Abstract
Background
This study investigated whether subgroups of alcohol dependent patients responded differently to naltrexone vs. placebo in the NIAAA COMBINE study. In particular, the A vs. B and the Early Onset vs. Late Onset typologies were examined. Relative to Type A alcoholics, Type Bs are characterized by greater severity, earlier onset, stronger family history, more childhood risk factors (e.g., conduct disorder), and greater frequency of comorbid psychiatric and substance use disorders.
Methods
COMBINE study participants were categorized as Type A or Type B using k-means cluster analysis and variables from 5 domains that have been shown to replicate the original Babor typology efficiently. Early Onset was defined as alcohol dependence beginning before age 25. For the planned analyses, the sample was reduced to the 618 participants receiving naltrexone alone or placebo, either with medical management (MM) alone or with MM plus the Combined Behavioral Intervention (CBI). The a priori primary outcome was percent heavy drinking days during treatment in the groups receiving MM without CBI.
Results
Among those receiving MM without CBI, Type A alcoholics had better drinking outcomes with naltrexone than placebo, whereas medication condition did not influence outcomes significantly in the Type Bs. Age of onset was not significantly related to outcome. For those receiving CBI, no significant effects were found for either typology.
Conclusions
In this sample, the beneficial effects of opioid antagonism were limited to Type A alcoholics receiving treatment in a medical management model. Future studies should investigate the relationship between clinically relevant genotypes, phenotypes such as typologies, and treatment response. More work is also needed to develop practical algorithms for phenotypic assignment.
Keywords: Alcoholism, pharmacotherapy, typology, naltrexone, randomized clinical trial
Introduction
Although the efficacy of naltrexone has been demonstrated in the majority of well-designed trials (Pettinati et al., 2006), and this medication has consistently been found superior to placebo in meta-analyses, effect sizes have been modest (Srisurapanont and Jarusuraisin, 2005). Also, some well-designed trials have yielded null findings (e.g. (Krystal et al., 2001)). The inconsistency of results and modest effect sizes across studies raise the question of whether there are individual characteristics that predict greater or lesser response to naltrexone. If identified, such characteristics could play a useful role in treatment matching.
Numerous alcoholism typologies have been proposed in an effort to identify relatively homogenous subtypes of alcoholics (Penick et al., 1999). These typologies are useful if they can be used to predict clinical course and response to treatment, including patient-treatment matching. Perhaps the most-studied alcoholism typology is that of Babor et al. (Babor et al., 1992). Relative to Type A alcoholics, Type Bs are characterized by greater severity, earlier onset, stronger family history, more childhood risk factors (e.g., conduct disorder), and greater frequency of comorbid psychiatric and substance use disorders. Studies have reported an effect of typology on outcome, independent of specific treatment, with worse outcomes in persons with Type B alcohol dependence (Babor et al., 1992, Yoshino and Kato, 1996). Differential response to psychosocial treatments based on typology was found in one study (Litt et al., 1992), but this finding was not replicated in Project MATCH (Litt and Babor, 2001b).
Considerable evidence indicates that the Babor A vs. B typology (Babor et al., 1992) can determine the strength of the response to pharmacologic treatments for alcoholism. Studies have demonstrated relatively poor response to SSRIs in persons with Type B alcohol dependence (Dundon et al., 2004, Pettinati et al., 2000, Kranzler et al., 1996). Consistent with this, a study using the alcoholism typology of Cloninger (Cloninger et al., 1988) found worse response to fluvoxamine in persons with Type II alcohol dependence. Cloninger's Type II shares features with Babor's Type B (Chick et al., 2004). In contrast, the effect of quetiapine on alcohol consumption also appears to depend strongly on an A vs. B typology, with a much larger medication effect observed in Type Bs (Kampman et al., 2007). To our knowledge, Babor's typology has never been applied to studies using naltrexone.
Age of onset (early vs. late) and the typology of Lesch (Lesch et al., 1989) have also been studied as possible predictors of response to pharmacologic treatment of alcohol dependence. Ondansetron, acamprosate, and naltrexone have all been reported to be more effective in early onset alcoholics (Kiefer et al., 2005, Johnson et al., 2000, Kiefer et al., 2008). However, for acamprosate, a pooled analysis of data from seven European studies failed to detect significant interactions of treatment (acamprosate vs. placebo) with age of onset, physiological dependence, familial alcoholism, anxiety, severity of craving, or gender (Verheul et al., 2005). Two reports suggest that the Lesch typology predicts response to acamprosate, with significant response limited to Types I and II (Lesch et al., 2001, Kiefer et al., 2005). For naltrexone, response was reported to be better in Types III and IV (Kiefer et al., 2005).
The purpose of this study was to assess whether the effect of the opioid antagonist naltrexone in the COMBINE study was moderated by participants' alcoholism subtype. In COMBINE naltrexone given with medical counseling was associated with significant but modest improvement in drinking outcomes (Anton et al., 2006). There would be great clinical value in identifying sub-groups of alcoholics that were more likely to benefit from naltrexone. The COMBINE data set contained adequate baseline characteristics to attempt the analysis with the Babor typology and with Age of Onset (Anton and Randall, 2005). The COMBINE study did not include all of the information necessary to apply the Lesch typology. In addition to the Kiefer et al. study reporting greater efficacy in early onset alcoholics (Kiefer et al., 2008), studies have reported larger effects of naltrexone in those with stronger family history, greater craving, other substance use disorder, and stronger antisocial personality traits (Rohsenow et al., 2007, Monterosso et al., 2001, Rubio et al., 2005). Therefore we hypothesized that naltrexone would have a stronger therapeutic effect in the more severe Type B and Early Onset alcoholics.
Materials and Methods
Brief Summary of the COMBINE Study
The rationale and methods of the COMBINE study have been described in detail elsewhere (Anton and Randall, 2005, COMBINE Study Research Group, 2003, Pettinati et al., 2005b). Briefly, COMBINE was designed with the goal of determining whether alcohol dependence treatment outcomes can be improved by combining specific pharmacotherapies and behavioral therapies. Overall, the study included 1383 participants at 11 sites. Participants were alcohol dependent male and female adult outpatients who had been drinking heavily during the preceding 90 days but were abstinent for at least 4 days at randomization and not experiencing significant alcohol withdrawal. Patients were excluded if they had serious mental illness, were currently dependent on any drug other than alcohol, nicotine, or marijuana, had any significant recent opioid use, had any medical condition that interfered with study participation, or required medication that would increase the potential risks of the study.
Participants were randomly assigned to one of 9 treatment conditions, and received 16 weeks of active treatment. Eight conditions consisted of all possible combinations of naltrexone vs. placebo, acamprosate vs. placebo, and CBI vs. no CBI (2 by 2 by 2 factorial design). All participants in these cells also received up to 9 sessions of manualized MM (Pettinati et al., 2004) along with the study medication. CBI consisted of up to 20 sessions of manualized individual therapy incorporating elements of Motivational Enhancement Therapy, Cognitive Behavioral Skills Training, and 12-step Facilitation (Miller, 2004). The 9th cell received CBI only, without study medication, placebo, or MM. Primary assessments were administered at baseline and at weeks 8, 16, 26, 52, and 68 post randomization, with abbreviated assessments at 1, 2, 4, 6, 8, and 12 weeks. The primary outcomes for the main trial were percent days abstinent per month during treatment and time to first relapse to heavy drinking (5 or more drinks in a day for men, 4 or more drinks in a day for women).
Typological Assignment for the Present Study
The original Babor typology is based on the 17 characteristics listed in Table 1. These domains can be thought of as representing 4 underlying constructs: vulnerability factors, alcohol involvement, chronicity of alcohol problems, and comorbid psychopathology. In practice, each study that has operationalized this typology has used a somewhat different set of variables. For example, Project MATCH used 14 variables to replicate the typology using cluster analysis, and then created a simplified criterion-based typology using 5 variables, which corresponded well to the assignment based on cluster analysis (Litt and Babor, 2001a). Based on examination of the variables that were available in the COMBINE database, we chose to use a set of 5 variables which Schuckit et al. (Schuckit et al., 1995) found to correlate most strongly with Babor's A/B typologic assignment in the COGA study sample of 1539 alcohol dependent persons. In that study typologic assignment based on these 5 variables produced clusters which were descriptively similar to those produced with the full 17 domains, and typologic assignment was strongly correlated (R = .66 in males, .87 in females, respectively) (Schuckit et al., 1995).
Table 1. Comparison of A vs. B typologic assignment in present study vs. Schuckit et al., 1995, and Babor et al., 1992.
| Domain | Present study | Schuckit et al. | Babor et al. |
|---|---|---|---|
| Standard drinks per drinking day | Form 90 (Miller and Del Boca, 1994) | Drinks per drinking day, past 6 months | Average alcohol consumption, 6 months prior to treatment |
| Relief drinking | Sum of withdrawal-related items from the Alcohol Dependence Scale (Skinner and Allen, 1982) (items 3, 5, 9, 11,14, 21) | 4-item index of relief drinking symptoms from the SSAGA (Bucholz et al., 1994) | 6-item scale rating salience of “relief drinking” |
| Number of severe or enduring alcohol related medical conditions | 8 items medical history and laboratory data: hypertension; heart disease, cirrhosis; stomach problems; low magnesium, potassium, or phosphate; any abnormally high liver enzyme; high bilirubin or low albumin; and high mean corpuscular volume or low platelets. | 5-item index of alcohol-related medical conditions requiring medical attention from the SSAGA | 84-item index of alcohol-related conditions, primarily from Cornell Medical Index (Brodman et al., 1949) |
| Physical consequences | Alcohol Dependence Scale (items 2, 4, 7, 8, 10, 13, 16, 17, 19, 20, 22) (Skinner and Allen, 1982) | 6-item index of alcohol-related physical problems from the SSAGA | 5-item scale of alcohol-related physical consequences during past 6 months (Hesselbrock et al., 1983) |
| Social and interpersonal consequences | Sum of social and interpersonal consequences scores from the Drinker Inventory of Consequences (Miller et al., 1995) | 7-item index of alcohol related social problems from the SSAGA | 5-item scale of alcohol related social consequences, past 6 months (Hesselbrock et al., 1983) |
| Additional Domains (different measures used by Babor et al. and Schuckit et al.) | family history, childhood psychiatric disorder, bipolar character dimension, age of onset, dependence syndrome, benzodiazepine use, polydrug use, lifetime severity, years heavy drinking, depressive symptoms, antisocial personality disorder symptoms, anxiety severity. | ||
SSAGA: Semi-Structured Assessment for the Genetics of Alcoholism
For the present study, baseline assessment data from COMBINE were used to create composite variables for the following five domains: 1. Standard drinks per drinking day; 2. Relief drinking; 3. Number of severe or enduring alcohol-related medical conditions; 4. Physical consequences; 5. Social and interpersonal consequences. Table 1 shows how each of these composite variables was constructed for the present study in comparison to the methodology of Schuckit et al. (Schuckit et al., 1995) and Babor et al. (Babor et al., 1992). Typological assignment was done using all COMBINE study participants for whom the necessary baseline data were available. K-means cluster analysis was used, with the 2-cluster solution selected a priori. Early vs. Late Onset alcoholics were defined by age of onset of alcohol dependence less than vs. greater than or equal to 25, from the Structured Clinical Interview for DSM IV (SCID) (First et al., 1997).
Statistics
Outcome measures and covariates were drawn from the COMBINE database. Drinking outcomes were derived from data collected with the Form 90 (Miller and Del Boca, 1994). The a priori primary outcome for our analyses was percent heavy drinking days (PHDD) from the Form 90 16-week data. This measure was chosen because it incorporates both frequency and intensity. Secondary outcomes were percent days abstinent (PDA), drinks per day (DPD), time to relapse to heavy drinking, and good clinical outcome (defined as abstinence or moderate drinking without problems). T tests, chi square tests, ANOVAs, ANCOVAs, MANCOVAs, and Cox regression methods were used for the various analyses as describes in the Results section.
Results
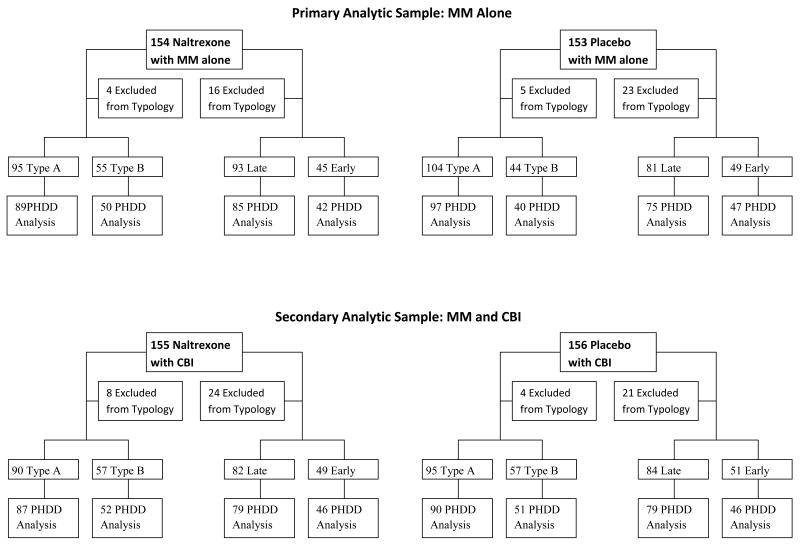
Figure 1 depicts the disposition of COMBINE study participants into the groups used for analyses in this study. Of the 1,383 study participants in the COMBINE study, 54 (4%) did not provide sufficient information to compute the Babor typology, and 191 (14%) had missing values for the age-of-onset variable. Of the participants classified using the Babor typology (N= 1329 or 96% of the original sample), 829 were assigned Type A and 500 Type B. Of the participants we were able to include for the age-of-onset typology (N= 1192 or 86% of the original sample), 439 were classified as early onset and 753 as late onset. No relationships were found between missing intake data and treatment group assignment. Table 2 provides intake demographic information and measures of alcohol use and consequences for the COMBINE sample divided by typologic classification. The pattern of differences seen between Types A and B was similar to that seen between early and late onset groups, but the differences were generally larger in magnitude in the Type A/B classification. The more severe category in both typologies (Type B, Early Onset), indicated that more impaired alcohol users were younger, less educated, less likely to be married, and more likely to be male. Likewise, both of the more severe subgroups reported more alcohol dependence symptoms and alcohol-related consequences. Interestingly, participants in both of the more severe categories reported drinking more drinks per drinking episode, but fewer drinking days relative to the less severely impaired participants.
Figure 1.
Disposition of COMBINE study participants in analyses of typological effects
Table 2. Intake Characteristics by Typologic Classification of the COMBINE Sample (S.D.).
| Babor Typology | Age of Onset | |||
|---|---|---|---|---|
| (N = 1,329) | (N = 1,192) | |||
| A | B | Late | Early | |
| (n = 829) | (n = 500) | (n = 753) | (n = 439) | |
| Age | 45.82 | 42.99* | 46.69 | 39.86* |
| (10.38) | (9.51) | (9.49) | (9.88) | |
| Education | 15.02 | 13.77* | 14.59 | 13.97* |
| (2.72) | (2.60) | (2.81) | (2.40) | |
| %Male | 66.8% | 71.9%* | 68.3% | 76.5%* |
| %Married | 47.0% | 34.9%* | 42.9% | 36.1%* |
| %non-Hispanic White | 78.5% | 74.8% | 75.4% | 73.1% |
| % Working | 68.6% | 48.9%* | 57.7% | 66.7%* |
| ADS Score | 13.47 | 21.87* | 16.14 | 18.28* |
| (5.38) | (7.10) | (7.06) | (7.50) | |
| SCID Dependence | 4.78 | 5.71* | 5.07 | 5.32* |
| (1.17) | (.97) | (1.21) | (1.18) | |
| % Fam Hx Alcohol | 71.7% | 80.4%* | 73.1% | 77.4% |
| Craving (OCDS) | 23.81 | 31.33* | 26.42 | 27.18 |
| (6.77) | (8.26) | (8.19) | (8.61) | |
| Motivation | 10.32 | 11.11* | 10.71 | 10.54 |
| (1.50) | (1.46) | (1.51) | (1.51) | |
| Consequences | 35.35 | 67.32* | 46.89 | 51.21* |
| (11.84) | (15.34) | (20.12) | (20.76) | |
| PDA | 24.14 | 26.13 | 24.30 | 29.07* |
| (24.83) | (25.48) | (24.62) | (26.03) | |
| DPDD | 9.52 | 17.28* | 12.08 | 14.20* |
| (4.48) | (9.88) | (7.33) | (8.98) | |
| PHDD | 63.10 | 69.94* | 66.15 | 63.41 |
| (29.39) | (26.75) | (28.65) | (28.33) | |
| % Med. Adherence | 89.32 | 88.76 | 89.38 | 88.53 |
| (17.56) | (17.65) | (17.42) | (18.84) | |
| # of MM sessions | 7.70 | 7.24* | 7.65 | 7.17* |
| (2.17) | (2.47) | (2.31) | (2.46) | |
p < .01
ADS: Alcohol Dependence Scale (Skinner and Allen, 1982)
OCDS: Obsessive Compulsive Drinking Scale (Anton et al., 1995)
A chi square test was done to assess the extent of agreement between the two typologies in classifying participants as less (Type A or late onset) or more (Type B or early onset) severely alcohol-impaired. Of the 1,143 participants with complete data, agreement between typologies was achieved with only 56.6% (n = 651) of the participants, χ2 (1) 8.89, p < .003. Both typologies placed 461 participants (40.3%) in the less severe group and 190 subjects in the more severe category (16.6%). Disagreements in classification included a similar number of false positives and negatives. Specifically, 233 (20.4%) participants were classified as Type A and early onset, while 259 (22.7%) were classified as Type B and late onset.
For the planned primary analyses, the sample was reduced to the participants in COMBINE study conditions of placebo + MM and naltrexone + MM (no CBI). Parallel secondary analyses were conducted in the two groups receiving placebo + MM+ CBI and naltrexone + MM + CBI. The reasoning for this was as follows. Results of the COMBINE study showed that the effect of naltrexone was limited to the patients who did not receive CBI (Anton et al., 2006). Therefore, we expected that naltrexone-by-typology interactions were more likely to be significant in the MM-only condition. Participants receiving acamprosate were excluded from the analyses because the aim of the study was to investigate whether typology moderated the effects of opioid antagonism. Including the subjects who received acamprosate would have introduced the possibility that effects of acamprosate in particular subgroups (i.e., acamprosate-by-typology or acamprosate-by-naltrexone-by-typology interactions) could obscure the effects we were interested in. Figure 1 depicts the disposition of COMBINE participants for the analyses reported here. With these sample sizes, with alpha = .05, statistical power to detect a clinically significant effect size of d = .21 was .80 for the Babor typology and .75 for the age-of-onset typology in the MM-only groups. With alpha set at .025 to account for the two primary contrasts, the corresponding powers were .71 and .65, respectively.
To test whether alcohol typology moderated the effectiveness of naltrexone (relative to placebo) during the 16-week treatment phase of the trial, we used multivariate repeated measures that jointly included four (1-4) monthly values of PHDD as the within-subject factor and two between-subject factors: Typology (2 levels, A vs. B, late vs. early onset) and medication group (2 levels, placebo versus naltrexone). Baseline PHDD was included as a covariate.
Table 3 reports the results of the two primary and two secondary repeated measure MANCOVAs with PHDD as the dependent variable. Among those receiving MM without CBI, A significant interaction was found between Babor typology and medication (p < .019), with no main effect of typology or medication. In contrast, for the age of onset typology, among the MM-only participants a main effect of medication was found (p < .035), but no effect of typology or typology-by-medication interaction. Among participants receiving CBI, for both typologies there was no significant main effect of medication or typology, and there was no significant interaction between medication and typology.
Table 3. Moderating Effect of Alcohol Typology on Percentage Heavy Drinking Days During Active Medication Phase.
| Primary Analyses: Naltrexone versus Placebo in Medical Management | |
| Babor Typology | |
| Medication main effect | F (1, 271) = 2.21, p < .14 |
| Babor Typology main effect | F (1, 271) = .53, p < .47 |
| Medication × Babor typology | F (1, 271) = 5.54, p < .019 |
| Age of Onset Typology | |
| Medication main effect | F (1, 244) = 4.48, p < .035 |
| Age of onset Typology main effect | F (1, 244) = 1.28, p < .26 |
| Medication × Age of Onset Typology | F (1, 244) = .02, p < .89 |
| Secondary Analyses: Naltrexone versus Placebo in Medical Management & CBI | |
| Babor Typology | |
| Medication main effect | F (1, 275) = .50, p < .48 |
| Babor Typology main effect | F (1, 275) = 1.55, p < .21 |
| Medication × Babor Typology | F (1,275) = .00, p < .98 |
| Age of Onset Typology | |
| Medication main effect | F (1, 245) = .22, p < .64 |
| Typology main effect | F (1, 245) = 2.31, p < .13 |
| Medication × Age of Onset Typology | F (1, 245) = 1.52, p < .22 |
To assess whether there were baseline differences that could account for the observed interactions between A vs. B typology and medication condition in the MM only groups, 2×2 ANOVAs were conducted for each of the continuous baseline variables included in Table 2, testing for the significance of the typology-by-medication interaction term. None of these was significant (p > .05). For the categorical variables in Table 1, 2 X 2 Chi square analyses were conducted for each level of each variable. Again, none of these was significant. Finally, additional MANCOVAs were performed with the Babor typology in the MM-only groups to assess whether participant characteristics not clearly related to typology could have accounted for the effect of the typology-by-medication interaction on PHDD. Age, gender, readiness for change (from the University of Rhode Island Change Assessment (URICA) (McConnaughy et al., 1983)), and baseline AA attendance (from the Form 90) were added one at a time to the model, along with their interaction with medication. In each case, the typology-by medication interaction remained significant.
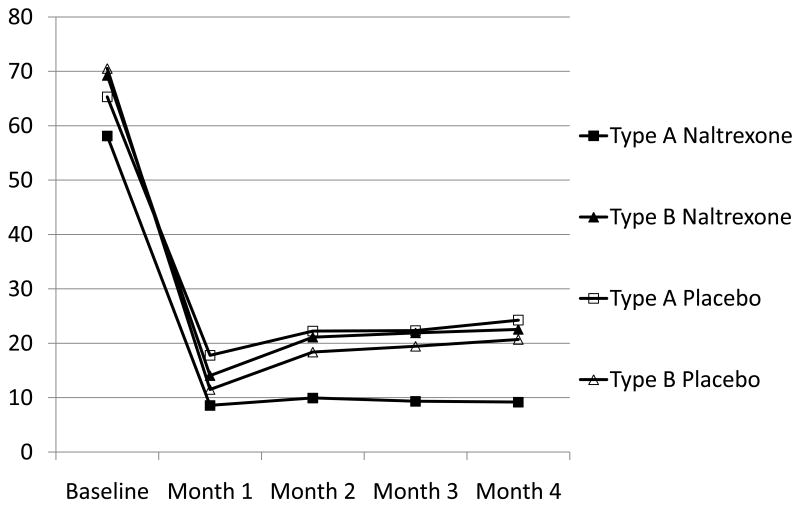
Figure 2 depicts the mean monthly values of percentage heavy drinking days for the four groups involved in the observed interaction of Babor typology and medication in the participants receiving MM without CBI. As shown, the most favorable mean response to naltrexone was reported by Type A alcoholics, and simple main effect tests indicated that this group reported significantly fewer heavy drinking episodes relative to the other three groups at the 2-, 3-, and 4-month time points (F (3, 275) = 2.99, p < .032, F (3, 275) = 3.25, p < .02, and F (3, 275) = 3.83, p < .01, respectively). Descriptively, the magnitude of the observed advantage of naltrexone for Type A alcoholics relative to the next best mean outcome was moderate, e.g. d = .48 at 4 months.
Figure 2.
Percent Heavy Drinking Days by Typology and Medication Condition
Additional and parallel repeated measure MANCOVAs were done to try to replicate the observed interaction in the MM condition, this time using secondary measures of alcohol use. A moderating effect was found with monthly PDA, again with Type A alcoholics faring better in the naltrexone group, F (1, 191) = 4.87, p < .013. Also similar to primary analyses, Type A alcoholics receiving placebo fared most poorly, with 15% lower mean values in abstinence frequency relative to Type A alcoholics receiving naltrexone. Using a measure of DPD, the moderating effect of typology was not significant, although a weak trend (p < .11) was observed favoring naltrexone in the type A group. Finally, Cox regression was conducted with days to first heavy drinking day (censored at 113 days) as the dependent measure. Main effects of group (placebo vs. naltrexone) and typology (Type A vs. B) were entered in step 1, and in step two the product term representing the group-by-typology interaction was entered. Neither the main effects nor the interaction term approached significance in this analysis.
To provide a more meaningful picture of the clinical significance of typology on response to naltrexone in the COMBINE study, Table 4 depicts naltrexone vs. placebo end-of-treatment (weeks 13-16) outcomes for Type A and Type B alcoholics on three key drinking variables analyzed above: PHDD, PDA, and DPD. Percent achieving Good Clinical Outcome are also reported for the four groups. Highly significant (p < .002) differences on all four outcomes were found between naltrexone- and placebo-treated participants with Type A alcoholism. For Type B alcoholics, naltrexone- and placebo treated participants were not significantly different on any of these outcomes. The Type A naltrexone group also had significantly (p < .02) better outcomes on all four measures than either of the Type B groups.
Table 4. End of Treatment Drinking Outcomes by Babor Typology: Naltrexone versus Placebo in the Medication Management Condition (no CBI)a.
| Type Ab | Type Bc | |||
|---|---|---|---|---|
| Naltrexoned | Placebo | Naltrexone | Placebo | |
| PHDD | 9.20 (18.05) | 24.27 (31.90) | 22.57 (36.93) | 20.71 (33.00) |
| PDA | 80.99 (25.25) | 61.74 (35.73) | 74.93 (38.11) | 78.04 (33.86) |
| DPD | 1.19 (2.44) | 2.58 (3.24) | 2.85 (5.04) | 3.68 (7.13) |
| Good Clinical Outcome | 83.6% | 56.8% | 64.6% | 62.2% |
Reported drinking means are unadjusted for baseline values, but significance tests in this table adjusted for baseline values (except for Good Clinical Outcome).
All pairwise contrasts between Type A drinkers who received Naltrexone and Placebo were significant, p < .002.
All pairwise contrasts between Type B drinkers who received Naltrexone or Placebo were not significant, smallest p was p < .62.
All pairwise contrasts between Type A Naltrexone and Type B Naltrexone or Type B placebo were significant, p < .02
Discussion
The primary finding in this secondary analysis is that COMBINE study participants in MM conditions (i.e. those not receiving CBI) who met criteria for having Type A alcoholism (lower risk/severity) had better within-treatment drinking outcomes with 100 mg/day naltrexone alone than with placebo, and that this medication advantage was not found in patients meeting criteria for Type B alcoholism (higher risk/severity). This pattern of results was consistent across clinically important outcome measures, with the exception that no effects of typology were found on time to first heavy drinking day. This suggests that less severe alcoholics are more likely to benefit from naltrexone in the context of low-intensity psychosocial treatment, and that this advantage is not related to any effect on the ability to maintain total abstinence. These findings did not appear to be confounded by differences in medication adherence. In contrast, age of onset did not have a measureable effect on response to naltrexone in terms of drinking outcomes. These results are consistent with other studies that there are subtypes of alcohol dependence that are worth identifying because they respond to pharmacotherapies differentially (Kampman et al., 2007, Chick et al., 2004, Johnson et al., 2000, Kranzler et al., 1996, Pettinati et al., 2000). Our findings are also consistent with the negative results of naltrexone trials such as that of Krystal et al. (2001) which recruited primarily high-severity alcoholics. However, they contrast with results of other studies that found stronger naltrexone effects in early onset alcoholics and among individuals with greater severity on a number of univariate markers of severity or risk.
Although this is the first published study to evaluate a medication directly related to opioid function (naltrexone) in Type A vs. B subjects, other studies have examined a variety of univariate characteristics as predictors of response to naltrexone. In contrast to our findings, these studies have generally found that patients with characteristics generally thought of as indicative of severity were more likely to respond to naltrexone, although the findings have not been entirely consistent. Monterosso et al. found better response (fewer heavy drinking days) in alcoholics with familial history of alcoholism or higher pre-treatment alcohol craving (Monterosso et al., 2001). Rubio et al. reported that naltrexone was effective (higher rate of total abstinence) in patients with a family history of alcoholism and those having another substance use disorder, but was not effective in the complementary groups of patients (Rubio et al., 2005). Kiefer et al. reported better naltrexone response (abstinence) in patients with higher levels of depression, but found that baseline craving did not predict response to naltrexone (Kiefer et al., 2005). Rohsenow et al. recently reported a secondary analysis in which family history of alcoholism and antisocial personality traits were independent moderators of response to naltrexone (in terms of heavy drinking days), with better response in participants who had these characteristics (Rohsenow et al., 2007). However, comorbid drug use did not predict response to naltrexone.
The reasons for these apparent inconsistencies are not immediately clear. There are many possibilities, as each of the other studies differed in significant respects from this analysis from the COMBINE study. Differences among the studies include differences demographics, drinking intensity, psychosocial treatment intensity, dose of naltrexone, analytic methods, and alcohol outcome variables. It is important to note that there is no direct contradiction between our findings and those listed above because none of them used the Babor typology. Further, the characteristics found to be predictive in the other studies, such as family history, age of onset, antisocial personality traits, craving, depression, and other substance use disorder, were not used in our operationalization of the Babor typology. These findings serve as a reminder that “severity” is multidimensional and that it is not yet clear what specific characterstics are most relevant to naltrexone response, or how these phenotypes are related to specific genotypes.
Regarding age of onset, secondary analyses of European studies (Kiefer et al., 2008, Kiefer et al., 2005, Rubio et al., 2005) have found a significant matching effect such that earlier onset alcoholics benefited more from naltrexone. However, a recent US study found no significant difference in naltrexone efficacy between early- and late-onset alcoholics (Rohsenow et al., 2007). Population differences and study design factors are possible explanations for this discrepancy. Such discrepancies are hardly unprecedented: European and North American findings for acamprosate main effects are markedly different, presumably due at least in part to differences in population and design (Johnson, 2008).
The reasons for the lack of significant effects of naltrexone in the CBI-treated participants are not clear, but are consistent with the main findings of the COMBINE study (Anton et al., 2006) in which naltrexone effects were significant only in the MM-only condition. Our findings showed that naltrexone was not effective in Type B alcoholics regardless of level of psychosocial treatment, whereas the effect of naltrexone in Type A alcoholics was limited to participants receiving MM without CBI. This suggests that the naltrexone effect in lower-severity alcoholics is masked by higher intensity psychosocial treatment and becomes apparent only with lower intensity addiction treatments e.g., brief medical management (Pettinati et al., 2005a, Pettinati et al., 2004). If so, that would strengthen the rationale for use of naltrexone to treat milder cases of alcohol dependence in primary care settings.
Work has begun to elucidate the relationships between genotype, phenotype, and response to naltrexone. For example, it has been reported that patients who have the OPRM1 Asp40 allele had better treatment outcomes with naltrexone than those without it this allele (Anton et al., 2008, Oslin et al., 2003), although conflicting results were reported from the VA Cooperative Study (Gelernter et al., 2007). In a study of non-treatment-seeking heavy drinkers, the Asp40 allele of the mu opioid receptor was associated with lower alcohol craving, stronger “high” from alcohol, and greater blunting of the reinforcing effects of alcohol by naltrexone (Ray and Hutchison, 2007). However, another recent study reported no significant effect of mu opioid receptor genotype on drinking or craving, but did find an effect of D4 dopamine receptor genotype on percent heavy drinking days (Tidey et al., 2008). Further work is needed to determine whether specific genotypes are associated with alcoholism subtype. Additional studies will also be necessary to investigate possible mediators (e.g., changes in reward or craving) of the enhanced response to naltrexone in type A alcoholics. It is not certain a priori that genotype is entirely responsible for differences in phenotype that are related to naltrexone response. Environment likely contributes to epigenetic changes that could ultimately determine response. Classification systems incorporating both specific genetic profiles and phenotypic characteristics (such as are currently used in treatment of cancer and other illnesses) may prove superior in predicting response and matching patients to treatments.
These exploratory analyses have several limitations deserving mention. The analysis was post-hoc, and the sample was not stratified by typology. Because the results were obtained in a clinical trial and not in a typical treatment setting, the findings may not readily generalize to some clinical settings, e.g., those that treat predominantly patients with polysubstance use. All of our drinking measures were based on self-report. However, data were collected by experienced staff who had received supervised training in the use of the Form-90 a semi-structured interview with excellent psychometric properties (Miller and Del Boca, 1994). Also, the blind was kept intact throughout the study and there would be no reason to suspect systematic bias in self-reports between medication and placebo conditions. Because of the repeated-measures techniques used, these results include only the participants who completed 16 weeks of treatment—as such these results speak directly to the efficacy of the medication when taken, but do not account for treatment drop-out.
Finally, the procedure in this study to determine type A vs. B is not identical to those used in other studies, and it is possible that the findings would have been different had a different procedure been applied or different variables chosen. The A vs. B typology has been operationalized in a variety of ways in its published applications. In fact, investigators have been forced to recreate the typology with each new sample because of differences in instruments used in the various studies, although the underlying hypothesized dimensions of severity are consistent. The Babor typology applied to the COMBINE study in the present paper used five measures approximating those that were empirically derived in an analysis using the original 17 constructs, conducted as part of the COGA study (Schuckit et al., 1995). This simplified method is more practical than methods with numerous measures requiring a more extensive analysis to derive the classification. However, Kampman and colleagues (Kampman et al., 2007) also empirically derived a simplified a 4-measure method for subtyping patients at pre-treatment into either Type A or B alcoholism. These 4 items are not the same as the 5 items derived by Schuckit et al. (Schuckit et al., 1995). It is not known how robust the findings of the present study are with respect to the details of the typologic assignment algorithm.
While the findings in the present study require replication in other samples, they suggest that a subgroup of patients with lower-severity alcohol dependence may have an enhanced clinical response to a course of naltrexone pharmacotherapy with medical counseling. Typologies, whether requiring one (early vs late onset) or over 10 measures (Babor's original typology), genotyping, and, potentially, more discriminating DSM criteria, will undeniably be proven to be useful in selection of treatments for alcohol dependent patients. Efforts to replicate the findings reported here for naltrexone treatment should be accompanied by further efforts to refine a subtyping procedure that can be readily applied in the clinic.
Acknowledgments
Data presented in this report were collected as part of the multisite COMBINE study sponsored by the National Institute on Alcohol Abuse and Alcoholism, in collaboration with the Combine Study Research Group. Preparation of this report was supported in part by NIAAA grants U10AA011716, U10AA011768 K02AA000326, and K24AA016555. A full listing of the staff of the COMBINE study can be found at http://www.cscc.unc.edu/combine/.
This paper is dedicated to the memory of James Hosking, Ph.D. who, prior to his untimely death, was the primary statistician and head of the coordinating center for the COMBINE study.
Footnotes
Trial registration: clinical trials.gov identifier NCT00006206
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, Locastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Randall CL. Measurement and choice of drinking outcome variables in the COMBINE Study. J Stud Alcohol Suppl. 2005:104–9. doi: 10.15288/jsas.2005.s15.104. discussion 92-3. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, Delboca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Chick J, Aschauer H, Hornik K. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one-year, double-blind, placebo-controlled multicentre study with analysis by typology. Drug Alcohol Depend. 2004;74:61–70. doi: 10.1016/j.drugalcdep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Gilligan SB, Von Knorring AL, Reich T, Bohman M. Genetic heterogeneity and the classification of alcoholism. Adv Alcohol Subst Abuse. 1988;7:3–16. doi: 10.1300/J251v07n03_02. [DOI] [PubMed] [Google Scholar]
- Combine_Study_Research_Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res. 2003;27:1107–22. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Dundon W, Lynch KG, Pettinati HM, Lipkin C. Treatment outcomes in type A and B alcohol dependence 6 months after serotonergic pharmacotherapy. Alcohol Clin Exp Res. 2004;28:1065–73. doi: 10.1097/01.alc.0000130974.50563.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, Krystal JH. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31:555–63. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, Diclemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. Jama. 2000;284:963–71. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, Tirado C, Oslin DW, Sparkman T, O'Brien CP. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27:344–51. doi: 10.1097/JCP.0b013e3180ca86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Helwig H, Tarnaske T, Otte C, Jahn H, Wiedemann K. Pharmacological relapse prevention of alcoholism: clinical predictors of outcome. Eur Addict Res. 2005;11:83–91. doi: 10.1159/000083037. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jimenez-Arriero MA, Klein O, Diehl A, Rubio G. Cloninger's typology and treatment outcome in alcohol-dependent subjects during pharmacotherapy with naltrexone. Addict Biol. 2008;13:124–9. doi: 10.1111/j.1369-1600.2007.00073.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–41. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lesch OM, Bonte W, Kefer RJ, R M, Musalek M, A N, Sprung R, Walter H. A new typology in chronic alcoholism and its biological markers. Alcohol Alcohol. 1989;24:380. [Google Scholar]
- Lesch OM, Riegler A, Gutierrez K, Hertling I, Ramskogler K, Semler B, Zoghlami A, Benda N, Walter H. The European acamprosate trials: conclusions for research and therapy. J Biomed Sci. 2001;8:89–95. doi: 10.1007/BF02255976. [DOI] [PubMed] [Google Scholar]
- Litt MD, Babor TF. Alcoholic typology as an attribute for matching clients to treatment. In: L R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses, Project MATCH Monograph Series. Vol. 8. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2001a. [Google Scholar]
- Litt MD, Babor TF. Alcoholic typology as an attribute for matching clients to treatment. In: Longabaugh R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2001b. [Google Scholar]
- Litt MD, Babor TF, Delboca FK, Kadden RM, Cooney NL. Types of alcoholics, II. Application of an empirically derived typology to treatment matching. Arch Gen Psychiatry. 1992;49:609–14. doi: 10.1001/archpsyc.1992.01820080017003. [DOI] [PubMed] [Google Scholar]
- Miller WR, editor. Combined Behavioral Intervention manual: A clinical research guide for therapists treating people with alcohol abuse and dependence. Vol. 1. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–8. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–68. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati HM, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with therapeutic response in alcohol dependent patients treated with naltrexone. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Penick EC, Nickel EJ, Powell BJ, Liskow BI, Campbell J, Dale TM, Hassanein RE, Noble E. The comparative validity of eleven alcoholism typologies. J Stud Alcohol. 1999;60:188–202. doi: 10.15288/jsa.1999.60.188. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SM, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. J Clin Psychopharmacology. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–9. [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: A psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of Studies on Alcohol. 2005a;15:170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan DM, Ernst DB, Rounsaville BJ. Medical Management (MM) treatment manual: A clinical research guide for medically trained clinicians providing pharmacotherapy as part of the treatment for alcohol dependence. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Pettinati HM, Zweben A, Mattson M, editors. The COMBINE Study: Conceptual, Methodological and Practical Issues in a Clinical Trial that Combined Medications and Behavioral Treatments. 2005b. [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–77. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Miranda R, Jr, Mcgeary JE, Monti PM. Family history and antisocial traits moderate naltrexone's effects on heavy drinking in alcoholics. Exp Clin Psychopharmacol. 2007;15:272–81. doi: 10.1037/1064-1297.15.3.272. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J, Palomo T. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40:227–33. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Shapiro E, Hesselbrock VM, Bucholz KK, Reich T, Nurnberger JI., Jr An evaluation of type A and B alcoholics. Addiction. 1995;90:1189–203. doi: 10.1046/j.1360-0443.1995.90911894.x. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–80. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, Mcgeary JE, Mackillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone's effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul R, Lehert P, Geerlings PJ, Koeter MW, Van Den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology (Berl) 2005;178:167–73. doi: 10.1007/s00213-004-1991-7. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Kato M. Prediction of 3-year outcome of treated alcoholics by an empirically derived multivariate typology. Am J Psychiatry. 1996;153:829–30. doi: 10.1176/ajp.153.6.829. [DOI] [PubMed] [Google Scholar]