Abstract
Background
There is growing evidence that GABA-B receptor agonists may be effective in the treatment of alcohol abuse or dependence. The primary goal of this study was to determine the safety of baclofen in combination with alcohol consumption in heavy drinkers. In addition, the effects of baclofen alone, and in combination with alcohol, on subjective effects, cognitive performance effects, as well as alcohol craving, were assessed.
Methods
Eighteen non-treatment seeking heavy social drinkers (mean of 28 drinks/week) who did not meet criteria for alcohol dependence participated. All individuals were tested using a double-blind double-dummy design with six 2-day inpatient phases. Baclofen (0, 40, and 80 mg) was administered 2.5 hours before alcohol (1.5 g/l body water or approximately 0.75 g/kg) or placebo beverages, given in 4 divided doses every 20 min.
Results
Baclofen, either alone, or in combination with alcohol, produced only modest increases in heart rate and blood pressure and no adverse effects were reported. Baclofen did not increase positive subjective effects (e.g., Stimulant effects, Drug Liking) but did increase sedation and impair performance. Even though both baclofen and alcohol impaired performance, for the most part performance was not impaired to a greater extent when baclofen was combined with alcohol. Among this population of non-dependent drinkers, baclofen did not alter alcohol craving or alcohol-induced positive subjective effects.
Conclusions
Baclofen alone has minimal abuse liability in heavy social drinkers and baclofen is relatively well tolerated and safe when given in combination with intoxicating doses of alcohol.
Keywords: Baclofen, GABA, Alcohol, Medication development, Heavy Social Drinkers
Alcoholism is a major public health problem in the United States, such that approximately 15.6 million people meet criteria for alcohol abuse or dependence, and over 800,000 received treatment for alcohol abuse or dependence in 2006 (SAMHSA, 2007). Despite the enormous impact of this disease on society, there are only three medications (disulfiram, naltrexone, and acamprosate) currently approved by the FDA for the treatment of alcohol dependence (e.g., Bouza et al., 2004; Heilig and Egli, 2006; Kiefer and Mann, 2005; Kranzler and Van Kirk, 2001) and given their limited efficacy there is a need for additional pharmacotherapies for alcohol dependence.
The interaction of alcohol with the gamma-amino butyric acid (GABA)-ergic neurotransmitter system is well known (Grant and Lovinger, 2005) and targeting GABA-ergic neurotransmission has been considered as a potential treatment strategy for alcohol dependence (Johnson et al., 2005; Heilig and Egli, 2006). Baclofen, a GABA-B receptor agonist, has attracted considerable attention as a potential medication not only for alcoholism (Addolorato et al., 2006a; Colombo et al., 2004; Heilig and Egli, 2006; Johnson et al., 2005; Kranzler, 2000), but also for other addictive disorders (Cousins et al., 2002).
There is encouraging preclinical evidence in laboratory rodents that baclofen decreases 1) alcohol withdrawal symptoms (Colombo et al., 2000; Knapp et al., 2007), 2) acquisition and maintenance of voluntary alcohol consumption (Colombo et al., 2000, 2002; Daoust et al., 1987), 3) alcohol consumption associated with alcohol deprivation (Colombo et al., 2003a, 2006), 4) alcohol self-administration (Anstrom et al., 2003; Besheer et al., 2004; Liang et al., 2006; Walker and Koob, 2007), and 5) responding for alcohol during extinction, suggestive of decreased motivation to obtain alcohol (Colombo et al., 2003b; Maccioni et al., 2008). However, not all preclinical studies have reported that baclofen produces a reduction in alcohol consumption or self-administration (e.g., Colombo et al., 2005; Czachowski et al., 2006; Moore et al., 2007; Petry, 1997; Smith et al., 1999; Tomkins and Fletcher, 1996). Nonetheless, the preclinical literature suggests that baclofen may have therapeutic efficacy for alcoholism.
Several studies have been conducted in humans to evaluate the efficacy of baclofen for the treatment of patients with alcohol problems. One of the first studies used an open-label design in 10 alcohol-dependent patients (Addolorato et al., 2000) and found that maintenance on 30 mg/day of baclofen for 4 weeks decreased alcohol craving in the 9 patients who completed the study, with 7 patients maintaining total abstinence. Similar positive findings were obtained in two subsequent studies, both of which maintained alcohol-dependent patients on 30 mg/day of baclofen (Addolorato et al., 2002a; Flannery et al., 2004). These findings were recently extended and confirmed in a larger randomized clinical trial among alcohol-dependent patients with liver cirrhosis who were treated with either baclofen (30 mg/day) or placebo for 12 weeks (Addolorato et al., 2007). In all of these studies, side effects of baclofen were minimal and retention was excellent. Further, there have been two case reports showing that high doses of baclofen (100–140 mg/day) were effective in reducing alcohol craving and consumption (Ameisen, 2005; Buckman, 2007). Lastly, several small pilot studies have reported that baclofen (30 mg/day) reduces alcohol withdrawal symptoms in alcohol-dependent patients (Addolorato et al., 2002b, 2003, 2006b).
Despite the promising preclinical and clinical findings to date suggesting that baclofen may be effective for treating alcohol dependence, there is a paucity of human laboratory studies assessing the interaction of baclofen and alcohol. Human behavioral laboratory studies can play an important role in the early stages of the medication development process for drug and alcohol abuse (Cousins et al., 2002; O’Brien and Gardner, 2005), particularly for assessing the safety of a candidate medication when administered alone and in combination with alcohol, as well as to elucidate the therapeutic mechanism. Baclofen is a centrally acting muscle relaxant approved by the FDA for the alleviation of signs and symptoms of spasticity; while the usual dose range is between 40–80 mg daily in divided doses, much higher doses have been used for the treatment of spasticity with a good safety profile (Aisen et al., 1992). Baclofen is also known to have anxiolytic effects (Breslow et al., 1989; Drake et al., 2003; Jamous et al., 1994), even among a population of alcoholic patients (Krupitsky et al.,1993), and these anxiolytic effects have been hypothesized to reduce alcohol craving and drinking. In fact, several studies have observed a reduction in anxiety in baclofen-treated alcohol-dependent patients (Addolorato et al., 2002a, 2006b, 2007;Flannery et al., 2004). Alternatively, baclofen may reduce alcohol craving and drinking by reducing the positive effects of alcohol, including the stimulant effects (Cousins et al., 2002), or by enhancing the negative effects of alcohol, including sedation. Despite these potential benefits, the sedative and anxiolytic effects of baclofen also raise concerns about the safety of baclofen in combination with alcohol and its abuse liability (Heilig and Egli, 2006).
The purpose of the present study was to comprehensively assess the acute behavioral and physiological effects of baclofen (0, 40, 80 mg) alone, and in combination with an intoxicating dose of alcohol (0.75 g/kg) in non-treatment seeking heavy social drinkers. Specifically, we wanted to address the issues related to the safety profile of baclofen alone and in combination with alcohol, as well as the behavioral effects of baclofen that might elucidate the nature of its putative pharmacotherapeutic effect.
SUBJECTS AND METHODS
Participant Selection
Research participants were recruited via advertisements in newspapers. All eligible participants received a detailed medical, psychological, and psychiatric evaluation. Laboratory testing included blood and urine analysis, urine drug toxicologies (opioids, cocaine, benzodiazepines, cannabinoids, and amphetamines), and an electrocardiogram. Participants were excluded from the study if they had any current physical disorder, were taking any prescription medication, had an Axis I psychiatric disorder other than alcohol abuse or nicotine dependence, or were pregnant. In order to be eligible for the study, participants were required to be regular drinkers of alcoholic beverages, consuming between 20–60 drinks per week based on standard drink units. They were not eligible if they met criteria for alcohol dependence or were seeking treatment for alcohol-related problems.
Participants signed a consent form describing the potential risks of participation including the side effects of alcohol intoxication, the side effects of baclofen and the unknown interaction of alcohol and baclofen. Volunteers were paid for their participation. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute. Of the 24 participants who started the study, data from 6 individuals were not used (3 due to schedule or noncompliance problems, 1 due to nausea and vomiting, 1 due to data collection problems), thus a total of 18 completers were included in the data analyses. Table 1 shows the demographic characteristics of the 18 participants who completed the study, as well as their current pattern of alcohol use.
Study Design and Procedures
Prior to admission, participants completed a training session and an alcohol pre-dose session, when they received a test dose of alcohol under conditions identical to the actual study. The study itself consisted of 6 inpatient experimental phases separated by at least 5 days to prevent carryover effects. Three doses of baclofen (0, 40, and 80 mg) were tested in combination with beverages containing either placebo or alcohol, and a double-dummy design was used. The sequence of experimental phases was randomized and counterbalanced across participants, with participants and raters blinded to the treatment conditions.
Participants were instructed to refrain from alcohol and drug use the day before the session. This was verified by urine and breathalyzer testing on arrival at the laboratory on the morning of each inpatient phase. Participants remained in private rooms equipped with a computer station, recliner chair, bed, and a TV/VCR unit. Audio and video equipment were used to permit continuous monitoring of participants by research staff located in the control room. Upon arrival to the laboratory at 900 hours, participants had a standard breakfast, and completed baseline measures and tasks at 1000 hours. An acute oral dose of baclofen (0, 40, or 80 mg) and a placebo beverage (double dummy design) were administered at 1130 hours. At 1400 hours, 2.5 hours after drug administration, at the estimated time of peak baclofen blood levels, participants began consuming the study beverages. Alcohol or placebo beverages were administered as four 150 ml beverages, spaced 20 minutes apart. Participants were given 30 seconds to consume each beverage. Breath Alcohol Concentration (BAC) was measured using a breathalyzer (Alcosensor III Intoximeter, St. Louis, MO), before the first beverage and at 20, 40, 60, 90, 120, 180, 240, 300, and 360 minutes after the initiation of drinking. Participants spent the night at the laboratory and were released the next morning.
Physiologic Measures
A blood pressure monitor (NBS Medical Services, Costa Mesa, CA) was used for the measurement of heart rate (HR), systolic and diastolic blood pressure (SBP and DBP). These measures were obtained 60 min before medication was administered, 60 min before drinking (90 after medication), 10 min after each beverage, as well as 90, 120, 180, 240 and 300 min after the initiation of drinking.
Assessment Batteries
The Subjective-Effects Battery consisted of the following scales: 1) The Alcohol Craving Scale (ACS) consists of 10 Likert-type statements that were adapted for alcohol based on Tiffany’s subtypes of craving for nicotine (Cox et al., 2001; Tiffany and Drobes, 1991). Participants were asked to rate, on a scale from 1 to 7, how strongly they agreed or disagreed with each statement. The ACS was scored to provide two factors: Factor 1 reflects intention and desire to drink, and anticipation of pleasure from drinking, and factor 2 reflects anticipation of relief from negative affect and urgent and overwhelming desire to drink. 2) The Visual Analog Scales (VAS) (Evans et al., 2000) is a series of forty-three lines presented on the computer screen one at a time. The participants rated each statement by placing a mark on a 100 mm point visual analog line, with the left extreme labeled “not at all” and the right extreme labeled “extremely.” The statements presented included items related to mood, drug effects, and physical symptoms. Individual VAS items were grouped into five clusters and analyzed separately. The clusters were: Personality Traits, Physical Symptoms, Bad Effects, Elevated Mood and Sedation (see Evans and Levin, 2002, for details). The ACS and the VAS were completed 60 min before medication was administered, 60 min before drinking (90 min after medication) and 60, 180 and 300 min after the initiation of drinking. 3) The Drug Effects Questionnaire (DEQ) consists of a series of 6 questions relating to drug effects (see Evans et al., 2000 for details). The DEQ was administered 90 and 60 min before drinking (i.e., 45 and 90 min after medication was administered) and 60, 180 and 300 min after the initiation of drinking.
The Alcohol-Effects Battery was completed immediately before the first alcohol administration, 10 minutes after each beverage, every half hour afterwards for the next 4 hours, and 6.5 hours after the completion of drinking. The battery consisted of the following: 1) The Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993) is a 14-item adjective rating scale that provides measures of alcohol’s effects on an 11-point scale from “not at all” (0) to “extremely” (10). The BAES contains two subscales measuring stimulant (BAES Stimulant) and sedative (BAES Sedative) effects of alcohol. 2) The Brief version of Visual Analog Scales (BVAS) consisted of the following 10 items: stimulated, anxious, high, tired, social, confused, dizzy, difficulty concentrating, nauseous, and mellow. Two additional VAS questions asked the participants to rate how many drinks they thought they had consumed from 0 (0 mm) to 10 drinks (100 mm) and to rate the taste of the beverage from 0 (“no taste”) to 100 (“very strong taste”). The Alcohol Effects Battery was administered 60 min before drinking (i.e., 90 min after medication was administered), 10 minutes after each beverage, as well as 120, 180, 240 and 300 min after the initiation of drinking.
The Performance Task Battery consisted of four computerized tasks and two non-computerized tasks to assess various aspects of learning, memory, vigilance, and psychomotor ability. The computerized tasks consisted of a 3-min Digit Symbol Substitution Test (DSST) (McLeod et al., 1982), a 10-min Divided-Attention Task (DAT) (Miller et al., 1988), a 3-min Digit Enter and Recall (Roache and Griffiths, 1987), and a Time Estimation Task (TET) that requires participants to estimate a single period of time by pressing the computer key when they feel that 34 seconds have elapsed. This computerized performance battery was completed 60 min before medication was administered, 60 min before drinking (90 after medication) and 60, 180 and 300 min after the initiation of drinking. In addition, to assess motor coordination, The Balance Task measured the participant’s ability to stand upright for a maximum of 30 seconds on each foot for a total of 60 seconds (Evans et al., 1990). Balance was measured 60 min before medication was administered, 60 min before drinking (90 after medication), 10 min after the second and third beverages, as well as 90, 120 and 240 min after the initiation of drinking. Lastly, the Word Recall and Recognition Task (see Evans et al., 2000 for details) measured immediate word recall after the completion of drinking, delayed word recall 2 and 4 hours after alcohol administration, and delayed recognition 4 hours after alcohol administration.
Drugs
Baclofen (0, 40, and 80 mg, purchased as 20 mg Lisoresal® tablets) tablets were prepared in colored gelatin capsules (size 0) with lactose powder as filler; placebo capsules contained only lactose powder. Each session at 11:30 am, four identically-appearing capsules were ingested with a 150 ml placebo beverage (see below). Alcohol dose was calculated based on the estimated total body water (TBW) of each participant to eliminate differences in alcohol pharmacokinetics between sexes (Watson et al., 1980). The volume of all beverages was held constant at 150 ml to control for any alcohol expectancy effects. The placebo and active beverage for a given individual were isocaloric (no more than a 10-calorie difference between beverages), using regular or low calorie tonic and juice and dextrose or Equal ® sweetener. The placebo beverage consisted of Canada Dry Tonic® water and Baja Bob’s® original low-calorie margarita mix, in a 3:1 ratio. The alcohol beverage consisted of the same mixture, with 100 proof Absolute® vodka added, as needed. Each beverage was topped with 1 ml of vodka and 1 drop of lime oil. Alcohol was given in four doses of 0.375 g/l body water each, spaced 20 minutes apart for a total of 1.5 g/l or approximately 0.75 g/kg of body weight. All capsules and beverages were consumed under the supervision of one of the investigators.
Data Analyses
Data analyses were conducted using SuperANOVA software. In the analyses three specific questions were addressed:) is baclofen safe and does baclofen have any abuse liability, either alone or in combination with alcohol?; 2) does baclofen reduce alcohol craving and/or the positive effects of alcohol?; 3) does baclofen enhance any of the adverse effects of alcohol, including sedation and performance impairment?
Despite using total body water to calculate alcohol doses, that were intended to minimize sex differences on breath alcohol levels, breath alcohol levels were actually lower in women than in men. Therefore, sex was included as a factor in all the analyses. Specifically, four-factor repeated measures analyses of variance (ANOVA) were conducted with Drug (0, 40, and 80 mg of baclofen), Alcohol (0 and 0.75 g/kg) and Time (varied depending on the measure) as the within-subjects factors and sex as the between-subjects factor. Planned contrasts were used to test a priori hypotheses. Specifically, we hypothesized that baclofen would 1) not increase measures related to abuse liability (e.g., Drug Liking), 2) decrease ACS scores and increase BAES Stimulation scores, and 3) increase sedation and impair performance. In all cases, Huynh-Feldt adjustments were used to reduce type I error and results were considered statistically significant at p<0.05.
RESULTS
Breath Alcohol Levels and Physiological Effects
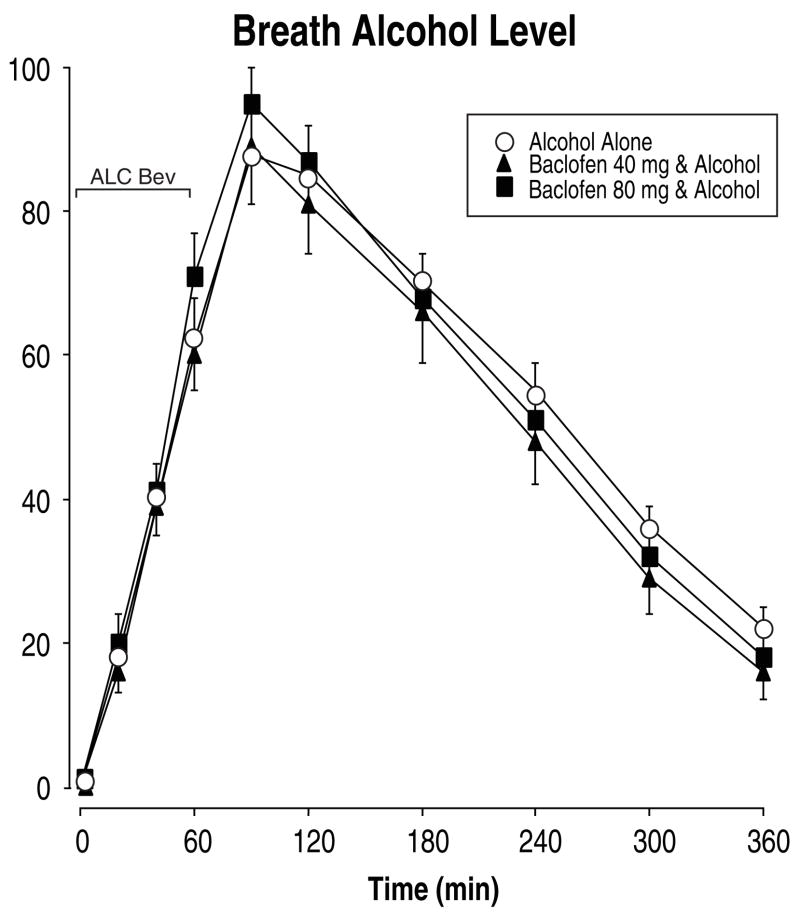
Figure 1 shows that alcohol administration significantly increased breath alcohol levels as a function of time within the session [F(10,160)=216.89, p<0.0001], with breath alcohol levels reaching a peak of 90 mg/dl 30 min after the completion of drinking. Pretreatment with baclofen did not alter breath alcohol levels. However, despite using total body water to calculate alcohol dose and control for sex differences in alcohol pharmacokinetics, there was a significant Time × Sex interaction [F(10,160)=4.50, p<0.008], such that women had lower peak breath alcohol levels than men (84 mg/dl and 94 mg/dl, respectively) and consequently breath alcohol levels decreased more rapidly in women over time. Therefore, for all other analyses, Sex was included as a factor.
Fig. 1.
Mean breath alcohol concentrations as a function of baclofen dose and time after drinking four alcoholic beverages (approximately 0.75 g/kg body weight). Only active doses of alcohol are shown since all measurements for placebo beverages were 0. Data points show means of 18 individuals; vertical bars show ± 1 S.E.M. Some error bars have been omitted for clarity and the absence of any bars indicates 1 S.E.M. fell within the area of the data symbol. ALC Bev = alcohol beverage; min = minutes.
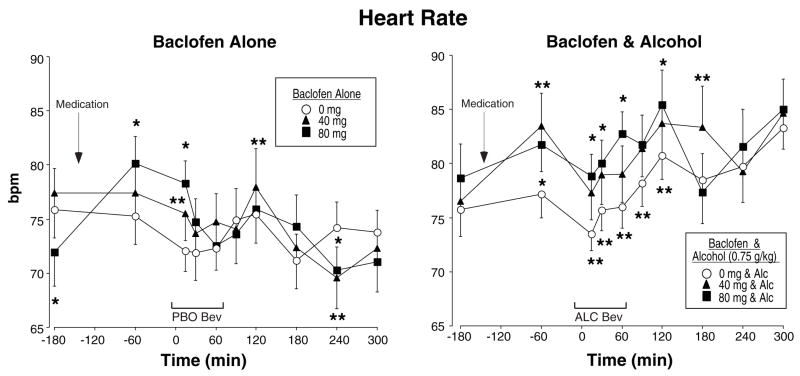
Figure 2 shows heart rate for baclofen alone (left panel) and in combination with alcohol (right panel) as a function of time during the sessions. Baclofen alone increased heart rate (Baclofen × Time interaction [F(18,288)=2.02, p<0.03]) and this tended to increase as a function of dose [F(2,32)=3.33, p<0.06], with a mean peak increase from 75 bpm following placebo to 80 bpm following 80 mg baclofen. Similarly, alcohol increased heart rate (Alcohol × Time interaction [F(9,144)=6.65, p<0.0001]) and the combination of baclofen with alcohol produced even greater increases in heart rate (Baclofen × Alcohol × Time interaction [F(18,288)=20.4, p<0.009]), particularly during the ascending limb of the breath alcohol curve. Baclofen alone also produced small, but statistically significant dose-related increases in systolic blood pressure [F(2,32)=10.21, p<0.001]; mean peak systolic blood pressure increased from 121 mmHg following placebo to 125 mmHg following 80 mg baclofen. There were no significant effects of alcohol on systolic blood pressure or any other interactions. The only significant effect for diastolic blood pressure was an Alcohol × Time interaction [F(9,144)=2.28, p<0.05], with diastolic pressure ranging between 62–72 mmHg throughout the session.
Fig. 2.
Mean heart rate after baclofen alone (left panel) or baclofen in combination with alcohol (right panel) as a function of baclofen dose and time. PBO Bev = Placebo beverage; ALC Bev = alcohol beverage; Alc = alcohol; bpm = beats per minute; min = minutes. See Figure 1 for details.
Alcohol Effects and Subjective Effects
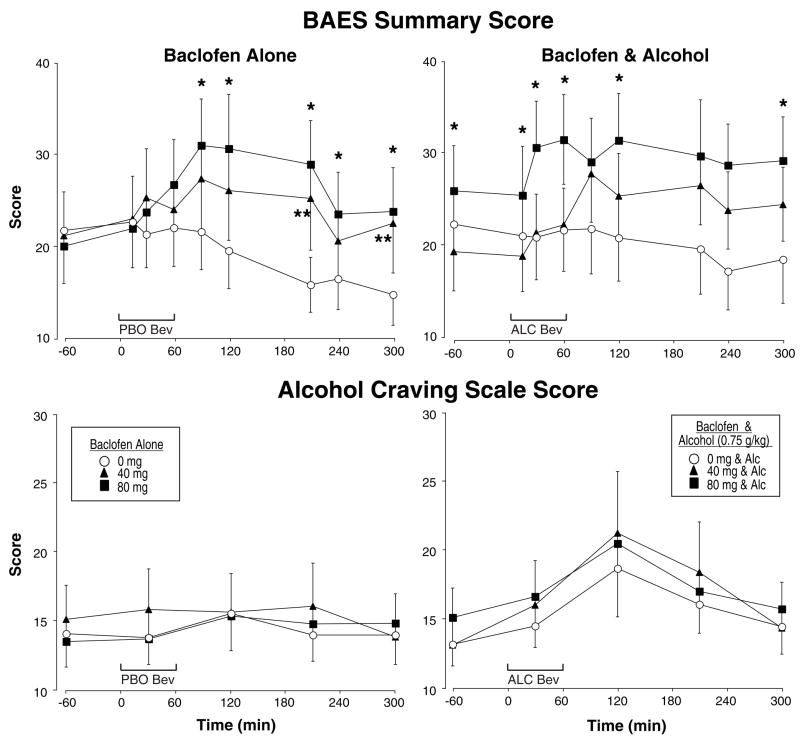
Figure 3 shows the BAES Summary scores separately for baclofen alone (top left panel) and in combination with alcohol (top right panel) as a function of time during the sessions. While baclofen alone did not alter BAES Stimulation scores, baclofen alone did significantly increase BAES Sedation [F(2,32)=5.11, p<0.02] and Summary scores [F(2,32)=5.41, p<0.02; see upper left panel of Figure 3] in a dose-related manner. As expected, alcohol significantly increased BAES Stimulation scores on the ascending limb of the breath alcohol curve (Alcohol × Time interaction [F(8,128)=3.38, p<0.03]), whereas alcohol increased BAES Sedation scores (Alcohol × Time interaction [F(8,128)=10.98, p<0.0001]) on the descending limb. As shown in the upper right panel of Figure 3, baclofen in combination with alcohol significantly increased BAES Summary scores (Baclofen × Alcohol × Time interaction [F(16,256)=2.20, p<0.04]) and the combination also tended to increase BAES Sedation scores (Baclofen × Alcohol × Time interaction [F(16,256)=1.99, p<0.06]), but not BAES Stimulation scores.
Fig. 3.
Mean BAES (Biphasic Alcohol Effects Scale) Summary Scores (range 0–70) and Alcohol Craving Scores (range 7–70) after baclofen alone (left panels) or baclofen in combination with alcohol (right panels) as a function of baclofen dose and time. * indicates a significant difference from 0 mg baclofen and 80 mg baclofen for a given time point, ** indicates a significant difference from 0 mg baclofen and 40 mg baclofen for a given time point. See Figure 1 and 2 for details.
The lower panels of Figure 3 also show total scores on the Alcohol Craving Scale. Overall, participants reported relatively low levels of alcohol craving throughout the study and there were no significant changes on the Alcohol Craving Scale total score, Factor 1 or Factor 2 as a function of time, alcohol administration, baclofen dose or any interactions. There was only a slight trend for alcohol to increase Factor 1 (anticipation of pleasurable effects of drinking) scores (Alcohol × Time interaction [F(4,64)=2.56, p<0.08]).
Based on the Brief Visual Analog Scale (BVAS), alcohol increased ratings of High [F(1,16)=9.25, p<0.008], as well as ratings of Nauseated, Tired, and Stimulated (Alcohol × Time interactions [Fs(4,64)>3.16, ps<0.03]), and tended to increase ratings of Social (Alcohol × Time interaction [F(4,64)=2.69, p<0.07]). Baclofen alone did not alter any items on the BVAS, and there were no significant interactions with alcohol, with one exception: ratings of High were increased more when baclofen was combined with alcohol (Baclofen × Alcohol × Time interaction [F(8,128)=2.76, p<0.04]). There were no other main effects or interactions on any other BVAS items, including Confused, Difficulty Concentrating, Mellow and Anxious. Alcohol increased ratings of “I had x number of drinks” (Alcohol × Time interaction [F(8,128)=4.93, p<0.02]), with participants reporting a mean of 3.0 drinks 60 minutes after the onset of drinking; baclofen alone or in combination with alcohol did not alter the reported number of drinks. On the VAS cluster scores, alcohol increased “Bad Effects,” “Elevated Mood,” and “Sedation” cluster scores across the session (Alcohol × Time interactions [Fs(4,64)>3.55, ps<0.02]). Baclofen alone did not significantly alter any VAS clusters, although “Elevated Mood” cluster scores tended to increase as a function of baclofen dose [F(2,32)=3.16, p<0.06]).
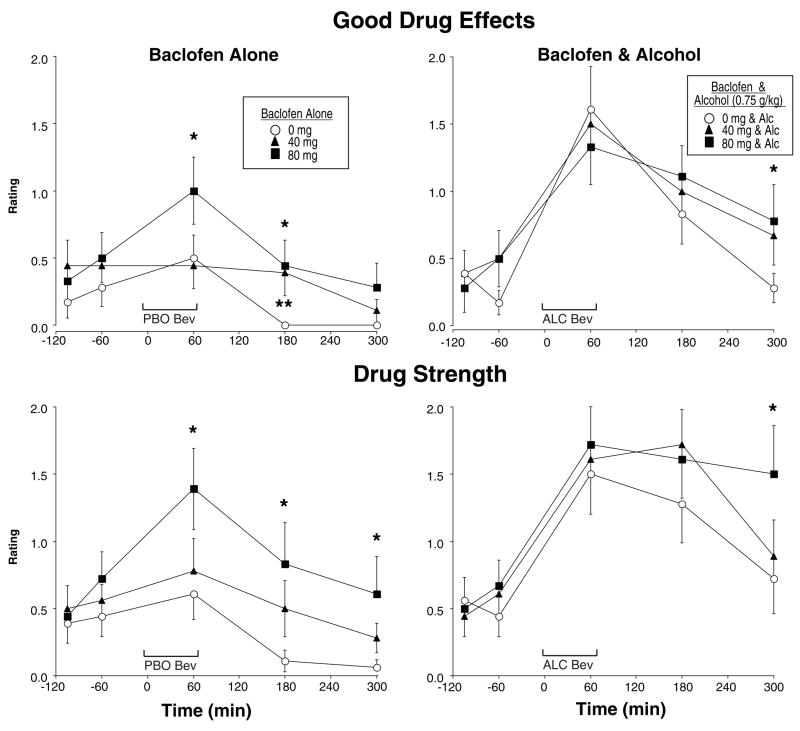
Figure 4 shows two items from the Drug Effect Questionnaire (DEQ). Baclofen alone tended to produce dose-related increases in ratings of Good Drug Effects [F(2,32)=3.35, p<0.07]), whereas alcohol produced clear increases in Good Drug Effects, particularly on the ascending limb of the breath alcohol curve (Alcohol × Time interaction [F(4,64)=7.79, p<0.0001]), but there were no interactions between alcohol and baclofen. As also shown in Figure 4, ratings of Drug Strength were increased by baclofen in a dose-related manner [F(2,32)=7.29, p<0.005]), as well as by alcohol (Alcohol × Time interaction [F(2,64)=2.54, p<0.07]), but there were no interactions between alcohol and baclofen. Baclofen alone also produced significant, but small, dose-related increases in Bad Drug Effects (Baclofen × Time interaction [F(8,128)=2.37, p<0.05]). Ratings of Drug Liking and Bad Drug Effects were both significantly increased after alcohol administration (Alcohol × Time interactions [Fs(4,64)>4.60, ps<0.01]) and there was a similar trend for Willing to Take Again (Alcohol × Time interaction [F(4,64)=2.58, p<0.06]). However, there were no interactions between alcohol and baclofen on any of these measures.
Fig. 4.
Mean ratings of Good Drug Effects and Drug Strength after baclofen alone (left panels) or baclofen in combination with alcohol (right panels) as a function of baclofen dose and time. Ratings for Good Drug Effects and Drug Strength could range from 0–4. See Figures 1–3 for details.
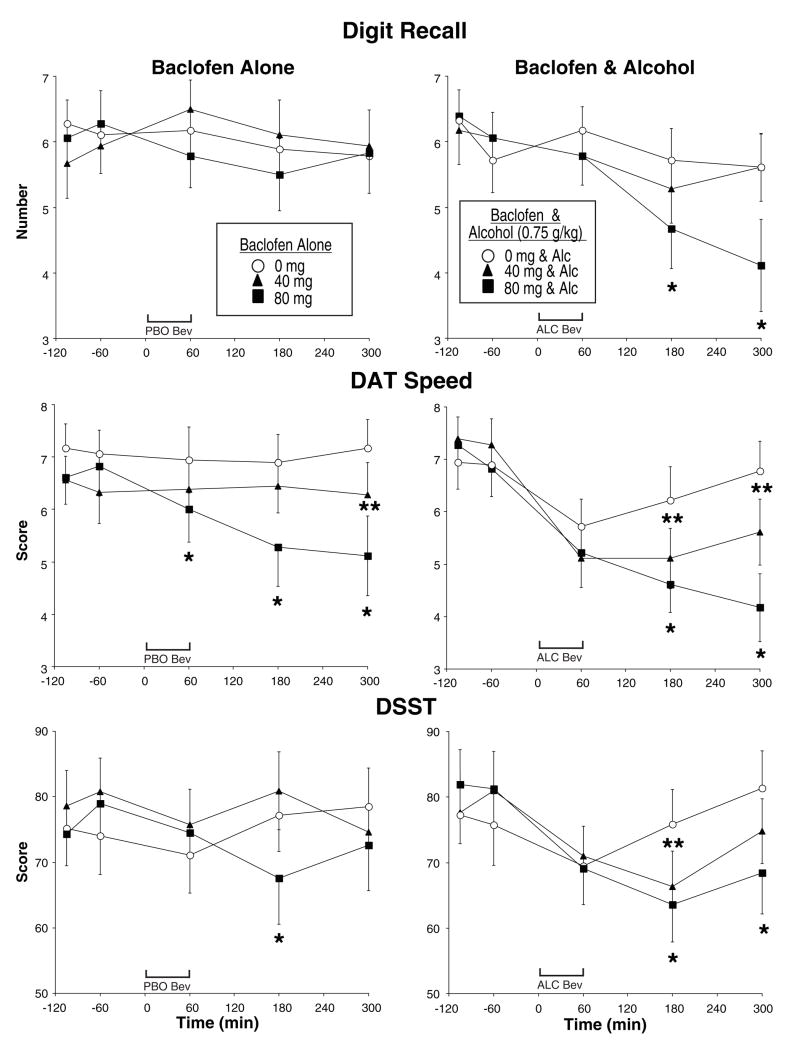
Performance Effects
Figure 5 shows the results from measures on three different performance tasks. Immediate digit recall (top panels) on the Digit Enter and Recall Task showed no main effects of balcofen or alcohol, such that neither drug alone impaired performance. However, when baclofen and alcohol were combined, immediate digit recall was impaired, particularly towards the end of the session following 80 mg of baclofen (Baclofen × Alcohol × Time interaction [F(8,128)=2.16, p<0.04]). A similar interaction was observed for delayed digit recall (Baclofen × Alcohol × Time interaction [F(8,128)=3.37, p<0.002]), but baclofen alone also impaired delayed digit recall in a dose-related manner [F(2,32)=4.62, p<0.02].
Fig. 5.
Mean scores on selected performance measures after baclofen alone (left panels) or baclofen in combination with alcohol (right panels) as a function of baclofen dose and time. DAT = Divided Attention Task; DSST = Digit Symbol Substitution Task. See Figures 1–3 for details.
All measures on the DAT were impaired by baclofen alone, including speed (see middle panels of Figure 5), tracking distance, hit latency, as well as the number of hits, misses and false alarms (main effects of Baclofen or Baclofen × Time interactions, Fs>2.95, ps<0.03). Alcohol also impaired every measure on the DAT (main effects of Alcohol or Alcohol × Time interactions, Fs>3.63, ps<0.04), except for tracking distance. Despite this, only DAT speed (Figure 5) was impaired more when balcofen and alcohol were combined (Baclofen × Alcohol × Time interaction [F(8,128)=2.46, p<0.02]).
As shown in the lower panels of Figure 5, performance on the DSST (total correct) was impaired by 80 mg baclofen (Baclofen × Time interaction [F(8,128)=4.29, p<0.001]) and by alcohol (Alcohol × Time interaction [F(4,64)=3.01, p<0.03]). Although there were no statistically significant interactions between baclofen and alcohol, based on the planned comparisons, DSST performance returned to baseline levels more slowly when baclofen and alcohol were combined. This same pattern was observed for the number attempted on the DSST. Similarly, performance on the balance task was significantly impaired by alcohol (Alcohol × Time interaction [F(6,96)=3.77, p<0.02]) and marginally impaired by balcofen (Baclofen × Time interaction [F(12,192)=1.97, p<0.06]), but there were no interactions between baclofen and alcohol. On the Word Recall Task, alcohol impaired immediate word recall, 2 hr and 4 hr delayed recall, and delayed word recognition [Fs(1,15)>7.45, ps<0.02], but there were no main effects of baclofen, and no interactions of baclofen with alcohol. Lastly, performance on the Time Estimation Task was not altered by baclofen, alcohol or the combination.
Sex Differences
In addition to women having lower breath alcohol levels than men, there were several other interactions with sex. For systolic blood pressure, there was an overall effect of sex [F(1,16)=4.99, p<0.05], with men having higher systolic blood pressure than women (mean of 126 and 117 bpm, respectively) and baclofen produced greater increases in systolic blood pressure in men than in women (Baclofen × Sex interaction F(2,32)=3.57, p<0.05]. On the BAES, women had lower Stimulation scores than men when baclofen was combined with alcohol (Baclofen × Alcohol × Sex interaction [F(2,32)=3.58, p<0.04]. For BAES Sedation scores, women reported significantly higher levels of sedation than men during the sessions (Sex × Time interaction [F(8,128)=3.12, p<0.02]) and women tended to report higher levels of sedation than men following baclofen (Baclofen × Sex interaction [F(2,32)=3.17, p<0.06]). Correspondingly, there was a trend for women to have higher BAES Summary scores than men (Sex × Time interaction [F(8,128)=2.22, p<0.08]). On BVAS ratings, the only significant interaction with sex was that women reported higher ratings of Dizzy following alcohol than men (Alcohol × Sex interaction [F(1,16)=5.05, p<0.04]), although ratings of Dizzy overall were quite low. There were several interactions with Sex on the VAS clusters. “Personality Traits” cluster scores were higher after alcohol in women compared to men (Alcohol × Time × Sex interaction [F(4,128)=3.27, p<0.03], “Bad Effects” cluster scores were higher after baclofen in women compared to men (Baclofen × Time × Sex interaction [F(8,128)=3.06, p<0.02] and tended to be higher in women after alcohol (Alcohol × Time × Sex interaction [F(4,128)=2.53, p<0.07]. On the DEQ, there was a trend for women to report higher ratings of Good Drug Effects than men (Sex × Time interaction [F(2,64)=2.54, p<0.07]), although following baclofen, ratings of Drug Liking tended to be lower in women (Baclofen × Sex interaction [F(2,32)=3.05, p<0.07]). In addition, women tended to report higher ratings on Drug Strength after alcohol (Alcohol × Sex interaction [F(1,16)=4.14, p<0.06]).
There were no sex differences on most of the performance tasks, with one exception: women tended to be more impaired than men on the Digit Enter and Recall Task. When baclofen and alcohol were combined, women were more impaired than men on delayed digit recall (Baclofen × Alcohol × Sex × Time interaction [F(8,128)=4.15, p<0.001]) and tended to be more impaired on immediate digit recall (Baclofen × Alcohol × Sex × Time interaction [F(8,128)=2.00, p<0.06]). For digit recognition, women tended to be more impaired than men after baclofen alone (Baclofen × Sex × Time interaction [F(8,128)=1.88, p<0.08]) and when baclofen was combined with alcohol (Baclofen × Alcohol × Sex interaction [F(2,32)=3.09, p<0.06]).
DISCUSSION
This is the first study to directly investigate the safety of baclofen when combined with intoxicating doses of alcohol. No adverse side effects were observed or reported despite the fact thatthe doses of baclofen tested were higher than those used in previous clinical trials. Therefore, in combination with the results obtained in alcohol-dependent patients (Addolorato et al., 2002a; Flannery et al., 2004), including those with alcohol-related liver damage (Addolorato et al., 2007), our laboratory study suggests that baclofen has an acceptable safety profile even among individuals who continue to drink.
Another goal of this study was to determine if baclofen has any abuse liability in heavy drinkers, either alone or in combination with alcohol. Although baclofen tended to produce dose-related increases on ratings of Good Drug Effect and Elevated Mood, no comparable increases on BAES Stimulation Scores and ratings of High or Drug Liking were observed. In contrast, alcohol produced increases on many of these ratings including High, Stimulated, Elevated Mood, Drug Liking and Good Drug Effect, similar to previous studies among moderate to heavy drinkers (Bisaga and Evans, 2004, 2006; McCaul et al., 2000). When baclofen was combined with alcohol, there were no significant interactions on any measures of abuse liability, with the exception that ratings of High were increased. Taken together, these results suggest that baclofen has minimal abuse liability in this population, even in the context of alcohol consumption. Similarly, other laboratory studies that assessed the interaction of baclofen with cocaine have not shown that baclofen has abuse potential among individuals who abuse cocaine (Haney et al., 2006; Lile et al., 2004).
Given the promising preclinical and clinical literature indicating that baclofen decreases alcohol intake and self-administration (Addolorato et al., 2000, 2002a, 2007; Besheer et al., 2004; Colombo et al., 2000, 2003a, b; Flannery et al., 2004; Walker and Koob, 2007), we hypothesized that baclofen would reduce many of the positive subjective effects produced by alcohol drinking among heavy drinkers in the laboratory. As described above, baclofen did not attenuate any of the positive subjective effects produced by alcohol, including ratings of High, Drug Liking and Good Drug Effect or the alcohol-induced increases on BAES Stimulation scores. The most promising clinical evidence to date among alcohol-dependent patients (Addolorato et al., 2000, 2002a, 2007; Flannery et al., 2004) indicates that baclofen may reduce alcohol consumption by reducing alcohol craving. However, a recent study in baclofen-treated patients found that a reduction in stress-related hormones, particularly aldosterone, was correlated with a reduction in alcohol craving (Leggio et al., 2008). In the present study, alcohol craving did not vary either as a function of alcohol consumption, baclofen administration, or the combination. There are several possible reasons why we failed to see any changes on alcohol craving. First, alcohol craving scores were relatively low throughout the study, making it difficult to detect reductions in alcohol craving, although some studies have been able to show reductions in alcohol craving even when baseline levels were low (Bisaga and Evans, 2004). The low alcohol craving was most likely due to the fact that participants in the present study were social drinkers and not alcohol dependent. For instance, previous laboratory studies have been able to show that non-treatment seeking alcohol-dependent individuals report greater alcohol craving than social drinkers (Drobes et al., 2004) and that medications like naltrexone, can reduce alcohol craving (e.g., Drobes et al., 2004) and alcohol self-administration among alcohol-dependent participants (e.g., O’Malley et al., 2002). Further, the design of the present study was to primarily assess safety, and only indirectly assess efficacy measures. Laboratory studies that use other procedures such as cue reactivity or behavioral economic procedures for assessing the reinforcing effects of alcohol may provide more useful information for understanding the relationship between alcohol craving and drinking behavior and the mechanisms of potential medications that cannot be obtained by simply measuring subjective craving (e.g., Drobes et al., 2003; O’Malley et al., 2002). Lastly, participants in this study were psychologically healthy and were not experiencing any anxiety. In contrast, alcohol-dependent patients in treatment not only have higher rates of anxiety and other affective disorders, but they often experience heightened anxiety and alcohol craving when attempting to reduce their alcohol use. Thus the anxiolytic effects of baclofen (Breslow et al., 1989; Knapp et al., 2007; Krupitsky et al., 1993), may be more effective at reducing alcohol craving in a treatment seeking population by reducing anxiety. In fact, several clinical trials with baclofen have observed both a reduction in alcohol craving and anxiety symptoms (Addolorato et al., 2002a; Flannery et al., 2004).
In preclinical studies, baclofen has been shown to increase the sedative and depressant effects of alcohol, as typically indicated by greater impairment on motor behavior (Besheer et al., 2004; Chester and Cunningham, 1999; Cott et al., 1976; Martz et al., 1983). However, preclinical (Besheer et al., 2004) and clinical evidence (Addolorato et al., 2000, 2007; Ameisen, 2005) also suggest that individuals tolerant to the effects of alcohol are less sensitive to the sedative effects of GABA-B receptor agonists. This is consistent with recent biochemical studies suggesting that presynaptic GABA-B receptor activity may play a role in regulating behavioral sensitivity to ethanol and stimulating GABA-B receptors decreases GABAergic effects of alcohol (Ariwodola and Weiner, 2004; Boehm et al., 2002). Therefore, another goal of this study was to determine if baclofen enhances the sedation and performance impairment produced by alcohol. Consistent with the sedative profile of baclofen and alcohol, both drugs increased BAES Sedation and BAES Summary scores when administered alone. Moreover, these ratings were increased more when baclofen and alcohol were combined. Other than BAES scores, baclofen produced minimal changes on other subjective effects questions indicative of adverse or side effects, with the exception that baclofen produced small increases on ratings of Bad Drug Effects, but these effects were not increased when combined with alcohol. With respect to performance impairment, baclofen alone impaired performance on several tasks requiring motor skills (DAT, DSST, balance), but did not affect memory skills (digit or word recall) or time estimation. Not surprisingly, alcohol alone impaired performance on all of the tasks except for digit recall and time estimation. In spite of the fact that both drugs impaired performance on several tasks, only immediate digit recall and one measure on the DAT (tracking speed) were significantly impaired to a greater extent when baclofen was combined with alcohol. Nevertheless, patients should be cautioned about taking baclofen when driving or engaging in other hazardous jobs or tasks since baclofen did impair performance and in some cases when baclofen was combined with alcohol, the duration of impairment was prolonged.
In the present study, one-third of the participants were women, allowing us to explore potential sex differences. Overall, minimal sex differences were observed in this study. While men had higher systolic blood pressure than women, these effects were small and consistent with previous studies (Recklehoff, 2001). Despite having lower breath alcohol levels, there was some evidence that women were slightly more affected by both alcohol and baclofen. Unfortunately, these differences are difficult to interpret since women had lower breath alcohol levels than men, despite the fact that alcohol doses were based on total body water using anthropomorphic equations designed to minimize differences between men and women (Watson et al., 1980). However, these anthropomorphic equations were not determined in heavy drinkers. In the present study, women reported drinking at the same level as the men (28 drinks/week) and therefore may have been more tolerant to the effects of alcohol that could have resulted in more rapid alcohol metabolism (Salaspuro et al., 1978). Thus, if breath alcohol levels had been more similar between men and women, it is possible that greater sex differences would have been observed, with women being more affected.
The present study had a number of strengths, including an inpatient setting, testing a range of doses of baclofen alone, and in combination with alcohol in the same participants, and the use of a double-dummy design with placebo beverages to control for alcohol expectancy effects, and the use of a cumulative dosing procedure (de Wit et al., 1989). Additionally, a comprehensive assessment battery was conducted multiple times each session to capture effects before alcohol, on the ascending limb, peak and descending limb of the breath alcohol curve. Despite these strengths, there were several limitations. The primary limitation is that the participants were not alcohol dependent or seeking treatment for their alcohol use. Second, the sample size was relatively small. Third, the interaction of baclofen and alcohol only involved acute administration and the effects of baclofen may be different after chronic administration. Fourth, the alcohol drinking was essentially experimenter-administered as opposed to allowing participants to self-administer alcohol beverages of their choice. Lastly, despite the best intentions, there were sex differences in breath alcohol levels, making it difficult to adequately interpret potential sex differences of baclofen or the interaction of baclofen with alcohol.
Taken together, the results of the present study suggest that baclofen alone has minimal abuse liability in this population and that baclofen is relatively well tolerated and safe when given in combination with intoxicating doses of alcohol. With respect to the potential efficacy of baclofen for reducing alcohol craving or other positive subjective effects of alcohol, no definitive conclusions can be drawn from the present study due to the design limitations and the participant population. However, this study indicates that future laboratory studies could be safety conducted to investigate whether baclofen would alter alcohol craving and actual alcohol consumption using self-administration or choice studies in alcohol-dependent individuals.
Table I.
Demographic Characteristics of Participants
| N or mean (range) | |
|---|---|
| Age (years) | 28.9 (21–44) |
| Gender (M = male, F = female) | 12 M/6 F |
| Race (White/Black/Hispanic/Asian) | 8/4/5/1 |
| Education (years) | 14.2 (12–18) |
| BMI (kg/m2) | 24.0 (19–30) |
| Alcohol Use | |
| Drinks* (week) | 28.2 (20–52) |
| Number of drinks per occasion | 5.4 (4–9) |
| Frequency of drinking (days/week) | 5.1 (4–7) |
| Diagnosis of alcohol abuse | 4 |
| SMAST score | 4.3 (0–16) |
| Current Other Drug Use | |
| Cigarettes (# smokers) | 10 |
| Marijuana (# users) | 7 |
Drinks are based on standard drink units
BMI = body mass index; SMAST = Short Michigan Alcohol Screening Test
Acknowledgments
This research was supported by research grants from the National Institute of Alcohol Abuse and Alcoholism (AA12599) to Dr. Evans, the National Institute on Drug Abuse to Dr. Bisaga (K23 DA00429). Participants resided on the Biological Studies Unit supported by MH 61274 from the National Institutes of Health. Portions of the data contained in this manuscript were presented at the Annual Meeting of the College on Drug Dependence in 2005. The authors thank Dr. Laslo Papp for use of the research unit, as well as Annie Rosenberg, Shannon Becker, Brendan Carroll and Kelly Livingston for their expert technical assistance.
References
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002a;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med. 2002b;112:226–229. doi: 10.1016/s0002-9343(01)01088-9. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, DeLorenzi G, Parente A, Caputo F, Janiri L, Capristo E, Rapaccini GL, Gasbarrini G. Suppression of alcohol delirium tremens by baclofen administration: a case report. Clin Neuropharmacol. 2003;26:258–262. doi: 10.1097/00002826-200309000-00010. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006b;119:276, e13–18. doi: 10.1016/j.amjmed.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Agabio R, Colombo G, Gasbarrini G. Baclofen: a new drug for the treatment of alcohol dependence. Int J Clin Pract. 2006a;60:1003–1008. doi: 10.1111/j.1742-1241.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Aisen ML, Dietz MA, Rossi P, Cedarbaum JM, Kutt H. Clinical and pharmacokinetic aspects of high dose oral baclofen therapy. J Am Paraplegia Soc. 1992;15:211–216. doi: 10.1080/01952307.1992.11761520. [DOI] [PubMed] [Google Scholar]
- Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40:147–150. doi: 10.1093/alcalc/agh130. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–908. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl) 2004;174:358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl) 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alc Depend. 2006;83:25–32. doi: 10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Breslow MF, Fankhauser MP, Potter RL, Meredith KE, Misiaszek J, Hope DG., Jr Role of gamma-aminobutyric acid in anitpanic drug efficacy. Am J Psychiatry. 1989;146:353–356. doi: 10.1176/ajp.146.3.353. [DOI] [PubMed] [Google Scholar]
- Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007;42:158–160. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav. 1999;63:325–331. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MAM, Pibiri F, Serra S, Vacca G, Gessa GL. Role of GABAB receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–414. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I--Preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, Addolorato G, Froestl W, Carai MA, Gessa GL. The GABA(B) receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol. 2002;37:499–503. doi: 10.1093/alcalc/37.5.499. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 2003a;70:105–108. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MAM, Gessa GL. Effect of the combination of naltrexone and baclofen, on acquisition of alcohol drinking behavior in alcohol-preferring rats. Drug Alcohol Depend. 2005;77:87–91. doi: 10.1016/j.drugalcdep.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Gessa GL. Baclofen-induced suppression of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats exposed to different alcohol concentrations. Eur J Pharmacology. 2006;550:123–126. doi: 10.1016/j.ejphar.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology (Berl) 2003b;167:221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Cott J, Carlsson A, Engel J, Lindqvist M. Suppression of ethanol-induced locomotor stimulation by GABA-like drugs. Naunyn-Schmiedeber’s Arch Pharmacol. 1976;295:203–209. doi: 10.1007/BF00505087. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABA(B) agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30:812–818. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Pierri J, Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology. 1989;98:113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F. GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol. 1987;4:469–472. doi: 10.1016/0741-8329(87)90087-5. [DOI] [PubMed] [Google Scholar]
- Drake RG, Davis LL, Cates ME, Jewell ME, Ambrose SM, Lowe JS. Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother. 2003;37:1177–1181. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. The effects of alprazolam and buspirone in light and moderate female social drinkers. Behav Pharmacol. 2002;13:427–439. doi: 10.1097/00008877-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–1255. [PubMed] [Google Scholar]
- Evans SM, Levin FR, Fischman MW. Increased sensitivity to alprazolam in females with a paternal history of alcoholism. Psychopharmacology (Berl) 2000;150:150–162. doi: 10.1007/s002130000421. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–1523. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid-and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–1821. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jamous A, Kennedy P, Psychol C, Grey N. Psychological and emotional effects of the use of oral baclofen: a preliminary study. Paraplegia. 1994;32:349–353. doi: 10.1038/sc.1994.59. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248–254. doi: 10.1097/01.alc.0000153542.10188.b0. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Mann K. New achievements and pharmacotherapeutic approaches in the treatment of alcohol dependence. Eur J Pharmacol. 2005;526:163–171. doi: 10.1016/j.ejphar.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol. 2000;35:537–547. doi: 10.1093/alcalc/35.6.537. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grienko AJ, Borodkin YS. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug Alcohol Depend. 1993;33:157–163. doi: 10.1016/0376-8716(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Miceli A, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Renin and adolosterone but not the natriuretic peptide correlate with obsessive craving in medium-term abstinent alcohol-dependent patients: a longitudinal study. Alcohol. 2008;42:375–381. doi: 10.1016/j.alcohol.2008.03.128. [DOI] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PEA, Hays LR, Rush CR. Bacofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology. 2004;171:441–449. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of gamma-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Bienkowski P, Carai MAM, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 2008;95:284–287. doi: 10.1016/j.drugalcdep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addition and its treatment: Human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishman-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5:183–194. [PubMed] [Google Scholar]
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Lorazepam and meprobamate dose effects in humans: Behavioral effects and abuse liability. J Pharmacol Exp Ther. 1987;243:978–988. [PubMed] [Google Scholar]
- Salaspuro MP, Lieber CS. Non-uniformity of blood ethanol elimination: its exaggeration after chronic consumption. Ann Clin Res. 1978;10:294–297. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293. Rockville, MD: 2007. Results from the 2006 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Smith BR, Boyle AE, Amit Z. The effects of the GABA(B) agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol. 1999;17:231–240. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Fletcher PJ. Evidence that GABA(A) but not GABA(B) receptor activation in the dorsal raphe nucleus modulates ethanol intake in Wistar rats. Behav Pharmacol. 1996;7:85–93. [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]