Abstract
Although it is now generally accepted that the thalamus is more than a simple relay of sensory signals to the cortex, we are just beginning to gain an understanding of how corticothalamic feedback influences sensory processing. Results from an increasing number of studies across sensory systems and different species reveal effects of feedback both on the receptive fields of thalamic neurons and on the transmission of sensory information between the thalamus and cortex. Importantly, these studies demonstrate that the corticothalamic projection cannot be viewed in isolation, but must be considered as an integral part of a thalamo-cortico-thalamic circuit which intimately interconnects the thalamus and cortex for sensory processing.
Introduction
Neurons in the sensory thalamus play an essential role in relaying information about the external environment to the cerebral cortex. At first glance, this role may seem relatively straightforward with the thalamus serving as a simple gate for information flow. A number of recent studies, however, reveal a much more complex picture of thalamic function. Notably, thalamic neurons receive robust input from corticothalamic feedback neurons thereby allowing the cortex to communicate continuously with the thalamus during sensory processing. As a consequence, the cortex has the opportunity to dynamically influence thalamic processing and ultimately shape the nature of its own input.
Corticothalamic feedback neurons reside in cortical layer 6 and give rise to axons that terminate both in the thalamus and in the layers of the cortex that receive thalamic input [Figure 1; reviewed in 1,2]. As a consequence, corticothalamic neurons are in a strategic position to influence sensory processing. In addition to providing monosynaptic input to relay neurons in the thalamus, the axons of corticothalamic neurons also provide input to two sources of polysynaptic inhibition onto relay neurons—local inhibitory neurons and neurons in the RTN [Figure 1; reviewed in 1,2]. Thus the influence of corticothalamic activity on thalamic function is expected to be complex, with both excitatory and inhibitory components. In vitro studies indicate that corticothalamic synapses have a low probability of release [3] and can experience facilitation and augmentation with high frequency stimulation [4,5]. Whether or not these effects exist in vivo and/or in the absence of anesthesia is unclear [6]. Nevertheless, there are clear differences in the properties of corticothalamic inputs onto relay neurons and RTN neurons, as EPSPs from corticothalamic synapses onto RTN neurons are sharper and display less facilitation leading to greater temporal precision [7]. Finally, corticothalamic activation of RTN neurons can evoke a poly-synaptic spreading of IPSPs within the RTN [8], which may further increase the temporal precision of RTN inhibition onto thalamic neurons.
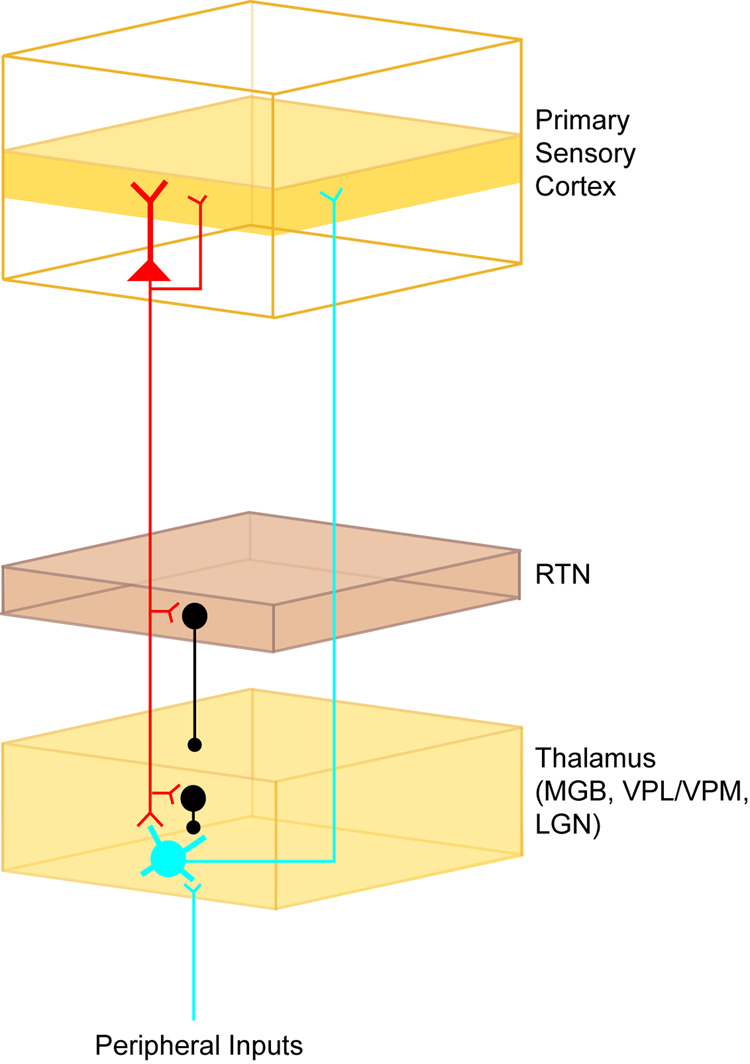
Figure 1.
Schematic diagram of thalamo-cortico-thalamic circuitry. Thalamocortical neurons (blue) receive peripheral inputs and project axons to layer 4 of primary sensory cortex. Corticothalamic neurons (red) receive local input from thalamocortical recipient layer 4 and provide output to layer 4 and to the thalamus. Corticothalamic projections also supply di-synaptic inhibition to thalamic neurons via RTN inhibitory neurons and local thalamic interneurons (black).
Because of the similarities in both the anatomy and cellular physiology of corticothalamic neurons, it is tempting to suggest that corticothalamic neurons and their projections perform similar functions across species and sensory modalities. Indeed, recent and exciting results from studies examining feedback in the visual, auditory and somatosensory systems support the notion of commonalities in corticothalamic function. Two prominent roles for corticothalamic feedback are (1) feedback serves to sharpen the receptive fields and/or shift the tuning of thalamic neurons, and (2) feedback serves to enhance the transmission of sensory signals from the periphery to cortex. In the sections below, we review these new developments in our understanding of corticothalamic function.
Corticothalamic feedback and thalamic receptive fields
Several studies examining sensory processing in the thalamic nuclei serving vision, audition, and somatosensation (LGN, MGB and VPM/VPL, respectively) identify a role for corticothalamic feedback in shaping the receptive fields of thalamic neurons. Because neurons in these nuclei have receptive fields that closely resemble those of their afferent input, the influence of feedback is arguably subtle. Still, feedback appears to enhance responses among selected ensembles of thalamic neurons that are tuned to the same properties as the active corticothalamic neurons.
A clear demonstration of this effect comes from research by Suga and colleagues on the auditory system of the bat. These studies led to the term ‘egocentric selection’ to describe a feedback-dependent shift in the tuning of MGB neurons toward the preferred frequency of active feedback neurons [9]. More recent work reveals similar egocentric effects of feedback in both the MGB and inferior colliculus of mice [10,11•].
In the somatosensory system of rodents, recent work suggests that corticothalamic projections evoke similar egocentric enhancement of thalamic activity. For instance, stimulation of barrels in primary somatosensory cortex increases the direction selectivity of VPM barreloid neurons tuned to the same direction and changes the selectivity of barreloid neurons tuned to different directions [12••]. Furthermore, stimulation of mismatched barrels suppresses VPM responses [12••,13]. These findings have been described by Simons and colleagues as evidence for a topographic organization for corticothalamic feedback [13], and while ‘egocentric’ and ‘topographic’ cannot be used interchangeably, the underlying mechanisms may be similar.
The corticothalamic projection in the visual system likely functions in a similarly topographic, if not also egocentric, manner [reviewed in 14]. For instance, corticothalamic feedback has been shown to sharpen the border of the classical receptive field [15–18, but see 19,20]. Similar effects have also been reported for the somatosensory thalamus [21]. Corticothalamic feedback has also been shown to selectively enhance the activity of groups of LGN neurons aligned along the orientation axis of their inputs from visual cortex [22•,23], analogous to the egocentric modification described above.
Thus strong evidence from the auditory, somatosensory and visual systems suggests that one of the roles of the corticothalamic pathway is to selectively enhance specific populations of thalamic neurons with similar or aligned response properties. This could occur at the level of single cells or ensembles of cells and is predicted to depend on the receptive field properties of feedback neurons and thalamic target neurons as well as the specificity of feedforward and feedback projections.
Corticothalamic feedback and thalamocortical communication
In addition to influencing the shape and strength of thalamic receptive fields, corticothalamic feedback may also enhance the transmission of sensory information from the thalamus to the cortex. This could occur by increasing the gain or responsiveness of thalamic neurons to sensory stimuli, improving the reliability of thalamic responses, and/or altering the temporal patterns of activity among individual thalamic neurons and neuronal ensembles.
Corticothalamic feedback has been shown to influence the gain and responsiveness of thalamic neurons at both local and global levels. At the local level, studies from the somatosensory, auditory and visual system show that corticothalamic feedback can selectively increase the gain of sensory responses among individual thalamic neurons [9,13,24–26]. At the global level, corticothalamic projections contribute to the neuronal circuitry involved with adjusting the responsiveness of thalamic neurons (and activity patterns) during sleep and wakefulness (reviewed in 27). Corticothalamic feedback is also likely to contribute to the effects attention can have on sensory responses in the thalamus [28–30]. Somewhat related, recent and exciting work from the somatosensory system indicates that corticothalamic feedback increases thalamic responses to painful stimuli and increases information flow between the thalamus and somatosensory cortex [31••,32••]. Although pain and attention are clearly not identical, similar mechanisms could be utilized by feedback to mediate such global effects.
Corticothalamic feedback may also influence thalamocortical communication by increasing the reliability of thalamic responses to sensory stimulation. Indeed, recent work in the visual system indicates that feedback can reduce the variability of LGN responses to repeated presentations of a visual stimulus [23,33]. Along these lines, thalamic neurons produce two categories of spikes—burst spikes and tonic spikes [34]. Although there is some controversy over the extent to which corticothalamic feedback influences these two response modes [35–38], recent work indicates that bursts spikes display more reliability and temporal precision than tonic spikes [39,40]. Much of the excitement surrounding the topic of burst spikes stems from results showing that burst spikes are more effective at driving cortical responses than tonic spikes [41]. In addition, it has been suggested that corticothalamic feedback generates conductance noise within the thalamus which can serve to mix burst and tonic firing thereby creating a more linear transfer function for thalamocortical inputs [42].
A growing body of work indicates that corticothalamic feedback may influence sensory processing over different time scales. For instance, several studies identify distinct classes of corticothalamic neurons that vary widely in the conduction latency of their feedback axons [43–47•]. Notably, these studies all describe a class of fast-conducting corticothalamic neurons with latencies for signal propagation between cortex and thalamus of 1–7 msec. These neurons are therefore particularly well suited for rapid modulation of ongoing thalamic activity. In the corticogeniculate pathway of the monkey, some of these neurons also receive monosynaptic, suprathreshold input from the thalamus, thereby completing the thalamo-cortico-thalamic loop with just a single cortical synapse [47•]. Perhaps most interesting of these observations, however, is the finding that corticothalamic neurons as a population operate at multiple speeds and are thus capable of modulating the temporal dynamics of thalamic neurons on multiple time scales.
Corticothalamic feedback may also serve to influence correlations in activity among large ensembles thalamic neurons. Along these lines, Destexhe et al [48] proposed a model whereby corticothalamic projections increase thalamic synchrony and consequently coherent oscillations in the cortico-thalamo-cortical loop. In support for this model, later work found that corticothalamic stimulation triggers different patterns of correlated activity within the thalamus dependent on stimulation mode and inhibitory activity in the RTN [49]; and that some patterns of thalamic activity result in greater synchronization across neurons via a redistribution of their spike timing [50].
Synchronized activity between the thalamus and cortex may also depend on membrane potential fluctuations occurring on slow (< 1 Hz) time scales, called Up/Down states, which bare similarities to wakeful activity [51]. For instance, recent work shows that thalamocortical activation can trigger cortical Up states [52,53]. Furthermore, there may be a link between membrane state fluctuations and correlated oscillations in the thalamus and cortex, as state-dependent corticothalamic activation of thalamic neurons may contribute to coherent oscillations [54]. Finally, as recent studies reveal increased neuronal responses to stimuli during specific membrane states [55–57••,58,59], the relationship between cortico-thalamo-cortical loops and correlated activity at slow and fast frequencies will be an important topic for further exploration.
Conclusions
Despite the clear gaps in our understanding of corticothalamic function and sensory processing in the thalamus, great strides have been made in the past decade. In addition to effects on the receptive field profiles of thalamic neurons, an increasing number of studies reveal an influence of feedback on the temporal properties of thalamic responses. Perhaps most important is the growing appreciation for the view that corticothalamic feedback does not operate in isolation, but rather as an integral part of a dynamic circuit linking the thalamus and sensory cortex. This circuit is likely to comprise multiple channels and cell types, each performing specific functions and operating with distinct neuronal ensembles to accomplish the complicated task of processing and communicating sensory information.
Abbreviations
- LGN
lateral geniculate nucleus
- MGB
medial geniculate body
- VPL
ventral posterior lateral nucleus
- VPM
ventral posterior medial nucleus
- RTN
reticular nucleus of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2nd Edition. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 2.Jones EG. The Thalamus. 2nd Edition. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 3.Granseth B, Lindström S. Unitary EPSCs of corticogeniculate fibers in the rat dorsal lateral geniculate nucleus in vitro. J Neurophysiol. 2003;89:2952–2960. doi: 10.1152/jn.01160.2002. [DOI] [PubMed] [Google Scholar]
- 4.Granseth B, Lindström S. Augmentation of corticogeniculate EPSCs in principal cells of the dorsal lateral geniculate nucleus of the rat investigated in vitro. J Physiol. 2004;556:147–157. doi: 10.1113/jphysiol.2003.053306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granseth B. Dynamic properties of corticogeniculate excitatory transmission in the rat dorsal lateral geniculate nucleus in vitro. J Physiol. 2004;556:135–146. doi: 10.1113/jphysiol.2003.052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci. 2005;25:7179–7190. doi: 10.1523/JNEUROSCI.1445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander GM, Fisher TL, Godwin DW. Differential response dynamics of corticothalamic glutamatergic synapses in the lateral geniculate nucleus and thalamic reticular nucleus. Neuroscience. 2006;137:367–372. doi: 10.1016/j.neuroscience.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Jones EG. Corticothalamic inhibition in the thalamic reticular nucleus. J Neurophysiol. 2004;91:759–766. doi: 10.1152/jn.00624.2003. [DOI] [PubMed] [Google Scholar]
- 9.Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Yan J. Modulation of the receptive fields of midbrain neurons elicited by thalamic electrical stimulation through corticofugal feedback. J Neurosci. 2007;27:10651–10658. doi: 10.1523/JNEUROSCI.1320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Yan Y. Corticothalamic feedback for sound-specific plasticity of auditory thalamic neurons elicited by tones paired with basal forebrain stimulation. Cereb Cortex. 2008 doi: 10.1093/cercor/bhm188. [Epub ahead of print, PMID 18203697]This landmark study demonstrates egocentric modification of MGN activity in the mouse following forebrain stimulation paired with tone presentation. Corticothalamic circuits likely underlie this modification as cortical inactivation blocks the measured effects.
- 12.Li L, Ebner FF. Cortical modulation of spatial and angular tuning maps in the rat thalamus. J Neurosci. 2007;27:167–179. doi: 10.1523/JNEUROSCI.4165-06.2007.This study elegantly illustrates egocentric modification of barreloid direction selectivity by stimulating and recording from matched and mismatched barrels/barreloids in the rodent somatosensory system. Barreloids matching barrels in whisker preference and directional tuning increased their activity while those with different directional tuning shifted toward that of the barrels.
- 13.Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron. 2004;41:639–651. doi: 10.1016/s0896-6273(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 14.Cudeiro J, Sillito AM. Looking back: corticothalamic feedback and early visual processing. Trends Neurosci. 2006;29:298–306. doi: 10.1016/j.tins.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987;329:727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- 16.Jones HE, Andolina IM, Oakely NM, Murphy PC, Sillito AM. Spatial summation in lateral geniculate nucleus and visual cortex. Exp Brain Res. 2000;135:279–284. doi: 10.1007/s002210000574. [DOI] [PubMed] [Google Scholar]
- 17.Rivadulla C, Martínez LM, Varela C, Cudeiro J. Completing the corticofugal loop: a visual role for the corticogeniculate type 1 metabotropic glutamate receptor. J Neurosci. 2002;22:2956–2962. doi: 10.1523/JNEUROSCI.22-07-02956.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb BS, Tinsley CJ, Barraclough NE, Easton A, Parker A, Derrington AM. Feedback from V1 and inhibition from beyond the classical receptive field modulates the responses of neurons in the primate lateral geniculate nucleus. Vis Neurosci. 2002;19:583–592. doi: 10.1017/s0952523802195046. [DOI] [PubMed] [Google Scholar]
- 19.Bonin V, Mante V, Carandini M. The suppressive field of neurons in lateral geniculate nucleus. J Neurosci. 2005;25:10844–10856. doi: 10.1523/JNEUROSCI.3562-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alitto HJ, Usrey WM. Origin and dynamics of extraclassical suppression in the lateral geniculate nucleus of the macaque monkey. Neuron. 2008;57:135–146. doi: 10.1016/j.neuron.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Jones HE, Andolina IM, Salt TE, Sillito AM. Functional alignment of feedback effects from visual cortex to thalamus. Nat Neurosci. 2006;9:1330–1336. doi: 10.1038/nn1768.This study shows that synapses from corticogeniculate neurons onto LGN cells in the cat are arranged in alignment with the orientation preferences of the feedback neurons. Interestingly, this synaptic arrangement also displays a phase-reversal of on and off subregions of the corticogeniculate receptive fields.
- 23.Andolina IM, Jones HE, Wang W, Sillito AM. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Natl Acad Sci USA. 2007;104:1685–1690. doi: 10.1073/pnas.0609318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S, Murray GM, Turman AB, Rowe MJ. Corticothalamic influences on transmission of tactile information in the ventroposterolateral thalamus of the cat: effect of reversible inactivation of somatosensory cortical areas I and II. Exp Brain Res. 1994;100:276–286. doi: 10.1007/BF00227197. [DOI] [PubMed] [Google Scholar]
- 25.Przybyszewski AW, Gaska JP, Foote W, Pollen DA. Striate cortex increases contrast gain of macaque LGN neurons. Vis Neurosci. 2000;17:485–494. doi: 10.1017/s0952523800174012. [DOI] [PubMed] [Google Scholar]
- 26.Cano M, Bezdudnaya T, Swadlow HA, Alonso JM. Brain state and contrast sensitivity in the awake visual thalamus. Nat Neurosci. 2006;9:1240–1242. doi: 10.1038/nn1760. [DOI] [PubMed] [Google Scholar]
- 27.Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Vanduffel W, Tootell RB, Orban GA. Attention-dependent suppression of metabolic activity in the early stages of the macaque visual system. Cereb Cortex. 2000;10:109–126. doi: 10.1093/cercor/10.2.109. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 30.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monconduit L, Lopez-Avila A, Molat JL, Chalus M, Villanueva L. Corticofugal output from the primary somatosensory cortex selectively modulates innocuous and noxious inputs in the rat spinothalamic system. J Neurosci. 2006;26:8441–8450. doi: 10.1523/JNEUROSCI.1293-06.2006.Using pharmacological techniques and single unit recordings, this elegant study demonstrates that feedback from somatosensory cortex enhances VPL responses to noxious stimuli.
- 32.Wang JY, Chang JY, Woodward DJ, Baccalá LA, Han JS, Luo F. Corticofugal influences on thalamic neurons during nociceptive transmission in awake rats. Synapse. 2007;61:335–342. doi: 10.1002/syn.20375.This study demonstrates that painful stimuli increase information flow and correlation strength between S1 and VPM in awake rats. In addition, it demonstrates that S1 activity precedes VPM activity suggesting a role of corticothalamic circuitry in mediating responses to pain.
- 33.Funke K, Nelle E, Li B, Worgotter F. Corticofugal feedback improves the timing of retino-geniculate signal transmission. Neuroreport. 1996;7:2130–2134. doi: 10.1097/00001756-199609020-00013. [DOI] [PubMed] [Google Scholar]
- 34.Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 35.Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyding D, Macklis JD, Neubacher U, Funke K, Wörgötter F. Selective elimination of corticogeniculate feedback abolishes the electroencephalogram dependence of primary visual cortical receptive fields and reduces their spatial specificity. J Neurosci. 2003;23:7021–7033. doi: 10.1523/JNEUROSCI.23-18-07021.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz O, Royal D, Sáry G, Chen X, Schall JD, Casagrande VA. Low-threshold Ca2+-associated bursts are rare events in the LGN of the awake behaving monkey. J Neurophysiol. 2006;95:3401–3413. doi: 10.1152/jn.00008.2006. [DOI] [PubMed] [Google Scholar]
- 38.de Labra C, Rivadulla C, Grieve K, Mariño J, Espinosa N, Cudeiro J. Changes in visual responses in the feline dLGN: selective thalamic suppression induced by transcranial magnetic stimulation of V1. Cereb Cortex. 2007;17:1376–1385. doi: 10.1093/cercor/bhl048. [DOI] [PubMed] [Google Scholar]
- 39.Alitto HJ, Weyand TG, Usrey WM. Distinct properties of stimulus-evoked bursts in the lateral geniculate nucleus. J Neurosci. 2005;25:514–523. doi: 10.1523/JNEUROSCI.3369-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denning KS, Reinagel P. Visual control of burst priming in the anesthetized lateral geniculate nucleus. J Neurosci. 2005;25:3531–3538. doi: 10.1523/JNEUROSCI.4417-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swadlow HA, Gusev AG. The impact of 'bursting' thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 42.Wolfart J, Debay D, Le Masson G, Destexhe A, Bal T. Synaptic background activity controls spike transfer from thalamus to cortex. Nat Neurosci. 2005;8:1760–1767. doi: 10.1038/nn1591. [DOI] [PubMed] [Google Scholar]
- 43.Harvey AR. Characteristics of corticothalamic neurons in area 17 of the cat. Neurosci. Letters. 1978;7:177–181. doi: 10.1016/0304-3940(78)90164-7. [DOI] [PubMed] [Google Scholar]
- 44.Tsumoto T, Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. J Comp Neurol. 1980;193:223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- 45.Swadlow HA, Weyand TG. Corticogeniculate neurons, corticotectal neurons, and suspected interneurons in visual cortex of awake rabbits: receptive-field properties, axonal properties, and effects of EEG arousal. J Neurophys. 1987;57:977–1001. doi: 10.1152/jn.1987.57.4.977. [DOI] [PubMed] [Google Scholar]
- 46.Briggs F, Usrey WM. Temporal properties of feedforward and feedback pathways between the thalamus and visual cortex in the ferret. Thalamus Relat Syst. 2005;3:133–139. doi: 10.1017/S1472928807000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs F, Usrey WM. A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J Neurosci. 2007;27:5431–5466. doi: 10.1523/JNEUROSCI.1035-07.2007.In the first physiological characterization of identified corticogeniculate neurons in the monkey, this study identifies distinct populations of corticogeniculate neurons on the basis of axonal conduction latency. Notably, corticogeniculate neurons with the fastest conducting axons receive direct, suprathreshold input from the LGN, thereby allowing information to travel from the thalamus to the cortex and back to the thalamus on a fast time scale.
- 48.Destexhe A, Contreras D, Steriade M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience. 1999;92:427–443. doi: 10.1016/s0306-4522(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 49.Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bal T, Debay D, Destexhe A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. J Neurosci. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic 'up' states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigas P, Castro-Alamancos MA. Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 54.Destexhe A. Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J Physiol Paris. 2000;94:391–410. doi: 10.1016/s0928-4257(00)01093-7. [DOI] [PubMed] [Google Scholar]
- 55.Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nature Neurosci. 2000;3:617–621. doi: 10.1038/75797. [DOI] [PubMed] [Google Scholar]
- 56.Sachdev RNS, Ebner FF, Wilson CJ. Effects of subthreshold Up and Down states on the whisker-evoked response in somatosensory cortex. J Neurophys. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- 57.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593.This elegant study examines the role of spike timing in synaptic transmission between the thalamus and somatosensory cortex in the rodent. Importantly, the study shows that while individual thalamocortical inputs are weak, synchronous inputs are significantly stronger.
- 58.Haider B, Duque A, Hasenstaub AR, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophys. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- 59.Hasenstaub AR, Sachdev RNS, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]