the sera of rAdD2E-immunized animals. This is consistent with the ELISA (Fig. 2) and immunoprecipitation (Fig. 3) data. Sera from the iDEN-2 virus-immunized animals also failed to manifest detectable neutralizing antibody titers after the initial injection. After a single booster immunization, the rAd vector elicited virus-neutralizing antibody titers comparable to those elicited by iDEN-2 virus. A second booster immunization with rAdD2E was accompanied by an increase in virus-neutralizing titers (from 17 to 73). This increase was comparable to that elicited by iDEN-2 virus after the second booster inoculation (from 15 to 106). The increases in neutralizing antibody titers accompanying the second booster inoculation in the two groups were within ∼1.5-fold of each other (73 versus 106). These data demonstrate that the rAdD2E is immunogenic and can elicit antibodies that can specifically recognize and neutralize DEN-2 virus.
TABLE 1.
Virus-neutralizing antibody titers in sera of immunized micea
| Immunization | Virus used for immunization (titer)
|
|||
|---|---|---|---|---|
| Noneb | cAdc | rAdd | iDEN-2e | |
| First | NDf | ND | ND | ND |
| Second | ND | ND | 17 | 15 |
| Third | ND | ND | 73 | 106 |
Aliquots of DEN-2 virus (250 TCID50) were preincubated with serial dilutions of heat-inactivated, pooled antisera and then used to infect BHK cells in 96-well plates (5 wells were assayed for each dilution of antiserum). The antiserum dilution corresponding to the 50% end points of virus infectivity was determined by cumulative averaging using the method of Reed and Muench as described by Burleson et al. (3); the reciprocal of this dilution is expressed as the neutralizing titer.
Mock immunization with PBS.
cAd lacking E1 (does not encode any dengue virus antigen).
rAdD2E (rAd lacking E1 and expressing DEN-2E virus protein).
Formalin-inactivated DEN-2 virus (from Biodesign International).
ND, No detectable virus-neutralizing antibody titer.
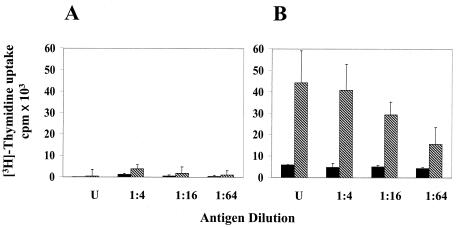
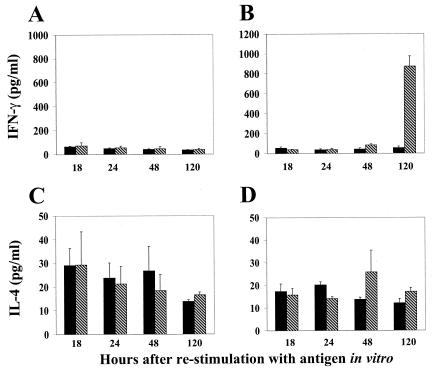
In addition to neutralizing antibodies, T-cell responses to dengue viruses are likely to be important in conferring protective immunity. To analyze T-cell response, spleen cells in culture (derived from cAd- and rAdD2E-primed mice) were restimulated with serial twofold dilutions of DEN-2 virus and monitored for proliferation responses and cytokine (gamma interferon [IFN-γ] and interleukin-4 [IL-4]) production. At all dilutions of DEN-2 virus used for in vitro restimulation, cAd-primed mouse splenocytes did not exhibit any proliferative response (Fig. 4A). In contrast, cells from rAdD2E-primed mouse spleens demonstrated a significant proliferation response (as evidenced by the magnitude of [3H]thymidine uptake) at all dilutions of DEN-2 virus used for in vitro restimulation (Fig. 4; compare panels B and A). Mock-stimulated spleen cell cultures, obtained from rAdD2E-primed mice, displayed a consistently minimal response (Fig. 4B). The proliferative response displayed by rAdD2E-primed mouse splenocytes was accompanied by IFN-γ secretion. These cells displayed significantly elevated levels of IFN-γ after 5 days of restimulation with DEN-2 virus; mock-stimulated cells did not secrete IFN-γ into the culture medium (Fig. 5B). When cAd-primed mouse spleen cell cultures were tested, IFN-γ was barely detectable in mock- and DEN-2 virus-stimulated culture supernatants (Fig. 5A). In contrast to the situation with IFN-γ, IL-4 was detected in the splenocyte culture supernatants during the entire course of the 5-day experiment. However, there were only marginal differences in the levels of secreted IL-4 between mock- and DEN-2 virus-stimulated spleen cell cultures regardless of whether they had been obtained from cAd- or rAdD2E-primed animals (Fig. 5, panels C and D). This leads to the conclusion that splenocytes from rAdD2E-primed mice respond to in vitro restimulation with DEN-2 virus by secreting elevated levels of IFN-γ without any significant change in the basal levels of IL-4 production. This may be indicative of a Th1 response, which is considered to be crucial for maturation of cytotoxic T lymphocytes that are necessary to eliminate virus-infected cells.
FIG. 4.
Determination of T-cell proliferative response. Splenocytes obtained from cAd-primed (A) and rAd-primed (B) mice were seeded in 96-well plates in Dulbecco's modified Eagle's medium-10% heat-inactivated fetal calf serum (2.5 × 105 cells in 0.1 ml/well). Cells were either mock stimulated (solid bars) or stimulated with live DEN-2 virus (hatched bars) (at several dilutions as indicated) for 5 days (U, undiluted virus equivalent to 1,000 TCID50). Each dilution was assayed in triplicate. Cells were pulsed with [3H]thymidine (1μCi/well) for 16 h at the end of the 5-day incubation period, and proliferation was quantitated by measuring the uptake of the radioisotope in a scintillation counter. Data depicted represent the mean values of three determinations (error bars represent SD).
FIG. 5.
IFN-γ and IL-4 responses to DEN-2 virus stimulation. Splenocytes obtained from cAd-primed (A and C) and rAd-primed (B and D) mice were either mock stimulated (solid bars) or stimulated with 1000 TCID50 of live DEN-2 virus (hatched bars) for 5 days. Aliquots of the culture supernatant were withdrawn at the time points shown, and the levels of IFN-γ (A and B) and IL-4 (C and D) were determined by solid-phase ELISA using commercially available murine IFN-γ and IL-4 kits (Quantikine M; R&D systems). Data depicted represent the mean values of three separate determinations (error bars represent SD).
In conclusion, our data show that the rAd vector expressing the ectodomain of DEN-2E virus protein is immunogenic in mice and is capable of inducing both B- and T-cell responses. This is the first report describing the use of a rAd vector as a possible dengue virus vaccine carrier. The Ad vector used in this work lacks early regions E1 and E3 and has an insert capacity of ∼7.5 kb, of which the CMV promoter-driven DEN-2E virus expression cassette occupies ∼2.3 kb. A vector lacking E1/E3/E4 (with an insert capacity of ∼11 kb) which can be propagated in 911E4 cells is available (15). Also, there are Ad vectors lacking all viral genes that have a maximum insert capacity of ∼35 kb and can be propagated in the presence of packaging-defective mutant Ad helpers (44). It is thus feasible to insert the major structural genes of all four of the dengue viruses into a single rAd vector. This aspect would make a rAd-based dengue virus vaccine an attractive alternative, especially since this strategy has the potential to eliminate the phenomenon of viral interference that tends to skew the immune response predominantly towards one serotype when the tetravalent vaccine is constituted from four monovalent viral vaccines (13, 21). Currently, work is under way to develop such a tetravalent rAd vector.
Acknowledgments
The work was supported by institutional core funds.
We thank Andrew Falconar and Bert Vogelstein for providing DEN-2 virus and the components of the AdEasy system, respectively. We are grateful to Radha Padmanabhan for advice on culturing dengue viruses and Pawan Sharma for his helpful suggestions. S.J. is a Senior Research Fellow supported by the Council of Scientific and Industrial Research, Government of India.
REFERENCES
- 1.Bhamarapravati, N., and Y. Sutee. 2000. Live attenuated tetravalent dengue vaccine. Vaccine 18:44-47. [DOI] [PubMed] [Google Scholar]
- 2.Bisht, H., D. A. Chugh, M. Raje, S. Swaminathan, and N. Khanna. 2002. Recombinant dengue virus type 2 envelope/hepatitis B surface antigen hybrid protein expressed in Pichia pastoris can function as a bivalent antigen. J. Biotechnol. 99:97-110. [DOI] [PubMed] [Google Scholar]
- 3.Burleson, F. G., T. M. Chambers, and D. L. Wiedbrauk. 1992. Virology: a laboratory manual, p. 53-61. Academic Press, New York, N.Y.
- 4.Casimiro, D. R., L. Chen, T.-M. Fu, R. K. Evans, M. J. Caulfield, M.-E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D.-M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in Rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, T. J., Y. Liang, D. A. Droll, J. J. Schlesinger, A. D. Davidson, P. J. Wright, and X. Jiang. 2003. Yellow fever virus/dengue-2 virus and yellow fever virus/dengue-4 virus chimeras: biological characterization, immunogenicity, and protection against dengue encephalitis in the mouse model. J. Virol. 77:3655-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., T. Maguire, and R. M. Marks. 1996. Demonstration of binding of dengue envelope protein to target cells. J. Virol. 70:8765-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crill, W. D., and R. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durbin, A. P., R. A. Karron, W. Sun, D. W. Vaughn, M. J. Reynolds, J. R. Perreault, B. Thumar, R. Men, C.-J. Lai, W. R. Elkins, R. M. Chanock, B. R. Murphy, and S. S. Whitehead. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 65:405-413. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca, B. A. L., S. Pincus, R. E. Shope, E. Paoletti, and P. W. Mason. 1994. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine 12:279-285. [DOI] [PubMed] [Google Scholar]
- 10.Gaydos, C. A., and J. C. Gaydos. 1999. Adenovirus vaccines, p. 609-628. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 3rd ed. W. B. Saunders Co., Philadelphia, Pa.
- 11.Gonin, P., W. Oualikene, A. Fournier, and M. Eloit. 1996. Comparison of the efficacy of replication-defective adenovirus and NYVAC poxvirus as vaccine vectors in mice. Vaccine 14:1083-1087. [DOI] [PubMed] [Google Scholar]
- 12.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guirakhoo, F., J. Arroyo, K. V. Pugachev, C. Miller, Z.-X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Soike, M. Ratterree, and T. P. Monath. 2001. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 75:7290-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guirakhoo, F., K. Pugachev, J. Arroyo, C. Miller, Z.-X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Draper, and T. P. Monath. 2002. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298:146-159. [DOI] [PubMed] [Google Scholar]
- 15.He, T.-C., S. Zhou, L. T. DaCosta, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hitt, M. M., and F. L. Graham. 2000. Adenovirus vectors for human gene therapy. Adv. Virus Res. 55:479-505. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, M. S. 2001. Adenoviruses, p. 2301-2326. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4rd ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 18.Huang, C. Y.-H., S. Butrapet, D. J. Pierro, G.-J. J. Chang, A. R. Hunt, N. Bhamarapravati, D. J. Gubler, and R. M. Kinney. 2000. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J. Virol. 74:3020-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 20.Irie, K., P. M. Mohan, Y. Sasaguri, R. Putnak, and R. Padmanabhan. 1989. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain). Gene 75:197-211. [DOI] [PubMed] [Google Scholar]
- 21.Kanesa-thasan, N., W. Sun, G. Kim-Ahn, S. Van Albert, J. R. Putnak, A. King, B. Raengsakulsrach, H. Christ-Schmidt, K. Gilson, J. M. Zahradnik, D. W. Vaughn, B. L. Innis, J.-F. Saluzzo, and C. H. Hoke, Jr. 2001. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 19:3179-3188. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, E. P., J. J. Greene, A. D. King, and B. L. Innis. 2000. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine 18:2549-2559. [DOI] [PubMed] [Google Scholar]
- 23.Kochel, T. J., K. Raviprakash, C. G. Hayes, D. M. Watts, K. L. Russell, A. S. Gozalo, I. A. Phillips, D. F. Ewing, G. S. Murphy, and K. R. Porter. 2000. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine 18:3166-3173. [DOI] [PubMed] [Google Scholar]
- 24.Konishi, E., M. Yamaoka, I. Kurane, and P. W. Mason. 2000. A DNA vaccine expressing dengue type 2 virus premembrane and envelope genes induces neutralizing antibody and memory B cells in mice. Vaccine 18:1133-1139. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn, R. J., W. Zhang, M. G. Rossman, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, C. J., M. Bray, R. Men, A. Cahour, W. Chen, H. Kawano, M. Tadano, K. Hiramatsu, I. Tokimatsu, A. Pletnev, S. Arakai, G. Shameem, and M. Rinaudo. 1998. Evaluation of molecular strategies to develop a live dengue vaccine. Clin. Diagn. Virol. 10:173-179. [DOI] [PubMed] [Google Scholar]
- 27.Leyssen, P., E. De Clercq, and J. Neyts. 2000. Perspectives for the treatment and infections with Flaviviridae. Clin. Microb. Rev. 13:67-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: The viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4rd ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 29.Markoff, L., X. Pang, H.-S. Houng, B. Falgout, R. Olsen, E. Jones, and S. Polo. 2002. Derivation and characterization of a dengue type 1 host range-restricted mutant virus that is attenuated and highly immunogenic in monkeys. J. Virol. 76:3318-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mégret, F., J. P. Hugnot, A. Falconar, M. K. Gentry, D. M. Morens, J. M. Murray, J. J. Schlesinger, P. J. Wright, P. Young, M. H. V. van Regenmortel, and V. Deubel. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue envelope glycoprotein. Virology 187:480-491. [DOI] [PubMed] [Google Scholar]
- 31.Men, R., L. Wyatt, I. Tokimatsu, S. Arakaki, G. Shameem, R. Elkins, R. Chanock, B. Moss, and C.-J. Lai. 2000. Immunization of rhesus monkeys with a recombinant of modified vaccinia virus Ankara expressing a truncated envelope glycoprotein of dengue type 2 virus induced resistance to dengue type 2 virus challenge. Vaccine 18:3113-3122. [DOI] [PubMed] [Google Scholar]
- 32.Men, R. H., M. Bray, C. J. Lai. 1991. Carboxy-terminally truncated dengue virus envelope glycoproteins expressed on the cell surface and secreted extracellularly exhibit increased immunogenicity in mice. J. Virol. 65:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morens, D. M. 1994. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 19:500-512. [DOI] [PubMed] [Google Scholar]
- 34.Polo, J. M., and T. W. Dubensky, Jr. 2002. Virus-based vectors for human vaccine applications. Drug Discov. Today 7:719-727. [DOI] [PubMed] [Google Scholar]
- 35.Putnak, R., D. A. Barvir, J. M. Burrous, D. R. Dubois, V. M. Dandrea, C. H. Hoke, J. C. Sadoff, and K. H. Eckels. 1996. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J. Infect. Dis. 174:1176-1184. [DOI] [PubMed] [Google Scholar]
- 36.Sabchareon, A., J. Lang, P. Chanthavanich, S. Yoksan, R. Forrat, P. Attanath, C. Sirivichayakul, K. Pengsaa, C. Pojjaroen-Anant, W. Chokejindachai, A. Jagsudee, J.-F Saluzzo, and N. Bhamarapravati. 2002. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am. J. Trop. Med. Hyg. 66:264-272. [DOI] [PubMed] [Google Scholar]
- 37.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 38.Staropoli, I., M.-P. Frenkiel, F. Mégret, and V. Deubel. 1997. Affinity-purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine 15:1946-1954. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson, J. 1998. Defective adenoviruses as novel vaccines for the flaviviridae. Clin. Diagn. Virol. 10:187-194. [DOI] [PubMed] [Google Scholar]
- 40.Sugrue, R. J., J. Fu, J. Howe, and Y.-C. Chan. 1997. Expression of the dengue virus structural proteins in Pichia pastoris leads to the generation of virus-like particles. J. Gen. Virol. 78:1861-1866. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z.-Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 42.Trirawatanapong, T., B. Chandran, R. Putnak, and R. Padmanabhan. 1992. Mapping of a region of dengue virus type-2 glycoprotein required for binding by a neutralizing monoclonal antibody. Gene 116:139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Most, R. G., K. Murali-Krishna, R. Ahmed, and J. H. Strauss. 2000. Chimeric yellow fever/dengue virus as a candidate dengue vaccine: quantitation of the dengue virus-specific CD8 T-cell response. J. Virol. 74:8094-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, Y., and S. Huang. 2000. Adenovirus technology for gene manipulation and functional studies. Drug Discov. Today 5:10-16. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead, S. S., B. Falgout, K. A. Hanley, J. E. Blaney, Jr., L. Markoff, and B. R. Murphy. 2003. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 77:1653-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, Z.-Y., L. S. Wyatt, W.-P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]