Abstract
Background
Estrogens may induce the proliferation of neoplastic cells by activating neo-angiogenesis.
Aim
To evaluate the effect of estrogens on the expression of vascular endothelial growth factor (VEGF) and related receptors (VEGF-R) in human cholangiocarcinoma and the role played by VEGF in mediating the proliferative effects of estrogens.
Methods
Seven biopsies of intra-hepatic cholangiocarcinoma and the HuH-28 cell lines were investigated. Cell proliferation was measured by both PCNA Western blot and MTS proliferation assay.
Results
By immunohistochemistry, biopsies of human cholangiocarcinoma stained positively for VEGF-A and VEGF-C and related receptors. HuH-28 cells expressed VEGF-A, -C, and VEGFR-1, -2, -3 and, their protein level was enhanced by 17β-estradiol in association with the stimulation of cell proliferation. 17β-Estradiol-stimulated proliferationof HuH-28 cells was blocked by 70% by VEGF-trap, a receptor-based VEGF inhibitor. 17β-Estradiol induced the secretion of VEGF in the supernatant of HuH-28 cells. The stimulatory effect of 17β-estradiol on the protein expression of VEGF-A, VEGF-C and VEGFR-1, -2, -3 was blocked by antagonists of ER (Ici182,780) or insulin-like growth factor 1-receptor (αIR3).
Conclusions
With the limitations of experiments performed in a cell line, our study indicates that VEGF plays a major role in mediating the proliferative effects of estrogens on human cholangiocarcinoma.
Keywords: Cholangiocarcinoma, Estrogens, IGF1, Proliferation, VEGF
1. Introduction
Estrogens induce the proliferation of normal and neoplastic cells expressing estrogen receptors (ER) by both genomic and non-genomic pathways where multiple signalling transduction pathways are involved [1–3]. In different types of cancer, estrogens synergize the effects of growth factors by acting at both receptor and post-receptor levels and, doing so, favour the growth, proliferation and spreading of tumour mass [4–5]. Neo-angiogenesis is a fundamental step in cancer growth which involves multiple mechanisms and complex loops of growth factors and cytokines [6]. Recently, a number of different observations indicate that estrogens play a major role in the induction of neo-angiogenesis in estrogen-sensitive cancers [7]. The vascular endothelial growth factor (VEGF), which is a main player in the mechanisms underlying neo-angiogenesis, has been considered to be responsible for the angiogenic action of estrogens in both normal and neoplastic tissues [8–9].
Cholangiocarcinoma is a malignant tumour arising from the epithelial cells (cholangiocytes) lining the biliary tree, characterised by a poor prognosis and scarce response to current therapies [10]. The incidence and mortality for cholangiocarcinoma are increasing worldwide and this is especially true for the intra-hepatic form of this cancer [11]. We have recently shown that intra-hepatic cholangiocarcinoma express ER-αand -β, insulin-like growth factor 1 (IGF1) and IGF1-R (receptor) and that estrogens cooperate with IGF1 and its receptor in modulating the growth of cholangiocarcinoma [12]. Recent studies suggest that, also in cholangiocarcinoma, neo-angiogenesis plays a key role in the growth and spreading of tumour mass [13–15].
The aim of our study was to evaluate the effect of estrogens on the expression of VEGF and related receptors (VEGFR) in human cholangiocarcinoma and the role played by VEGF in mediating the proliferative effects of estrogens in this cancer.
2. Materials and methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. Human recombinant VEGFA was purchased from Sigma (St. Louis, MO). The IGF-IR blocking antibody, αIR3, was obtained from Oncogene-DBA (DBA Italia, srl, Segrate, Milan, Italy). VEGF-TRAPR1R2 was obtained from Regeneron Pharmaceuticals Inc. (Tarrytown, NY).
2.1. Immunohistochemistry (IHC) on human liver biopsies
We studied seven patients (3F/4M, age 65–75 years) with intra-hepatic cholangiocarcinoma presenting as a single mass lesion within the liver. US-guided liver biopsies were investigated. As normal controls, we investigated 5 liver biopsies with a normal histology from patients (3F/2M, age 67–74 years) submitted to laparotomy. Fragments of intra-hepatic cholangiocarcinoma and normal liverwere processed for common liver histomorphology (haematoxylin–eosin and Masson’s stains) and for immunohistochemistry (IHC). For IHC, sections were mounted on glass slides coated with 0.1% poly-L-lysine. After deparaffination, endogenous peroxidase activity was blocked by a 30-min incubation in methanolic hydrogen peroxide (2.5%). Endogenous biotin was blocked by Biotin Blocking System (Dako, code X0590, Dako, Copenhagen, Denmark). Sections were hydrated in graded alcohol, rinsed in PBS (pH 7.4), and were incubated overnight at 4 °C with antibodies for VEGF-A (sc-152, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), VEGF-C (sc-1881, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), VEGF-R1 (sc-9029, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, VEGF-R2 (sc-6251, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and VEGF-R3 (sc-321, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Samples were then rinsed with PBS for 5 min, incubated for 10 min at room temperature with secondary link biotinylated antibody (biotinylated anti-rabbit, anti-mouse, anti-goat, ABC system; DAKO LSAB1, Copenhagen, Denmark), incubated with streptavidin–peroxidase for 10 min, and finally were developed with DAB. Negative controls were performed by applying specific nonimmune serum. Light microscopy and IHC observation were taken by Olympus BX-5 1 Light Microscopy (Tokyo, Japan) with a Videocam (Spot Insight, Diagnostic Instrument Inc. Sterling Heights, MI) and processed with an Image Analysis System (IAS, Delta Sistemi, Roma, Italy). LM and IHC observations were independently performed by three pathologists in a blind fashion.
2.2. Experiments on HuH-28 cell line
The study was performed on HuH-28 cell lines derived from intra-hepatic cholangiocarcinoma [16] and acquired from Cancer Cell Repository, Tohoku University, Japan. Media and serum for cell culture were obtained from Gibco BRL (Gaithersburg, MD). HuH-28 cells were maintained in CRML 1066 medium containing 10% fetal bovine serum. To evaluate cell proliferation, HuH-28 cells, cultured in the appropriate medium (MEM Eagle) containing 10% fetal bovine serum, were deprived of serum for 24 h. Then, cells were maintained in serum-deprived conditions for an additional 6 h (controls = C) or exposed to 17β-estradiol, IGF1 and/or receptor antagonists for additional 6 h. Specifically, cell medium was replaced with fresh serum-free where the tested agent was added. 17β-Estradiol and Ici182,780 were dissolved in DMSO while IGF1 and αIR3 were dissolved in saline as a stock solution which was then added (dilution 1:100,000) into serum-free culture medium. Viability of HuH-28 cells (trypan blue exclusion) was unaffected by 6 h incubation with Ici182,780 (1 μg/ml, n = 10) or αIR3 (1 μM = 10). In these experimental conditions, proliferation was assessed by a commercially available colorimetric cell proliferation assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, MTS Kit, Promega, Madison, WI), by following the manufacturer’s instructions. Proliferation index was calculated as the ratio (multiplied × 100) between cell numbers in un-stimulated and stimulated cultures as described [17]. In selected experiments, proliferation was also evaluated by PCNA protein expression (Western blot).
2.3. Western blot analysis
For Western blot analysis, cells were solubilised in lysis buffer containing 15 mM Tris–HCl (pH 7.4), 5 mM EDTA, 100 mM NaCl, Igepal 1%, 2 mM PMSF (phenyl methyl sulfonyl fluoride), 2 mM benzamidine and 1% aprotinin on ice for 30 min. After the centrifugation at 10,000 × g for 20 s at 4 °C, the supernatant was recovered and protein concentration was determined with the Bio-Rad Protein Assay-Dye Reagent (Bio-Rad Laboratories GmbH). Cell extracts (10 μg) were diluted in 6× LSB (Laemly sample buffer) containing 0.3 M 2-mercaptoethanol and resolved by 10% SDS-polyacrylamide gel electrophoresis. Western blotting was performed as described [18,19] by using the following primary antibodies: (1) anti-PCNA, specific mouse monoclonal antibody (1:350 dilution; Santa Cruz); (2) anti-VEGF-A (1:200 dilution; Santa Cruz); (3) anti-VEGF-C; rabbit polyclonal antibody (1:2000 dilution; Zymed); (4) anti-VEGFR-1 mouse monoclonal antibody (1:100 dilution; Sigma); (5) anti-VEGFR-2 rabbit polyclonal antibody (1:1250 dilution: Upstate Biotechnology); (6) anti-VEGFR-3 rabbit polyclonal antibody (1:200 dilution; Santa Cruz); (7) anti-β actin, mouse monoclonal antibody (1:1500 dilution; Sigma). As secondary antibodies, anti-mouse IgG peroxidase conjugated (Sigma; 1:2000) or anti-rabbit IgG peroxidase conjugated (1:10,000; Sigma) or anti-goat IgG peroxidase conjugated (1:10,000; Sigma) were used. The intensity of the bands was determined by scanning video densitometry (Ultra Violet Products, Cambridge, England) and expressed as arbitrary densitometric units normalised to β-actin expression (i.e. tested protein/β-actin × 100).
2.4. Measurement of VEGF secretion from HuH-28 cells
The amount of VEGF in the supernant of HuH-28 cells was measured by enzyme-linked immunoadsorbent assay kit according to the manufacturer instructions (Pierce Biotech-nology Inc., Rockford, IL). HuH-28 cells maintained in the appropriate culture medium added with 10% fetal bovine serum, were starved without serum for 24 h and then left without serum for 6 h (controls = C) or exposed for additional 6 h to 17β-estradiol (10 nM) in the presence or absence of the ER antagonist, Ici182,780, or IGF1-R blocking antibody (αIR3). Subsequently, VEGF concentration was measured in the supernatant.
2.5. Statistical analysis
Data are presented as arithmetic mean ± standard errors. Statistical analysis was conducted by using one-way analysis of the variance with pair wise comparison by the Fisher’s protected-least-significant-difference test. In all cases, p < 0.05 was considered significant.
3. Results
3.1. Immunohistochemistry (IHC) on human liver biopsies
Biopsies of intra-hepatic cholangiocarcinoma revealed an intense positivity for VEGF-A (Fig. 1B) and VEGF-C (Fig. 1C) and for VEGF-R1 (Fig. 2D), VEGF-R2 (Fig. 2E) and VEGF-R3 (Fig. 2F) which involved almost all neoplastic cells and this was homogeneously found in 7/7 patients examined. Cholangiocytes lining intra-hepatic bile ducts of the normal liver (n = 5) were negative for VEGF-A and VEGF-C (Fig. 1A) but showed positivity for VEGF-R1 (Fig. 2A), VEGF-R2 (Fig. 2B) and VEGF-R3 (Fig. 2C).
Fig. 1.
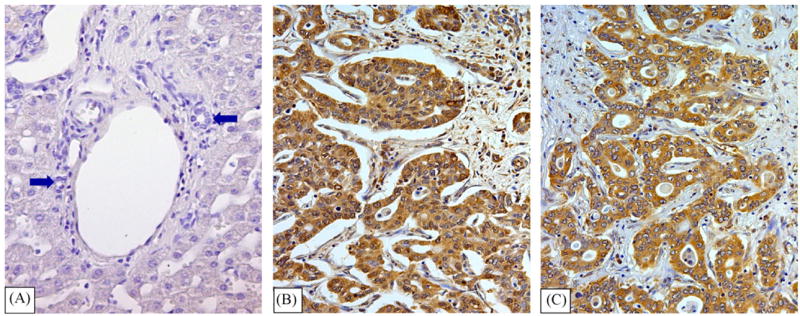
Immunohistochemistry for VEGFA and VEGFC in intra-hepatic cholangiocarcinoma. Cholangiocytes of intra-hepatic bile ducts (arrows) of the normal liver were negative for both VEGF-A (not shown) and VEGF-C (A). Intra-hepatic cholangiocarcinoma shows a strong positivity for VEGF-A (B) and VEGF-C (C) (LM original magnification 20×).
Fig. 2.
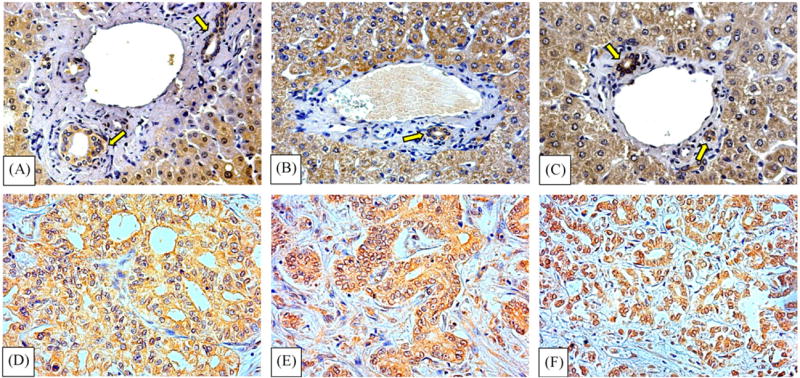
Immunohistochemistry for VEGF receptors in normal human liver and intra-hepatic cholangiocarcinoma. Top: biopsies of normal liver showing positivity for VEGF-R1 (A), VEGF-R2 (B) and VEGF-R3 (C) in cholangiocytes lining intra-hepatic bile ducts (arrows). Hepatocytes are also positive for VEGF receptors. Bottom: biopsies of intra-hepatic cholangiocarcinoma showing positivity for VEGF-R1 (D), and VEGF-R2 (E) and VEGF-R3 (F) (LM original magnification × 20).
HuH-28 cells expressed VEGF-A, VEGF-C, VEGFR-1, VEGFR-2 and VEGFR-3 as evaluated by Western blot (Figs. 3 and 4). HuH-28 cells were starved without serum for 24 h and then exposed for 6 h to 17β-estradiol in the presence or absence of receptor antagonists. 17β-Estradiol (10 nM) induced in HuH-28 cells a significant (p < 0.01) increase in the protein level of VEGF-A (+34 ± 1%, Fig. 3A), VEGF-C (+57 ± 4%, Fig. 3B), VEGFR-1 (+70 ± 5%, Fig. 4A), VEGFR-2 (+517 ± 12%, Fig. 4B) and VEGFR-3 (+102 ± 10%, Fig. 4C). The effect of 17beta;-estradiol on VEGF-A, VEGF-C and their receptors was almost completely inhibited by the specific ER antagonist, Ici182,780 or the IGF1-R blocking antibody, αIR3 (Figs. 3 and 4). In the absence of 17β-estradiol, neither Ici182,780 nor αIR3 showed a significant effect on the protein expression of VEGF-A, VEGF-C, VEGFR-1, VEGFR-2 and VEGFR-3 (n = 3; not shown).
Fig. 3.
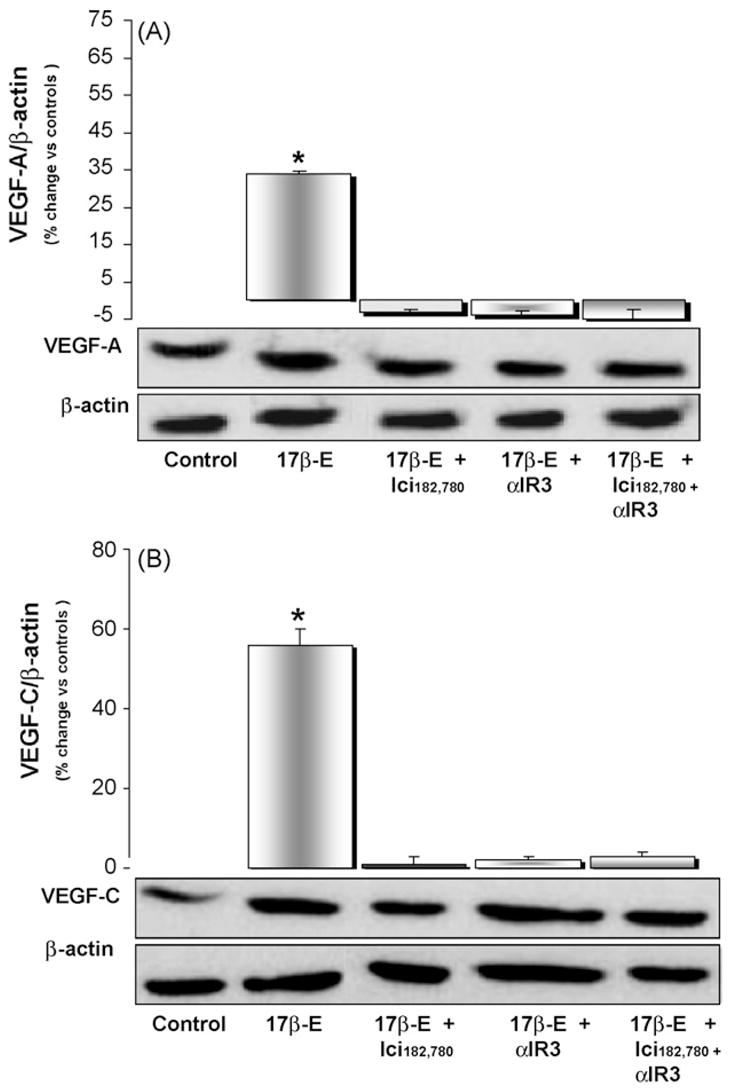
Western blot analysis of VEGF-A (A) and VEGF-C (B) in HuH-28 cell line. HuH-28 cells maintained in the appropriate culture medium (see Section 2) added with 10% fetal bovine serum were starved without serum for 24 h, and then left without serum for 6 h (controls = C) or exposed for 6 h to 17β-estradiol (17β-E, 10 nM) in the presence or absence of ER antagonist, Ici182,780 (1 μM) or IGF1-R blocking antibody (αIR3, 1 μg/ml). After the treatment, cells were solubilised in lysis buffer and than the cell extract was resolved by 10% SDS-polyacrylamide gel electrophoresis. The protein level was determined by evaluating the intensity of the bands by scanning video densitometry, normalised to β-actin expression (i.e. tested protein/β-actin) and expressed as percent change with respect to controls. 17β-Estradiol enhanced the protein level of VEGF-A (A) and VEGF-C (B) and its effect was blocked by the ER antagonist, Ici182,780, or IGF1-R blocking antibody (αIR3). *p < 0.01 vs. controls, 17β-E + Ici182,780, 17β-E + αIR3 or 17β-E + Ici182,780 + αIR3. n = 12 independent experiments.
Fig. 4.
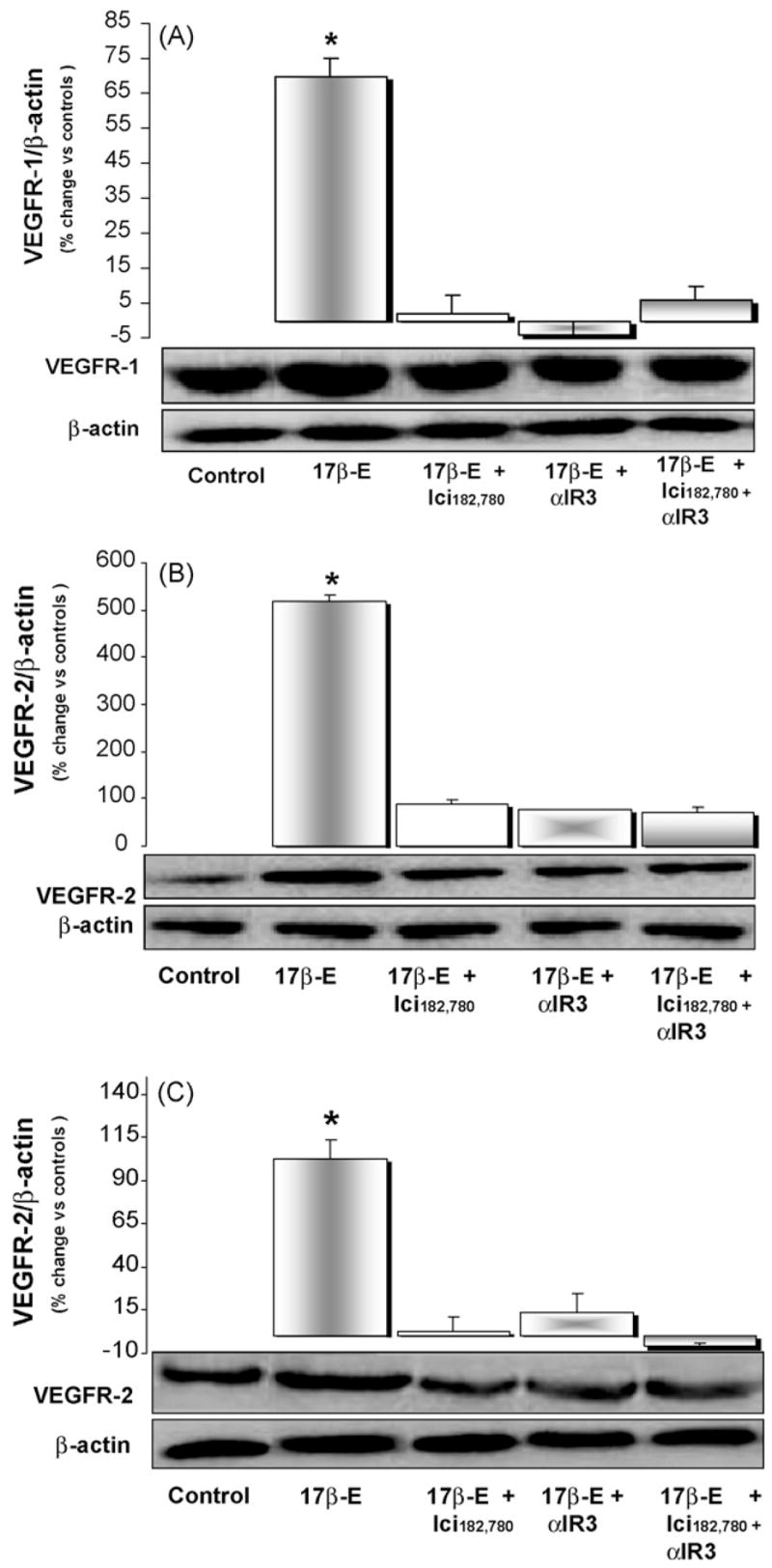
Western blot analysis of VEGFR-1 (A), VEGFR-2 (B) and VEGFR-3 (C) in HuH-28 cell line. HuH-28 cells maintained in the appropriate culture medium (see Section 2) and added with 10% fetal bovine serum were starved without serum for 24 h and then left without serum for 6 h (controls = C) or exposed for 6 h to 17β-estradiol (17β-E, 10 nM) in the presence or absence of ER antagonist, Ici182,780 (1 μM) or IGF1-R blocking antibody (αIR3, 1 μ/ml). 17β-Estradiol enhanced the protein level of VEGFR-1 (A), VEGFR-2 (B) and VEGFR-3 (C) and its effect was blocked by the ER antagonist, Ici182,780, or IGF1-R blocking antibody (αIR3). *p < 0.01 vs. controls, 17β-E + Ici182,780, 17β-E + αIR3 or 17β-E + Ici182,780 + αIR3. n = 9 independent experiments.
When HuH-28 cells, starved without serum for 24 h, were exposed to rVEGF-A (recombinant VEGF-A, 10 ng/ml) for 6 h, proliferation was enhanced although into a lower (p < 0.05) extent than after 17β-estradiol and these were evaluated by both PCNA Western blot (Fig. 5A) and MTS proliferation assay (Fig. 5B). The effect of rVEGF-A on proliferation was inhibited by VEGF-TRAPR1R2 (0.1 μg/ml), a receptor-based VEGF inhibitor, which also was inhibited by approx. 70% the proliferative effect of 17β-estradiol as evaluated by both PCNA Western blot (Fig. 5A) and MTS proliferation assay (Fig. 5B).
Fig. 5.
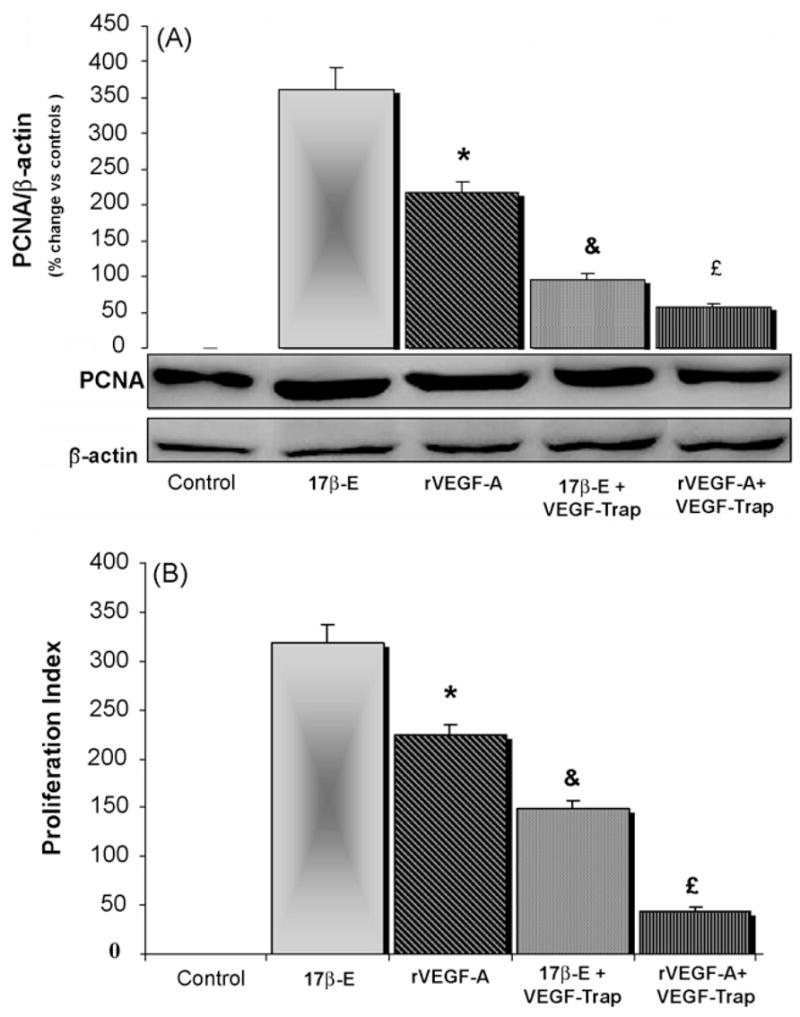
Effect of rVEGF-A on the proliferation of HuH-28 cells. HuH-28 cells maintained in the appropriate culture medium (see Section 2) added with 10% fetal bovine serum were starved without serum for 24 h and then left without serum for 6 h (controls = C) or exposed for 6 h to rVEGF-A (recombinant VEGF-A, 10 ng/ml) or 17β-estradiol (17β-E, 10 nM) inthe presence or absence of VEGF-TRAPR1R2 (0.1 3g/ml), a receptor-based VEGF inhibitor. Cell proliferation was evaluated by Western blot for PCNA (A) or MTS proliferation assay (B). A = PCNA Western blot. After the treatment, cells were solubilised in lysis buffer and than the cell extract was resolved by 10% SDS-polyacrylamide gel electrophoresis. The protein level was determined by evaluating the intensity of the bands by scanning video densitometry, normalised to β-actin expression (i.e. tested protein/β-actin) and expressed as percent change with respect to controls. rVEGF-A enhanced PCNA protein expression into a lower extent than 173-estradiol. VEGF-TRAPR1R2 inhibited the proliferative effect of rVEGF-A and 17β-estradiol. n = 5 independent experiments. *p < 0.05 vs.17β-estradiol; &p < 0.05 vs. 17β-E alone; £p < 0.5 vs. rVEGF-A alone. (B) For proliferation assay HuH-28 cells starved without serum for 24 h were left without serum for 6 h (controls = C) or exposed for 6 h to serum, rVEGF-A (recombinant VEGF-A, 10 ng/ml) or 17β-estradiol (10 nM) in the presence or absence of VEGF-TRAPR1R2. Proliferation index was calculated as the ratio (multiplied × 100) between cell number (MTS assay) in stimulated and unstimulated (Control) cultures. rVEGF-A enhanced proliferation index into a lower extent than 173-estradiol. VEGF-TRAPR1R2 inhibited the proliferative effect of rVEGF-A and 17β-estradiol. n = 4 independent experiments for each protocol. *p < 0.05 vs. 17β-estradiol; &p < 0.05 vs. 17β-E alone; £p < 0.05 vs. rVEGF-A alone.
We next measured (ELISA) VEGF-A concentration in the supernatant of HuH-28 cells exposed for 6 h to 17β-estradiol in the presence or absence of receptor antagonists. After 6 h exposure to 17β-estradiol, VEGF-A in the supernatant increased from 0 to 180 × 15 pg/ml (n = 5) and this was partially inhibited by the specific ER antagonist, Ici182,780 (VEGF = 95 × 7 pg/ml, n = 5) or the blocking antibody against IGF1-R, αIR3 (VEGF = 120 × 11 pg/ml, n = 5) and completely blocked only when the two antagonists were added together (VEGF = 4 × 3 pg/ml, n = 5).
4. Discussion
The main results of our study indicate that: (1) biopsies of human intra-hepatic cholangiocarcinoma were positive, at the IHC study, for both VEGF-A and VEGF-C and related receptors; (2) the HuH-28 cell line, derived from intra-hepatic cholangiocarcinoma [16] express VEGF-A, VEGF-C, VEGFR-1 VEGFR-2 and VEGFR-3 and their protein level was markedly enhanced by 17β-estradiol in association with the stimulation of cell proliferation; (3) the 17β-estradiol induced the increase of VEGF and VEGFR protein level was inhibited by ER or IGF1-R specific antagonists; (4) rVEGF-A stimulated the proliferation of HuH-28 cells starved without serum; (5) 17β-estradiol induced the secretion of VEGF in the supernatant of HuH-28 cells which was inhibited by ER or IGF1-R antagonists; (6) approximately 70% of the proliferative effect of 17β-estradiol was inhibited by VEGF-trap a receptor-based VEGF inhibitor. Altogether, our findings indicate that VEGF and related receptors play a major role in mediating the proliferative effect of estrogens on human cholangiocarcinoma.
The study was performed in a human cell line (HuH-28) derived from intra-hepatic cholangiocarcinoma [16] which expressed both ER and IGF1-R [12]. This cell line markedly proliferated when exposed to 17β-estradiol or IGF1, which also inhibited apoptosis with additive effects [12]. Estrogens exerted their proliferative effects by acting on both ER and IGF1-R with a sort of functional coupling of the two receptors, specifically ER-α and IGF1-R, and this was previously shown in different neoplastic and non-neoplastic cell types [18–19]. In the present study we evaluated the involvement of VEGF and related receptors in the estrogen-indu0ced proliferation of HuH-28 cells. This topic is of current interest since VEGF and neo-angiogenesis have been demonstrated to play a major role in the growth and spreading of different cancers including cholangiocarcinoma [6,8,13,14,20]. Most importantly, anti-VEGF and anti-angiogenic strategies are currently under clinical evaluation to delay or stop cancer progression [6].
We firstly showed that HuH-28 cells express the three different VEGF receptors other than proteins for VEGF-A and VEGF-C. In previous studies, VEGF but not VEGF receptors were found in three different cell lines derived from extra-hepatic cholangiocarcinoma [14,21]. In human studies, IHC positivity for VEGF was demonstrated in surgical samples of intra-hepatic [13] and extra-hepatic cholangiocarcinoma [14,21]. Specifically, more than 70% of surgical samples from intra-hepatic cholangiocarcinoma showed immunohistochemical positivity for VEGF-C, which represented an independent and important prognostic factor suspected to play an important role in the lymph node metastasis [13]. In our study 7/7 biopsies of patients affected by intra-hepatic cholangiocarcinoma showed positivity for VEGF-A and VEGF-C and related receptors with a staining involving almost all neoplastic cells. In normal liver biopsies, used as controls, cholangiocytes were negative for VEGF-A and VEGF-C but positive for related receptors and, therefore, positivity for VEGF-A and VEGF-C (but not VEGF-receptors) characterises neoplastic with respect to normal cholangiocytes.
17β-Estradiol induced a significant increase in the protein expression of all the three VEGF receptors with a prominent effect on VEGFR-2 (>5-fold increase) which is a preferred receptor for both VEGF-A and VEGF-C other than being implicated in neoplastic growth [8,9,13,14,20]. The effect of 17β-estradiol was mediated by either ER or IGF1-R since it was inhibited by two specific receptor antagonists (Ici182,780 and αIR3 ). Since the specificity of αIR3 for IGF1 [22] and of Ici182,780 for ER [23] is absolute our findings indicate a sort of interplay between ER and IGF1-R in mediating the effect of 17β-estradiol on the induction of VEGFs and related receptors. This is consistent with the proliferative effect of 17β-estradiol on HuH-28 cells that also require intact ER and IGF1-R [12]. The VEGF induced by 17β-estradiol in HuH-28 cells is secreted in the supernatant allowing the supposition that, through autocrine mechanisms, this growth factor may have a role in the proliferative effect of 17β-estradiol. On the other hand, VEGF per se plays a major role in modulating the proliferation of normal cholangiocytes as recently demonstrated in experimental studies [24,25].
To directly evaluate the role played by VEGF in the proliferative effect of 17β-estradiol, we investigated whether VEGF-TRAPR1R2, a receptor-based VEGF inhibitor [26], was able to block the effect of 17β-estradiol. We found that at least 70% of the proliferative effect was inhibited by VEGF-TRAPR1R2 and this was demonstrated by both PCNA and proliferation assay. Therefore, our study with the limitations of experiments performed in a cell line suggests that estrogens induce the synthesis and secretion of VEGF which markedly contribute in the activation of proliferative machinery. It is conceivable that the VEGF secreted by proliferating neoplastic cells other than sustaining proliferation may also play an active role in tumour neo-angiogenesis. In fact, several findings obtained in normal and neoplastic tissues indicate that the induction of VEGF is involved as mechanism of the pro-angiogenic action of estradiol [8,9,27,28]. In conclusion, we demonstrated that VEGF plays a major role in mediating the proliferative effects of estrogens on human intra-hepatic cholangiocarcinoma and that strategies based on the antagonism of ER and/or VEGF could help in delaying the progression of this cancer.
Practice points
Biopsies of human intra-hepatic cholangiocarcinoma express, at the immunohistochemical analysis, VEGF and VEGF-receptors while normal intra-hepatic bile ducts failed to express VEGF.
In HuH-28 cell lines, derived from human intra-hepatic cholangiocarcinoma, VEGF and related receptors were induced by estrogens in association with the enhancement of cell proliferation.
The estrogen effects on HuH-28 cell lines was inhibited by approx. 70% by VEGF-trap, a receptor-based VEGF inhibitor, suggesting that the VEGF system plays a major role in mediating the proliferative effects of estrogens on human cholangiocarcinoma.
Research agenda
Comparative evaluation of tumoural neovasculature, expression of estrogen receptors and VEGF should be performed in surgically resected intra-hepatic cholangiocarcinoma to definitively demonstrate the role of estrogens and angiogenesis in this cancer.
The prognostic impact of VEGF, VEGF-receptors and estrogen receptors on intra-hepatic cholangiocarcinoma survival and recurrence after surgical resection is demanding.
The combination of estrogen receptor antagonists and VEGF antagonists should be tested in pilot studies on the treatment of intra-hepatic cholangiocarcinoma non-eligible for surgical resection.
Acknowledgments
A. Mancino and M.G. Mancino equally contributed to this manuscript. We thank F. Lucarelli and Cinzia Tesse for their technical assistance in immunoblotting and Tracie Dorn-busch for English Editing.
Grant Support: This work was supported by a grant award to Dr. Alpini from Scott & White Hospital and The Texas A & M University System, by a VA Merit Award, a VA Research Scholar Award and an NIH grant DK 58411 to Dr. Alpini. D. Alvaro is supported by MIUR grants: PRIN 2005: 2005067975 002. E. Gaudio is supported by MIUR grants (PRIN 2005: 2005067975 001) and Faculty funds.
List of abbreviations
- ER
estrogen receptors
- 17β-E
17β-estradiol
- IGF1
insulin-like growth factor 1
- IGF1-R
IGF1-receptor
- PCNA
proliferating cellular nuclear antigen
- VEGF
vascular endothelial growth factor
- VEGF-R
VEGF-receptor
Footnotes
Conflict of interest statement
None declared.
References
- 1.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Hata H, Hamano M, Watanabe J, Kuramoto H. Role of estrogen and estrogen-related growth factor in the mechanism of hormone dependency of endometrial carcinoma cells. Oncology. 1998;55(Suppl 1):35–44. doi: 10.1159/000055257. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K, Inoue S. Estrogen receptors and their downstream targets in cancer. Arch Histol Cytol. 2004;67:435–42. doi: 10.1679/aohc.67.435. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Sex steroid hormones act as growth factors. J Steroid Biochem Mol Biol. 2002;83:31–5. doi: 10.1016/s0960-0760(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 5.Wimalasena J, Meehan D, Dostal R, Foster JS, Cameron M, Smith M. Growth factors interact with estradiol and gonadotropins in the regulation of ovarian cancer cell growth and growth factor receptors. Oncol Res. 1993;5:325–37. [PubMed] [Google Scholar]
- 6.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 7.Hyder SM. The role of steroid hormones on the regulation of vascular endothelial growth factor. Am J Pathol. 2002;161:345–6. doi: 10.1016/s0002-9440(10)64186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Harkonen PL. Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells. Mol Cell Endocrinol. 1999;149:29–40. doi: 10.1016/s0303-7207(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura J, Lu Q, Aberdeen G, Albrecht E, Brodie A. The effect of estrogen on aromatase and vascular endothelial growth factor messenger ribonucleic acid in the normal nonhuman primate mammary gland. J Clin Endocrinol Metab. 1999;84:1432–7. doi: 10.1210/jcem.84.4.5641. [DOI] [PubMed] [Google Scholar]
- 10.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 11.Blendis L, Halpern Z. An increasing incidence of cholangiocarcinoma, why? Gastroenterology. 2004;127:1008–9. doi: 10.1053/j.gastro.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser S, Alpini G, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–88. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol. 2006;29:138–42. doi: 10.1097/01.coc.0000204402.29830.08. [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, Kondo M, et al. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525–32. [PubMed] [Google Scholar]
- 15.Benckert C, Jonas S, Cramer T, Von Marschall Z, Schafer G, Peters M, et al. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63:1083–92. [PubMed] [Google Scholar]
- 16.Kusaka Y, Tokiwa T, Sato J. Establishment and characterization of a cell line from a human cholangiocellular carcinoma. Res Exp Med (Berl) 1988;188:367–75. doi: 10.1007/BF01851205. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–33. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 18.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 19.Adesanya OO, Zhou J, Samathanam C, Powell-Braxton L, Bondy CA. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proc Natl Acad Sci USA. 1999;96:3287–91. doi: 10.1073/pnas.96.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara S, Yano H, Higaki K, Takayama A, Akiba J, Shiota K, et al. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res. 2001;20:97–113. doi: 10.1016/s1386-6346(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 22.Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;2:6589–97. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- 23.Diel P, Smolnikar K, Michna H. The pure antiestrogen ICI 182780 is more effective in the induction of apoptosis and down regulation of BCL-2 than tamoxifen in MCF-7 cells. Breast Cancer Res Treat. 1999;58:87–97. doi: 10.1023/a:1006338123126. [DOI] [PubMed] [Google Scholar]
- 24.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–82. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–17. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 27.Koos RD, Kazi AA, Roberson MS, Jones JM. New insight into the transcriptional regulation of vascular endothelial growth factor expression in the endometrium by estrogen and relaxin. Ann NY Acad Sci. 2005;1041:233–47. doi: 10.1196/annals.1282.037. [DOI] [PubMed] [Google Scholar]
- 28.Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, et al. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene. 2004;23:1052–63. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]


