Abstract
Salmonella enterica serovar Typhi vaccine strain CVD 908-htrA was genetically engineered for stable plasmid-based expression of protective antigen of anthrax toxin (PA83) fused with the export protein ClyA (ClyA-PA83). The priming potential of CVD 908-htrA expressing ClyA-PA83 was assessed in 12 rhesus and 20 cynomolgus macaques immunized mucosally (intranasally) on days 0 and 14. A parenteral boost with purified PA83 plus alum was given to rhesus macaques on days 42 and 225; cynomolgus monkeys were boosted only once, 3 months after priming, with either PA or licensed anthrax vaccine (Biothrax®). Monkeys primed with S. Typhi expressing ClyA-PA83 developed high levels of serum toxin neutralization activity (TNA) antibodies (> 1.3 ×103 ED50), 7 days after boosting, while unprimed controls lacked serum TNA (0 ED50). The success in non-human primates of this anthrax vaccine strategy based on heterologous mucosal prime followed by parenteral subunit vaccine boost paves the way for clinical trials.
Keywords: anthrax, vaccine, heterologous prime-boost, immunization, protective antigen, Salmonella Typhi
Introduction
Deliberate bioterrorist release of Bacillus anthracis spores in 2001 resulted in 22 confirmed anthrax cases, 11 inhalational (5 fatal) and 11 cutaneous [1]. The anthrax vaccine licensed by the Food and Drug Administration, BioThrax® (previously called Anthrax Vaccine Adsorbed, AVA), derives from a cell-free supernatant of an attenuated, non-encapsulated B. anthracis strain, formulated with aluminum adjuvant [2]. Six subcutaneous injections of BioThrax® over 18 months are recommended, followed by annual boosters, to elicit sustained immunity [3].
Serum toxin neutralizing activity (TNA) antibodies constitute a correlate of protection against inhalational anthrax, based on spore challenges in rabbits and non-human primates [4-8]. The primary immunogen of BioThrax® that elicits TNA responses is the eukaryotic cell-binding Protective Antigen (PA) component of anthrax toxin [9-11].
Although placebo-controlled trials and post-licensure surveillance have failed to attribute severe adverse reactions to BioThrax® [10-12], the public perception of this vaccine is not positive [13-16]. Absent a tangible bioterror threat, target populations (e.g., laboratory workers, decontaminators, “first responders”) are reluctant to receive parenteral anthrax vaccine. Efforts are ongoing to produce less reactogenic, more immunogenic anthrax vaccines for US civilians based on highly purified full-length PA83 [8, 17-19] and to stockpile these vaccines. We propose a novel strategy in which mucosally-administered, well-tolerated, attenuated S. Typhi live vector vaccine strains expressing PA83 would stimulate strong immunologic priming and memory so that subsequently, in the face of a bioterror event, primed individuals given a single dose of PA83-based vaccine (BioThrax® or purified PA83) would rapidly (within a few days) attain protective serum TNA levels.
Materials and Methods
Culture conditions and construction of vaccine strains
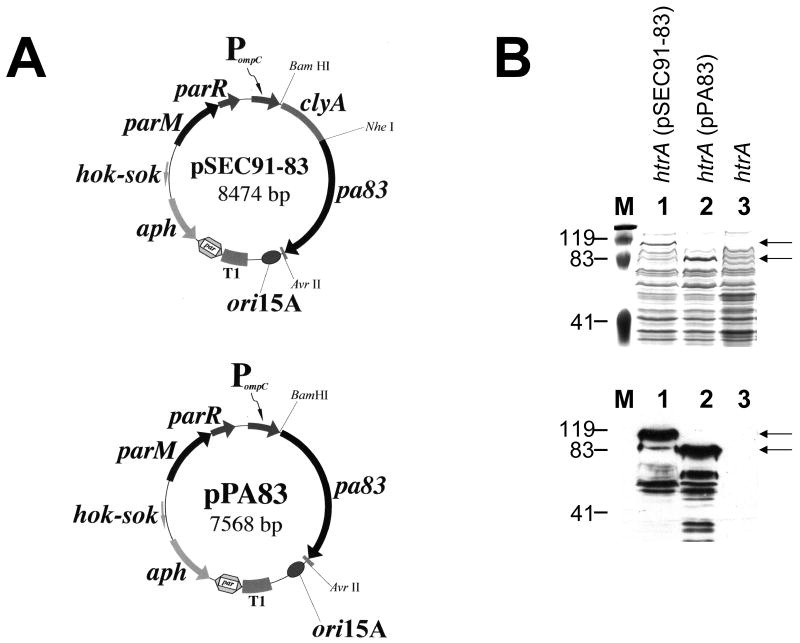
Plasmids were constructed by standard techniques [20]. CVD 908-htrA, a safe, immunogenic derivative of wild type strain Ty2, with deletions in aroC, aroD, and htrA [21], was the live vector. A prokaryotic codon-optimized 2223 base pair synthetic gene (GenBank accession number EU818794) encoding the 735 residues of PA83 was chemically synthesized as a Nhe I - Avr II cassette by GenScript Corp., and encoded a protein with an expected molecular mass of 83.0 kDa. This cassette was inserted in-frame into the unique Nhe I site at the carboxyl terminus of the antigen export protein ClyA, encoded by genetically stabilized expression plasmid pSEC91 [22], creating pSEC91-83 (Fig. 1, panel A). Insertion of the identical cassette into pSEC91 cleaved with Spe I and Nhe I removed clyA to create isogenic construct pPA83 expressing cytoplasmic PA83 (Fig. 1A). Both constructs were confirmed by DNA sequence analysis. After electroporation of these plasmids into CVD 908-htrA, expression of ClyA-PA83 and unfused PA83 was examined by western immunoblotting [22], using goat anti-PA IgG (List Biological) and horseradish peroxidase (HRP)-labeled rabbit anti-goat IgG (Kirkegaard & Perry Labs; KPL).
Figure 1.
Plasmid-based expression of Protective Antigen (PA83) in CVD 908-htrA live vectors. (A) Genetic maps of the isogenic expression plasmids encoding ClyA-PA83 and PA83 immunogens. Abbreviations: PompC, modified osmotically controlled ompC promoter from E. coli; clyA, encodes cytolysin A (ClyA) from S. Typhi; pa83, encodes full-length B. anthracis Protective Antigen, codon-optimized for expression in S. Typhi; clyA∷pa83, encodes pa83 fused to the carboxyl terminus of ClyA; ori15A, origin of replication from pACYC184, providing an expected expression plasmid copy number of ∼15 per chromosomal equivalent; aph, encodes the aminoglycoside 3′-phosphotransferase conferring resistance to kanamycin; T1, transcriptional terminator from the rrnB ribosomal RNA operon of E. coli; hok-sok, post-segregational killing locus from the multiple antibiotic resistance R-plasmid pR1; parM and parR, comprise the parA active partitioning system from pR1. (B) B. anthracis PA expression in vaccine constructs. Coomassie brilliant blue-stained SDS-polyacrylamide gel (top) and western immunoblot analysis (bottom) of whole cell lysates from either CVD 908-htrA(pSEC91-83) (lane 1), CVD 908-htrA(pPA83) (lane 2), or empty CVD 908-htrA live vector as a control (lane 3). Immunoblot membranes were probed with polyclonal antiserum raised against PA83, as described in Materials and Methods. Arrows indicate the expected molecular masses for ClyA-PA83 (116.6 kDa) and PA83 (83.0 kDa), respectively.
Immunoelectron microscopy
Bacterial suspensions were placed on Formvar-carbon coated nickel grids and incubated with blocking buffer containing 5% BSA-c and 0.1% cold water fish skin gelatin (Aurion) in PBS, followed by mouse anti-PA and goat anti-mouse IgG conjugated to 10 nm gold particles (Aurion). Grids stained with 1% ammonium molybdate were examined in a JEOL 1200 EX transmission electron microscope.
Immunizations
a) Rhesus macaques
Twelve rhesus macaques (Macaca mulatta) weighing 4.3-5.9 kg were assembled into 3 groups of 4 animals each with similar weight and sex distributions. The groups were then randomly allocated to be immunized mucosally (intranasally, i.n.) on days 0 and 14 with CVD 908-htrA(pSEC91-83), CVD 908-htrA(pPA83) or CVD 908-htrA not carrying an expression plasmid (empty live vector). After ketamine anesthesia (10 mg/kg), animals were placed in a sitting position and 50 μl of vaccine suspension containing between 4.7×109 and 1.1×1010 colony forming units (hereafter referred to as ∼5 × 109 CFU) in PBS were instilled into the nares (25 μl/nare). Monkeys were boosted intramuscularly (i.m.) on day 42 with purified PA83 (42.5 μg) adsorbed to 0.75 mg of alum (VaxGen; developmental lot #8). A second PA83 boost (VaxGen, developmental lot #041906) was given to all monkeys six months later (day 225).
b) Cynomolgus macaques
Twenty Macaca fascicularis weighing from 2.5 – 4.0 kg were assembled by weight and sex into 2 similar groups of 12 and 8 animals. Half of the animals per group were randomly allocated to be immunized i.n. on days 0 and 14 with between 3.2×109 and 8.0×109 CFU (hereafter referred to as ∼5 × 109 CFU) of CVD 908-htrA(pSEC91-83), while the others received CVD 908-htrA carrying pSEC91 without PA genes (empty expression plasmid). Three months after mucosal priming (day 100), one half of the animals in each group were randomly allocated to be boosted i.m. with 85 μg of PA83 adsorbed to 0.75 mg of alum (VaxGen, developmental lot # VXG-0827) and the other half subcutaneously with 0.5 ml of BioThrax® (Emergent Biosolutions, lot FAV119). Blood was collected prior to and after immunization.
Studies were approved by the University of Maryland, School of Medicine Institutional Animal Care and Use Committee.
Antibody responses
Serum TNA antibodies were measured by the assay of Quinn and colleagues [8, 23, 24]. Titers were calculated using an end-point algorithm and reported as the reciprocal of a serum dilution that resulted in 50% neutralization of toxin-mediated cytotoxicity (ED50). Serum IgG to PA was measured by ELISA. Plates were coated with PA83 (List Biological) at 2 μg/ml in PBS and blocked with 10% dry-milk in PBS. Duplicate samples were tested in serial dilutions. HRP-labeled anti-monkey IgG (KPL) and anti-human IgG1-4 (The Binding Site) were used as conjugates, followed by TMB substrate (KPL). Anti-PA IgG titers were calculated by interpolation of regression corrected Absorbance values of experimental samples into a standard curve, and reported in μg/ml. Anti-PA IgG subclass titers were calculated through linear regression analysis as the inverse of the dilution that produces an Absorbance value of 0.2 above the blank. Respiratory secretions were not collected to measure SIgA anti-PA.
Statistical analysis
Antibody titers in selected groups were compared by Mann-Whitney test. P≤.05 was considered significant. No adjustment was made for multiple comparisons because with the small numbers of animals per group we elected not to control the overall Type I error rate at <.05. Statistical analyses, including linear regression, were performed using Sigma Stat 3.0 (SPSS Inc.).
Results
Expression of PA83 in S. Typhi
Whole cell lysates of CVD 908-htrA(pSEC91-83) and CVD 908-htrA(pPA83) expressing, respectively, PA83 fused to the export protein ClyA or unfused PA83 (Fig. 1A), were separated on SDS-polyacrylamide gels. After staining with Coomassie brilliant blue (Fig. 1B, top panel), protein bands approximating the expected molecular masses for ClyA-PA83 and unfused PA83 (116.6 kDa and 83.0 kDa, respectively) were observed. western blotting with anti-PA83 polyclonal antiserum also detected proteins of ∼ 117 and 83 kDa (Fig. 1B, lower panel). Smaller protein species were detected in these lysates, likely resulting from proteolytic cleavage of PA83.
Immunoelectron microscopy
Immune labeling with gold particles observed by electron microscopy confirmed that ClyA-PA83 fusions were exported out of the live vector cytoplasm. CVD 908-htrA(pSEC91-83) exhibited a high density of gold particles on the cell surface (Fig. 2A). In contrast, no gold particles were seen on the surface of CVD 908-htrA expressing cytoplasmic PA83 (Fig. 2B) or empty CVD 908-htrA live vector (Fig. 2C) incubated with antibodies specific for PA, or CVD 908-htrA(pSEC91-83) incubated with non-immune mouse sera (Fig. 2D).
Figure 2.
Expression of PA on the surface of S. Typhi CVD 908-htrA(pSEC91-83) and CVD 908-htrA(pPA83) by immunogold staining. Immunoelectron micrographs of (A) S. Typhi CVD 908-htrA(pSEC91-83), (B) S. Typhi CVD 908-htrA(pPA83) and (C) S. Typhi CVD 908-htrA incubated with mouse PA-specific antibodies and gold-labeled anti-mouse antibody. (D) CVD 908-htrA(pSEC91-83) incubated with negative serum and gold-labeled anti-mouse antibody. Bar: 0.25 μm.
Antibody responses to PA83 in rhesus macaques
Figure 3A displays serum TNA responses. No monkeys manifested rises in serum TNA antibodies following priming alone. Nevertheless, the animals primed with S. Typhi expressing PA83 (ClyA-PA83 or unfused) developed TNA responses by 7 days (earliest time point tested) after parenteral boosting with purified PA (median titers [MT] 1,701 and 225, respectively, vs. 0 in unprimed controls, P=.029). TNA responses peaked on day 56, two weeks after the boost, with MT of 6,755 and 2,255 ED50, respectively, for the ClyA-PA83 and unfused PA83 groups. At all time points from day 49 through 239 the monkeys primed with PA (ClyA and cytoplasmic data pooled) had significantly higher titers than the unprimed monkeys. Beyond the first month post-boost, titers declined rather slowly but remained elevated for over 6 months. TNA titers also decreased in the unprimed animals, remaining only slightly above the detection limit. TNA titers in monkeys primed with S. Typhi expressing ClyA-PA83 were ∼ 2- to 5-fold higher than animals primed with unfused PA83; on days 56 and 189 the differences in TNA titers between these two groups approached statistical significance (P=.057).
Figure 3.
Toxin neutralization activity (TNA) titers (A) and anti-PA IgG (B) in rhesus macaques primed intranasally with ∼5 × 109 CFU of either CVD 908-htrA(pSEC91-83), CVD 908-htrA(pPA83) or CVD 908-htrA empty live vector on days 0 and 14, and boosted intramuscularly on day 42 (and again on day 225) with PA83 adsorbed to alum (VaxGen). Arrows indicate the immunizations. Data points represent median titers (MT) of TNA or median concentrations (MC) of IgG anti-PA with bars representing 95% Confidence Intervals. Statistical tests (Mann Whitney) compared titers of individual animals in the different groups on the various time points, and statistical significance designations are defined as follows: *, P=.029 for animals primed with S. Typhi CVD 908-htrA carrying either (pSEC91-83) or (pPA83) versus unprimed control animals that received S. Typhi CVD 908-htrA without plasmid; $, P=.029 for CVD 908-htrA(pSEC91-83) vs control, and P=0.057 for CVD 908-htrA(pPA83) vs control; #, P=.029 for CVD 908-htrA(pSEC91-83) vs control, and P=.20 for CVD 908-htrA(pPA83) vs control.
Figure 3B shows the kinetics of the serum IgG anti-PA responses. With this serologic assay, modest anti-PA titers were observed after priming with S. Typhi expressing either form of PA, with 50% of animals seroconverting after receiving two doses of live vector. A rapid, strong anamnestic serum anti-PA response was observed in these animals on day 49, 7 days after parenteral boosting on day 42 with purified PA. In contrast, no responses were detected on day 49 in unprimed monkeys who had received S. Typhi not encoding PA83. Median concentrations (MC) of anti-PA IgG in macaques primed with ClyA-PA83, unfused PA83 and empty live vector were 604, 226 and 0.41 μg/ml, respectively, at day 49. Peak responses in monkeys primed with PA83-expressing live vectors appeared on day 56 and were > 10-fold higher than the peak responses of unprimed animals (P=.008), whose peak titers occurred two weeks later on day 70. At all time points, antibody levels were ∼ 2-fold higher in animals primed with CVD 908-htrA expressing the ClyA-PA83 versus strains expressing unfused PA83; however, only at day 99 was the difference significant (P=.029).
The rhesus macaques received a second parenteral PA83 immunization on day 225 (six months after the first parenteral boost). TNA and IgG anti-PA responses were similar to those observed after the first parenteral boost with PA (Fig. 3A & 3B). In animals primed with ClyA-PA83, TNA titers remained elevated long after boosting, with a MT on day 314 (the last time point measured) of 5,945 ED50; serum TNA levels fell more precipitously in animals primed with unfused PA83 (MT=1,119 ED50) (P=.029 vs. ClyA-PA83 animals) and in unprimed animals (MT = 768 ED50).
Antibody responses to PA83 in cynomolgus macaques
The second experiment, in cynomolgus macaques, had three objectives: 1) to confirm the reproducibility of the mucosal prime-parenteral boost immunization strategy using a different non-human primate model; 2) to test the capacity of this vaccine to prime memory B cells that would respond with a potent anamnestic humoral response to a more delayed (3 months after priming) PA83 boost; and 3) to compare the relative potency of different parenteral boosting agents (purified PA83 vs BioThrax®) to elicit anamnestic serum TNA responses.
Two cohorts of cynomolgus macaques containing 12 and 8 monkeys each were primed with CVD 908-htrA(pSEC91-83) or CVD 908-htrA carrying empty expression plasmid pSEC91, respectively. Three months later, one half of the animals in each cohort were boosted parenterally with purified PA83 and the other half with BioThrax®. The kinetics of the TNA and IgG anti-PA responses in the cynomolgus macaques (Fig. 4A & 4B) were remarkably similar to those seen in rhesus macaques. After mucosal priming with CVD 908-htrA(pSEC91-83), 83% of the monkeys manifested significant rises in serum IgG anti-PA83, whereas unprimed animals immunized with CVD 908-htrA(pSEC91) failed to show detectable IgG anti-PA83.
Figure 4.
Toxin neutralization activity (TNA) titers (A) and anti-PA IgG (B) in cynomolgus macaques primed intranasally with ∼5 × 109 CFU of CVD 908-htrA(pSEC91-83) or CVD 908-htrA(pSEC91) (empty expression plasmid) on days 0 and 14, and boosted on day 100 either with PA83 adsorbed to alum (VaxGen) given intramuscularly or Biothrax® given subcutaneously. Arrows indicate each immunization. Data points represent median titers (MT) of TNA or median concentrations (MC) of IgG anti-PA with bars representing 95% Confidence Intervals. In Fig. 4A statistical tests (Mann Whitney) compared titers of individual animals in the different groups on the various time points. Statistical significance designations are defined as follows: *, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals, and P=.038 for CVD 908-htrA(pSEC91-83)-primed animals boosted with Biothrax® vs control animals; $, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals, and P=.114 for CVD 908-htrA(pSEC91-83)-primed animals boosted with Biothrax® vs control animals. In Fig. 4B, statistical significance designations are defined as follows: $, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals, and P=.114 for CVD 908-htrA(pSEC91-83)-primed animals boosted with Biothrax® vs control animals; #, P=.038 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals, and P=.114 for CVD 908-htrA(pSEC91-83)-primed animals boosted with Biothrax® vs control animals.
Cynomolgus macaques primed with CVD 908-htrA(pSEC91-83) exhibited a rapid and strong antibody recall response on day 107, 7 days after boosting (day 100) with either PA83 or BioThrax®, despite the longer interval between mucosal priming and parenteral boosting. The serum TNA titers in cynomolgus monkeys measured on day 107, one week after boosting either with PA83 or BioThrax®, showed that none of the unprimed monkeys who received CVD 908-htrA(pSEC91) had TNA antibodies. In contrast, 11/12 monkeys primed with CVD 908-htrA(pSEC91-83) demonstrated TNA antibodies following boosting with PA83 (6/6 responders, MT = 2,255 ED50, P=.01 vs. unprimed) or BioThrax® (5/6 responders, MT = 519 ED50, P=.038). There was no statistically significant difference in the TNA response in monkeys given VaxGen PA83 versus those given BioThrax® at any time point. One monkey primed with CVD 908-htrA(pSEC91-83) failed to respond to the BioThrax® boost, despite seroconverting for anti-PA after live vector priming. This animal was immunized identically to other animals in this group, and there were no records of health related or technical issues that could account for the lack of response in this animal.
IgG subclass profiles
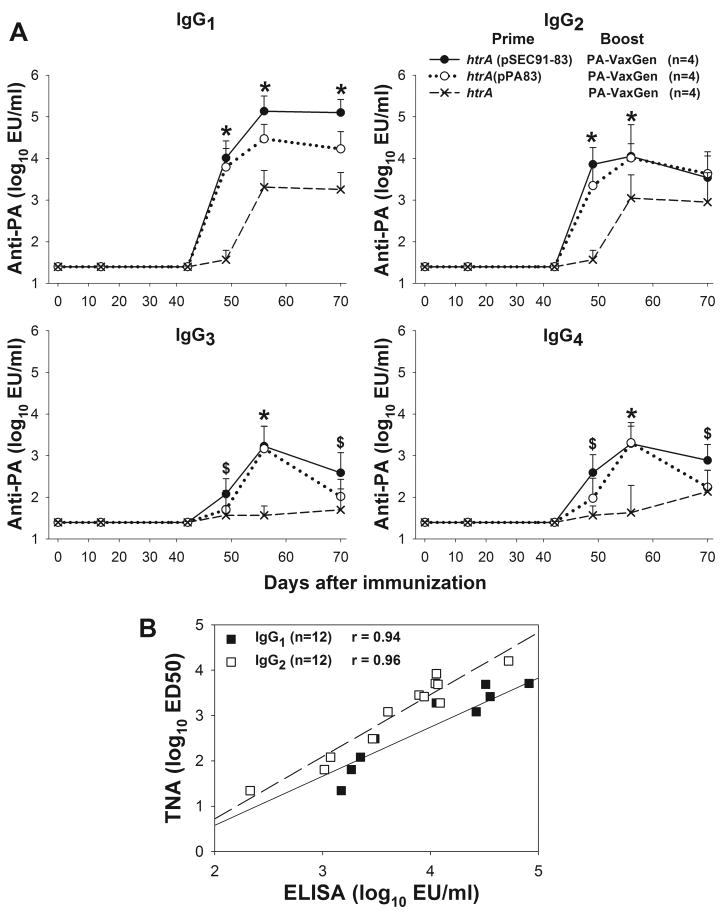
IgG subclass anti-PA profiles observed in rhesus and cynomolgus monkeys after the parenteral boost with PA83-containing vaccine are shown in Figures 5 and 6. There were no significant rises in any anti-PA IgG subclasses following priming alone but all animals exhibited rises in anti-PA of all subclasses after the parenteral boost, peaking 14 days later. Higher IgG1 and IgG2 levels were observed in monkeys primed with PA83-expressing CVD 908-htrA compared with animals who received empty live vector; rises in IgG3 and IgG4 anti-PA were also observed, albeit at much lower levels (Fig. 5A & 6A). An excellent correlation was found in both experiments between IgG1 and IgG2 titers and TNA, with r = 0.94 and r = 0.96, respectively, for rhesus macaques (P<.001, Figure 5B) and r = 0.92 and r = 0.93, respectively, for cynomolgus macaques (P<.001, Figure 6B).
Figure 5.
Anti-PA IgG subclass profile in rhesus macaques primed with S. Typhi expressing PA83 and boosted with PA83-alum according to the immunization schedule shown in Figure 3. (A) Kinetics of IgG1-4 anti-PA responses. Data points represent median antibody concentrations (MC) with bars representing 95% Confidence Intervals. Statistical tests (Mann Whitney) compared titers of individual animals in the different groups on the various time points. Statistical significance designations are defined as follows: *, P=.029 for animals primed with S. Typhi CVD 908-htrA carrying either (pSEC91-83) or (pPA83) versus unprimed control animals that received S. Typhi CVD 908-htrA without plasmid; $, P=.029 for CVD 908-htrA(pSEC91-83) vs control. (B) Linear regression analysis of IgG1 and IgG2 levels (x-axis) and toxin neutralization activity (y-axis) (P<.001). Data points represent titers of individual animals on day 56 (peak responses).
Figure 6.
Anti-PA IgG subclass profile in cynomolgus macaques primed with S. Typhi expressing ClyA-PA83 and boosted either with PA83 and BioThrax® according to the immunization schedule described in Figure 4. (A) Kinetics of IgG1-4 anti-PA responses. Data points represent median antibody concentrations (MC) with bars representing 95% Confidence Intervals. Statistical tests (Mann Whitney) compared titers of individual animals in different groups on the various time points, and statistical significance designations are defined as follows: *, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with either PA-containing vaccine vs unprimed animals; $, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals, P=.038 for CVD 908-htrA(pSEC91-83)-primed animals boosted with Biothrax® PA vs unprimed animals; #, P=.01 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals; Ψ, P=.038 for CVD 908-htrA(pSEC91-83)-primed animals boosted with VaxGen PA vs unprimed animals. (B) Linear regression analysis of IgG1 and IgG2 levels (x-axis) and toxin neutralization activity (y-axis) (P< 0.001). Data points represent titers of individual animals measured on day 113 (peak responses).
Discussion
Devising a strategy to protect potentially high risk sub-groups of the US civilian population in the event of a deliberate bioterrorist release of anthrax spores, as occurred in 2001, poses practical dilemmas. Since the only licensed anthrax vaccine requires multiple spaced doses and many weeks to achieve protective antibody levels, this argues for prophylactic immunization for such groups. However, poor public perception of the safety of the currently licensed parenteral anthrax vaccine renders many members of high-risk groups reluctant to undergo pre-incident immunoprophylaxis [14]. If another spore release occurs and the risk of anthrax becomes tangible, attitudes would change and members of these groups would likely seek vaccination. The challenge then would be to confer protection rapidly. Disappointingly, heretofore in clinical trials the new parenteral PA vaccines have not diminished the lag until protective levels of TNA appear [18, 19].
In a novel approach to resolve this dilemma, we propose a “hybrid” vaccination strategy in which a well tolerated attenuated S. Typhi live vector strain administered mucosally pre-incident is used to prime the immune system and elicit strong immunologic memory such that subsequently, at the time of an incident, parenteral administration of a single dose of PA-based vaccine (BioThrax® or purified PA) will rapidly (within a few days) elicit protective levels of serum TNA. This strategy assumes that the reluctance of high risk groups to receive a parenteral anthrax vaccine for priming discourages that option and that a well tolerated oral priming vaccine will achieve much higher compliance [25, 26]. Herein we provide highly encouraging, preliminary evidence to support the feasibility of this approach.
S. Typhi strain CVD 908-htrA was chosen as the live vector because it has been well tolerated and immunogenic in Phase 2 clinical trials, both as a live oral typhoid vaccine and as a live vector [21, 27]. Monkeys were immunized i.n. rather than orally, the natural route by which live typhoid vaccines are given [21, 28], because the narrow host restriction of S. Typhi makes it difficult to infect experimental animals orally. This caveat aside, these monkey experiments provide an unequivocal proof-of-principle of the effectiveness of mucosal priming. Monkeys primed mucosally with S. Typhi expressing PA83 exhibited > 100-fold rises in serum TNA and IgG anti-PA antibodies a mere 7 days after receiving a single parenteral boost with PA-based vaccine. In contrast, no TNA response (and only negligible IgG anti-PA response) was observed 7 days after parenteral administration of PA vaccine to unprimed animals.
Additional observations attest to the robustness of mucosal priming with S. Typhi live vector expressing PA83: i) the S. Typhi live vectors functioned in two species of macaques; ii) strong rapid anamnestic responses ensued whether the parenteral boost was administered with BioThrax® or purified PA; iii) mucosally-primed rhesus macaques maintained elevated serum TNA antibody titers for multiple months, with only a slow decline, following a single parenteral boost with PA83 (Figure 3); iv) the responses in mucosally-primed animals to a parenteral boost with PA83-containing vaccine were as robust after a lag of 3 months as after one month. The novel observation that monkeys can be successfully immunized intranasally with S. Typhi, thereby overcoming that serovar's notorious host restriction when given orally, can accelerate S. Typhi live vector vaccinology. Indeed, these encouraging data pave the way for clinical trials of this strategy in humans who would receive oral priming with S. Typhi vector. Assuming that initial human clinical trials corroborate the results of the monkey studies, subsequent clinical trials would establish the effectiveness of a single oral priming dose and would examine much longer lag times (e.g., 6, 12 and 24 months) before administering the single parenteral PA boost. Human trials will also aim to detect serum TNA at earlier time points than 7 days after boosting.
Immunoelectron microscopy studies confirmed that ClyA-PA83 fusions were exported to the outer surface of CVD 908-htrA(pSEC91-83) (Fig. 2A), an important observation since foreign antigens exported to the surface of or external to Salmonella live vectors are generally more immunogenic than the same antigen expressed within the cytoplasm or periplasm [22, 29-31]. This is true for PA83 [32] and domain 4 of PA [22]. The ClyA export system appears to be particularly effective. Mice immunized orally with S. Typhimurium expressing ClyA-PA83 [32] were protected against B. anthracis aerosol spore challenge, whereas similar constructs involving fusion of PA83 to an Escherichia coli hemolysin A (HlyA) plasmid-based secretion system failed to protect [32]. The experiments in rhesus macaques reported herein shed some light on the relative effectiveness of the ClyA-PA83 export system when used in a heterologous mucosal prime-parenteral boost strategy. Rhesus macaques primed with S. Typhi expressing ClyA-PA83 fusions exhibited 2- to 5-fold higher serum TNA antibody titers at all time points following administration of a dose of parenteral purified PA (Fig. 3) than monkeys primed with unfused PA83; however, the differences were not significant.
The antibody responses that developed after parenteral boosting were long-lasting and could be rapidly increased further with a second boost with PA83 given ∼ six months after the initial parenteral boost (Fig. 3). This suggests the presence of live vector-primed memory B cells ready to increase antibody production upon subsequent recall immunizations. Similar kinetics of serum IgG and TNA responses were reported by Pittman in humans immunized with multiple parenteral doses of AVA [33].
Because of the limited number of monkeys available, we could not include a comparator group who were primed parenterally with PA-based vaccine before boosting with an additional parenteral dose. Nevertheless, the lack of serologic data from such a control group is not deemed to be a significant shortcoming, for several reasons. First, the rationale for pursuing the mucosal priming strategy is that pre-incident parenteral priming simply does not appear to be a viable option. Second, our heterologous prime-boost immunization strategy produced peak serum TNA levels in rhesus monkeys (MT = 6,755 ED50) that were very similar to those reported by Williamson et al. who immunized rhesus macaques parenterally with two doses of PA83 (50 μg/dose) 4 weeks apart [8]; these monkeys exhibited serum TNA antibody levels of approximately 5,000 ED50 6 weeks after the boost and were fully protected against aerosol challenge with the spores of B. anthracis Ames strain. Third, as seen in Figure 3, for rhesus macaque controls receiving empty S. Typhi live vector on day 0 and i.m. booster doses of PA on days 42 and 225, the serum TNA antibody MT on day 239 (14 days after boost) is similar to the day 56 TNA MT for macaques primed mucosally with the ClyA-PA S. Typhi construct and boosted i.m. with PA on day 42.
Monkeys primed with S. Typhi expressing PA83 exhibited mainly rises in IgG1 and IgG2 subclasses, which correlated with serum TNA. IgG1 binds complement and all three Fcγ cellular receptors, mediates bactericidal and opsonophagocytic activity [34] and is associated with toxin neutralization in humans. The contribution of IgG2, which does not bind cell receptors, is less clear. Williamson et al. reported rises in IgG1 and IgG2 but not IgG3 and IgG4 anthrax antibodies in rhesus macaques immunized with PA83-alum [8]. IgG1, IgG2, and low levels of IgG3 anthrax antibodies were also described in humans who received multiple doses of AVA, and in patients with inhalational anthrax from the 2001 bioterrorist spore disseminations [35]. Appreciable levels of IgG3 and IgG4 were produced only when mucosal priming with S. Typhi expressing PA83 preceded the parenteral PA83 boost (Fig. 5A and 6A), suggesting that live vector priming may modulate responses to the parenteral boost. The broader response elicited by live vector priming prior to parenteral PA administration may enhance protection.
The results of our monkey studies support the feasibility of a modified pre-exposure vaccination strategy wherein a well-tolerated S. Typhi live vector anthrax vaccine is administered to the high risk population to prime them immunologically to mount rapid, protective serum TNA responses within a few days of receiving a single dose of PA-based vaccine. Such an approach would diminish the total number of doses needed to achieve protection in priority groups (diminishing pressure on stockpiles of vaccine) and could create protected cohorts within a few days of administration of the boost.
Acknowledgments
The authors thank Mr. James Panek and Dr. Marc Gurwith of VaxGen for providing the developmental PA83 lots for immunization and Dr. Christopher K. Allen and Dr. Susan Garges for providing the BioThrax® vaccine. We also thank Dr. Ivan Tatarov and Ms. Theresa M. Alexander (Veterinary Resources, University of Maryland) for assistance in immunization experiments, and Dr. Aldo Resendiz-Albor, Dr. Seema Thayil and Ms. Mardi Reymann (Applied Immunology Section, CVD) for outstanding technical support.
Grant support: This research was supported, in part, by grants R01 AI29471 and the Mid-Atlantic Regional Center for Excellence (MARCE) for Biodefense and Emerging Infectious Research cooperative agreement grant U54 AI57168, awarded to Myron M. Levine and by R01 AI065760 to Marcela F. Pasetti.
Footnotes
Potential conflicts of interest:
Magaly Chinchilla, no conflicts.
Marcela F. Pasetti, no conflicts.
Jin Yuan Wang, no conflicts.
Licheng Zhao, no conflicts.
Ivonne Arciniega-Martinez, no conflicts.
David J. Silverman, no conflicts.
James E. Galen holds patents on the plasmid maintenance system and the ClyA antigen export system.
Myron M. Levine was a member of the Board of Directors of VaxGen, Inc. while much of this work was ongoing.
Presentations at meetings: James Galen presented interim data at the Regional Center of Excellence Annual Meetings in March, 2005 (Galveston, TX); March, 2006 (New York, NY); April, 2007 (St. Louis, MO); and April, 2008 (Chicago,IL), as well as Annual Conference on Vaccine Research meetings of the National Foundation for Infectious Diseases in May, 2006 (abstract S18) and April, 2007 (abstract S20), Baltimore, MD. Magaly Chinchilla presented interim data at the 106th General Meeting of the American Society for Microbiology; May, 2006 (abstract E-021), Orlando, FL; and the Annual Meeting of the Federation of Clinical Immunology Societies; June, 2008 (abstract Su.38), Boston, MA.
References
- 1.Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–28. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cieslak TJ, Kortepeter MG, Eitzen EM., Jr . Vaccines against agents of bioterrorism. In: Levine MM, Kaper JB, Rappuoli R, Liu MA, Good MF, editors. New Generation Vaccines. 3. New York: Marcel Dekker, Inc; 2004. pp. 1067–79. [Google Scholar]
- 3.Hanson JF, Taft SC, Weiss AA. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin Vaccine Immunol. 2006;13:208–13. doi: 10.1128/CVI.13.2.208-213.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale L, Blanset D, Lowy I, et al. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect Immun. 2006;74:5840–7. doi: 10.1128/IAI.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phipps AJ, Premanandan C, Barnewall RE, Lairmore MD. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol Mol Biol Rev. 2004;68:617–29. doi: 10.1128/MMBR.68.4.617-629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22:422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Pitt ML, Little S, Ivins BE, et al. In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol. 1999;87:304. doi: 10.1046/j.1365-2672.1999.00897.x. [DOI] [PubMed] [Google Scholar]
- 8.Williamson ED, Hodgson I, Walker NJ, et al. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect Immun. 2005;73:5978–87. doi: 10.1128/IAI.73.9.5978-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brey RN. Molecular basis for improved anthrax vaccines. Adv Drug Deliv Rev. 2005;57:1266–92. doi: 10.1016/j.addr.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Grabenstein JD. Countering anthrax: vaccines and immunoglobulins. Clin Infect Dis. 2008;46:129–36. doi: 10.1086/523578. [DOI] [PubMed] [Google Scholar]
- 11.Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL. The Anthrax Vaccine: Is it safe? Does it work? Washington, D.C.: National Academy Press; 2002. [PubMed] [Google Scholar]
- 12.Payne DC, Rose CE, Jr, Aranas A, et al. Assessment of anthrax vaccination data in the Defense Medical Surveillance System, 1998-2004. Pharmacoepidemiol Drug Saf. 2007;16:605–11. doi: 10.1002/pds.1395. [DOI] [PubMed] [Google Scholar]
- 13.Martin SW, Tierney BC, Aranas A, et al. An overview of adverse events reported by participants in CDC's anthrax vaccine and antimicrobial availability program. Pharmacoepidemiol Drug Saf. 2005;14:393–401. doi: 10.1002/pds.1085. [DOI] [PubMed] [Google Scholar]
- 14.Fowler GL, Baggs JM, Weintraub ES, Martin SW, McNeil MM, Gust DA. Factors influencing laboratory workers' decisions to accept or decline anthrax vaccine adsorbed (AVA): results of a decision-making study in CDC's anthrax vaccination program. Pharmacoepidemiol Drug Saf. 2006;15:880–8. doi: 10.1002/pds.1302. [DOI] [PubMed] [Google Scholar]
- 15.Nass M. The Anthrax Vaccine Program: an analysis of the CDC's recommendations for vaccine use. Am J Public Health. 2002;92:715–21. doi: 10.2105/ajph.92.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S.General Accounting Office. Anthrax Vaccine GAO's Survey of Guard and Reserve Pilots and Aircrew. Washington, D.C.: U.S. General Accounting Office; 2002. Report No.: GAO-02-445. [Google Scholar]
- 17.Little SF, Ivins BE, Webster WM, Norris SL, Andrews GP. Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine. Vaccine. 2007;25:2771–7. doi: 10.1016/j.vaccine.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin. 2007;3:205–11. doi: 10.4161/hv.3.5.4459. [DOI] [PubMed] [Google Scholar]
- 19.Gorse GJ, Keitel W, Keyserling H, et al. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine. 2006;24:5950–9. doi: 10.1016/j.vaccine.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, MacCallum P, Russell D. Molecular Cloning: A Laboratory Manual. Third. Cold Spring Harbor: Cold Spring Harbor laboratory Press; 2001. [Google Scholar]
- 21.Tacket CO, Sztein MB, Wasserman SS, et al. Phase 2 Clinical Trial of Attenuated Salmonella enterica Serovar Typhi Oral Live Vector Vaccine CVD 908-htrA in U.S. Volunteers. Infect Immun. 2000;68:1196–201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galen JE, Chinchilla M, Zhao L, et al. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live vector vaccine strain CVD 908-htrA. Infect Immun. 2004;72:7096–106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn CP, Dull PM, Semenova V, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–36. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Soroka SD, Taylor TH, Jr, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333:89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Crockett M, Keystone J. “I hate needles” and other factors impacting on travel vaccine uptake. J Travel Med. 2005;12 1:S41–6. doi: 10.2310/7060.2005.12056. S41-S46. [DOI] [PubMed] [Google Scholar]
- 26.Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68:341–4. [PubMed] [Google Scholar]
- 27.Tacket CO, Galen J, Sztein MB, et al. Safety and Immune Responses to Attenuated Salmonella enterica Serovar Typhi Oral Live Vector Vaccines Expressing Tetanus Toxin Fragment C. Clin Immunol. 2000;97:146–53. doi: 10.1006/clim.2000.4924. [DOI] [PubMed] [Google Scholar]
- 28.Levine MM, Taylor DN, Ferreccio C. Typhoid vaccines come of age. Pediatr Infect Dis J. 1989;8:374–81. doi: 10.1097/00006454-198906000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kang HY, Curtiss R., III Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol Med Microbiol. 2003;37:99–104. doi: 10.1016/S0928-8244(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 30.Hess J, Gentschev I, Miko D, et al. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci, USA. 1996;93:1458–63. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galen JE, Levine MM. Can a ‘flawless’ live vector vaccine strain be engineered? Trends Microbiol. 2001;9:372–6. doi: 10.1016/s0966-842x(01)02096-0. [DOI] [PubMed] [Google Scholar]
- 32.Stokes MG, Titball RW, Neeson BN, et al. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect Immun. 2007;75:1827–34. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittman PR, Norris SL, Barrera Oro JG, Bedwell D, Cannon TL, McKee KT., Jr Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine. 2006;24:3654–60. doi: 10.1016/j.vaccine.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 34.Calvas P, Apoil P, Fortenfant F, et al. Characterization of the three immunoglobulin G subclasses of macaques. Scand J Immunol. 1999;49:595–610. doi: 10.1046/j.1365-3083.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 35.Semenova VA, Schmidt DS, Taylor TH, Jr, et al. Analysis of anti-protective antigen IgG subclass distribution in recipients of anthrax vaccine adsorbed (AVA) and patients with cutaneous and inhalation anthrax. Vaccine. 2007;25:1780–8. doi: 10.1016/j.vaccine.2006.11.028. [DOI] [PubMed] [Google Scholar]