Abstract
Cholera toxin is the principal factor causing the profuse intestinal fluid secretion that is characteristic of cholera. The DNA sequences of the cholera toxin subunit B structural genes from 45 Vibrio cholerae O1 strains isolated in 29 countries over a period of 70 years were determined by automated DNA sequencing of polymerase chain reaction-generated amplicons. Three types of cholera toxin B subunit gene (ctxB) were identified. Genotype 1 was found in strains of classical biotype worldwide and El Tor biotype strains associated with the U.S. Gulf Coast, genotype 2 was found in El Tor biotype strains from Australia, and genotype 3 was found in El Tor biotype strains from the seventh pandemic and the recent Latin American epidemic. All base changes correspond to an amino acid substitution in the B subunit of the cholera toxin. Heterogenicity in the B subunit could have implications for vaccine development and diagnostic tests for cholera toxin and antitoxin. We conclude that this technology provides timely and potentially useful epidemiological information.
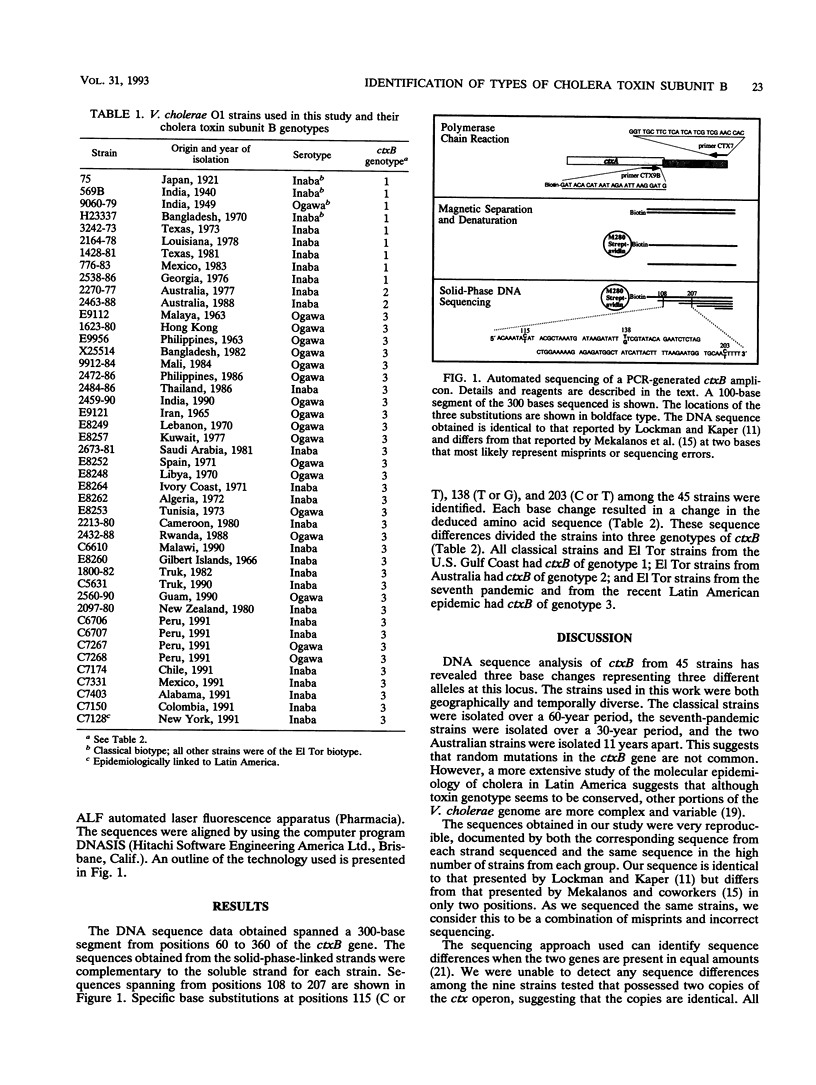
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brytting M., Wahlberg J., Lundeberg J., Wahren B., Uhlén M., Sundqvist V. A. Variations in the cytomegalovirus major immediate-early gene found by direct genomic sequencing. J Clin Microbiol. 1992 Apr;30(4):955–960. doi: 10.1128/jcm.30.4.955-960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Evins G. M., Cook W. L., Almeida R., Hargrett-Bean N., Wachsmuth K. Genetic diversity among toxigenic and nontoxigenic Vibrio cholerae O1 isolated from the Western Hemisphere. Epidemiol Infect. 1991 Aug;107(1):225–233. doi: 10.1017/s0950268800048846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- DePaola A., Capers G. M., Motes M. L., Olsvik O., Fields P. I., Wells J., Wachsmuth I. K., Cebula T. A., Koch W. H., Khambaty F. Isolation of Latin American epidemic strain of Vibrio cholerae O1 from US Gulf Coast. Lancet. 1992 Mar 7;339(8793):624–624. doi: 10.1016/0140-6736(92)90917-r. [DOI] [PubMed] [Google Scholar]
- Dubey R. S., Lindblad M., Holmgren J. Purification of El Tor cholera enterotoxins and comparisons with classical toxin. J Gen Microbiol. 1990 Sep;136(9):1839–1847. doi: 10.1099/00221287-136-9-1839. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Popovic T., Wachsmuth K., Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol. 1992 Aug;30(8):2118–2121. doi: 10.1128/jcm.30.8.2118-2121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling M. G., Holmes R. K. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol Microbiol. 1991 Jul;5(7):1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Mapping epitopic regions of cholera toxin B-subunit protein. Mol Immunol. 1991 Aug;28(8):865–876. doi: 10.1016/0161-5890(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Study of epitopes of cholera enterotoxin-related enterotoxins by checkerboard immunoblotting. Infect Immun. 1990 Jul;58(7):2352–2360. doi: 10.1128/iai.58.7.2352-2360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H., Kaper J. B. Nucleotide sequence analysis of the A2 and B subunits of Vibrio cholerae enterotoxin. J Biol Chem. 1983 Nov 25;258(22):13722–13726. [PubMed] [Google Scholar]
- McCarthy S. A., McPhearson R. M., Guarino A. M., Gaines J. L. Toxigenic Vibrio cholerae O1 and cargo ships entering Gulf of Mexico. Lancet. 1992 Mar 7;339(8793):624–625. doi: 10.1016/0140-6736(92)90918-s. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Cholera toxin: genetic analysis, regulation, and role in pathogenesis. Curr Top Microbiol Immunol. 1985;118:97–118. doi: 10.1007/978-3-642-70586-1_6. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Tamplin M. L., Ahmed M. K., Jalali R., Colwell R. R. Variation in epitopes of the B subunit of El Tor and classical biotype Vibrio cholerae O1 cholera toxin. J Gen Microbiol. 1989 May;135(5):1195–1200. doi: 10.1099/00221287-135-5-1195. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Bopp C. A., Fields P. I., Carrillo C. Difference between toxigenic Vibrio cholerae O1 from South America and US gulf coast. Lancet. 1991 May 4;337(8749):1097–1098. doi: 10.1016/0140-6736(91)91744-f. [DOI] [PubMed] [Google Scholar]
- Wahlberg J., Albert J., Lundeberg J., Cox S., Wahren B., Uhlén M. Dynamic changes in HIV-1 quasispecies from azidothymidine (AZT)-treated patients. FASEB J. 1992 Jul;6(10):2843–2847. doi: 10.1096/fasebj.6.10.1634047. [DOI] [PubMed] [Google Scholar]


