Abstract
Many microorganisms encode immune evasion molecules to escape host defenses. Herpes simplex virus type 1 glycoprotein gC is an immunoevasin that inhibits complement activation by binding complement C3b. gC is expressed on the virus envelope and infected cell surface, which makes gC potentially accessible to blocking antibodies. Mice passively immunized with gC monoclonal antibodies prior to infection were protected against herpes simplex virus challenge only if the gC antibodies blocked C3b binding. Mice treated 1 or 2 days postinfection with gC monoclonal antibodies that block C3b binding had less severe disease than control mice treated with nonimmune immunoglobulin G (IgG). Mice immunized with gC protein produced antibodies that blocked C3b binding to gC. Immunized mice were significantly protected against challenge by wild-type virus, but not against a gC mutant virus lacking the C3b binding domain, suggesting that protection was mediated by antibodies that target the gC immune evasion domain. IgG and complement from subjects immunized with an experimental herpes simplex virus glycoprotein gD vaccine neutralized far more mutant virus defective in immune evasion than wild-type virus, supporting the importance of immune evasion molecules in reducing vaccine potency. These results suggest that it is possible to block immune evasion domains on herpes simplex virus and that this approach has therapeutic potential and may enhance vaccine efficacy.
Viruses have evolved clever strategies to evade many aspects of host defense, including the complement system, antibodies, interferon, T cells, cytokines, and programmed cell death (1, 28). Understanding viral evasion mechanisms may allow for development of novel approaches to combat infectious diseases.
Herpes simplex virus type 1 (HSV-1) establishes latent infection in humans and reactivates periodically to produce fever blisters (herpes labialis). Reactivation occurs in immune individuals, which is indicative of the virus' ability to evade immune attack. HSV-1 encodes an immediate-early protein, ICP47 that inhibits CD8+ T-cell responses by preventing HSV-1 antigen presentation with major histocompatibility complex class I molecules (10, 32). HSV-1 glycoproteins gE and gI form a complex that functions as an immunoglobulin G (IgG) Fc receptor, blocking IgG Fc-mediated functions such as complement activation and antibody-dependent cellular cytotoxicity (4). HSV-1 glycoprotein gC binds complement component C3b and prevents complement proteins C5 and properdin from interacting with C3b (Fig. 1) (6, 15, 27). These gC-mediated activities protect the virus from complement-mediated injury and are important virulence factors in vivo (8, 9, 11, 12, 15, 18, 20).
FIG. 1.
Model of gC- and gE-mediated immune evasion. gC binds C3b and blocks C5 and properdin (P) binding to C3b, which inhibits complement activation. IgG binds by its Fab domain to its target (shown as gD) and by its Fc end to gE-gI, which blocks Fc-mediated activities, including complement activation.
No HSV vaccines are Food and Drug Administration approved. Recent studies with a glycoprotein gD subunit vaccine in previously uninfected subjects showed that it was ineffective at protecting subjects from acquiring the virus; however, it was effective at preventing HSV-2 genital lesions in women, but not men (25). These results raise hopes for developing an effective HSV subunit vaccine, but indicate that additional approaches are likely required to improve vaccine efficacy. One such approach is to devise strategies to prevent the virus from evading innate or acquired immune responses.
Glycoproteins gC and gE are expressed on the virus envelope and at the infected cell surface; therefore, these evasion molecules may be accessible to antibodies that bind to critical domains and block their function. HSV-1 infection in mice induces gC antibodies that inhibit C3b binding, which makes the murine model useful for evaluating effectiveness of vaccines or therapies that prevent immune evasion. To our knowledge, these are the first studies to report blocking immune evasion in vivo and represent a novel approach to prevention and treatment based on understanding microbial evasion strategies.
MATERIALS AND METHODS
Viruses.
Wild-type (WT) HSV-1 strain NS and mutant strains NS-gE339, NS-gCΔC5/P, NS-gCΔC3, and NS-gCΔC3,gE339 were described previously (7, 17, 18, 21). NS-gE339 has 4 amino acids inserted at gE amino acid 339, resulting in loss of IgG Fc binding. NS-gCΔC5/P has a deletion of gC amino acids 33 to 123, which is the domain involved in blocking C5 and properdin binding to C3b. NS-gCΔC3 deletes gC amino acids 275 to 367, leading to a loss of C3b binding, without affecting regions of the molecule thought to mediate attachment to heparan sulfate (26, 29). NS-gCΔC3,gE339 combines the gC and gE mutations into one virus. Purified virus pools were prepared on a 5 to 65% sucrose gradient as previously described (8).
Antibodies.
Murine monoclonal antibody (MAb) 140A-B4 (referred to hereafter as MAb 140) is an IgG2a gC antibody, and MAb 267-F7 (referred to hereafter as MAb 267) is an IgG2b gC antibody. MAb 1C8 is an IgG2a gC antibody whose characteristics have been previously described (6). Each of the MAb isotypes used in this study is known to activate mouse complement (22). Serum was obtained from subjects that participated in a GlaxoSmithKline HSV gD2 vaccine trial (25). Subjects were seronegative to HSV-1 and -2 prior to vaccination. Samples were obtained 1 month after completing three immunizations with either gD2 vaccine or adjuvant alone (placebo group) given at 0, 1, and 6 months (25).
Neutralization assays.
Neutralization assays were performed with IgG purified from human serum of subjects immunized with an experimental HSV-2 gD vaccine or with a placebo control (25). Ten percent serum from a donor seronegative for HSV-1 and -2 served as the source of complement. Vaccine IgG was used at a concentration that neutralized virus 50% (0.3 log10) in the absence of complement, while IgG from placebo recipients was used at comparable concentrations. IgG and complement was added to virus for 1 h at 37°C, and titers determined by plaque assay on Vero cells as previously described (18). For some experiments, neutralization assays were performed with 250 μg of murine IgG per ml purified from bac-gC457t-immunized mice or with murine MAbs 1C8, 140, and 267.
Rosette inhibition assays.
Vero cells were infected with WT HSV-1 at a multiplicity of infection (MOI) of 2 for 20 h. IgG was added to the infected cells for 1 h at room temperature. C3b-coated sheep erythrocytes were added for 1 h at 37°C and viewed by light microscopy for rosettes (8). Cells with 4 or more erythrocytes bound were considered positive.
Flow cytometry.
To detect antibody binding, Vero cells were infected at an MOI of 2 with WT virus or mutant gC virus NS-gCΔC3 or NS-gCΔC5/P for 20 h at 37°C and incubated with 1 μg of MAb 1C8, 140, or 267 and fluorescein isothiocyanate (FITC)-labeled F(ab′)2 sheep anti-mouse IgG.
Passive immunization of IgG in the murine flank model.
BALB/c female mice (Charles River, Wilmington, Mass.) or C3 knockout mice 5 to 7 weeks old were passively immunized intraperitoneally (i.p.) with 100 μg of purified gC MAb 1C8, 267, or 140 or nonimmune murine IgG (Sigma, St. Louis, Mo.). The mice were anesthetized, and the flanks of the mice were shaved and chemically denuded. Sixteen to 20 h later, mice were infected with purified HSV-1 WT virus by adding 10 μl containing 5 × 104 or 5 × 105 PFU and scratching the skin with the bevel of a 30-gauge needle. Mice were scored for zosteriform disease on days 3 to 7 postinoculation as follows: erythema or swelling with no vesicles was assigned 0.5 point, and individual vesicles were scored as 1 point each with a total maximum daily score of 10. If lesions coalesced, up to 10 points was assigned based on the size of the lesions (19).
Treatment with MAb 1C8 or nonimmune murine IgG after HSV-1 infection.
BALB/c mice were infected with WT virus at 5 × 104 PFU by scratch inoculation. On days 1 to 3 postinfection, mice were inoculated i.p. with 100 or 1,000 μg of MAb 1C8 or 1,000 μg of nonimmune murine IgG.
Immunization with bac-gC457t.
Female BALB/c mice were immunized i.p. three times at 2-week intervals with 10 μg of baculovirus-expressed gC protein, gC457t (26). The first immunization was in complete Freund's adjuvant, and subsequent immunizations were in incomplete Freund's adjuvant. The gC protein was purified by affinity chromatography with MAb 1C8.
Statistical analysis.
Student's t test was used to calculate P values. The lower limit of detection of virus in neutralization assays is 1.3 log10. Virus titers that were undetectable were assigned a value of 1.3 log10 for statistical analyses and when plotted in Fig. 7.
FIG. 7.
Neutralization assays using IgG purified from gD2 vaccine sera. (Left panel) Neutralization of WT and gC/gE double-mutant virus NS-gCΔC3,gE339 (labeled ΔgC/gE339) using PBS alone (□), 10% complement alone obtained from a donor seronegative for HSV-1- and -2 (░⃞), purified IgG from vaccine recipients (▨), or IgG plus complement (▪). Results represent the mean ± standard error of five separate samples, each tested in duplicate. For statistical purposes, neutralization of WT or NS-gCΔC3/gE339 virus was calculated as the difference between PBS and titers of IgG plus complement (P < 0.0001). (Right panel) Neutralization of WT and gC/gE mutant viruses by using purified IgG from placebo recipients. Results shown are the mean ± standard error of three separate samples, each tested in duplicate. No significant differences were detected between the two viruses using IgG from placebo recipients.
RESULTS
Murine MAbs can block C3b binding to gC.
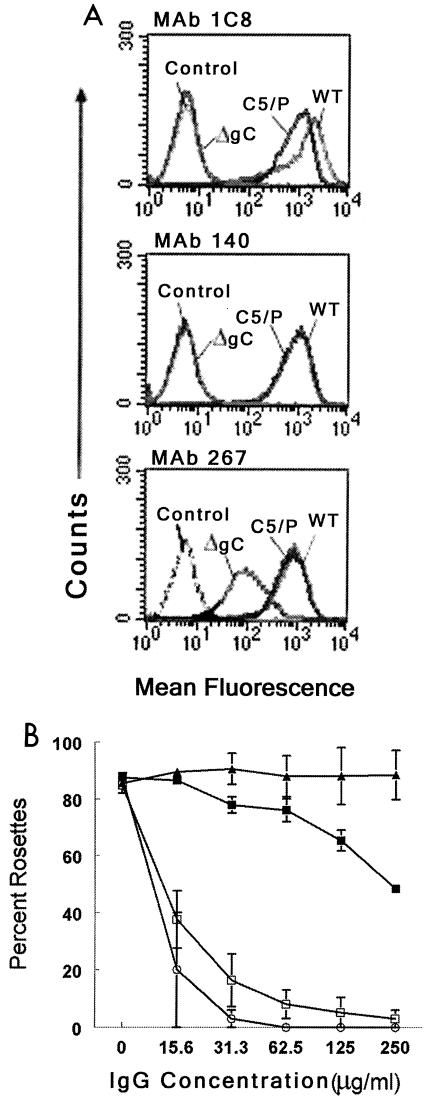
We focused our studies on blocking immune evasion mediated by HSV-1 gC. Flow cytometry assays were performed to identify murine MAbs that bind to different domains on gC expressed at the cell surface of infected cells. MAbs were incubated with cells infected with WT virus, a mutant virus lacking the C3b binding domain, or a mutant virus lacking the amino-terminal C5 and properdin domain (Fig. 2A) (12, 17). MAbs 1C8 and 140 failed to bind to the mutant virus lacking the C3b domain. In contrast, MAb 267 bound to this virus, although at reduced levels compared with WT virus, suggesting that MAb 267 recognizes a different domain on gC than MAbs 1C8 or 140 and that MAb 267 interacts little, if at all, with the C3b binding domain.
FIG. 2.
Studies using murine MAbs. (A) Flow cytometry to detect binding of MAbs 1C8, 140, and 267 to cells infected with HSV-1 WT and mutant viruses. WT is strain NS; ΔgC is NS-gCΔC3, which lacks amino acids 275 to 367 involved in C3b binding; C5/P is strain NS-gCΔC5/P, which has a deletion of gC amino acids 33 to 123 involved in blocking C5 and properdin (P) binding to C3b (12, 13, 15, 17). “Control” refers to cells that reacted with FITC conjugate alone. (B) Rosetting assay to measure MAb inhibition of C3b binding to gC. Cells were infected with WT virus and incubated with nonimmune murine IgG (▴) MAb 267 (▪), 1C8 (□), or 140 (○). The percentage of cells that bound ≥4 erythrocytes was determined. Each result is the average of two separate experiments, except for MAb 1C8, for which each result is the mean ± standard error of five determinations.
Rosetting assays with C3b-coated erythrocytes were performed to determine if MAb 1C8, 140, or 267 blocks C3b binding to cells infected with virus. MAbs 1C8 and 140 blocked C3b binding in a dose-dependent manner, while MAb 267 had little effect (Fig. 2B).
MAbs 1C8, 140, and 267 were evaluated for neutralizing activity against WT virus. The antibodies were tested at the highest concentrations available, which were 350 μg/ml for 267 and 1 mg/ml for 1C8 and 140. None of the MAbs neutralized WT virus, defined as reducing virus titer by ≥50% (0.3 log10). This result suggests that the MAbs do not neutralize virus in the absence of complement and that differences among the MAbs described below are not related to antibody neutralizing activity.
Passive immunization of mice with gC MAbs.
We previously reported that the gC domain that binds C3b is a virulence factor in mice and is more potent than the C5/P domain (17). Therefore, we evaluated whether blocking the C3b domain by using MAbs would modify virulence. Mice were passively immunized with gC MAb 1C8 or 140, which blocks gC-C3b interaction, or with MAb 267, which fails to block C3b binding. We postulated that the blocking MAbs 1C8 and 140 would protect the mice because they prevent complement inactivation by gC, which should enhance complement activity in host defense.
Mice were injected i.p. with 100 μg of IgG and infected the next day with 5 × 104 PFU of WT virus. The dose of 100 μg was chosen based on preliminary experiments that showed no significant difference in protection between 100 and 500 μg. In this model, disease occurs first at the inoculation site, and virus then spreads along nerves to spinal ganglia, where virus infects additional neurons and returns to the skin along nerves to cause zosteriform disease (19, 24). None of the antibodies significantly modified infection at the inoculation site; however, zosteriform disease was significantly reduced in mice that received MAb 1C8 or 140 (Fig. 3A, left panel). To further define the role of complement in protection, C3 knockout mice were passively immunized with gC MAb 1C8 or with control antibody MAb 267 and infected the next day with WT virus at 5 × 104 PFU. MAb 1C8 offered no protection in these mice (Fig. 3A, right panel), supporting the hypothesis that antibodies that bind to the gC complement-binding domain protect in a complement-dependent fashion.
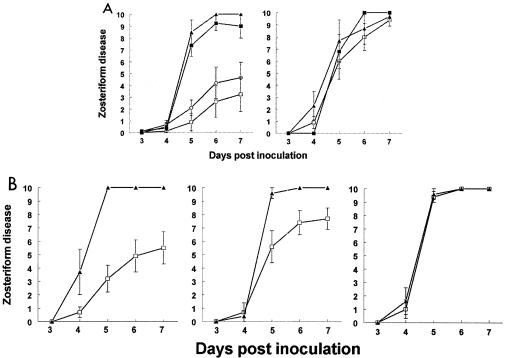
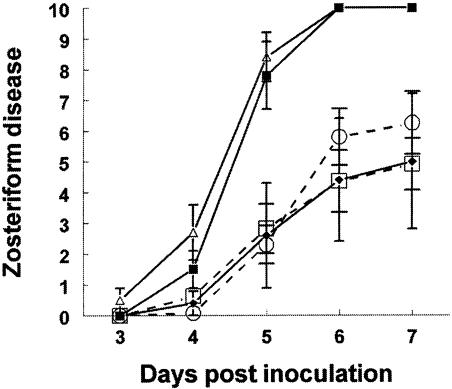
FIG. 3.
Passive immunization protection studies in the murine flank model. (A) (Left panel) Five-to-6-week-old BALB/c females were passively immunized with 100 μg of nonimmune murine IgG (▴; n = 6), MAb 267 (▪; n = 8) (anti-gC MAb that binds gC but does not block C3b binding), MAb 140 (○; n = 9), or MAb 1C8 (□; n = 8) (anti-gC MAbs that bind gC and block C3b binding). Sixteen to 20 h later, mice were infected with 5 × 104 PFU. P < 0.01 for comparison of scores for MAbs 1C8 and 267 on days 5 to 7. P < 0.01 for comparison of scores for MAbs 140 and 267 on days 5 and 6, and P = 0.02 for comparison on day 7. (Right panel) C3 knockout mice were passively immunized with nonimmune murine IgG (▴; n = 6), MAb 267 (▪; n = 8), or MAb 1C8 (□; n = 8) and challenged with WT virus at 5 × 104 PFU. (B) Protection against disease by passive antibody transfer 1 to 3 days postinfection. BALB/c mice were infected with WT virus at 5 × 104 PFU and passively immunized with 1 mg of nonimmune murine IgG (▴) or MAb 1C8 (□). (Left panel) Passive transfer 1 day post-virus infection with nonimmune IgG (n = 7) or MAb 1C8 (n = 10). P < 0.01 for comparison on days 5 to 7. (Middle panel) Passive transfer 2 days postinfection with nonimmune IgG (n = 5) or MAb 1C8 (n = 7). P = 0.02 for comparison on day 5 and P = 0.03 for days 6 and 7. (Right panel) Passive transfer 3 days postinfection with nonimmune IgG (n = 5) or MAb 1C8 (n = 7). Error bars represent ± standard error.
MAb 1C8 modifies disease severity when administered postinfection.
BALB/c mice were infected with WT virus at 5 × 104 PFU and passively immunized 1, 2, or 3 days post-virus inoculation with 1 mg of MAb 1C8 or nonimmune IgG as a control. MAb 1C8 afforded significantly greater protection than nonimmune IgG on days 1 and 2 (Fig. 3B, left and middle panels, respectively), but not on day 3 (Fig. 3B, right panel). When the same experiments were repeated with a lower MAb dose (100 μg/ml), differences were significant on day 1 only (results not shown). These findings indicate that antibody can modify disease even when administered 2 days after infection.
Active immunization of mice with bac-gC457t.
Mice were immunized i.p. three times at 2-week intervals with bac-gC457t, a gC protein truncated prior to the transmembrane domain and purified from supernatant fluids of baculovirus-infected cells (26). Mice were immunized with adjuvant alone as a control. Mice were challenged with 5 × 105 PFU of WT virus or gC mutant virus, NS-gCΔC3, defective in C3b binding.
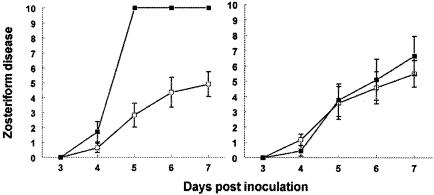
We postulated that if immunization protects because antibodies bind to and block gC evasion domains, then the vaccine should defend against challenge by WT virus, but not gC mutant virus lacking these domains. The results support our hypothesis. Compared with mock-immunized controls, bac-gC457t protected against challenge with WT (Fig. 4, left panel), while no differences were detected after challenge with gC mutant virus (Fig. 4, right panel). As previously reported, disease scores were lower in mice infected with the gC mutant virus than in those infected with WT virus because the mutant virus is more susceptible to complement-mediated attack (17). These experiments suggest that gC immunization protects against WT virus challenge, but not against challenge with a gC mutant virus that is defective in C3b binding.
FIG. 4.
Immunization with bac-gC457t and challenge of mice with WT or gC mutant virus. (Left) BALB/c mice were mock immunized (▪; n = 7) or injected with bac-gC457t (□; n = 11) and challenged with WT virus at 5 × 105 PFU. P < 0.001 for comparison of mock versus gC-bac457t immunized on days 5 to 7. (Right) Mice were mock immunized (▪; n = 9) or immunized with bac-gC457t (□; n = 12) and challenged with gC mutant virus NS-gCΔC3 at 5 × 105 PFU. No significant differences were detected between groups.
To further evaluate whether protection is related to blocking gC evasion domains, IgG was purified from gC-immunized mice and tested for rosette inhibition. IgG inhibited C3b rosettes in a dose-dependent fashion, indicating that bac-gC457t immunization induces blocking antibodies (Fig. 5A).
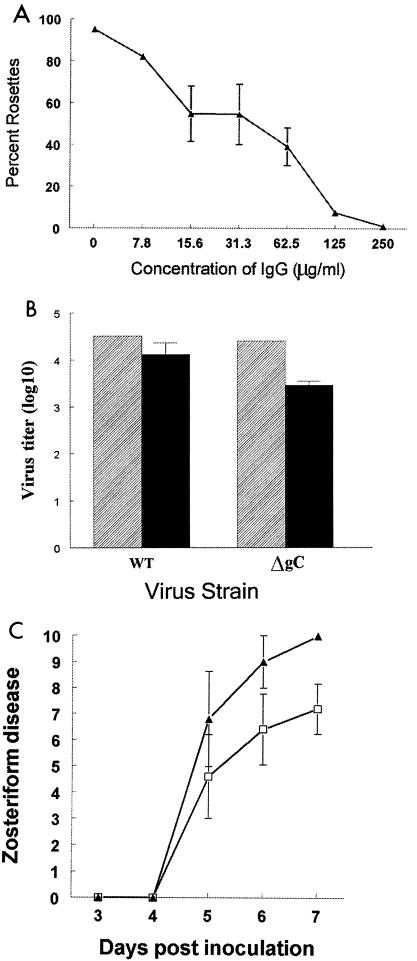
FIG. 5.
Characterization of antibodies to bac-gC457t produced in mice. (A) Rosette inhibition by murine antibodies to bac-gC457t. Cells were infected with WT virus and incubated with IgG purified from mice immunized with bac-gC457t. The percentage of cells that bound ≥4 erythrocytes was determined. Results are the average ± standard error of IgG from two immunized mice. (B) Neutralization of WT and gC mutant virus by murine antibodies to bac-gC457t. WT or gC mutant virus NS-gCΔC3 (labeled ΔgC) was incubated with PBS (▨) or 250 μg of IgG per ml purified from bac-gC457t-immunized mice (▪). Results are the average ± standard error of IgG from two mice. No significant differences were detected in virus neutralization. (C) Passive transfer of antibodies to bac-gC457t protects mice against WT virus challenge. One day prior to infection with 5 × 104 PFU of WT virus, mice were inoculated with IgG pooled from eight mice that had been immunized with bac-gC457t (□; n = 5) or with nonimmune murine IgG (▴; n =5). Antibodies to bac-gC457t provide significant protection against WT virus challenge on day 7 (P = 0.02). Error bars represent ± standard error.
One possibility for the greater effect of immunization against WT virus is that antibodies produced may be primarily directed against the C3b binding domain that is deleted in the gC mutant virus. Therefore, neutralizing activity of bac-gC457t-induced antibodies was compared against WT virus or gC mutant virus. The antibodies had little neutralizing activity against either virus (Fig. 5B), suggesting that results shown in Fig. 4 cannot be explained by greater neutralization of antibody against WT than gC mutant virus. Rather, the results are consistent with the hypothesis that antibodies are more effective at inhibiting WT virus because they block gC evasion domains.
Passive immunization experiments were performed to evaluate vaccine-induced antibodies independent of T-cell responses. IgG was purified from immunized mice and transferred into naïve mice that were then challenged with 5 × 104 PFU of WT virus. The IgG protected against challenge (Fig. 5C), supporting the role of gC antibodies in mediating this effect.
Magnitude of protection provided by active and passive immunization.
Experiments were performed to define the magnitude of protection provided by gC immunization or passive transfer of gC antibody. Mice were infected with WT virus at titers ranging from 5 × 103 to 5 × 105 PFU. These mice received no treatments (no passive antibodies or immunization) prior to infection to establish disease scores in the absence of interventions. For comparison, zosteriform disease scores were determined in mice passively immunized with MAb 1C8 or immunized with bac-gC457t and infected with WT virus at 5 × 105 PFU (Fig. 6). The immunized mice had disease scores similar to those infected with 5 × 103 PFU without interventions. Therefore, immunization with MAb 1C8 and gC reduced zosteriform disease to a degree equivalent to decreasing the dose of inoculated virus by 100-fold.
FIG. 6.
Magnitude of protection provided by passive transfer of MAb 1C8 or immunization with bac-gC457t. BALB/c mice were infected over a 100-fold range of inoculation titers with WT virus at 5 × 105 PFU (▵; n = 10), 5 × 104 PFU (▪; n = 5), or 5 × 103 PFU (⋄; n = 5). None of these mice were immunized or treated by passive antibody transfer. Additional mice were passively immunized 1 day prior to infection with 100 μg of MAb 1C8 (○; n = 12) or immunized three times at 2-week intervals with bac-gC457t (□; n = 11) and infected with WT virus at 5 × 105 PFU. Error bars represent ± standard error.
Neutralization assays using human gD vaccine sera.
We previously reported HSV-1 neutralization studies using human IgG pooled from thousands of normal donors. The IgG was added to human complement and tested in a neutralization assay against a gC mutant virus defective in C3b binding, a gE mutant virus defective in IgG Fc binding, or a gC/gE double-mutant virus defective in both C3b and IgG Fc binding. Complement increased the neutralizing activity of antibody against the gC or gE mutant virus compared with WT virus; however, the most impressive result was a marked increase in complement-enhanced neutralization of the gC/gE double-mutant virus compared with WT virus (18).
As an extension of these studies, we evaluated whether gC and gE immunoevasins modify antibody and C neutralization of HSV-1 when the source of antibody was IgG purified from serum of subjects vaccinated with an experimental HSV-2 glycoprotein gD vaccine (25). As a control, IgG was purified from placebo recipients who received adjuvant alone. All vaccine and placebo recipients were seronegative to HSV-1 and -2 prior to vaccination. Vaccine IgG was used at a concentration 12.5 to 50 μg/ml, which neutralized virus 50% (0.3 log10) in the absence of complement. These IgG concentrations were selected because antibody binds to virus; however, neutralizing activity is modest enough that if complement adds to the effect, it can be measured in the assay (complement-enhanced antibody neutralization assay) (18). IgG from subjects receiving the placebo vaccine was also used at concentrations of 12.5 to 50 μg/ml. Vaccine or placebo IgG was combined with 10% serum from a donor seronegative for HSV-1 and -2, which served as the source of complement, and tested for its ability to neutralize WT HSV-1 strain NS or a mutant virus defective in both gC-mediated C3b binding and gE-mediated IgG Fc binding (NS-gCΔC3,gE339) (18). The combination of immune IgG and complement neutralized infectivity of the gC/gE mutant virus 2.2 log10 compared with phosphate-buffered saline (PBS). In contrast, immune IgG and complement had only a minimal effect (0.3 log10) on WT virus (Fig. 7, left panel). Complement and IgG from placebo subjects failed to neutralize either virus (Fig. 7, right panel). The protection provided by gC and gE against antibody and complement is consistent with the model shown in Fig. 1. These results support the concept that blocking evasion domains on WT virus may improve efficacy of an HSV vaccine.
DISCUSSION
HSV-1 gC and gE are immunoevasins that contribute to virulence (17, 18, 21). Studies using human gD2 vaccine sera demonstrate that HSV-1 immunoevasins can have a dramatic impact on neutralizing activity of vaccine sera, which is consistent with our previous findings with pooled human IgG that showed gC and gE together are more potent immunoevasins than either alone (18). In this study, we evaluated blocking the gC C3b binding domain; however, added benefit seems likely if the gC C5/P and gE IgG Fc domains are also blocked (17, 18).
Unlike many immunoevasins that function intracellularly, gC and gE are expressed on the virus and the infected cell surface, making them potentially accessible to blocking antibodies. Studies with human and simian immunodeficiency viruses suggest that viral glycoprotein domains important in pathogenesis may remain hidden from antibodies (16, 23). Whether gC and gE evasion domains will be accessible to blocking antibodies during HSV infection in humans is currently unknown. However, in the murine zosteriform model, blocking antibodies produced during immunization reduced WT virus virulence by approximately 100-fold, suggesting that blocking evasion domains is an approach worth pursuing.
gC passive antibody therapy and gC immunization had a greater effect on zosteriform disease than on inoculation site disease. These results suggest that it may be difficult to modify early HSV-1 events, such as inoculation site disease. Passive antibody therapy was effective even when given 2 days after infection, which is consistent with the conclusion that gC evasion has a greater effect on later events. Additional studies, such as analyzing viral load in ganglia, will be required to determine whether antibody therapy modifies the ability of virus to reach the ganglia or affects virus transport from ganglia back to skin. Our prior studies showed that gC-mediated immune evasion had little impact on disease scores until 5 days postinfection, which is consistent with a role for complement relatively late in acute infection (17). One explanation for these observations may be that complement concentrations may be too low at infection sites until virus-induced tissue injury develops. Another consideration is that complement serves as a bridge between innate and acquired immunity and that it takes several days to mount acquired immune responses (2, 3, 5, 30).
The results of several experiments support the conclusion that gC antibodies prevent disease because they block C3b binding. First, both MAbs that blocked C3b binding to virus in vitro were more protective in vivo than a MAb that failed to block this interaction. Second, mice that were immunized with bac-gC457t were protected from zosteriform disease caused by WT virus but not from disease caused by a gC mutant virus defective in C3b binding. Third, the gC MAb 1C8 failed to protect C3 knockout mice from zosteriform disease, suggesting that protection is complement dependent. This result also suggests that protection is not mediated by antibody-dependent cellular cytotoxicity, an immune function mediated by mononuclear cells, which are intact in C3 knockout mice. Fourth, the MAbs or bac-gC457t-induced antibodies are not capable of neutralizing HSV-1 in the absence of complement; therefore, differences cannot be ascribed to antibody neutralization alone. These MAbs are only weakly neutralizing even in the presence of complement and only become highly neutralizing if the gC C5/P domain is deleted (H. Friedman, unpublished observation). These results suggest that antibodies are likely mediating protection by interacting with infected cells rather than by neutralizing cell-free virus. Fifth, passive transfer of bac-gC457t-induced antibodies protected against disease, and this antibody blocked C3b binding. Protection was at reduced levels compared with active immunization with bac-gC457t, suggesting that T-cell responses may contribute to protection provided by gC immunization. Alternatively, the concentration of IgG used in passive transfer experiments may have been low. Taken together, the evidence strongly supports the conclusion that blocking gC evasion domains reduces virulence of HSV-1.
An important consideration is whether antibodies that block immune evasion can be used for treatment of serious HSV infections or whether immune evasion proteins can be included as components of an HSV vaccine. HSV encephalitis and neonatal infection are life-threatening diseases (14, 31). Antibody therapy that prevents immune evasion may improve host defense and be effective when give in combination with antiviral drugs. For vaccine development, blocking immune evasion is an appealing concept, not as the sole antigen, but if used in combination with potent B- and T-cell immunogens. The goal is to induce effective immune responses while also preventing the pathogen from evading those responses.
Acknowledgments
K.A.J. and J.M.L. contributed equally to this study.
J. Moorhead, K. Tyler, and L. Pizer from the University of Colorado kindly provided MAbs 140 and 267.
This work was supported in part by NIH Public Health Service grants RO1 HL28220, AI33063, and DE14152 from the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll, M. C. 2000. The role of complement in B cell activation and tolerance. Adv. Immunol. 74:61-88. [DOI] [PubMed] [Google Scholar]
- 3.Da Costa, X. J., M. A. Brockman, E. Alicot, M. Ma, M. B. Fischer, X. Zhou, D. M. Knipe, and M. C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 96:12708-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fearon, D. T., and R. H. Carter. 1995. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13:127-149. [DOI] [PubMed] [Google Scholar]
- 6.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 7.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143:266-273. [DOI] [PubMed] [Google Scholar]
- 8.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636-1641. [PubMed] [Google Scholar]
- 10.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 11.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162:331-337. [DOI] [PubMed] [Google Scholar]
- 12.Hung, S. L., C. Peng, I. Kostavasili, H. M. Friedman, J. D. Lambris, R. J. Eisenberg, and G. H. Cohen. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299-312. [DOI] [PubMed] [Google Scholar]
- 13.Hung, S.-L., S. Srinivasan, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1992. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 66:4013-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimberlin, D. W., C. Y. Lin, R. F. Jacobs, D. A. Powell, L. M. Frenkel, W. C. Gruber, M. Rathore, J. S. Bradley, P. S. Diaz, M. Kumar, A. M. Arvin, K. Gutierrez, M. Shelton, L. B. Weiner, J. W. Sleasman, T. M. de Sierra, S. J. Soong, J. Kiell, F. D. Lakeman, R. J. Whitley, and Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2001. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 108:223-229. [DOI] [PubMed] [Google Scholar]
- 15.Kostavasili, I., A. Sahu, H. M. Friedman, R. J. Eisenberg, G. H. Cohen, and J. D. Lambris. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 158:1763-1771. [PubMed] [Google Scholar]
- 16.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 17.Lubinski, J., L. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 190:1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 76:9232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 166:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuberger, M. S., and K. Rajewsky. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012-1016. [DOI] [PubMed] [Google Scholar]
- 23.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 24.Simmons, A., and A. A. Nash. 1984. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J. Virol. 52:816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, G. Dubin, and G. GlaxoSmithKline Herpes Vaccine Efficacy Study. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [Online.] [DOI] [PubMed] [Google Scholar]
- 26.Tal-Singer, R., C. Peng, M. Ponce de Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal-Singer, R., C. Seidel-Dugan, L. Fries, H. P. Huemer, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 1991. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J. Infect. Dis. 164:750-753. [DOI] [PubMed] [Google Scholar]
- 28.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 29.Trybala, E., T. Bergstrom, B. Svennerholm, S. Jeansson, J. C. Glorioso, and S. Olofsson. 1994. Localization of a functional site on herpes simplex virus type 1 glycoprotein C involved in binding to cell surface heparan sulphate. J. Gen. Virol. 75:743-752. [DOI] [PubMed] [Google Scholar]
- 30.Verschoor, A., M. A. Brockman, D. M. Knipe, and M. C. Carroll. 2001. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J. Immunol. 167:2446-2451. [DOI] [PubMed] [Google Scholar]
- 31.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 32.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]