Abstract
Steroid-free regimen is increasingly employed in kidney transplant recipients across transplant centers. However, concern remains because of unknown impact of such approach on long-term graft and patient survival. We studied outcomes of steroid-free immunosuppression in a population-based U.S. cohort of kidney transplant recipients.
All adult solitary kidney transplant recipients engrafted between January 1, 2000 and December 31, 2006 was stratified according to whether they were selected for steroid-free or steroid-containing regimen at discharge. Multivariate Cox regression models were used to estimate graft and patient survival. The impact of practice pattern on steroid use at individual transplant centers was analyzed.
Among 95,755 kidney transplant recipients, 17.2 % of them were steroid-free at discharge (n=16,491). Selection for steroid-free regimen was associated with reduced risks for graft failure and death at 1 year (HR 0.78, 95% CI 0.72-0.85, and 0.73, 95% CI 0.65-0.82, respectively, p<0.0001) and 4 years (HR 0.83, 95% CI 0.78-0.87, and 0.76, 95% CI 0.71-0.83, respectively, p<0.0001). This association was mostly observed at individual centers where less than 65% of recipients were discharged on steroid-containing regimen.
De novo steroid-free immunosuppression as currently practiced in the US appears to carry no increased risk of adverse clinical outcomes in the intermediate term.
Keywords: kidney transplantation, steroid free immunosuppressive regimens, survival analysis
Introduction
Steroid therapy has been a core component of transplant immunotherapy since early stages of clinical kidney transplantation and credited for some role in prevention and treatment of acute rejection [1-4]. However, chronic steroid therapy is associated with numerous adverse effects, including worsening hypertension and dyslipidemia, increased susceptibility to infection, development of diabetes mellitus, osteoporosis, weight gain, etc [5-7]. These adverse effects may have contributed to the development and worsening of cardiovascular disease in kidney transplant recipients [8]. Thus, the effort to develop steroid-free immunosuppression has continued for nearly three decades. Such enthusiasm waned in the mid 1980s following the results of the Multicenter Study of 523 kidney transplant recipients in Canada in the 1980s and other studies which showed increased risk of acute rejection and graft loss in the absence of steroid in low risk kidney transplant recipients [9-12].
The introduction of more effective anti-rejection drugs, notably, mycophenolate mofetil and thymoglobulin in the late 1990s reinvigorated the testing of newer combinations of immunosuppressive agents with early withdrawal or avoidance of steroid. More recent experiences with early steroid withdrawal have yielded comparable results with steroid containing regimens [13-18]. The FREEDOM Trial showed no differences in composite endpoint of acute rejection rate, recipient and graft survival at 12 months between steroid-withdrawal and steroid-containing regimens, but found a significant increase in incidence of early acute rejection in the steroid-withdrawal group [17]. On the other hand, steroid-withdrawal group in the FREEDOM Trial was associated with a small reduction in the rate of metabolic complications, as seen in some other studies [15, 17]. There is no conclusive data on whether the use of steroid-free regimen in kidney transplantation leads to improvement in patient and graft survival principally because prior studies lacked the necessarily large sample size and long duration of follow-up to yield definitive results on the endpoints of death and graft failure. Concern remains whether steroid-free regimen could lead to slow deterioration of renal allograft function and allograft loss over the years, thus counterproductive of any potential benefits observed in various clinical trials during short time follow-up.
The present study is a retrospective cohort evaluation of US transplant registry data to address the following questions: (1) whether steroid-free regimen was associated with a different rate of short and intermediate term patient and graft survival, respectively, (2) which types of patients were selected for steroid-free regimen and whether they were systematically different from recipients treated with steroid-containing regimen, (3) what is the trend in the use of steroid-free regimen and (4) whether there were differences in the induction and maintenance regimen between recipients treated with and without maintenance steroid.
Materials and Methods
Data source
The Scientific Registry of Transplant Recipients (SRTR) provided data collected by the Organ Procurement and Transplantation Network (OPTN) from all US kidney transplant programs. The study population consisted of subjects aged ≥ 18 years at the time of transplantation who received a solitary kidney transplant from either a deceased or living donor between January 1, 2000 and December 31, 2006 in the United States and who were alive with a functioning graft at discharge from the transplant surgery and had at least one maintenance immunosuppresion drug reported at the time of discharge.
Analytic methods
Subjects were classified as being treated with a steroid-free maintenance immunosuppression if it was recorded on the transplant registration form that maintenance immunosuppression does not include any steroid and if the recipient’s list of immunosuppressive medications determined at the time of discharge from the transplant surgery did not include steroid. This definition was not conditioned on the use of steroid while recipients were still in the hospital and data on changes in maintenance regimen that occurred after initial discharge was not used to classify study subjects. Thus, this is an observational with “as treated” analysis using the maintenance immunosuppressive regimen at the time of discharge from the initial transplant hospitalization as the basis of subject stratification. Subjects were followed with interval data collection at 6 and 12 months posttransplantation and annually thereafter until the occurrence of death, graft failure or end of study period (December 31, 2006). The relationship between steroid-free immunosuppression on graft and patient survival was estimated with Cox proportional hazards regression models of time to graft failure and time to death with an indicator variable for “discharge without steroid” as a covariate. Covariates included in all Cox regression models are grouped as recipient, donor or transplant characteristics. The recipient variables included induction regimen, non-steroid regimen, age, race/ethnicity, gender, source of payment (public, private or missing), body mass index, primary diagnosis as etiology of end-stage renal disease (ESRD), hepatitis C status (antibody positivity), years of ESRD prior to transplant, peak panel reactive antibodies (PRA), previous transplant, and functional status at transplant. Donor characteristics common to both living and deceased donor included age, race/ethnicity and gender. For living donor it also included the relationship between donor and recipient, while for deceased donor it included, in addition, the cause of death, history of diabetes mellitus and hypertension, hepatitis C status (antibody positivity), serum creatinine at the time of donation, donation after cardiac death (DCD) and expanded criteria donor (ECD). Covariates related to the transplant included the ratio of donor’s weight to recipient’s, HLA mismatch, and cold ischemia time. For deceased donor, the indicators for machine preservation of the kidney and donor service area (DSA) were also included. For the graft survival analysis, death with a function graft was censored since the purpose of this analysis was to illustrate the relationship between steroid use and graft outcome.
Since the decision to treat a recipient with or without steroid-free maintenance regimen is both a function of the recipient characteristics and the practice pattern at individual transplant centers, we examined the “center effect” of steroid practice pattern with an indicator variable in which the fraction of transplant recipients at a particular transplant center that were discharged without steroid from the calendar year in which each index kidney transplant was performed. This center-level variable was created for each subject in the study and incorporated into Cox regression model of individual-level data, that is, recipients were clustered within transplant center.
Subjects were also classified according to the induction immunosuppression given at the time of transplantation as well as according to the other maintenance immunosuppressive drugs received at discharge from the initial transplant hospitalization. The induction regimens were classified as yes and no, with yes category including rabbit anti-thymocyte globulin (Thymoglobulin, Genzyme, Cambridge,MA), anti-IL-2 receptor antibodies (baxiliximab, Simulect, Norvatis, Basel, Switzerland, and daclizumab, Zanepax, Roche Pharmaceuticals, Nutley, NJ), and alemtuzumab (Campath, Bayer Healthcare Pharmaceuticals, Wayne, NJ). The maintenance regimens were classified into combination of tacrolimus (Prograf, Astella, Tokyo, Japan) with mycophenolate mofetil (MMF) (Cellcept, Roche Pharmaceuticals, Nutley, NJ), cyclosporine with MMF, tacrolimus with either sirolimus (Rapamune, Wyeth, Madison, NJ) or everolimus (Certican, Norvatis, Basel, Switzerland), and cyclosporine with either sirolimus or everolimus. All others included a large number of combinations being used in the context of clinical trials and were grouped as “all other regimens”.
All analyses were performed using SAS version 9.1.
Results
The study cohort consisted of 95,755 recipients who received a solitary kidney transplant from living or deceased donors between January 1, 2000 and December 31, 2006 and who met inclusion criteria described in the methods section. Of these, 17.2% (n=16,491) were discharged on steroid-free maintenance immunosuppression (group 1) and the remaining 82.8% (n=79,264) were discharged with a maintenance regimen that included a steroid preparation (group 2). Table 1 shows the baseline recipient and donor characteristics of the two study groups. There were notable differences in some of the characteristics related to recipients and donors between the two study groups. Subjects discharged on steroid-free regimen had a higher mean age (49.9 ± 13.5 vs. 48.5 ± 13.4, p<0.0001) and weight (BMI: 27.3 ± 5.6 vs. 26.9 ± 5.4, p<0.0001) at transplantation, included fewer African American recipients (21.1% vs. 24.4%, p<0.0001) but had a higher prevalence of diabetes mellitus as cause of ESRD (24.9% vs. 22.8%, p<0.0001). Subjects discharged on steroid-free regimen had spent less time on dialysis (3.1 ± 4.0 years vs. 3.8 ± 4.6, p<0.0001), had an improved functional status at transplantation (NYHA I and II: 89.7% vs. 84.6%, p<0.0001), were more likely to be first transplant recipients (90.7% vs. 85.4%, p<0.0001) and more likely to have received their kidney transplant from a living donor (47.3% vs. 40.3%, p<0.0001).
Table 1.
Descriptive statistics for selected recipient, donor and transplant characteristics
| Group 1 N=16,491 | Group 2 N=79,264 | p | |
|---|---|---|---|
| Recipient Characteristics | |||
| Age (years ± S.D.) | 49.9 ± 13.5 | 48.5 ± 13.4 | <0.0001 |
| Male (%) | 61.4 | 59.7 | <0.0001 |
| African American (%) | 21.1 | 24.4 | <0.0001 |
| Primary diagnosis | <0.0001 | ||
| DM (%) | 24.9 | 22.8 | |
| HTN (%) | 22.8 | 22.0 | |
| GN (%) | 25.3 | 27.7 | |
| PCKD (%) | 10.6 | 9.3 | |
| Others (%) | 16.4 | 18.2 | |
| 1st transplant (%) | 90.7 | 85.4 | <0.0001 |
| Duration on dialysis (years) | 3.1 ± 4.0 | 3.8 ± 4.6 | <0.0001 |
| Body mass index (BMI) at transplant | 27.3 ± 5.6 | 26.9 ± 5.4 | <0.0001 |
| Peak panel reactive antibody (PRA) | <0.0001 | ||
| < 10% | 71.1 | 70.2 | |
| 10-79% | 21.1 | 20.2 | |
| > 80% | 6.5 | 8.6 | |
| Missing | 1.3 | 1.1 | |
| Functional status (NYHA I and II)(%) | 89.7 | 84.6 | <0.0001 |
| HCV positive serology (%) | 4.5 | 5.0 | 0.004 |
| Donor Characteristics | |||
| Age (years ± S.D.) | 39.5 ± 14.5 | 40.3 ± 14.9 | <0.0001 |
| Living donor (%) | 47.3 | 40.3 | <0.0001 |
| Male (%) | 50.4 | 52.0 | <0.0001 |
| African American (%) | 12.4 | 12.6 | 0.524 |
| Cold ischemia* (hours) | 16.9 | 16.2 | <0.0001 |
| Donor HCV positive serology* (%) | 1.7 | 1.4 | 0.008 |
| History of hypertension* (%) | 27.7 | 23.1 | 0.008 |
| History of diabetes mellitus* (%) | 6.1 | 4.8 | 0.007 |
| Serum creatinine* (mg/dl) | 1.13 | 1.07 | <0.0001 |
| Donation after cardiac death (DCD)* (%) | 7.8 | 5.1 | <0.0001 |
| Extended criteria donor (ECD)* (%) | 10.3 | 9.7 | <0.0001 |
| Recipient-Donor Characteristics | |||
| Donor-Recipient weight ratio | 0.931 | 0.922 | 0.086 |
| HLA matching (% of zero mismatch) | 12.9 | 13.2 | 0.261 |
| Donor-Recipient relationship ** | <0.0001 | ||
| (% of biologically related) | 64.1 | 66.4 | |
for deceased donor only
for living donor only
The recent trend in the use of steroid-free regimen at discharge among US transplant centers is illustrated in Figure 1. In 2000, 3.7 % of recipients were discharged on steroid-free maintenance regimen and by 2006, 32.6% were on steroid-free maintenance regimen at discharge.
Figure 1.
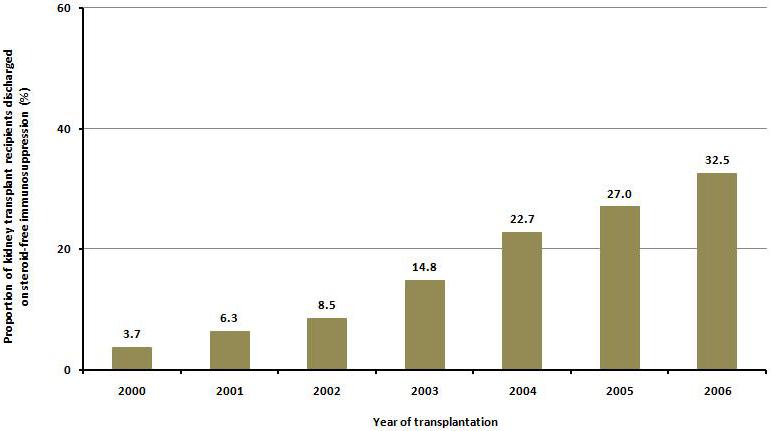
Trends in steroid-free immunosuppression at discharge for kidney transplant recipients in U.S. transplant centers, 2000-2006.
The adjusted death-censored graft survival at 1 and 4 years was 96.4% and 84.6% for group 1, 95.4% and 82.4% for group 2, respectively. The adjusted patient survival at 1 and 4 years was 98.3% and 92.5% for group 1, 97.7% and 90.7% for group 2, respectively. The risk of graft failure for recipients on steroid-free maintenance regimen (group 1) was 22% and 17% lower than those with steroid at 1 and 4 years, respectively (p<0.0001). Similarly, the risk of death for recipients on steroid-free maintenance regimen (group 1) was 27% and 24% lower at 1 and 4 years, respectively, compared with recipients who were discharged on steroid-containing maintenance regimen (p<0.0001) (Table 2).
Table 2.
Graft and patient survival by use of steroid at discharge
| Outcome | Group 1 Adjusted Survival (S.E.) N=16,491 | Group 2 Adjusted Survival (S.E.) N=79,264 | HR (95% CI) [reference=group 2] |
|---|---|---|---|
| 1-year graft survival | 96.4% (0.1%) | 95.4% (0.1%) | 0.78* (0.72, 0.85) |
| 1-year patient survival | 98.3% (0.1%) | 97.7% (0.1%) | 0.73* (0.65, 0.82) |
| 4-year graft survival | 84.6% (0.4%) | 82.4% (0.2%) | 0.83* (0.78, 0.87) |
| 4-year patient survival | 92.5% (0.3%) | 90.7% (0.2%) | 0.76* (0.71, 0.83) |
p<0.0001
In sub-analysis in which living and deceased donor kidney transplant recipients were evaluated with separate multivariate Cox regression models, the observed reduced risk for graft loss and death in the steroid-free regimen group was more pronounced in living donor kidney transplant recipients compared to the recipients of deceased donor allograft. The risk for death-censored graft failure was 32% lower for living and 18% lower for deceased donor kidney recipients at 1 year and 24% lower for living and 14% lower for deceased donor kidney transplant recipients at 4 years posttransplant (p=0.04 for the difference at 4 years). Similarly, the risk of death was 37% lower for living and 24% lower for deceased donor kidney recipients at one year and 35% lower for living and 21% lower for deceased donor kidney recipients at four years (p=0.002 for the difference at four years)(Table 3).
Table 3.
Risk of graft failure and patient death for living and deceased donor kidney transplant recipient by use of steroid at discharge
| Hazard Ratio | 95% CI | p for difference LD* vs. DD** | ||
|---|---|---|---|---|
| Graft | 1 year | DD: 0.82 | 0.74, 0.90 | 0.145 |
| LD: 0.68 | 0.58, 0.81 | |||
| 4 years | DD: 0.86 | 0.81, 0.92 | 0.042 | |
| LD: 0.76 | 0.69, 0.84 | |||
| Patient | 1 year | DD: 0.76 | 0.67, 0.87 | 0.139 |
| LD: 0.63 | 0.50, 0.79 | |||
| 4 years | DD: 0.79 | 0.72, 0.86 | 0.002 | |
| LD: 0.65 | 0.56, 0.76 |
living donor transplant
deceased donor transplant
The pattern on steroid usage at individual transplant centers was significantly associated with both patient and graft survival. Using transplant centers with 95 to 100% steroid usage as the reference group, the decrease in death censored graft failure appeared to be greater among the centers where steroid-free regimen was used more selectively. In centers where the use of steroid-free regimen ranged from 36% to 80% of all kidney transplant recipients in the index year, the risk of graft failure was 24% to 29% lower at one year and 15% to 21% lower at four years (p<0.01 for both), whereas in centers that used steroid-free regimen in more than 80% of all kidney transplant recipients in the index year, a lower risk of graft failure was only seen at one year (12% lower than reference group, p=0.01) but not four years (5% lower than reference group, p=0.17). Similarly, in centers where steroid-free regimen was utilized in 35% or less of all transplant recipients in the index year, the difference in the risk of graft failure between recipients discharged on steroid-free regimen and those discharged on steroid-containing regimen was much smaller (Figure 2a). Contrawise, increase in the use of steroid-free maintenance regimen at the center level was associated with higher patient survival. The risk of death was significantly lower in the transplant programs where more than 35% of kidney transplant recipients were placed on steroid-free regimen with a hazard ratio of death ranging from 0.73 to 0.80 at one year (p=0.02 and p<0.001) and 0.76 to 0.84 (p=0.02 and p<0.001) at four years compared to recipients discharged on steroid containing regimen (Figure 2b).
Figure 2.
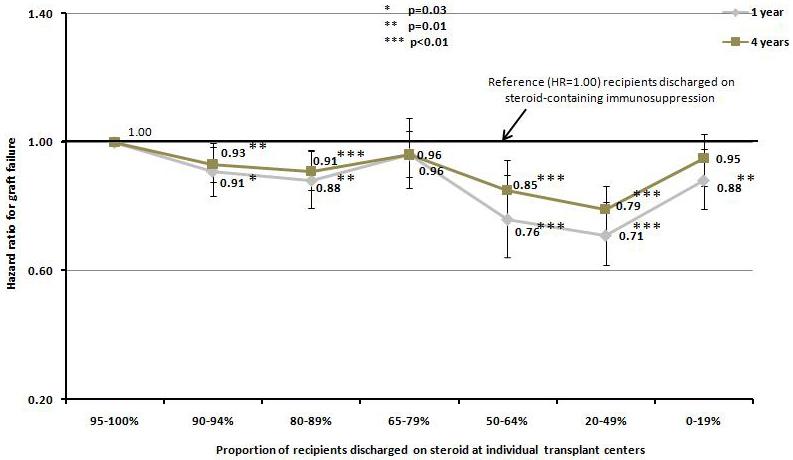
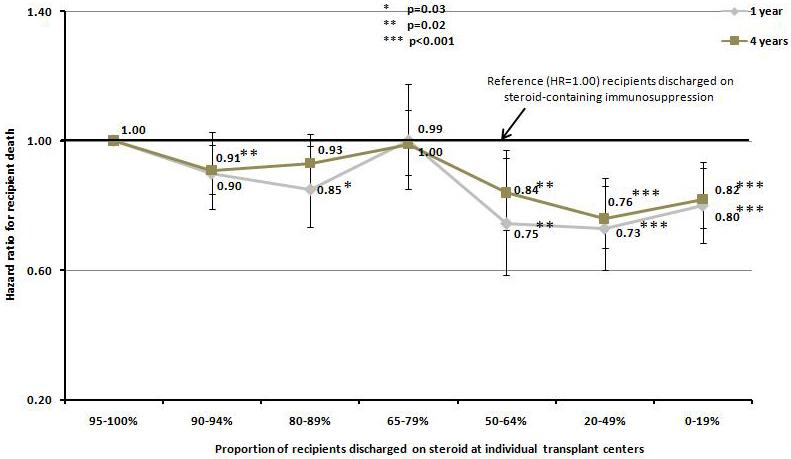
Hazard ratio for death censored graft failure a), and patient death b), at one and four years according to the percentage of steroid use at discharge among individual transplant centers.
The use of steroid-free maintenance immunosuppressive regimen was associated with a higher likelihood of monoclonal or polyclonal antibody induction therapy (80.6% in group 1 vs. 66.3% in group 2, p<0.0001)(Figure 3a and 3b). The most commonly used induction agent in steroid-free patients was rabbit anti-thymocyte globulin (41.8%) followed by alemtuzumab (17.0%), basiliximab and daclizumab (13.9%) and others (8.0%). Compared to no antibody induction therapy, antibody induction therapy was not associated with a significant difference in the 1- or 4-year risks of graft failure or death. When analyzed individually, the use of alemtuzumab was associated with 22% and 29% increase in risk for death censored graft failure at 1 and 4 years (p=0.004 and p<0.0001, respectively), but not death (Figure 4a and 4b).
Figure 3.
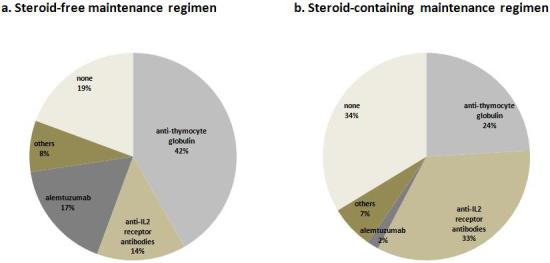
Induction usage among kidney transplant recipients discharged without steroid a), and with steroid b).
Figure 4.
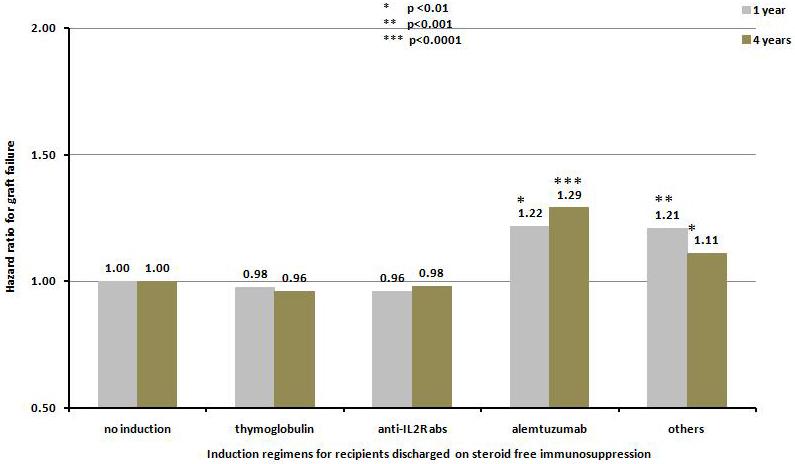
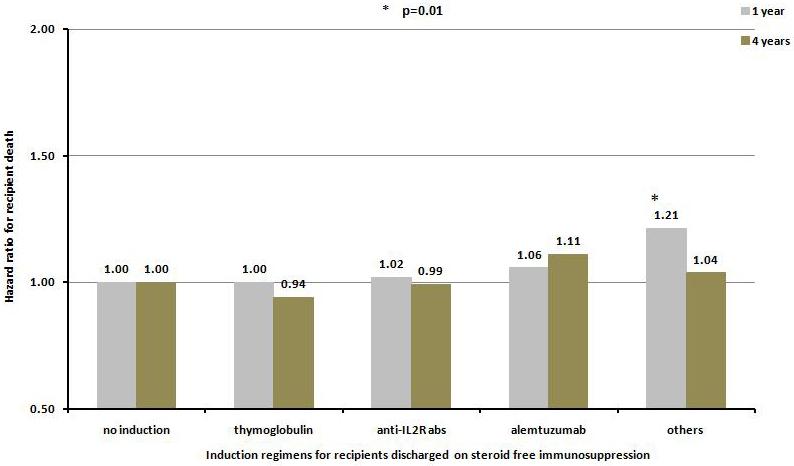
Hazard ratio for death censored graft failure a), and patient death b), at one and four years for kidney transplant recipients receiving no steroid at discharge according to various induction agents.
The most frequently used maintenance regimen, in addition to steroid-free, was the combination of tacrolimus and mycophenolate mofetil. Using the combination of tacrolimus and mycophenolate mofetil as reference group, the combinations of tacrolimus and sirolimus/everolimus, cyclosporine and mycophenolate mofetil, cyclosporine and sirolimus/everolimus, and all others were associated with increased risks for graft failure and death at 1 and 4 years after kidney transplantation (Figure 5a and 5b).
Figure 5.
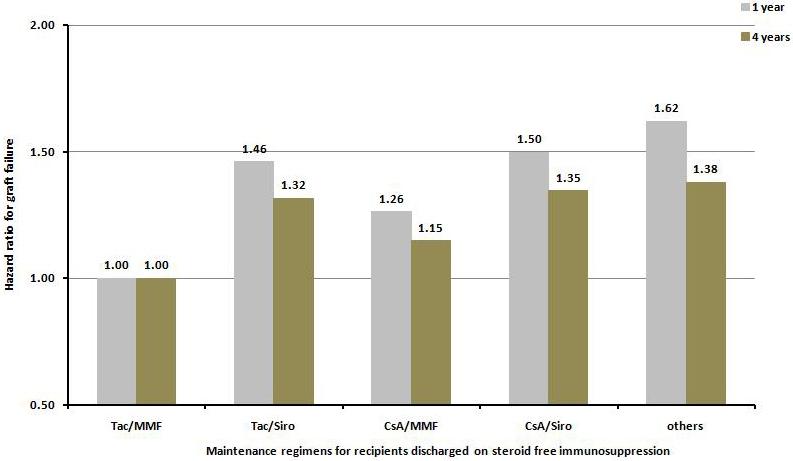
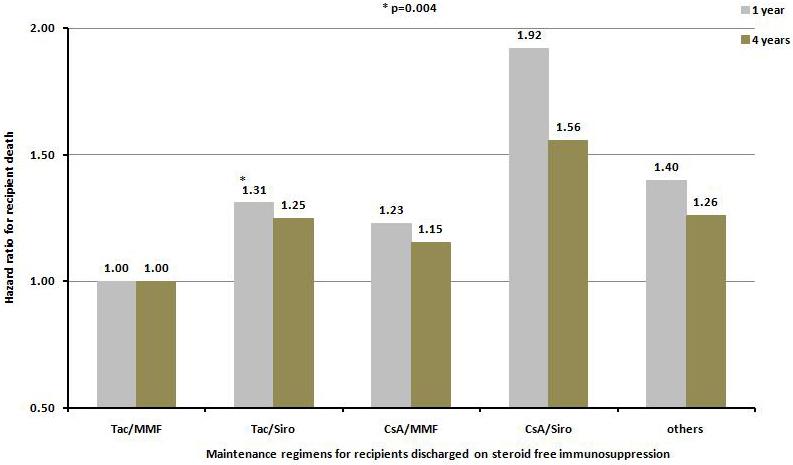
Hazard ratio for death censored graft failure a), and patient death b), at one and four years for kidney transplant recipients receiving no steroid at discharge according to the maintenance immunosuppressive regimen at discharge. All regimens are significantly different from the reference group Tac/MMF at p value <0.0001 except where indicated otherwise.
Discussion
The plausible benefits of steroid-free maintenance immunosuppression in solid organ transplantation as documented in the literature include reduced incidence of hypertension, dyslipidemia and hyperglycemia, reduced propensity towards excessive posttransplant weight gain and minimization of cosmetic, ocular and musculoskeletal complications. These potential benefits have been confirmed to a variable extent in several clinical trials in kidney transplant recipients [13-17]. However, steroid-free immunosuppressive regimen may constitute inadequate immunosuppression in certain populations of recipients and several clinical trials have documented an increased incidence of early acute rejection and long-term deterioration in graft function with use of steroid-free maintenance protocol [17-20].
The current registry-based retrospective study confirms a significant trend towards the increased use of steroid-free maintenance immunosuppression between 2000 and 2006 in clinical transplant centers across the United States. This trend was associated with a variable pattern of steroid use as individual transplant centers discharging from 0 to100% of their kidney transplant recipients on a steroid-free maintenance regimen. The factors governing such variability in steroid use across centers were not explored in the current study but it probably reflects a combination of recipient characteristics and the provider-specific pattern of clinical practice. The wide variability in the use of steroid-free protocol in the current study has important implications for patient outcomes and the design of future clinical trials as this study clearly demonstrated that the pattern of steroid use across individual centers is itself an independent predictor of both patient and graft survival. This effect of center practice (“center effect”) on outcomes also indicates that there may be an aggregate threshold at each center that demarcates the ranges of use of steroid-free regimen associated with better patient and graft survival. For example, centers where less than 35% or more than 80% of recipients were discharged on steroid-free regimen did not have a demonstrable association between steroid-free regimen and graft survival rates. It is open to speculation whether center policy or patient selection, or both is the dominant driver in centers where the use of steroid-free regimen appears to be in the intermediate range.
The recent increase in the use of steroid-free regimen may have been influenced by the availability of more potent immunosuppressive agents with fewer adverse effects such as derivatives of mycophenolic acid, anti IL2R-bloccking monoclonal antibodies and anti-thymocyte globulin. These relatively newer agents have altered the trend in both the induction and maintenance immnusuppression strategies in a manner that appears to be related to whether or not a steroid-free regimen should be employed. As an illustration, the use of steroid-free regimen in the current study was associated with a significantly higher use of thymoglobulin inductions (41.8% vs. 24.1% thymoglobulin use for steroid-free versus steroid-containing regimen, p<0.0001). Thus, it seems that apart from recipient characteristics, anticipated long-term maintenance immunosuppression governs the initial selection of an induction regimen. In this respect, studies examining the impact and costs associated with induction regimen may need to take into account the long-term regimen and outcomes associated with the induction regimen to be able to capture a comprehensive picture of the regimen being evaluated.
In the setting of randomized clinical trials comparing steroid-free versus steroid-containing maintenance regimens in kidney transplant, most but a few studies in the literature showed equivalence in both graft and patient survival between the two regimens. A few studies showed some salutary effects of steroid sparing on posttransplant metabolic and musculoskeletal complications [14, 15, 17]. These studies have reported first biopsy proven acute rejection rates ranging from 10-30% in recipients treated steroid-free immunosuppression and tended to occur earlier across various clinical trials [16, 17, 21-23] with a second or subsequent rejection rates as high as 32% after a successfully treated first rejection episode [24]. When protocol biopsy was performed, the cumulative incidence of subclinical acute rejection and chronic allograft nephropathy was reported to be 16-27% and 10-24% respectively, among kidney transplant recipients of steroid-free regimen using a combination of tacrolimus and MMF or tacrolimus and sirolimus, at the end of two years follow-up [25]. It remains an open question whether modest increase in acute rejection associated with steroid-free maintenance regimen, observed in some of those clinical trials, will have long lasting deleterious effects on the recipient and the allograft.
The present study is the first registry data analysis to show that selection for de novo steroid-free maintenance immunosuppression does not appear to lead to any significant decrement in patient and graft survival after accounting for all the major confounding factors resident in the donor, recipient and transplant center. Indeed deceased and living donor transplant recipients experienced a 21% and 35% lower 4-year mortality rate, respectively, when discharged on a steroid-free regimen compared to those discharged on steroid-containing regimen. Both the short and intermediate term graft survival rates were also higher, albeit slightly, in the steroid-free regimen group with 1-year and 4-year graft survival rates of 96.4% and 84.6% compared to 95.4% and 82.4% in the group discharged on a steroid-containing regimen. However, due to the presence of selection bias that is inherent to the retrospective nature of current study, the relationship between improved patient and graft survival and the use of steroid-free regimen cannot be construed and, in all likelihood, it may not be due to the effect of steroid-free immunosuppression for several reasons: (1) there is a significant difference in important covariates (age, race, number of transplant, co-morbidity, functional status, etc.) between recipients treated with steroid-free regimen and those who receive a steroid-containing regimen and multivariate statistical adjustment for these differing baseline covariates may not completely eliminate residual confounding; (2) unmeasured important clinical characteristics between recipients in the two comparison groups may have contributed to the differences in graft and patient survival rates (blood pressure, presence and severity of cardiovascular disease, level of glycemic controls in the diabetics, etc); (3) it is likely that recipients who were constitutionally at lower risk for adverse posttransplant outcomes were selected for the steroid-free regimen thus making the study findings a confirmation of the physician’s astute clinical judgment rather than a demonstrable benefit of steroid-free regimen and (4) it is also possible that steroid-containing group included kidney transplant recipients who did not do well initially, thus steroid was kept in place (such as patients with DGF, etc.). In any event, selecting patients for steroid-free regimen have somehow ensured better patient and graft outcomes.
In general, previous clinical trials of steroid-free regimen in kidney transplantation have included sample size and duration of follow-up that do not permit a meaningful evaluation of the endpoints of patient survival, allograft survival or cardiovascular event rates which if chosen as endpoints of clinical trials makes the studies prohibitively expensive and infeasible. Thus, a registry study as reported herein offers a unique methodological advantage to test the association between treatment regimens and outcomes on a large and diverse cohort of subjects in the “field”. That is, in a situation where the treatment is applied as intended outside the contrived experimental setting which is a necessary requirement of clinical trials. Contrariwise, the clinical events occurred around the time of initial transplant surgery, not captured by registry data, may have dictated the decision whether or not steroid will be used, thus further patient selection bias which cannot be solved with any analytic method. In addition, the current study is also limited by lack of granularity of some important and influential details that could have an effect on the outcomes being studied. For example, the outcome analysis was based on steroid use at discharge in a quasi experimental “as treated” fashion. This approach while methodologically valid ignores the possible changes in maintenance regimen that might have occurred after discharge. It is well known from clinical trials that 25-30% of recipients discharged on steroid-free regimen may end up on a steroid-containing regimen during follow-up, mostly due to occurrence of rejection. However, this should have diluted the strength of observed association. Similarly, some recipients who were discharged on a steroid-containing regimen may have ended up with a steroid-free regimen for a significant fraction of their follow-up time as some transplant centers perform steroid withdrawal late after transplantation. Furthermore, clinical indications may have led to withdrawal of steroid after discharge which would not have been captured in the current study. Nothing withstanding the potential for misclassification bias of subjects inherent to the current study, the impact on the results is likely to be minimal since the misclassification is non-differential which tends to deflate the results towards the null [26].
In conclusion, the present data should not be interpreted to mean that steroid free regimen is superior to the alternative as the absolute differences in graft and patient survival rates were small, albeit, statistically significant with large relative risks. The main thrust of the findings is that when applied judiciously in selected kidney transplant recipients, steroid-free regimen from the time of discharge following the initial transplant surgery is not associated with worse allograft and recipient outcomes. It is unlikely that a clinical trial testing the efficacy of steroid-free regimen on long term outcomes will be mounted in the near future for feasibility reasons but having shown that steroid-free regimen is not harmful and in fact could be beneficial in some subgroups of recipients, it is now necessary to design prospective clinical studies that would identify kidney transplant recipients who are not likely to benefit from steroid-free regimen.
References
- 1.Hume DM, et al. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955;34(2):327–82. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray JE, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–23. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 3.Reemtsma K, et al. Reversal of Early Graft Rejection after Renal Heterotransplantation in Man. JAMA. 1964;187:691–6. doi: 10.1001/jama.1964.03060230019006. [DOI] [PubMed] [Google Scholar]
- 4.Bell PR, et al. Reversal of acute clinical and experimental organ rejection using large doses of intravenous prednisolone. Lancet. 1971;1(7705):876–80. doi: 10.1016/s0140-6736(71)92441-x. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807–18. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 6.Arner P, et al. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care. 1983;6(1):23–5. doi: 10.2337/diacare.6.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Julian BA, et al. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544–50. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11(9):1735–43. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair NR, The Canadian Multicentre Transplant Study Group Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts. CMAJ. 1992;147(5):645–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Hricik DE, et al. Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol. 1993;4(6):1300–5. doi: 10.1681/ASN.V461300. [DOI] [PubMed] [Google Scholar]
- 11.Almawi WY, et al. Pretreatment with glucocorticoids enhances T-cell effector function: possible implication for immune rebound accompanying glucocorticoid withdrawal. Cell Transplant. 1999;8(6):637–47. doi: 10.1177/096368979900800610. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, et al. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11(10):1910–7. doi: 10.1681/ASN.V11101910. [DOI] [PubMed] [Google Scholar]
- 13.Vincenti F, et al. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant. 2003;3(3):306–11. doi: 10.1034/j.1600-6143.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Matas AJ, et al. Prednisone-free maintenance immunosuppression-a 5-year experience. Am J Transplant. 2005;5(10):2473–8. doi: 10.1111/j.1600-6143.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar MS, et al. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation. 2006;81(6):832–9. doi: 10.1097/01.tp.0000203558.34739.c6. [DOI] [PubMed] [Google Scholar]
- 16.Rostaing L, et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005;79(7):807–14. doi: 10.1097/01.tp.0000154915.20524.0a. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti F, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–16. doi: 10.1111/j.1600-6143.2007.02057.x. [DOI] [PubMed] [Google Scholar]
- 18.Hricik DE, et al. Long-term graft outcomes after steroid withdrawal in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2007;83(3):277–81. doi: 10.1097/01.tp.0000251652.42434.57. [DOI] [PubMed] [Google Scholar]
- 19.Woodle ES, et al. Multivariate analysis of risk factors for acute rejection in early corticosteroid cessation regimens under modern immunosuppression. Am J Transplant. 2005;5(11):2740–4. doi: 10.1111/j.1600-6143.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 20.Vitko S, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005;80(12):1734–41. doi: 10.1097/01.tp.0000188300.26762.74. [DOI] [PubMed] [Google Scholar]
- 21.Kandaswamy R, et al. A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients--an interim analysis. Am J Transplant. 2005;5(6):1529–36. doi: 10.1111/j.1600-6143.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman DB, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: comparison with basiliximab induction--long-term results. Am J Transplant. 2005;5(10):2539–48. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 23.Borrows R, et al. Five years of steroid sparing in renal transplantation with tacrolimus and mycophenolate mofetil. Transplantation. 2006;81(1):125–8. doi: 10.1097/01.tp.0000189716.50701.2d. [DOI] [PubMed] [Google Scholar]
- 24.Humar A, et al. Steroid avoidance regimens: a comparison of outcomes with maintenance steroids versus continued steroid avoidance in recipients having an acute rejection episode. Am J Transplant. 2007;7(8):1948–53. doi: 10.1111/j.1600-6143.2007.01883.x. [DOI] [PubMed] [Google Scholar]
- 25.Anil Kumar MS, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation. 2005;80(6):807–14. doi: 10.1097/01.tp.0000173378.28790.0b. [DOI] [PubMed] [Google Scholar]
- 26.Copeland KT, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–95. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]


