Abstract
Fear extinction, which involves learning to suppress the expression of previously learned fear, requires N-methyl-D-aspartate receptors (NMDARs) and is mediated by the amygdala and ventromedial prefrontal cortex (vmPFC). Like other types of learning, extinction involves acquisition and consolidation phases. We recently demonstrated that NR2B-containing NMDARs (NR2Bs) in the lateral amygdala (LA) are required for extinction acquisition, but whether they are involved in consolidation is not known. Further, although it has been shown that NMDARs in the vmPFC are required for extinction consolidation, whether NR2Bs in vmPFC are involved in consolidation is not known. In this report, we investigated the possible role of LA and vmPFC NR2Bs in the consolidation of fear extinction using the NR2B-selective antagonist ifenprodil. We show that systemic treatment with ifenprodil immediately after extinction training disrupts extinction consolidation. Ifenprodil infusion into vmPFC, but not the LA, immediately after extinction training also disrupts extinction consolidation. In contrast, we also show pre-extinction training infusions into vmPFC has no effect. These results, together with our previous findings showing that LA NR2Bs are required during the acquisition phase in extinction, indicate a double dissociation for the phase-dependent role of NR2Bs in the LA (acquisition, not consolidation) and vmPFC (consolidation, not acquisition).
Keywords: conditioning, infralimbic, learning, NMDA, plasticity, prelimbic
Introduction
Survival and mental health depend on learning when to fear and also when not to fear. Much is known about the brain mechanisms engaged when learning about danger from fear conditioning studies (LeDoux 2000; Maren 2001; Rodrigues et al. 2004). However, our understanding of learning when not to fear, assessed by extinction of conditioned fear, is more limited (Sotres-Bayon et al. 2004; Myers and Davis 2007; Quirk and Mueller 2008).
N-methyl-D-aspartate receptors (NMDARs) are crucial for many forms of learning and underlying synaptic plasticity (Martin et al. 2000). Because extinction involves learning, it makes sense that systemic blockade of NMDARs impairs (Santini et al. 2001) and systemic augmentation of this receptor facilitates fear extinction (Walker et al. 2002). However, distinct functional roles of NMDARs depend on subunit composition (Cull-Candy and Leszkiewicz 2004). The 2B subunit in particular confers NMDARs with key features for the induction of synaptic plasticity (Barria and Malinow 2005; Sobczyk et al. 2005) and appears to be particularly involved in learning and associated plasticity in a number of structures (Tang et al. 1999; Ge et al. 2007), including the amygdala and medial prefrontal cortex (Blair et al. 2005; Zhao et al. 2005). Further, unlike drugs that have higher affinity for NR2As, drugs that target the NR2B-containing NMDARs (NR2Bs) do not appear to have confounding effects on fear learning and expression (Rodrigues et al. 2001). NR2B pharmacological manipulation may therefore constitute a relatively selective tool for studying the contribution of NMDAR-mediated plasticity to extinction.
The particular involvement of NMDARs, including NR2Bs, in a specific brain structure is likely to be determined by the particular phase of extinction in which the animal is engaged. Fear extinction is mediated by brain structures that include the amygdala and ventromedial prefrontal cortex (vmPFC) (Sotres-Bayon et al. 2004; Myers and Davis 2007; Quirk and Mueller 2008). And like other forms of learning, extinction involves acquisition and consolidation phases. Blockade of NMDARs in the vmPFC with antagonist (±)-3-(2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) was recently found to impair the consolidation but not acquisition of extinction (Burgos-Robles et al. 2007). In our recent study, we found that selective blockade of NR2Bs, using ifenprodil, in the lateral amygdala (LA) before extinction training impaired fear extinction acquisition (Sotres-Bayon et al. 2007). Whether NR2Bs in LA and/or vmPFC are involved in extinction consolidation, and/or whether NR2Bs in vmPFC are involved in extinction acquisition, is not known. Despite the independent conclusions of existing studies, the lack of direct comparisons of the relative contribution of different brain structures to extinction, using the same drug and identical experimental procedures, prevents an understanding of their specific role in the different phases of fear extinction. In fact, dissociation of the role of amygdala and vmPFC, while suggested by previous work, has not been directly tested.
Here, we performed experiments involving ifenprodil treatment to investigate whether NR2Bs in vmPFC and LA play different roles in extinction. We first determined whether systemic treatment with ifenprodil affected extinction consolidation and then examined the effects of local blockade in LA or vmPFC.
Materials and Methods
Subjects
Adult male Sprague–Dawley rats (Hilltop Lab Animals, Scottdale, PA), weighing 325–350 g upon arrival, were individually housed in transparent polyethylene cages and maintained on a 12-h light/dark cycle (lights on at 7:00 AM) within a temperature- and humidity-controlled environment. Food and water were available ad libitum throughout the duration of the experiments. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the New York University Animal Care and Use Committee.
Behavioral Procedures
Apparatus and Stimuli
Rats underwent habituation, fear conditioning, and fear extinction in 1 of 4 identical chambers constructed of aluminum and Plexiglas walls (rat test cage, Coulbourn Instruments, Allentown, PA), with metal stainless steel rod flooring that was attached to a shock generator (Model H13-15; Coulbourn Instruments). The chambers were lit with a single house light, and each chamber was enclosed within a sound isolation cubicle (Model H10-24A; Coulbourn Instruments). An infrared digital camera, mounted on top of each chamber, allowed videotaping during behavioral procedures for later behavioral scoring. In addition, an overhead 24-cell, 3-dimensional infrared activity sensor continuously monitored (temporal resolution of 20 ms) all movement in the chamber, and the data were recorded on a computer equipped with Coulbourn Instruments LabLinc Habitest Universal Linc System. The computer also controlled stimulus presentation with Graphic State 2 software (Coulbourn Instruments). Chamber grid floors, trays, and walls were thoroughly cleaned with water and dried between sessions. Rats were allowed to freely explore the chamber for 4 min before each behavioral procedure (i.e., habituation, fear conditioning, and extinction training/testing sessions).
Fear Conditioning Procedure
Conditioning was conducted in groups of 4 rats at a time, each in a different chamber (see above). All rats were first exposed to 5 habituation trials (tone-alone presentations) on Day 0, followed by 7 conditioning trials (tone–footshock pairings) on Day 1. The conditioned stimulus was a 30-s, 5-kHz, 80-dB sound pressure level sine wave tone, which coterminated with a 1-s, 0.7-mA footshock unconditioned stimulus during fear conditioning. Mean intertrial interval was 4 min (2–6 min range) throughout habituation and fear conditioning. Freezing, a measure of conditioned fear, was continuously recorded during the conditioning session and later scored to determine the degree to which rats acquired the conditioned association (see Measurement of Freezing Behavior). After conditioning, rats were returned to their home cages and to the colony room.
Extinction Procedures
Rats that showed ≤50% freezing during fear conditioning (average of conditioning trials 2–7) were excluded from the subsequent phases of the study. Rats that satisfied the freezing criterion (>50% freezing) during conditioning were assigned to either an experimental or control group, matched for freezing during fear conditioning. Three different experiments were conducted in rats that met this criterion. Because rats were subjected to behavioral procedures 4 at a time, special care was taken to test 2 experimental and 2 control rats in each batch of 4. Freezing was recorded continuously during the 20 extinction training trials and 5-trial test sessions. Consistent with the fear conditioning procedure, throughout extinction sessions (training and test) mean intertrial interval was 4 min (2–6 min range).
Measurement of Freezing Behavior
Freezing was used to measure the conditional emotional fear response and was defined as the cessation of all movement with the exception of respiration-related movement and nonawake or rest body posture (Blanchard RJ and Blanchard DC 1969; McAllister WR and McAllister DE 1971; Fanselow 1994). Freezing was videotaped and later scored offline with a digital stopwatch by recording the total time spent freezing during every 30-s tone. Freezing was scored blind with respect to the treatment group.
In addition, online assessment of freezing was obtained using activity/inactivity data collected from the overhead infrared activity monitor (see Apparatus and Stimuli). These data were converted to freezing values using a custom MATLAB (MathWorks, Natick, MA) code, where freezing was defined as continuous inactivity lasting at least 2 s. These values were then transformed to freezing percentage and used exclusively to match groups after conditioning.
Drugs
Ifenprodil tartrate salt (Sigma-Aldrich, St. Louis, MO), a noncompetitive, selective NR2B-containing NMDAR antagonist (Chenard and Menniti 1999; Williams 2001; Nikam and Meltzer 2002), was dissolved in distilled water for systemic studies and for intra-LA and intra-vmPFC infusions was dissolved in artificial cerebrospinal fluid that contained 2% (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO), adjusted to pH = 7.4 using hydrochloric acid. A new sealed vial of drug was used each time, and all solutions were prepared the same day they were used.
Intra-LA and Intra-vmPFC Local Infusions
Cannulae were surgically implanted to locally infuse ifenprodil or its vehicle into the LA or vmPFC. Rats were anesthetized with a mixture of ketamine (100 mg/kg, intraperitoneally [i.p.]; Ketaject) and xylazine (6.0 mg/kg i.p.; Xyla-Ject) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Supplemental doses of the mix were given as needed to maintain a deep level of anesthesia. Body temperature was maintained with a heated gel pad. The skull was exposed, and small holes were drilled. Using a stereotaxic apparatus, stainless steel guide cannulae (LA: 22 gage; vmPFC: 26 gage; Plastics One, Roanoke, VA) fitted with infusion cannulae (LA: 28 gage; vmPFC: 33 gage) that extended (amygdala: 1.5 mm; vmPFC 1.0 mm) beyond the base of the guide were positioned (LA: bilaterally; vmPFC: midline unilateral) above the LA (5.8 mm anterior, 5.2 mm lateral and 2.2 mm dorsal from the interaural line) or infralimbic cortex (2.8 mm anterior, −1.0 mm lateral and −4.1 mm dorsal from bregma; angled 11° toward the midline in the coronal plane)(Paxinos and Watson 1998). The guide cannulae were secured to the skull using surgical screws and acrylic dental cement. Infusion cannulae were replaced with dummy cannulae and cut to extend 0.5 mm beyond the guide cannulae to prevent clogging. Antibiotic ointment was applied to prevent infections. After surgery, rats were administered buprenorphine hydrochloride (0.02 mg/kg, i.p.; buprenex) and atipamezole (1.0 mg/kg, i.p.; Antisedan) for analgesia and reversal of the anesthetic, respectively.
After 7 days recovery from surgery, rats were subjected to habituation, fear conditioning, and then to extinction training a day later (see above). Immediately after extinction training, rats received bilateral intra-LA infusions (0.25 μl/side) or midline intra-vmPFC (0.50 μl) of either vehicle or ifenprodil (LA: 1.0 μg/side; vmPFC: 2.0 μg/midline). Also, to control for possible effects of NMDAR blockade in the vmPFC during extinction acquisition, another group of rats were given midline intra-vmPFC ifenprodil (2 μg/0.5 μl) or vehicle but before extinction training. Solutions were infused in freely moving rats at a rate of 0.15 μl/min through infusion cannulae attached to a 1.0-μl Hamilton syringe via polyethylene tubing (PE-10, Harvard Apparatus, South Natick, MA). Cannulae were left in place for an additional 60 s after the infusion to allow for diffusion of the solution away from the cannula tip, after which the dummy cannulae were replaced and the rat was returned to its home cage and brought back to the colony room.
Histology
To verify the intra-LA and intra-vmPFC placement of the injection cannula tips, rats were anesthetized following completion of the behavioral procedures with an overdose of chloral hydrate (25%, 1 ml/100 g) and transcardially perfused with 10% buffered formalin. Brains were removed and stored in 10% buffered formalin with 30% sucrose. Subsequently, brains were blocked and cut in 40-μm sections through the amygdala and prefrontal cortex using a cryotome. After standard histological Nissl staining, sections were examined on a light microscope for injector tip localization into the LA and vmPFC. Only data from rats that had either bilateral placement within 0.25 mm of the LA (as in Blair et al. 2005) or midline placements within the infralimbic vmPFC were included in the study—decisions to include or exclude animals were made without knowledge of the experimental results.
Experimental Design
Three studies were performed to assess the contribution of NR2B-containing NMDARs to fear extinction consolidation. The first experiment involved systemic injections, and the last 2 experiments involved intracerebral microinfusion: intra-LA and intra-vmPFC microinfusions.
Systemic Injections of Ifenprodil Immediately after Fear Extinction Training
In the first experiment, we evaluated the effects of systemic injection of ifenprodil immediately after extinction training. Immediately after 20 extinction training trials (Day 2; one day after conditioning), rats were injected i.p. with 5 mg/kg ifenprodil (n = 11) or vehicle (n = 12) and taken to their home cages. The next day (Day 3), rats received 5 extinction test trials, drug free.
Intra-LA Infusions of Ifenprodil Immediately after Extinction Training
In this experiment, we evaluated the effects of local infusion of ifenprodil into the LA immediately after extinction training. We tested the effect of an intra-LA ifenprodil dose that has been shown to be sufficient to block the acquisition of both fear conditioning and fear extinction (1.0 μg/0.25 μl/side) (Rodrigues et al. 2001; Blair et al. 2005; Sotres-Bayon et al. 2007). Immediately after 20 extinction training trials (Day 2; one day after conditioning) rats received bilateral intra-amygdala infusion of ifenprodil (n = 11) or vehicle (n = 11). The next day (Day 3), as in the previous experiments, rats received 5 extinction test trials, drug free.
Intra-vmPFC Infusions of Ifenprodil before and after Extinction Training
In the last experiment, we evaluated the effects of local infusion of ifenprodil into the vmPFC before and after extinction training. We tested the effect of intra-vmPFC ifenprodil (2 μg/0.5 μl midline). Immediately after 20 extinction training trials (Day 2; one day after conditioning) rats received midline intra-vmPFC infusion of ifenprodil (n = 8) or vehicle (n = 7). The next day (Day 3), as in the previous experiments, rats received 5 extinction test trials, drug free. Additionally, to control for possible effects of NMDAR blockade in the vmPFC during extinction acquisition, another group of rats were given midline intra-vmPFC ifenprodil (2 μg; n = 8) or vehicle (n = 7) but before extinction training.
No-Extinction Controls
To compare the extent of potential extinction impairment during retention test, a separate group of rats were conditioned on Day 1, but did not receive tone-extinction training on Day 2. Instead, these rats were exposed only to the context (for the same time other groups received tone-extinction training, ∼90 min) and received vehicle treatment. For the systemic study, rats received i.p. injections with vehicle (distilled water) immediately after context exposure (n = 4). For the intracerebral studies, a separate group of rats received local vehicle (artificial cerebrospinal fluid that contained 2% (2-hydroxypropyl)-β-cyclodextrin) infusions into the LA (n = 4) and into the vmPFC (n = 5), immediately after context exposure. Data from the 2 No-Extinction (No-Ext) retention control groups that received intracerebral vehicle infusions were combined in the subsequent analysis because there was no statistical difference (P > 0.05).
Data Analysis
Behavioral data from each experiment (percent freezing) were analyzed using analysis of variance (ANOVA), with group as a between-subjects factor and trial as within-subjects’ factor. Significant ANOVA results were followed up using Fisher's least significant difference post hoc mean comparison test. Statistica 8 (StatSoft, Tulsa, OK) was used for the analyses. All data are presented as mean ± standard error of the mean.
Results
We assessed whether systemic, intra-LA and intra-vmPFC administration of the NR2B subunit–selective antagonist ifenprodil affects fear extinction. We found that both systemic and intra-vmPFC, but not intra-LA, ifenprodil treatment after extinction learning impairs extinction retrieval.
Posttraining Systemic Activation of NR2Bs Is Required for Fear Extinction
As extinction training progressed, all rats showed a gradual reduction in freezing, reaching negligible freezing levels by the end of extinction training. In the extinction retention test, conducted 24 h after extinction training, rats given posttraining vehicle exhibited low levels of freezing during the test trials, indicating successful retention of extinction learning. In contrast, rats given posttraining ifenprodil (5 mg/kg) exhibited relatively high levels of freezing, similar to No-Ext controls, indicating that ifenprodil impaired the retention of fear extinction. Results (by trial) are shown in Figure 1a. Note that we use the 5 mg/kg dose because a higher dose (e.g., 10 mg/kg) impairs fear expression, presumably by affecting routine synaptic transmission (Rodrigues et al. 2001).
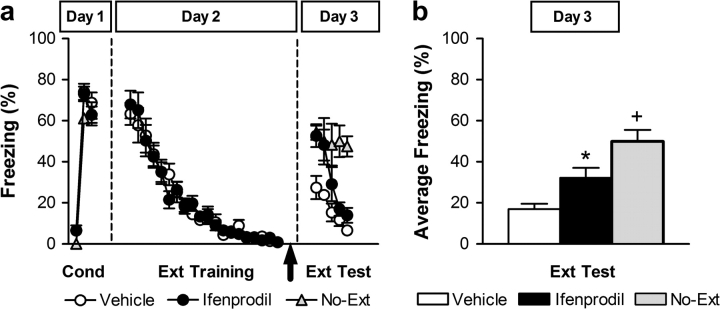
Figure 1.
Fear extinction requires posttraining NR2B activation. (a) Percent freezing during conditioning (first trial and averages of 2–4 and 5–7 tone–footshock trials on Day 1), trial-by-trial extinction training (20 tone-alone trials on Day 2), and extinction test (5 tone-alone trials on Day 3). Vehicle (white circles) or ifenprodil (black circles) injections were administered immediately after extinction training (arrow); No-Ext control group (No-Ext; gray triangles). (b) Averaged percent freezing across all extinction test trials for vehicle-treated (white bar), ifenprodil-treated (black bar), and No-Ext (gray bar) rats. *P < 0.05 relative to control groups (vehicle and No-Ext) in the same session. +P < 0.05 relative to ifenprodil and vehicle groups in the same session.
Extinction retention test results (all trials) were statistically evaluated using a repeated measures ANOVA with group (vehicle, ifenprodil, No-Ext) as a between-subjects factor and trials as within-subjects factors (ANOVA: significant main effects of group [F2,24 = 11.27, P < 0.0005], trial [F4,96 = 13.06, P < 0.0001], and a significant group × trial interaction [F8,96 = 3.16, P < 0.005]). Post hoc comparisons between groups showed that ifenprodil-treated rats exhibited significantly higher freezing during extinction retention test as compared with vehicle-treated rats (P < 0.01) and significantly lower as compared with No-Ext controls (P < 0.05) (Fig. 1b). Thus, ifenprodil given immediately after extinction training significantly impaired the consolidation of fear extinction learning.
As in previous studies, differences between experimental groups in extinction retrieval were assessed analyzing the first extinction test trials, whereas re-extinction learning differences were evaluated analyzing subsequent extinction test trials (Quirk et al. 2000; Burgos-Robles et al. 2007). Breakdown of the group × trial interaction into the group simple effects at each trial, followed by post hoc mean comparisons, showed that freezing levels on the first trials (1–3) were similar in both ifenprodil-injected and No-Ext controls rats, but significantly higher than vehicle, indicating a complete failure to retrieve extinction at the start of the session. In contrast, ifenprodil-treated rats also showed significantly lower freezing levels than No-Ext control rats in the last trials (4–5) (P < 0.005), indicating rapid re-extinction learning. This suggests that blocking NR2Bs during the extinction consolidation phase impairs plasticity necessary for the retrieval of the extinction memory.
Posttraining Activation of NR2Bs in LA Is Not Required for Fear Extinction
As in the previous experiment, all rats showed gradual reduction in freezing as extinction training progressed, reaching very low freezing levels by the end of extinction training. In the extinction test, conducted 24 h after extinction training, rats given posttraining intra-LA vehicle (0.25 μl/side) exhibited low levels of freezing during the test trials, indicating successful retention of extinction learning. Similarly rats given posttraining intra-LA ifenprodil (1.0 μg/0.25 μl/side) exhibited relatively low levels of freezing during test. Coronal drawings of infusion sites are shown in Figure 2a and results (by trial) are shown in Figure 2b. It is important to emphasize that we used a dose (1.0 μg) that impairs fear conditioning (Rodrigues et al. 2001) and that also produces the maximal behavioral impairment on extinction acquisition (Sotres-Bayon et al. 2007).
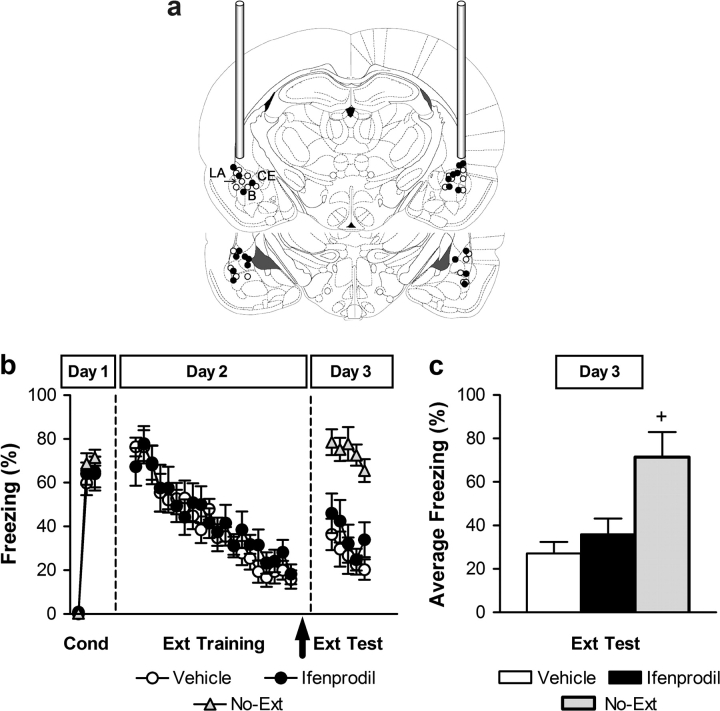
Figure 2.
Fear extinction does not require posttraining NR2B activation in the LA. (a) Coronal drawings show the localization of injector tips (top to bottom relative to bregma: −2.80 mm and −3.60 mm [adapted from Paxinos and Watson 1998]) from rats infused with vehicle (white circles) or ifenprodil (1 μg; black circles). B: basal amygdala; CE: central amygdala. (b) Percent freezing during conditioning (first trial and averages of 2–4 and 5–7 tone–footshock trials on Day 1), trial-by-trial extinction training (20 tone-alone trials on Day 2), and extinction test (5 tone-alone trials on Day 3). Vehicle (white circles) and ifenprodil (black circles) injections were administered immediately after extinction training (arrow); No-Ext control group (gray triangles). (c) Averaged percent freezing across all extinction retention trials for vehicle-treated (white bar), ifenprodil-treated (black bar), and No-Ext (gray bar) rats. +P < 0.05 relative to ifenprodil and vehicle groups in the same session.
The extinction retention test results (all trials) were statistically evaluated using a repeated measures ANOVA with group (vehicle, ifenprodil, No-Ext) as a between-subjects factor and trials as a within-subjects factor (ANOVA: significant main effects of group [F2,28 = 15.37, P < 0.005], trial [F4,112 = 3.34, P < 0.005], and nonsignificant group × trial interaction [P > 0.05]). Post hoc comparisons between groups showed that, as expected, vehicle-treated rats exhibited significantly lower freezing during retention test as compared with No-Ext controls (P < 0.0001). In contrast, ifenprodil-treated rats were not significantly different from vehicle-treated rats (P > 0.05) but were significantly different from No-Ext controls (P < 0.001) (Fig. 2c). Thus, ifenprodil into the LA, given immediately after extinction training, did not impair consolidation of fear extinction.
Posttraining Activation of NR2Bs in the vmPFC Is Required for Fear Extinction
In this experiment, we decided to infuse ifenprodil into the vmPFC to test its role in the phase of extinction consolidation because the pattern of results with systemic ifenprodil (impaired extinction retrieval and faster re-extinction) resemble those routinely found in extinction studies involving vmPFC manipulations (lesions [Quirk et al. 2000; Lebron et al. 2004] as well as NMDAR [Burgos-Robles et al. 2007] and noradrenergic blockade [Mueller et al. 2008]).
Similar to our previous experiments, all rats showed a gradual reduction in freezing during extinction training that resulted in very low freezing levels by the end of extinction training. One day after extinction training, in the extinction–retention test, rats given posttraining vehicle exhibited low levels of freezing during the test trials, indicating successful retention of extinction learning. In contrast, rats given posttraining ifenprodil (2.0 μg/0.5 μl/midline) into the vmPFC exhibited relatively high levels of freezing, similar to No-Ext controls, indicating that ifenprodil intra-vmPFC impaired the consolidation of fear extinction. Coronal drawings of infusion sites are shown in Figure 3a and results (by trial) are shown in Figure 3b.
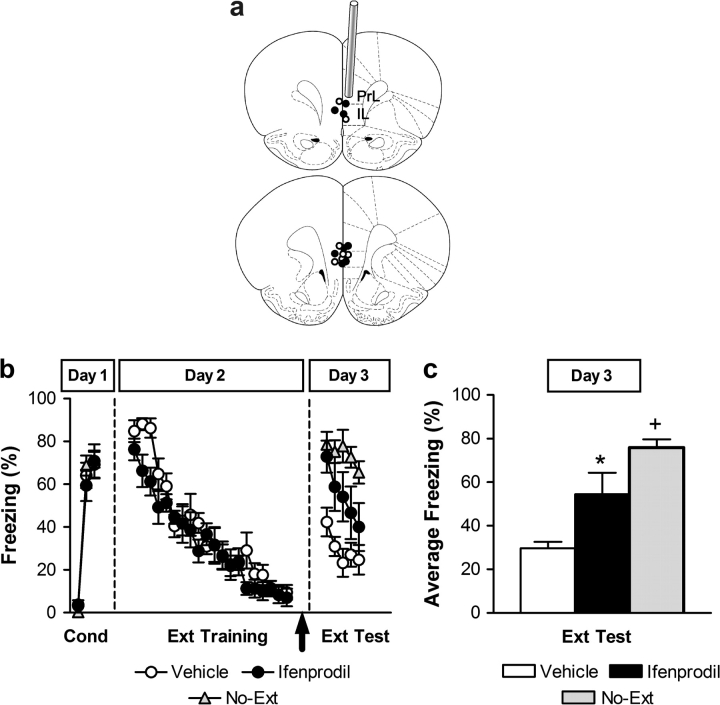
Figure 3.
Fear extinction requires posttraining NR2B activation in the vmPFC. (a) Coronal drawings show the localization of injector tips (top to bottom relative to bregma: +3.20 mm and +2.70 mm (adapted from Paxinos and Watson 1998) from rats infused with vehicle (white circles) or ifenprodil (2 μg; black circles). IL: infralimbic cortex; PrL: prelimbic cortex. (b) Percent freezing during conditioning (first trial and averages of 2–4 and 5–7 tone–footshock trials on Day 1), trial-by-trial extinction training (20 tone-alone trials on Day 2), and extinction test (5 tone-alone trials on Day 3). Vehicle (white circles) and ifenprodil (black circles) injections were administered immediately after fear extinction (arrow); No-Ext control group (No-Ext; gray triangles). (c) Averaged percent freezing across all extinction retention trials for vehicle-treated (white bar), ifenprodil-treated (black bar), and No-Ext (gray bar) rats. *P < 0.05 relative to control groups (vehicle and No-Ext) in the same session. +P < 0.05 relative to ifenprodil and vehicle groups in the same session.
The extinction–retention test results (all trials) were statistically evaluated using a repeated measures ANOVA with group (vehicle, ifenprodil, No-Ext) as a between-subjects factor and trials as within-subjects factors (ANOVA: significant main effects of group [F2,21 = 10.60, P < 0.001] and trial [F4,84 = 8.10, P < 0.0001], but a nonsignificant group × trial interaction [P > 0.05]). Post hoc comparisons between groups showed that ifenprodil-treated rats exhibited significantly higher freezing during extinction retention test as compared with vehicle-treated rats (P < 0.05) and lower compared with No-Ext controls (P < 0.05) (Fig. 3c). Thus, ifenprodil into the vmPFC, given immediately after extinction training, impairs the retrieval of fear extinction.
Activation of vmPFC NR2Bs during Training Is Not Required for Fear Extinction
Previous studies using pre-extinction training systemic injections of the nonsubunit-selective NMDAR antagonist CPP have implicated NMDARs in fear extinction retention but not in its acquisition (Santini et al. 2001; Suzuki et al. 2004). However, systemic results from our previous report, also using pre-extinction training manipulations, indicate that NMDARs containing the 2B subunit are required for fear extinction acquisition and the intra-LA results indicate that activation of these receptors in the LA is required (Sotres-Bayon et al. 2007). Further, we also noted that our systemic effect was stronger than the intra-LA effect, suggesting that besides amygdala, NR2Bs in another structure may also participate. Although substantial evidences shows that the vmPFC is involved in fear extinction consolidation/retrieval (Quirk et al. 2006), recent findings also suggest the vmPFC also plays a role in extinction acquisition (Zushida et al. 2007). Therefore, as a control for potential effects of NMDAR blockade in the vmPFC during extinction acquisition, in this experiment, we assessed whether blockade of NR2B-containing NMDARs in the vmPFC affects extinction learning by locally infusing ifenprodil into the vmPFC before extinction training.
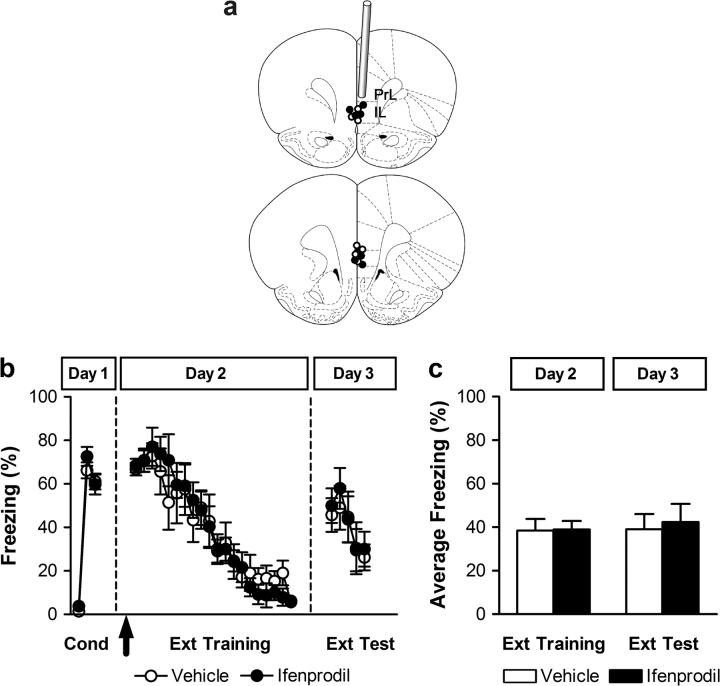
Rats infused with vehicle or ifenprodil (0 and 2.0 μg/0.5 μl, respectively) into the vmPFC, 15 min before extinction training, exhibited similar tone-elicited freezing during the first trial of extinction training indicating that the fear memory was acquired and expressed to the same extent in all groups. Over the course of the 20 extinction training trials, vehicle- and ifenprodil-treated rats showed a gradual reduction in freezing levels, with virtually no freezing by the end of extinction training. Accordingly, both control and drug groups behaved similarly also during the drug-free extinction test the next day. Coronal drawings of infusion sites are shown in Figure 4a and results (by trial) are shown in Figure 4b. Statistical analysis confirmed that both groups showed the same level of freezing throughout the experiment (Fig. 4c). These results suggest that NR2B-containing NMDARs in the vmPFC are not necessary for the acquisition of extinction learning and hence further support the notion that NMDAR-mediated activity in the vmPFC is not required for the acquisition but crucially engaged posttraining during the consolidation phase of fear extinction.
Figure 4.
Fear extinction does not require NR2B activation in the vmPFC during training. (a) Coronal drawings show the localization of injector tips (top to bottom relative to bregma: +3.20 mm and +2.70 mm (adapted from Paxinos and Watson 1998) from rats infused with vehicle (white circles), ifenprodil (2 μg; gray circles). IL: infralimbic cortex; PrL: prelimbic cortex. (b) Percent freezing during conditioning (first trial and averages of 2–4 and 5–7 tone-footshock trials on Day 1), trial-by-trial extinction training (20 tone-alone trials on Day 2), and extinction test (5 tone-alone trials on Day 3). Vehicle (white circles), ifenprodil (black circles) injections were administered before extinction training (arrow). (c) Averaged percent freezing across all extinction training and extinction retention trials for vehicle-treated (white bars), and ifenprodil-treated (black bars) rats.
Discussion
In this study and a previous one (Sotres-Bayon et al. 2007), we evaluated the effects of systemic injections of the NR2B antagonist ifenprodil as well as local infusions in the LA and vmPFC. Our systemic results show that pretraining blockade of NR2Bs disrupts extinction acquisition (Sotres-Bayon et al. 2007), whereas posttraining blockade disrupts its consolidation (this study). In local infusion studies, we found that NR2B pretraining blockade in the LA disrupted extinction acquisition (Sotres-Bayon et al. 2007), whereas posttraining infusions in the LA had no effect (this study). On the other hand, NR2B pretraining blockade in the vmPFC had no effect on extinction acquisition, whereas posttraining infusions in the vmPFC disrupted its consolidation (this study). Together, these results show a double dissociation of the role of NR2Bs in the LA (acquisition, not consolidation) and vmPFC (consolidation, not acquisition) in fear extinction.
NR2B Contribution in Extinction
Evidence for the timing of NMDAR-mediated contributions to fear extinction learning has been unclear. NMDARs are known to play an important role in memory acquisition during training, mediated via synaptic plasticity induction (Riedel et al. 2003). However, emerging evidence indicates that for several forms of learning, this receptor is also required after training during memory consolidation phase (Shimizu et al. 2000; Cui et al. 2005; McDonald et al. 2005; Schicknick and Tischmeyer 2006) (however, see Day and Morris 2001). Using NMDAR antagonist CPP, previous studies suggested that NMDARs systemic blockade before extinction training prevents the consolidation of this learning, but not its acquisition, consistent with the view that NMDARs are key for memory consolidation (Santini et al. 2001; Suzuki et al. 2004). However, using the NR2B subunit–selective antagonist ifenprodil, we recently found that the same treatment attenuates the initial acquisition, and consequently the subsequent retrieval of extinction, consistent with the notion that NMDARs are crucial for the initial acquisition of the extinction memory (Sotres-Bayon et al. 2007). Therefore, to directly examine the role of NR2Bs in extinction consolidation, here we used posttraining injections and found that ifenprodil impairs subsequent performance, supporting a role for NR2B activation during consolidation. Further analysis revealed that ifenprodil-treated rats showed impaired retrieval of extinction followed by faster re-extinction rates, suggesting that blocking NR2Bs during the extinction consolidation phase may impair plasticity necessary for the retrieval of the extinction memory. Thus, it appears that NMDARs are required for both the acquisition and consolidation phase of the extinction experience.
Further, here we examined whether the consolidation effect is mediated via the vmPFC and/or the LA. Our data show that activation of NR2Bs within the vmPFC, but not in the LA, is required after extinction training, indicating a role for vmPFC NR2Bs during the consolidation phase of the extinction experience.
NR2B Contribution in vmPFC after Extinction Training
Converging evidence using a variety of techniques (lesion, local inactivation, recording, stimulation as well as metabolic mapping in rodents, and functional imaging in humans) indicates that the vmPFC is engaged after extinction training occurs (Quirk et al. 2006; Rauch et al. 2006; Sotres-Bayon et al. 2006; Quirk and Mueller 2008). Thus, it is not surprising that NMDARs in the vmPFC have been involved after, rather than during, extinction training. Notably, in a recent report, Burgos-Robles et al. (2007) found that NMDAR-dependent bursting in the vmPFC is required for the initial 2 h of posttraining processing of extinction. They further suggested that the observed bursting could be generated by instructive input signals from hippocampus (Corcoran et al. 2005; Hugues et al. 2006); amygdala activity is another candidate input signal in this phenomenon (Garcia et al. 1999; Ishikawa and Nakamura 2003). However, Burgos-Robles et al. (2007) did not distinguish between NMDAR subtypes that are known to confer this receptor with distinct functional properties. That this may be important is suggested by results of recent studies showing that NR2Bs in mPFC are necessary for long-term potentiation and depression as well as contextual fear memory (Toyoda et al. 2005; Zhao et al. 2005). Consistent with these latter findings, we show that blockade of NR2Bs in vmPFC impairs posttraining processing of extinction.
Local infusion into the vmPFC of NMDAR antagonists ifenprodil (this report), as well as CPP (Burgos-Robles et al. 2007), immediately after extinction training impairs extinction retention, suggesting its role in extinction consolidation. Relative to each other, ifenprodil has higher affinity for NR2B, and CPP for the NR2A subunit (Lozovaya et al. 2004), suggesting NMDA-dependent processing in the vmPFC is required. It is important to emphasize that rats given posttraining ifenprodil manipulations in this study showed savings in the rate of re-extinction, as indicated by the systemic results and the trend seen with infusions into vmPFC. In fact, this is consistent with prefrontal extinction deficits found in previous studies using lesions (Quirk et al. 2000; Lebron et al. 2004) as well as NMDAR (Burgos-Robles et al. 2007) and noradrenergic blockade (Mueller et al. 2008). In contrast, no such trend was evident following infusions in LA (Sotres-Bayon et al. 2007). This suggests that NR2B-mediated processing after extinction training may be involved with the learning that is needed for extinction retrieval rather than simply the consolidation of the original “extinction memory trace.” Further, because the return of fear at extinction test followed by rapid re-extinction is reminiscent of contextual renewal, it suggests that the vmPFC may encode contextual information related to extinction rather than extinction per se. Indeed, induction of extinction-mediated plasticity in the vmPFC after extinction training may involve reactivation or “rehearsal” strategies (Wang et al. 2006), in extinction circuits that may include the amygdala and hippocampus (Hobin et al. 2003; Sotres-Bayon et al. 2004).
What exactly does it mean that vmPFC activity after extinction training is NMDA dependent? Consistent with the traditional role of NMDARs in the induction of neural plasticity during the acquisition of learning, NMDARs in the vmPFC may be required after training to acquire an important aspect of the extinction experience. At this time point (after extinction training), the vmPFC may encode the new reinforcement contingencies by putting together relationships between different input sources (e.g., inputs from amygdala and hippocampus), which in turn may later (during extinction retrieval) serve to shift behavioral strategies to rapidly inhibit the previously learned fear response. This interpretation is parsimonious with a cellular consolidation view where both amygdala and vmPFC encode, at particular times, different aspects of the extinction experience. Thus, induction of extinction-mediated plasticity in the vmPFC may be required during the consolidation phase (immediately after training) of the extinction experience.
NR2B Contribution in the Amygdala during Extinction Training
The amygdala is an obvious place to consider when searching for fear extinction-related changes, given its known role in fear learning and expression (LeDoux 2000). In contrast to accumulated evidence that suggests a role of the amygdala in fear extinction acquisition (Davis et al. 2003; Lin et al. 2003; Herry et al. 2006; Sotres-Bayon et al. 2007), its role in extinction consolidation has been explored sparsely. Thus, the importance of the negative effect of postextinction LA infusions of ifenprodil (this study) becomes clear when comparing to the positive effect of postextinction vmPFC infusions (this study) and of pre-extinction infusions reported in our previous study using the same dose and identical behavioral procedures (Sotres-Bayon et al. 2007).
Although here we found that amygdala NR2Bs are not necessary for posttraining extinction consolidation, this does not mean that molecular processes within the amygdala are uninvolved in this process. In fact, a few recent studies suggest that the amygdala plays an important role in extinction consolidation (Akirav et al. 2006; Berlau and McGaugh 2006). Notably, a recent study found that amygdala brain-derived neurotrophic factor (BDNF) signaling is required for consolidation but not acquisition of extinction (Chhatwal et al. 2006). Interestingly, BDNF activity appears to involve NR2As but not NR2Bs (Chen et al. 2007). This raises the possibility that while extinction acquisition requires NR2B receptors in the amygdala (Sotres-Bayon et al. 2007), extinction consolidation may depend on NR2A activation in the amygdala, thereby resulting in BDNF upregulation. Nonetheless, because the role of NR2A in extinction has not been explored, it is still possible that amygdala contribution to fear extinction consolidation is NMDAR independent. Further experiments are necessary to understand the molecular mechanisms in the amygdala engaged during extinction consolidation.
NR2B Contribution to Extinction: Amygdala during Training and vmPFC after Training
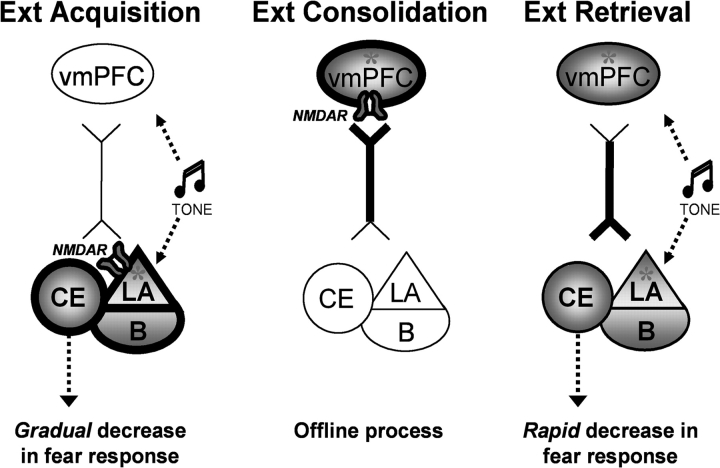
An important goal in extinction research is to understand the neural and molecular mechanisms that underlie each of the phases of the extinction experience. It is clear that a network of brain regions, including amygdala and vmPFC, are involved in encoding extinction learning as well as storage of the extinction memory necessary for its later retrieval in the appropriate environmental conditions. However, little is known regarding when and how each of these structures contributes to each phase of the extinction process. Our findings suggest that NR2B activation is engaged in the vmPFC and amygdala at different times in the extinction learning process: during extinction training in the amygdala and after extinction training in the vmPFC. Thus, we propose that the amygdala gradually encodes extinction, via NR2Bs, during extinction training (within-session extinction) (Sotres-Bayon et al. 2007) but not afterward, while the vmPFC encodes extinction offline, via NMDARs, after extinction training has occurred (between-session extinction). This does not exclude that other molecular processes within the amygdala and vmPFC are required to consolidate extinction learning; it simply emphasizes that in the phase of extinction processing after training only NR2B-mediated processing in the vmPFC, but not in the amygdala, is crucial. Finally, when presented with the appropriate environmental conditions (extinction test), the information stored in the extinction circuit (including amygdala and vmPFC) allows for rapid reduction of fear responses (see Fig 5).
Figure 5.
Model for extinction learning phases: NR2B contribution. Extinction acquisition (within-session): the lateral amygdala (LA) gradually encodes extinction via NR2B-containing NMDARs, leading to gradual decrease in fear response. Extinction consolidation (between-session): the vmPFC encodes extinction learning offline via NMDARs (including NR2Bs), instructed in part by amygdala input. Extinction retrieval: events that induced plasticity (“extinction memory trace”; asterisk) during and after extinction training are engaged in ventromedial prefrontal cortex (vmPFC)-induced suppression of amygdala output, leading to rapid reduction of fear responses. Ext: extinction; B: basal amygdala; CE: central amygdala.
In conclusion, our findings highlight the importance of timing and brain localization of NMDA-mediated processing in fear extinction learning. Future research will undoubtedly seek to further understand the molecular events that lead to fear extinction and how the neural mechanism engaged in different brain structures, including amygdala and vmPFC, interact to encode the extinction memory, stabilize it during consolidation, and ultimately coordinate its expression.
Funding
National Institute of Mental Health (R01 MH46516, R37 MH38774, K05 MH067048, P50 MH58911, and R21 MH072279) to J.E.L.
Acknowledgments
We thank Dr Christopher K. Cain, Dr Gregory J. Quirk, and Anthony Burgos-Robles for comments on an earlier version of this manuscript. Conflict of Interest: None declared.
References
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Blair HT, Sotres-Bayon F, Moita MA, Ledoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133:561–569. doi: 10.1016/j.neuroscience.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27:542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des. 1999;5:381–404. [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Lindl KA, Mei B, Zhang S, Tsien JZ. Requirement of NMDA receptor reactivation for consolidation and storage of nondeclarative taste memory revealed by inducible NR1 knockout. Eur J Neurosci. 2005;22:755–763. doi: 10.1111/j.1460-9568.2005.04257.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Day M, Morris RG. Memory consolidation and NMDA receptors: discrepancy between genetic and pharmacological approaches. Science. 2001;293:755. doi: 10.1126/science.293.5531.755a. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Ann Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McAllister WR, McAllister DE. Behavioral measurement of conditioned fear. In: FR Brush., editor. Aversive conditioning and learning. New York: Academic Press; 1971. pp. 105–179. [Google Scholar]
- McDonald RJ, Hong NS, Craig LA, Holahan MR, Louis M, Muller RU. NMDA-receptor blockade by CPP impairs post-training consolidation of a rapidly acquired spatial representation in rat hippocampus. Eur J Neurosci. 2005;22:1201–1213. doi: 10.1111/j.1460-9568.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nikam SS, Meltzer LT. NR2B selective NMDA receptor antagonists. Curr Pharm Des. 2002;8:845–855. doi: 10.2174/1381612024607072. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney (Australia): Academic Press; 1998. [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicknick H, Tischmeyer W. Consolidation of auditory cortex-dependent memory requires N-methyl-D-aspartate receptor activation. Neuropharmacology. 2006;50:671–676. doi: 10.1016/j.neuropharm.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, Ledoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of the prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Zhuo M. Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. Eur J Neurosci. 2005;22:485–494. doi: 10.1111/j.1460-9568.2005.04236.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]