Abstract
The global eradication of poliomyelitis will require substantial changes in immunization practices. One of the proposed scenarios includes cessation of vaccination with live oral poliovirus vaccine (OPV) and the creation of an OPV stockpile for emergency response in case of the reintroduction of poliovirus into circulation. We describe here a retrospective analysis of the cessation of OPV usage in a region of the Byelorussian Republic of the former Soviet Union in 1963 to 1966. During this period, a widespread circulation and evolution of independent lineages of vaccine-derived polioviruses took place in the region. Some of these lineages appeared to originate from OPV given to 40 children in the community during this period of essentially no vaccinations. The data demonstrate very high risks associated with both the local cessation of OPV vaccination and the proposed use of OPV to control a possible reemergence of poliovirus in the postvaccination period. The high transmissibility of OPV-derived viruses in nonimmune population, documented here, and the known existence of long-term OPV excretors should be also considered in assessing risks of the synchronized global cessation of OPV usage.
The remarkable success of the Program for Global Eradication of Poliomyelitis, which has resulted in a >99% reduction of the incidence of paralytic poliomyelitis and an immense decrease in the circulation of wild polioviruses, mandates the formulation of an optimal strategy for the “endgame” (8, 19, 27, 30) that must include both vaccination policy and emergency response measures. Among the options under consideration is the complete and coordinated cessation of vaccination with the live oral poliovirus vaccine from Sabin strains (OPV [currently the most widely used type of polio vaccine]) soon after the world is certified free from circulating wild polioviruses (32). Major advantages of this strategy are obvious: it would prevent cases of vaccine-associated paralytic poliomyelitis, which occur at a rate of one case per ∼106 of the first doses of the vaccine distributed (32), and would save substantial financial and other resources that could be redirected toward solving other important public health problems. On the other hand, stopping vaccination will inevitably result in a dramatic growth in the nonimmune population and create an opportunity for a rapid spread of poliovirus should it ever be unintentionally or purposefully reintroduced into the population. This risk can only be assessed in large-scale experiments that are ethically unacceptable. However, important lessons can be learned from certain past epidemiological situations, which may be regarded as possible scenarios for the future. We describe here a retrospective analysis of such a situation.
MATERIALS AND METHODS
Virus isolation and preliminary characterization.
For virus isolation, HeLa cells or primary cultures of human embryonic cells were used. Restriction fragment length polymorphism analysis was performed as described previously (15).
DNA oligonucleotide microarray analysis.
DNA oligonucleotide microarray analysis was as described in detail elsewhere (7). Briefly, genotype-specific oligoprobes uniformly distributed throughout the genome were designed for selective detection of each of the Sabin strains. The 5′ ends of each oligonucleotide were modified by adding an aminolink group (TFA Aminolink CE reagent; PE Applied Biosystems) during the automated oligonucleotide synthesis to enable covalent immobilization on the aldehyde-coated glass surface. The probes specific for a given genotype were spotted as separate rows on silylated (aldehyde-coated) glass slides (Cell Associates, Inc.) by using a contact microspotting robot (Cartesian Technologies, Inc.). Five individual microarrays for the recombination analysis were spotted onto one slide for parallel analysis of five samples. Fluorescently labeled single-stranded DNA (ssDNA) for microarray hybridization was prepared from the PCR-amplified full-length double-stranded viral cDNA containing biotinylated primers on the 5′ ends by using streptavidin-coated magnetic beads (GenoVision). The ssDNA probes were labeled by a Micromax ASAP RNA labeling kit (Perkin-Elmer). Immediately prior to hybridization, fluorescently labeled probes were dried, reconstituted in Micromax ASAP hybridization buffer II and denatured for 1 min at 95°C. The final concentration of each probe in the hybridization solution did not exceed 0.2 μM. Aliquots (5 μl) were applied to the microarray area, which was then covered with a plastic coverslip, followed by incubation in an ArrayIt incubation chamber for at least 30 min at 45°C. Microchip images were taken by using a ScanArray 5000 (GSI Lumonics) confocal fluorescent scanner.
Genome sequencing.
Conditions for reverse transcription-PCR amplification and cycle sequencing were as described previously (21), with primers spanning the genome. Sequencing was performed in both directions, and every nucleotide position was sequenced at least once from each strand. Terminal sequences were determined by using the 5′ RACE (rapid amplification of cDNA ends) and 3′ RACE system kits (Life Technologies, Gaithersburg, Md.) according to the manufacturer's instructions.
The partial sequence of the isolate P2S/Mog66-4 has been previously reported (strain P2-V/236/66) (16).
Serological analysis.
Twofold dilutions of human sera were assayed for their ability to neutralize 100 50% tissue culture infective doses of wild-type poliovirus types 1, 2, and 3 (strains Lugovskoy, Smirnov, and Saukett, respectively).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here are available from the GenBank nucleotide sequence database under accession numbers AY278549 to AY278553.
RESULTS
General epidemiological description.
OPV immunization in Byelorussia (a republic within the former USSR) was started in 1959. From 1961 to 1966, two rounds of a mass nationwide campaign of immunization with trivalent vaccine (i.e., containing all of the three viral serotypes) were carried out during the winter-spring period; all children from the ages of 2 months (1961 and 1962 and 1965 onwards) or 3 months (1963 and 1964) up to 10 years (1962 and 1963) or 7 years (1964 to 1966) were immunized, with the coverage of >90%. To evaluate the duration of OPV-induced immunity (and under the theory prevailing at the time that OPV strains could not regain neurovirulence [cf. reference 28]), children in the city of Mogilev and Mogilev District (a total population of ∼160,000) were not immunized from March 1963 to March 1966, except for 40 children in six nurseries that received a dose of trivalent OPV each in March 1965 (9). We present here evidence of the widespread circulation of the OPV-derived polioviruses within a nonimmunized community.
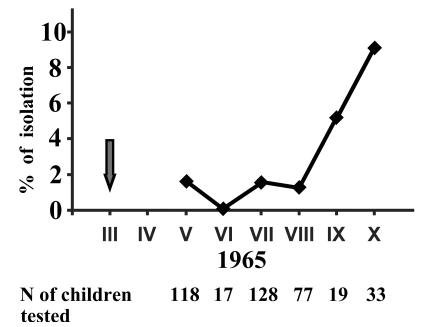
Attempts to isolate poliovirus from unvaccinated children started in May of 1965, i.e., roughly 2 years after the cessation of vaccination and 2 months after the limited reintroduction of OPV. During the 6-month observation period (May to October 1965), type 2 poliovirus was isolated from 9 of 392 randomly selected healthy unvaccinated children less than 3 years old (Fig. 1). The isolations were made in nurseries other than those where OPV was reintroduced, as well as from children not attending any such facilities. The frequency of positive isolations increased during the autumn, going in parallel with the frequency of isolation of nonpolio enteroviruses (data not shown). In addition, a type 2 poliovirus was isolated from a case of facial nerve paralysis (which is usually not associated with poliovirus infection) in January 1966. No polioviruses of the other two serotypes were isolated.
FIG. 1.
Isolation of poliovirus type 2 from unvaccinated children younger than 3 years old in the Mogilev District from May to October 1965. The arrow corresponds to the time of intentional limited introduction of OPV into six nurseries.
A serum survey for anti-poliovirus antibodies had also been conducted (9). Here, we were interested in unvaccinated children born at least 1 year after cessation of the OPV usage (and hence containing no maternal antibodies), i.e., the cohorts of 1 to 2 years of age in 1965 and 1 to 3 years of age in 1966 (the latter group were investigated before the resumption of vaccination). Of 57 such children (none of whom were from the nurseries where OPV was intentionally reintroduced in 1965), poliovirus type 2 neutralizing antibodies with titers of 1:8 or higher were detected in 21 children (37%), supporting the notion of wide circulation of this serotype. In addition, antibodies to poliovirus serotypes 1 and 3 were detected in nine (15%) and eight (14%) of these children, respectively. Although the investigated group was rather small, the results suggest that infection with all of the three poliovirus serotypes frequently occurred during the nonvaccination period.
Molecular characterization of the isolate genomes.
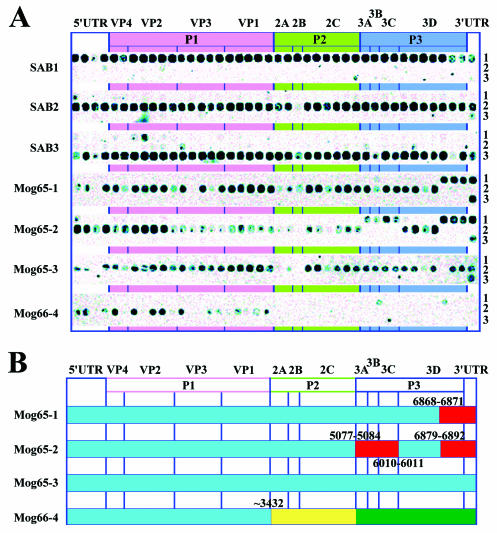
Of 10 virus isolates isolated in Mogilev in 1965 and 1966 from unvaccinated persons, 4 were available for characterization by current techniques (Table 1). The isolates came from three healthy children and one case of facial nerve paralysis. Restriction fragment length polymorphism data suggested that three of four isolates (P2S/Mog65-1, P2S/Mog65-2, and P2S/Mog66-4) had recombinant genomes with 5′-untranslated and capsid protein-encoding regions derived from Sabin 2 and that the remaining portions of the genome were derived from Sabin 1 (P2S/Mog65-1 and P2S/Mog65-2) or an unknown virus (P2S/Mog66-4) (data not shown). Oligonucleotide microarray analysis supported these conclusions (Fig. 2A) and demonstrated that the parts of the genome sequences encoding nonstructural proteins of the two former isolates had indeed originated from Sabin 1 (in isolate P2S/Mog65-2, there were two noncontiguous blocks of Sabin 1-derived sequences), whereas the origin of the “foreign” sequence in isolate P2S/Mog66-4 could not be traced by this technique either. In addition, the microarray data revealed that the relevant genome parts of the isolates diverged markedly from their Sabin 2 ancestor, as evidenced by the disappearance or weakening of hybridization with a number of genotype-specific oligonucleotide probes, isolate P2S/Mog66-4 exhibiting the highest level of divergence. Sequencing of the entire genomes of the isolates fully confirmed the above data. The structure of the genomes of these isolates is presented schematically in Fig. 2B.
TABLE 1.
Poliovirus strains isolated from unvaccinated children in Mogilev in 1965 and 1966
| Isolate number | Isolate name | Date of isolation (day.mo.yr) | Age of the patient (yr, mo) | Diagnosis |
|---|---|---|---|---|
| 20003 | P2S/Mog65-1 | 20.05.65 | 1, 8 | Healthy |
| 20077 | P2S/Mog65-2 | 11.08.65 | 2 | Healthy |
| 20120 | P2S/Mog65-3 | 06.10.65 | 2 | Healthy |
| 21043 | P2S/Mog66-4 | 24.01.66 | 1, 11 | Facial nerve paralysis |
FIG. 2.
Genome structure of the isolates. (A) DNA oligonucleotide microarray analysis. The figures on the right side of the panel correspond to the serotype specificity of the oligonucleotide rows. (B) Schematic representation of the sequencing results. The Sab1 and Sab2 derived sequences are in red and blue, respectively. The sequence distantly related to the corresponding part of the P1W/Bar65 genome is in green, and the sequence of unknown origin is in yellow. The coordinates of the crossover sites are indicated.
The non-Sabin part of the isolate P2S/Mog66-4 genome had a typical enterovirus nucleotide sequence. In a search for the possible origin of this RNA part, we compared its sequence with relevant regions of a variety of polioviruses and other enteroviruses available in the GenBank but did not find any reliable clues. However, interesting results were obtained when we compared the nucleotide sequence of P2S/Mog66-4 with that of a wild poliovirus type 1 (isolate 19276 or P1W/Bar65) isolated during the same period (19 August 1965) in another city of Byelorussia (Baranovichi, Brest Region) on day 33 after the onset of poliomyelitis. The 22-month-old patient came to Baranovichi from Kustanai (Kazakhstan) already presenting with the paralytic form of the illness. Isolates P2S/Mog66-4 and P1W/Bar65 appeared reasonably closely related (diverged by only 9%) within the 2,259-nucleotide interval of the P3 region (Table 2), suggesting the existence of a not-very-distant common ancestor for this part of their genomes. The 3′-untranslated regions of the two isolates also appeared to be more similar to each other than to those of any of the three Sabin serotypes, but the small length of this region and its very high conservation precluded any definite conclusions in this regard. On the other hand, there was no obvious relatedness between the two genomes in the central P2 region. It may be speculated that isolate P2S/Mog66-4 was a double recombinant, inheriting its P3 and P2 segments from a wild poliovirus of type 1 and from an unknown enterovirus, respectively.
TABLE 2.
Divergence of nucleotide sequences of different regions of the genome of isolate P2S/Mog66-4 from those of isolate P1W/Bar65 and of the three Sabin strainsa
| Virus | Divergence (%) of genomic region (length in nucleotides):
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2A (400) | 2B (291) | 2C (987) | 3A (261) | 3B (66) | 3C (549) | 3D (1,383) | 3′UTR (71 and 72) | P2 (1,678) | P3 (2,259) | |
| Bar65 | 24.5 | 21.6 | 19.6 | 9.6 | 0 | 6 | 10.6 | 2.8 | 21.1 | 9.0 |
| Sab1 | 23.3 | 23.7 | 20.3 | 16.5 | 16.7 | 15.8 | 14.8 | 5.6 | 21.6 | 15.3 |
| Sab2 | 20.3 | 20.3 | 18.6 | 18.4 | 24.2 | 15.7 | 15.7 | 5.6 | 19.3 | 16.2 |
| Sab3 | 20.8 | 20.3 | 18.5 | 14.9 | 19.7 | 15.3 | 14.8 | 4.2 | 19.4 | 15.1 |
The lengths of appropriate regions are indicated in parentheses in the column headings. Only 400 3′-terminal (non-Sabin) nucleotides of the 2A coding region were used for the calculations of values representing 2A and P2. The lengths of the 3′-untranslated regions (3′UTR) in Sab1 and Sab2 RNA are 72 and 71 nucleotides, respectively.
Isolates were of different “ages” and represented independent evolutionary lineages.
To gain insights into the possible origin of the isolates, we sought to estimate the time of their divergence from the Sabin strains. To this end, we needed data on the rate of accumulation of synonymous substitutions in the sequence encoding VP1 capsid protein of poliovirus. Because no such data for Sabin 2 were available, we used previously determined rates for Sabin 1 as an estimate: 3.28 × 10−2 (13) and 2.85 × 10−2 (1, 6) synonymous substitutions per synonymous site per year. These values were used to calculate the “ages” of the VP1-coding sequence of four isolates (Table 3). For isolates P2S/Mog65-3 and P2S/Mog66-4 there was a good correlation between the VP1 “age” and the time elapsed between their isolation and intentional OPV reintroduction in March 1965. However, isolate P2S/Mog65-1 appeared to be 3- to 3.5-fold “older,” and isolate P2S/Mog65-2 appeared to be 4- to 5-fold “younger” compared to their ages calculated under the assumption that they had originated from the reintroduced OPV. Although the precision of the estimation of the time of divergence of these isolates from their Sabin 2 predecessor was not very high due to a relatively small number of mutations accumulated, the results strongly suggested that the isolates started their independent evolution before, during, and after the limited intentional reintroduction of OPV.
TABLE 3.
Calculated “ages” of the VP1-coding region of the isolatesa
| Isolate name | Ks for VP1 coding regionb | “Age” (mo) | Time (mo) after OPV reintroduction |
|---|---|---|---|
| P2S/Mog65-1 | 1.6 | 6-7 | 2 |
| P2S/Mog65-2 | 0.3 | 1.1-1.2 | 5 |
| P2S/Mog65-3 | 1.6 | 6-7 | 7 |
| P2S/Mog66-4 | 2.1 | 8-9 | 10 |
Two other lines of evidence also demonstrated that all four isolates belonged to independent lineages. First, the recombinant genomes of three isolates had different crossover sites, whereas the fourth isolate was not a recombinant (Fig. 2B). Second, only a very small proportion of the mutations that had accumulated in the shared, i.e., Sabin 2-derived, portion of the isolate genomes (5′ untranslated and capsid protein-coding sequences) was common to any two isolates (Table 4). One of them was the A481→G in the 5′-untranslated region, while the other corresponded to nonsynonymous mutations at position 2909, resulting in the replacement of Ile143 of VP1 by another amino acid residue. Mutation A481→G and the mutation affecting Ile143 were not infrequently found in the Sabin 2 derivatives (2, 25, 26; unpublished data), suggesting that they may have an adaptive character. No common synonymous substitutions were present in the P1-coding region of the isolates.
TABLE 4.
Number of mutations in the 5′-untranslated region-P1 coding region (versus Sabin 2) shared by the isolatesa
| Isolate name | No. of mutations shared
|
||
|---|---|---|---|
| P2S/Mog65-2 | P2S/Mog65-3 | P2S/Mog66-4 | |
| P2S/Mog65-1 | 2 | 2 | 3 + 1 |
| P2S/Mog65-2 | 2 | 1 | |
| P2S/Mog65-3 | 1 | ||
Underlined numbers correspond to mutations A481→G (in the 5′-untranslated region) shared by all four isolates and U2909→C (corresponding to the replacement of Ile143 of VP1 by Thr) shared by the isolates P2S/Mog65-1, P2S/Mog65-2, and P2S/Mog65-3. In isolate P2S/Mog66-4, the same U residue mutated into A, resulting in the Ile143→Asn change. The total numbers of nucleotide substitutions in the 5′-untranslated region-P1 coding region, compared to the Sabin 2 ancestor, are 18, 6, 22, and 46 for the isolates P2S/Mog65-1, P2S/Mog65-2, P2S/Mog65-3, and P2S/Mog66-4, respectively.
DISCUSSION
The results reported here clearly show that local cessation of vaccination against poliomyelitis carries a high risk of the extensive dissemination of polioviruses and of OPV-derived polioviruses in particular. The rapid growth of cohorts of nonimmune children creates a fertile ground for such a development. Widespread circulation of derivatives of Sabin 2 virus was documented here by both virus isolation and a high prevalence of serotype-specific antibodies. Evidence for the circulation of Sabin 1 and Sabin 3 viruses in the population studied here was not so strong but is certainly deserving of attention, the more so since circulation of derivatives of the former in an inadequately vaccinated population has already been reported (4, 17). Remarkably, the latter examples concerned the circulation of OPV-derived polioviruses in developing countries with tropical climates and high population densities, whereas we documented here the circulation of such viruses in a European country with a moderate population density and a temperate climate.
Where did the reemerging viruses come from? A likely source was the OPV given to children in six nurseries during the nonimmunization period. Indeed, the “age” of the VP1-coding sequence of two isolates (P2S/Mog65-3 and P2S/Mog66-4) was consistent with their origin from the above source. On the other hand, there are reasons to suggest that the rapidly increasing poliovirus circulation also had sources other than the intentionally reintroduced OPV. The apparent “age” of isolate P2S/Mog65-1 was consistent with its divergence from Sabin 2 prior to March 1965, when OPV had been intentionally reintroduced into the population, whereas isolate P2S/Mog65-2 appeared to originate from a more recent unintentional import of a Sabin 2 virus. Furthermore, the presence in the genome of isolate P2S/Mog66-4 of a segment derived from a wild poliovirus is a clear indication of the circulation of non-OPV polioviruses within the rapidly expanding niche.
What lessons can be learned from this highly illuminating case? The first lesson is obvious, and it calls for strict adherence to a specially developed exit strategy. Among other things, this strategy must clearly answer questions related to immunization practices. What type of vaccine, if any, should be used for immunizations after eradication is complete? If vaccination can be stopped, is there any transition policy necessary to do it safely? Finally, a sound contingency plan must be developed for emergency response in case of reemergence of poliomyelitis. In developing this optimal strategy, we must face the reality that the eradication of poliomyelitis will inevitably result in decreased focus on maintaining an adequate vaccination coverage, which may create significant nonimmune “isles” surrounded by the OPV-rich environment. In view of the Mogilev experience described here, this cannot be considered an acceptable strategy, and the proposal to synchronously stop worldwide OPV vaccination (30) makes more sense than allowing uncoordinated and prolonged leveling off of OPV use. However, the abrupt withdrawal of all protection against poliomyelitis also carries substantial risks. In the long run, stopping all vaccinations will create a meta-stable epidemiological situation, in which the population of the entire world will be fully susceptible to poliomyelitis, so the safety can only be ensured by very strict containment measures. In the short term, the strategy is based on the assumption that OPV is excreted by the primary vaccinees only for relatively short periods of time, e.g., up to 2 to 3 months. However, the excretion may sometimes last significantly longer and, in very rare cases of immunodeficient persons, it may continue for years (1, 2, 13, 18, 22). The long-term excretors could represent a serious and increasing hazard for the growing susceptible population. Implicit in the simultaneous across-the-world-cessation of OPV usage also is the notion that spread of OPV-derived strains would be limited and of short duration. Although the transmissibility of these strains themselves is indeed relatively limited (10, 23), it is efficient enough to ensure the accumulation of mutations and the emergence of vaccine-derived strains with increased fitness and in some cases substantial pathogenicity and transmissibility (4-7, 17, 29). As demonstrated here, independent lineages of OPV-derived viruses could readily circulate in nonimmune populations, affecting a very significant proportion of the susceptible persons. Thus, the two key assumptions underlying the complete cessation of polio vaccinations are, to say the least, questionable. It should be admitted that there is no full consensus even among the authors of this study regarding the risk/benefit ratio of the simultaneous global cessation of OPV usage. We believe, however, that the data presented here should be taken into consideration in the process of making the far-reaching decisions on the endgame strategy.
Finally, an important and, we believe, unambiguous lesson concerns the use of OPV to control the possible reintroduction of poliovirus in the postvaccination era, which was also proposed (8, 11). The rapid dissemination of OPV derivatives within the nonimmune Mogilev population clearly demonstrates the inherent risks of such an option and demands consideration of alternative tactics. The inactivated polio vaccine (see, for example, references 24 and 25) is a possible substitute, but it does not induce intestinal immunity and may not be sufficiently effective in raising population immunity in the tropics to be counted on as a panacea. Another possibility is the development of more genetically stable attenuated strains of poliovirus. Although perhaps theoretically possible (25), their licensure is hardly practically feasible because of the enormous size of the clinical trials that would be needed to prove unambiguously that the new vaccine(s) is safer than the current OPV. Since, while OPV was in use in the United States, there was only a single-digit number of adverse reactions each year (3), to produce a statistically reliable comparison the clinical trial of the new live vaccine should probably involve the entire country. Moreover, some previous unfortunate experience (e.g., with the strain USOL-d-Bac) (see reference 25) is likely to discourage vaccine manufacturers from embarking on this endeavor. Therefore, new solutions should be considered. They may include improvement of the current inactivated polio vaccine by making it more efficacious in tropical countries and reducing its cost, as well as exploring entirely new approaches. These might include the development of a polio vaccine based on nonpathogenic enteric bacteria expressing engineered surface or secretable antigens or harboring viral cDNA (12, 14, 20, 31). Hopefully, such a vaccine may turn out to be safe, inexpensive, and effective. Obviously, the creation of a new vaccine could replace current vaccination policies and safely bring us into the world without OPV. Since the development and licensure may take considerable time, the creation of a coherent endgame strategy with explicit analysis of the needs for the new vaccines is an urgent priority.
Acknowledgments
This work was supported by grants from the World Health Organization, the U.S. Defense Advanced Research Project Agency, the National Vaccine Program Office, INTAS, and the Russian Foundation for Basic Research.
REFERENCES
- 1.Bellmunt, A., G. May, R. Zell, P. Pring-Akerblom, W. Verhagen, and A. Heim. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178-184. [DOI] [PubMed] [Google Scholar]
- 2.Butinelli, G., V. Donati, S. Fiore, J. Marturano, A. Plebani, P. Balestri, A. R. Soresina, R. Vivarelli, F. Delpeyroux, J. Martin, and L. Fiore. 2003. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J. Gen. Virol. 84:1215-1221. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Paralytic poliomyelitis-United States, 1980-1994. Morb. Mortal. Wkly. Rep. 46:79-83. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2001. Public health dispatch: acute flaccid paralysis associated with circulating vaccine-derived poliovirus-Philippines, 2001. Morb. Mortal. Wkly. Rep. 50:874-875. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2001. Circulation of a type 2 vaccine-derived poliovirus-Egypt, 1982-1993. Morb. Mortal. Wkly. Rep. 50:41-51. [PubMed] [Google Scholar]
- 6.Cherkasova, E. A., E. A. Korotkova, M. L. Yakovenko, O. E. Ivanova, T. P. Eremeeva, K. M. Chumakov, and V. I. Agol. 2002. Long-term circulation of vaccine-derived poliovirus resulting in paralytic disease. J. Virol. 76:6791-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherkasova, E., M. Laassri, V. Chizhikov, E. Korotkova, E. Dragunsky, V. I. Agol, and K. Chumakov. 2003. Microarray analysis of evolution of RNA viruses: evidence of circulation of virulent highly divergent vaccine-derived polioviruses. Proc. Natl. Acad. Sci. USA 100:9398-9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowdle, W. R., and S. L. Cochi. 2002. Global eradication of poliovirus: history and rationale, p. 473-480. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. American Society for Microbiology, Washington, D.C.
- 9.Feldman, E. V., V. I. Votyakov, I. B. Kardash, L. A. Gracheva, L. F. Telekovets, and L. P. Ralko. 1970. Summary of the study of the immunological structure of and spread of polioviruses in a population not immunized with OPV during a 3-year period, p. 187-201. In M. P. Chumakov (ed.), Enterovirus infections, vol. 14. Proceedings of the Institute of Poliomyelitis and Viral Encephalitides, Moscow, Russia.
- 10.Fine, P. E., and I. A. Carneiro. 1999. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 150:1001-1021. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P. E., R. W. Sutter, and W. A. Orenstein. 2001. Stopping a polio outbreak in the post-eradication era, p. 129-147. In F. Brown (ed.), Progress in polio eradication: vaccine strategies for the end game, vol. 105. S. Karger, Basel, Switzerland. [PubMed]
- 12.Galen, J. E., and M. M. Levine. 2001. Can a “flawless” live vector vaccine strain be engineered? Trends Microbiol. 9:372-376. [DOI] [PubMed] [Google Scholar]
- 13.Gavrilin, G. V., E. A. Cherkasova, G. Y. Lipskaya, O. M. Kew, and V. I. Agol. 2000. Evolution of circulating wild poliovirus and of vaccine-derived poliovirus in an immunodeficient patient: a unifying model. J. Virol. 74:7381-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentschev, I., G. Dietrich, S. Spreng, A. Kolb-Maurer, V. Brinkmann, L. Grode, J. Hess, S. H. Kaufmann, and W. Goebel. 2001. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine 19:2621-2628. [DOI] [PubMed] [Google Scholar]
- 15.Georgescu, M. M., F. Delpeyroux, M. Tardy-Panit, J. Balanant, M. Combiescu, A. A. Combiescu, S. Guillot, and R. Crainic. 1994. High diversity of poliovirus strains isolated from the central nervous system from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 68:8089-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillot, S., V. Caro, N. Cuervo, E. Korotkova, M. Combiescu, A. Persu, A. Aubert-Combiescu, F. Delpeyroux, and R. Crainic. 2000. Natural genetic exchanges between vaccine and wild poliovirus strains in humans. J. Virol. 74:8434-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kew, O. M., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C. J. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. van der Avoort, M. S. Oberste, D. Kilpatrick, S. Cochi, M. Pallansch, and C. de Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356-359. [DOI] [PubMed] [Google Scholar]
- 18.Kew, O. M., R. W. Sutter, B. K. Nottay, M. J. McDonough, D. R. Prevots, L. Quick, and M. A. Pallansch. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kew, O. M., and M. Pallansch. 2002. The mechanism of poliovirus eradication, p. 481-491. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. American Society for Microbiology, Washington, D.C.
- 20.Lee, J. S., K. S. Shin, J. G. Pan, and C. J. Kim. 2000. Surface-displayed viral antigens on Salmonella carrier vaccine. Nat. Biotechnol. 18:645-648. [DOI] [PubMed] [Google Scholar]
- 21.Liu, H. M., D. P. Zheng, L. B. Zhang, M. S. Oberste, M. A. Pallansch, and O. M. Kew. 2000. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J. Virol. 74:11153-11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, J., G. Dunn, R. Hull, V. Patel, and P. D. Minor. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74:3001-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mas Lago, P., V. M. Caceres, M. A. Galindo, H. E. Gary, Jr., M. Valcarcel, J. Barrios, L. Sarmiento, I. Avalos, J. A. Bravo, R. Palomera, M. Bello, R. W. Sutter, M. A. Pallansch, and C. A. de Quadros. 2001. Persistence of vaccine-derived poliovirus following a mass vaccination campaign in Cuba: implications for stopping polio vaccination after global eradication. Int. J. Epidemiol. 30:1029-1034. [DOI] [PubMed] [Google Scholar]
- 24.Melnick, J. L. 1996. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 655-712. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Press Publishers, Philadelphia, Pa.
- 25.Minor, P. D., and J. Almond. 2002. Poliovirus vaccines: molecular biology and immune response, p. 381-390. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. American Society for Microbiology, Washington, D.C.
- 26.Muzychenko, A. R., G. Yu. Lipskaya, S. V. Maslova, Y. V. Svitkin, E. V. Pilipenko, B. K. Nottay, O. M. Kew, and V. I. Agol. 1991. Coupled mutations in the 5′-untranslated region of the Sabin poliovirus strains during in vivo passages: structural and functional implications. Virus Res. 21:111-122. [DOI] [PubMed] [Google Scholar]
- 27.Nomoto, A., and I. Arita. 2002. Eradication of poliomyelitis. Nat. Immunol. 3:205-208. [DOI] [PubMed] [Google Scholar]
- 28.Sabin, A. B. 1985. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J. Infect. Dis. 151:420-436. [DOI] [PubMed] [Google Scholar]
- 29.Shulman, L. M., Y. Manor, R. Handsher, F. Delpeyroux, M. J. McDonough, T. Halmut, I. Silberstein, J. Alfandari, J. Quay, T. Fisher, J. Robinov, O. M. Kew, R. Crainic, and E. Mendelson. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 38:3729-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Technical Consulting Group to the World Health Organization on the Global Eradication of Poliomyelitis. 2002. “Endgame” issues for the Global Polio Eradication Initiative. Clin. Infect. Dis. 34:72-77. [DOI] [PubMed] [Google Scholar]
- 31.Thole, J. E., P. J. van Dalen, C. E. Havenith, P. H. Pouwels, J. F. Seegers, F. D. Tielen, M. D. van der Zee, N. D. Zegers, and M. Shaw. 2000. Live bacterial delivery systems for development of mucosal vaccines. Curr. Opin. Mol. Ther. 2:94-99. [PubMed] [Google Scholar]
- 32.Wood, D. J., R. W. Sutter, and W. R. Dowdle. 2000. Stopping poliovirus vaccination after eradication: issues and challenges. Bull. W. H. O. 78:347-357. [PMC free article] [PubMed] [Google Scholar]