Abstract
Objective
To examine the association between Attention Deficit Hyperactivity Disorder (ADHD) symptomatology and parent-reported sleep problems among preschoolers aged 2 to 5 years.
Method
1,073 parents of preschoolers aged 2–5 years attending a large pediatric clinic completed the Child Behavior Checklist 1½–5. A stratified probability sample of 193 parents of high scorers and 114 parents of low scorers were interviewed with the Preschool Age Psychiatric Assessment (PAPA). Poisson regression was used to test the association between parent-reported sleep problems and ADHD symptomatology, as well as psychiatric and demographic covariates.
Results
When considered without reference to other psychiatric disorders, elevated hyperactive-impulsive symptomatology was positively associated with parent reported problems including sleep assistance, parasomnias, and dyssomnias; however, all of these effects were attenuated to non-significance once psychiatric comorbidity was controlled. In contrast, elevated inattentive symptomatology (especially at lower levels of hyperactive-impulsive symptoms) was positively associated with daytime sleepiness even after psychiatric comorbidity was controlled.
Conclusions
Neither hyperactive-impulsive nor inattentive ADHD symptomatology was uniquely related to parent-reported problems involving sleep assistance, parasomnias, or dyssomnias. However, inattentive symptomatology was uniquely related to daytime sleepiness, above and beyond commonly occurring patterns of psychiatric comorbidity, sleep duration, and demographic factors.
Keywords: ADHD, sleep, slow cognitive tempo, preschoolers, psychopathology
Clinicians have long noted an association between attention deficit/hyperactivity disorder (ADHD) and sleep problems, and this association has received intermittent research attention for nearly four decades. In a comprehensive review of studies published between 1970 and 1998, Corkum and colleagues concluded that numerous methodological problems vitiated our ability to draw any firm conclusions regarding the association between ADHD symptoms and sleep disturbances.1 These problems included poorly defined and variable methods for measuring/diagnosing ADHD, inconsistencies in the type of sleep problems considered, the use of small sample sizes (limiting generalizability and increasing type II errors), and the omission or use of inadequate controls for potential confounding variables, such as psychiatric comorbidity and medication effects.1
A recent meta-analysis by Cortese and colleagues identified 13 studies—five that used subjective (parent report) and eight that used objective indicators of sleep problems—that that met the following criteria: (1) measured ADHD using criteria specified in the third (revised) or fourth editions of the Diagnostic and Statistical Manual (DSM), (2) adequately controlled for (or excluded cases due to) psychiatric comorbidity, (3) included a comparison group, and (4) did not include participants who were being treated with medications targeting sleep problems.2 The authors concluded that ADHD youth did not differ from controls with respect to sleep onset latency or sleep architecture parameters (e.g., REM sleep). In contrast, ADHD youth exhibited greater daytime sleepiness, more movement during sleep, and higher scores on an apnea-hypopnea index, which is consistent with a previous study that reported linked sleep disordered breathing and ADHD.3 Thus, when the methodological shortcomings previously noted by Corkum and colleagues were attended to, some specific associations between ADHD and sleep problems persisted.
Because the current study made use of parent reported sleep problems, we now focus on the five studies that used parent reports of sleep problems in ADHD youth that were included in Cortese et al’s (2006) review.4–8 Although all five studies represent the best of available research, including some attention to comorbidity and medication use, they shared at least three limitations. First, all five studies were based on psychiatric clinic samples of ADHD youth. To the extent that clinic referred youth were not representative of the general population, the reported associations between ADHD and sleep problems may have been biased.9, 10 The current study used a screen-stratified sample of preschool-aged children who were representative of the larger population of children attending primary care pediatric services. Moreover, because psychostimulant medications that are typically used to treat ADHD symptomatology have side effects related to sleep (e.g,. insomnia), our use of preschool-aged sample, where medication use was rare, helped guard against the possibility of a medication confound with respect to the association between ADHD and sleep problems.
Second, there was substantial between and within study variation with respect to child age in the previous studies. Given evidence that many of the sleep problems that were considered (including those in which differences between ADHD and comparison were reported) vary systematically with age, this may have accounted for inconsistencies in the presence and magnitude of sleep problems reported across studies.11 For example, of 1038 children who were seen in a general pediatric outpatient clinic, rates of parent-reported excessive daytime sleepiness increased from 10.6% of preschool-aged children (2.0–4.9 years), to 16.8% of early elementary (5.0–7.9 years), 20.7% of late elementary (8.0–10.9 years), and 20% of middle-school aged (11–13.9 years) youth. By contrast, parent-reported rates of sleep terrors decreased from 39.1% of preschoolers, to 22% of early elementary, 19.7% of late elementary, and 12.9% of middle school-aged youth. Another merit of our use of an exclusively preschool-aged sample was control for normative developmental variation in sleep problems.11
Third, in most cases, group comparisons focused on ADHD youth versus controls, with no distinction regarding ADHD subtypes and/or comparisons of the relative contributions of inattentive versus hyperactive-impulsive symptomatology in the prediction of sleep problems. This is an important limitation, given clinical and empirical evidence indicating that daytime sleepiness is primarily related to inattentive symptomatology, whereas sleep disordered breathing and movement during sleep is primarily related to hyperactive-impulsive symptomatology.5, 12, 13 Therefore, study differences in subtype distributions (and their concomitant differential comorbidity patterns) may account for inconsistencies in their results.14, 15 The current study will consider hyperactive-impulsive and inattentive symptomatology separately, as well as attending to psychiatric comorbidity.
Following the conclusions of the Cortese et al. (2006), we hypothesized that although ADHD would exhibit bivariate associations with a range of sleep problems when considered in isolation, only the association of daytime sleepiness would remain after controlling for psychiatric comorbidity and sleep duration. Morever, we hypothesized that the association between ADHD and daytime sleepiness would be most pronounced for inattentive symptomatology. Although the results of Cortese et al. (2006) also provide a basis for hypothesizing unique associations between ADHD and sleep-disordered breathing and movement during sleep, these outcomes were not measured because they were not relevant to the original aims of the study as a whole.
Method
We examined the association between ADHD and sleep problems using a screen-stratified sample of preschool-aged children who are representative of the larger population of children attending primary care pediatric services. This sample was initially derived in order to provide a one-week test-retest reliability evaluation of the Preschool Aged Psychiatric Assessment (PAPA), a structured parent-report psychiatric interview about preschool aged children. Results relevant to the reliability and validity of the PAPA, as well as other studies that have used these data to describe the phenomenology of preschool psychiatric disorders have been published elsewhere.16–18 We present a summary pertinent to the analyses conducted here (see Figure 1).
Figure 1.
Overview of Study Design
Study design
Data from the first of the two repeated interviews were employed in the present analyses. The study used a psychopathology screen-stratified design, with oversampling of those with high screen scores, and stratification by gender, age (2, 3, 4 and 5 year-olds), and race (African American and non-African American). The use of sampling weights in these analyses permits unbiased pediatric clinic estimates to be computed from such a stratified sample.
Over the 18 months of data collection, 1,191 parents were approached by a screener, who explained the study and sought informed consent for completion of the Child Behavior Checklist (CBCL) 1½--5 and, if invited, the PAPA.19 Of these, 1,073 were screened: 20 refused, and 98 were excluded, either because the accompanying adult was not English speaking (n = 48) or could not provide legal consent (n = 21), or the child had autism, mental retardation, or another pervasive developmental disorder (n = 14), or had a sibling already enrolled (n = 15). 307 children “screened high” (i.e. obtained a CBCL total symptom T-score ≥ 55). 766 “screened low” (i.e. obtained a CBCL total symptom T-score < 55). Stratifying by age, gender, and race, 80% of screen highs were randomly selected for PAPA interviews, as well as well as 20% of “screen lows.” We continued to request participation in the interview phase from members of each age × gender × ethnicity group until their particular cell was full. PAPA interviews were conducted by interviewers who were blind to the child’s screen status. The majority of interviews were conducted in the participant’s home. Two children whose parents reported current use of stimulant medications were dropped from all analyses because (1) these medications are the primary form of treatment for ADHD and (2) a well known side effect of these medications is insomnia. Focusing exclusively on stimulant medication is consistent with other reports 6.This provided a completely stimulant naïve sample within which to evaluate the effects of ADHD symptomatology on sleep problems (N = 305).
Sample characteristics
Demographic characteristics of the screened sample, the interviewed sample, and surrounding Durham County where the study was conducted are presented in Table 1. 86% of the interviews were conducted with the child’s biological mother. No significant differences in gender, age, or Medicaid status emerged between screen-refusers and participants or study-refusers and participants, so the sample weights were not adjusted for study-refusal.see 17
Table 1.
Demographic characteristics of the PTRTS subjects compared to the surrounding community
| Overall N | 1,073 PTRTS 307 |
|||
|---|---|---|---|---|
| Screen | TRT | 223,314 Durham Countyb | ||
| Gender | Female | 49% a | 46% a | 48% |
| Male | 51% | 54% | 52% | |
| Age | 2 year olds | 30% | 30% | N/A |
| 3 year olds | 21% | 24% | ||
| 4 year olds | 26% | 24% | ||
| 5 year olds | 23% | 22% | ||
| Race/ethnicity | AA/black | 58% | 55% | 40% |
| White/non-Hispanic | 32% | 35% | 48% | |
| Hispanic | 2% | 2% | 8% | |
| Asian | 2% | 1% | 3% | |
| Native American | 0.3% | 0.3% | 0.3% | |
| Other | 6% | 7% | 6% | |
| Medicaid/Medicare | 43% | 54% | 33% | |
| Headstart/Early Headstart | 5% | 9% | 4% | |
| Family income < $15,000/yr | 25% | 31% | 17% | |
| Full time parental employment | 63% | 63% | 61% | |
| Parent education | Some HS | 14% | 9% | 22% |
| HS graduate | 20% | 30% | 28% | |
| Some college | 35% | 30% | 27% | |
| 4 yr. college or > | 32% | 31% | 23% | |
Note TRT = Test Rest-test; AA=African American; PTRTS=PAPA test-retest study; HS=high school
Unweighted percentages
Information from the 2000 Census Report (factfinder.census.gov)
The Preschool Age Psychiatric Assessment (PAPA)
The PAPA is a parent-report interviewer-based structured psychiatric assessment involving a range of mandatory questions and probes, supplemented by further detailed exploratory probing to ensure that the ratings appropriately represent the child’s problems.16 When symptoms (e.g., restlessness) were reported, their frequency, duration and onset dates were also collected for a three-month primary period, in order to determine whether they met the criteria for the symptoms of various DSM-IV diagnoses. Symptom and diagnostic algorithms implementing the DSM-IV criteria were written in SAS. These SAS algorithms were run on the raw PAPA data to generate data on psychiatric symptoms and disorders. As far as possible, the PAPA symptom algorithms followed the Child and Adolescent Psychiatric Assessment algorithms. 20 However, (1) DSM-IV symptoms that are not applicable to young children were excluded (e.g., for CD five out of the 15 possible CD symptoms were excluded), (2) Research Diagnostic Criteria – Preschool Age RDC-PA, Task Force, 21 developmentally modified DSM-IV criteria were used for depression,22 and (3) the high prevalence of certain behaviors in preschoolers indicated a need to modify the cutpoints for the symptoms. For instance, because the frequency of ODD symptoms such as “often loses temper” is higher in preschoolers than in older children, the ODD algorithm was modified so that each ODD symptom reflected the top 10% of frequency for preschoolers based on PAPA data. Thus, we maintained the CAPA’s 90th percentile frequency cutoff conceptualization of ODD symptomatology by modifying the criterial frequency levels.23 A similar approach was taken for the CD symptoms of assaults and lying. The one week test-retest symptom scale reliabilities ranged from .61 (GAD) to .81 (ADHD), comparable to those of interviews for older children and adults.
Sleep Outcomes
Fifteen items from the PAPA sleep module were identified as potential outcomes. Exploratory factor analyses of the tetrachoric correlation matrix of these 15 dichotomously-scored sleep items indicated that a four factor model was preferable. The items from each factor were summed to create four outcome variables, including sleep assistance (2 items; sleeps with family members, reluctance to sleep alone), parasomnias (5 items; night waking, nightmares, night terrors, rises to check on family members, sleep walking), dyssomnias (4 items; bedtime resistance, difficult to rouse in morning, morning irritability, morning sluggishness), and daytime sleepiness (4 items; takes unscheduled naps, seems sleepy during day, easily tired, easily fatigued). This organization of items and naming conventions used here are similar those derived from a previous principal components analysis.4 It also closely corresponds to four of the five characteristics that are common to most parent report scales of sleep problems (i.e. B.E.A.R.S.; Bedtime resistance, Excessive daytime sleepiness, Awakening at night, Regularity/pattern/duration of sleep, Snoring).24 Despite consistency across studies that have utilized empirical strategies for grouping sleep problems, it is not clear that the grouped symptoms share a neurophysiologic basis.
Psychopathology
The independent variables were ADHD Inattentive (IN) and hyperactive-impulsive (HI) symptom counts (a maximum of 9 possible symptoms, each). Three symptom count variables representing the sum of (1) Oppositional Defiant (8 symptoms possible) and Conduct Disorder (9 possible) symptoms, (2) Social Phobia (2 possible), Specific Phobia (6 possible), Generalized Anxiety (6 possible), and Separation Anxiety (8 possible) symptoms, and (3) Dysthymia/Major depression (9 possible) symptoms were used as covariates. Symptoms counts, rather than diagnoses, were used because our primary interest was evaluating the differential effects of subcomponents of ADHD (hyperactivity-impulsivity, inattention) on sleep outcomes; however, no children met full diagnostic criteria for ADHD Inattentive type. To be clear, 20 children (weighted = 3.0%) met full diagnostic criteria for ADHD; 8 (weighted 1.4%) met criteria for ADHD Combined type and 12 (weighted 1.6%) met criteria for ADHD Hyperactive-Impulsive type. Although 4 (weighted 0.6%) additional children met the symptom criterion for ADHD Inattentive type, they did not meet full diagnostic criteria due to a lack of impairment.
Analytic Strategy
Poisson regression models were used to test the association between symptom counts and sleep outcomes, because the outcome scale scores were roughly Poisson-distributed. Most readers are familiar with Ordinary Least Squares (OLS) regression models. Poisson regression models differ from OLS models in two ways. First, the outcome is assumed to follow a Poisson not a Normal distribution. Second, whereas OLS models are linear in their coefficients (i.e., an identity link function relates predictors to outcomes), Poisson models are nonlinear in their coefficients (i.e., a logarithmic identity function relates predictors to outcomes). The basic Poisson regression model can be conceptualized as relating the log of the outcome (here sleep problem counts) to a set of predictors (here psychiatric symptom counts and covariates). By exponentiating both sides of the equation, the predictors can be interpreted with respect to the original scale of the outcome (i.e., we can make inferences about symptom counts, not logged symptom counts). We report exponentiated regression coefficients in the text and tables, which represent ratios of the group means (labeled MR for means ratio). Finally, in order to take into account the effects of the sampling design (i.e., over-sampling children who screened high on behavior problems) on both the parameter and variance estimates, weighted analyses with empirical variance estimates using the generalized estimating equations approach (GEE) were implemented in SAS PROC GENMOD.
Two models were estimated for each of the four sleep outcomes. The first model included the ADHD symptom count variables (HI and IN), their interaction, plus sleep duration and demographic variables (child age, race, sex) as covariates. When the interaction term was not significant, it was dropped and the model was re-estimated. The second model added Oppositional Defiant/Conduct Disorder, Anxiety Disorder, and Depression symptom count variables as additional covariates.
Results
Weighted descriptive statistics for all symptom count, as well as sleep outcome, variables for the entire sample are provided in Table 2. Although the mean number of symptoms and sleep problems was relatively low, the full range of behaviors was observed for each variable.
Table 2.
Weighted descriptive statistics for symptom count and sleep outcome variables (total sample)
| Mean | Count (weighted %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10+ | ||
| ADHD- IN | 0.6 | 76.8 | 8.1 | 6.0 | 3.5 | 1.3 | 1.5 | 1.3 | 0.5 | 0.8 | 0.1 | -- |
| ADHD- HI | 1.2 | 48.3 | 24.6 | 12.0 | 4.7 | 2.1 | 3.4 | 1.6 | 1.8 | 0.9 | 0.7 | -- |
| ODD/CD | 2.1 | 27.2 | 26.5 | 9.7 | 13.1 | 10.2 | 4.7 | 3.4 | 1.5 | 2.8 | 0.5 | 0.5 |
| Anxiety | 1.4 | 33.8 | 33.7 | 12.4 | 7.9 | 5.7 | 3.2 | 1.7 | 1.2 | 0.4 | 0.1 | -- |
| Depression/Dysthymia | 0.7 | 71.5 | 3.5 | 17.8 | 3.7 | 2.4 | 0.8 | 0.1 | 0.0 | 0.1 | -- | -- |
| Sleep Assist | 0.7 | 50.7 | 23.8 | 25.5 | -- | -- | -- | -- | -- | -- | -- | -- |
| Parasomnia | 1.0 | 36.4 | 33.6 | 22.5 | 6.1 | 1.3 | -- | -- | -- | -- | -- | -- |
| Dyssomnia | 0.6 | 62.8 | 25.9 | 5.5 | 4.9 | 0.8 | -- | -- | -- | -- | -- | -- |
| Daytime Sleepiness | 0.2 | 83.0 | 14.2 | 2.1 | 0.6 | 0.1 | -- | -- | -- | -- | -- | -- |
Note: ADHD – Attention Deficit/Hyperactivity Disorder; IN – Inattentive; HI – Hyperactive Impulsive; ODD – Oppositional Defiant Disorder; CD – Conduct Disorder
Parasomnias
We initially tested whether there was an association between ADHD symptom counts and parasomnias. The results are summarized in Table 3. Higher levels of HI symptoms were predictive of more parasomnias (MR = 1.09, p = .018). Hence, holding all other predictors constant, for each additional HI symptom the number of parasomnia symptoms increased by a factor of 1.09. Among covariates, only child race was a significant predictor. African American children exhibited fewer parasomnias than Caucasian children (MR = 0.73, p = .006). However, once we controlled for ODD/CD, anxiety, and depression/dysthymia symptom counts, the association between HI and parasomnias was no longer significant (MR = 1.04, p = .29). Increased levels of anxiety symptoms were associated with more parasomnias (MR = 1.17, p < .0001). The effect of race remained (MR = 0.72, p = .003).
Table 3.
Prediction of Parasomnias and Dyssomnias.
| Parasomnia | Dyssomnia | |||||||
|---|---|---|---|---|---|---|---|---|
| MR | (95% CI) | MR | (95% CI) | MR | (95% CI) | MR | (95% CI) | |
| Duration (hours) | 1.07 | 0.97 – 1.18 | 1.05 | 0.95 – 1.16 | 0.82* | 0.71 – 0.96 | 0.83** | 0.72 – 0.95 |
| Age (years) | 1.02 | 0.93 – 1.13 | 1.05 | 0.96 – 1.16 | 1.14 | 0.98 – 1.33 | 1.17* | 1.01 – 1.36 |
| Male | 0.95 | 0.77 – 1.18 | 1.01 | 0.82 – 1.25 | 0.88 | 0.62 – 1.24 | 0.99 | 0.71 – 1.39 |
| African Amer. | 0.73** | 0.59 – 0.91 | 0.72** | 0.58 – 0.89 | 1.19 | 0.82 – 1.73 | 1.23 | 0.86 – 1.77 |
| ADHD (IN) | 0.97 | 0.88 – 1.07 | 0.94 | 0.85 – 1.03 | 0.97 | 0.85 – 1.11 | 0.91 | 0.79 – 1.04 |
| ADHD (HI) | 1.09* | 1.02 – 1.18 | 1.04 | 0.96 – 1.13 | 1.19** | 1.07 – 1.33 | 1.09 | 0.97 – 1.23 |
| ADHD (IN X HI) | --- | --- | --- | --- | --- | --- | --- | --- |
| ODD/CD | --- | --- | 0.99 | 0.93 – 1.06 | --- | --- | 1.05 | 0.96 – 1.15 |
| Anxiety | --- | --- | 1.17*** | 1.10 – 1.25 | --- | --- | 1.22*** | 1.10 – 1.35 |
| Dep/Dys | --- | --- | 1.03 | 0.94 – 1.12 | --- | --- | 1.03 | 0.91 – 1.18 |
Note: + p < .10,
p <= .05,
p < .01,
p < .001;
MR – Mean Ratio; ADHD – Attention Deficit/Hyperactivity Disorder; IN – Inattentive; HI –Hyperactive Impulsive; ODD – Oppositional Defiant Disorder; CD – Conduct Disorder
Dyssomnias
Next, we tested whether there was an association between ADHD symptom counts and dyssomnias. The results are summarized in Table 3. HI symptoms were predictive of dyssomnias (MR = 1.19, p = .001). Among covariates, longer sleep durations were associated with fewer dyssomnias (MR = 0.82, p = .01). However, once we controlled for ODD/CD, anxiety, and depression/dysthymia symptom counts, the association between HI and dyssomnias was no longer significant (MR = 1.09, p = .13). Moreover, elevated levels of anxiety counts were associated with more dyssomnias (MR = 1.22, p < .0001). The effect of sleep duration remained (MR = 0.83, p = .008). An age effect also emerged (MR = 1.17, p = .04), indicating that older children had more dyssmonias than did younger children.
Sleep Assistance
Next, we tested whether there was an association between ADHD symptom counts and sleep assistance problems. The results are summarized in Table 4. There was a trend for higher levels of HI symptoms to be associated with greater sleep assistance (MR = 1.09, p = .07), as well as a trend for males to require less sleep assistance (MR = 0.77, p = .052). However, once we controlled for ODD/CD, anxiety, and depression/dysthymia symptom counts as additional covariates, the association between HI and sleep assistance problems was no longer significant (MR = 1.01, p = .85). There was a trend for higher levels of IN symptoms to be associated with less sleep assistance (MR = 0.88, p = .06). Moreover, higher levels of anxiety symptoms were significantly associated with more sleep assistance (MR = 1.31, p < .0001).
Table 4.
Prediction of Sleep Assistance and Daytime Sleepiness
| Sleep Assistance | Daytime Sleepiness | |||||||
|---|---|---|---|---|---|---|---|---|
| MR | (95% CI) | MR | (95% CI) | MR | (95% CI) | MR | (95% CI) | |
| Duration (hours) | 1.01 | 0.90 – 1.13 | 0.96 | 0.85 – 1.07 | 1.24+ | 0.99 – 1.57 | 1.21+ | 0.99 – 1.48 |
| Age (years) | 0.91 | 0.81 – 1.02 | 0.96 | 0.85 – 1.07 | 0.97 | 0.76 – 1.23 | 0.93 | 0.74 – 1.16 |
| Male | 0.77+ | 0.60 – 1.00 | 0.87 | 0.67 –1.11 | 1.56 | 0.91 – 2.67 | 1.80* | 1.11 – 2.90 |
| African Amer. | 1.16 | 0.89 – 1.53 | 1.14 | 0.89 – 1.48 | 1.40 | 0.80 – 2.45 | 1.42 | 0.85 – 2.35 |
| ADHD (IN) | 0.92 | 0.81 – 1.04 | 0.88+ | 0.78 – 1.00 | 1.64*** | 1.35 – 1.99 | 1.47*** | 1.20 – 1.79 |
| ADHD (HI) | 1.09+ | 0.99 – 1.19 | 1.01 | 0.92 – 1.12 | 1.11 | 0.94 – 1.31 | 1.0 | 0.85 – 1.18 |
| ADHD (IN X HI) | --- | --- | --- | --- | .94** | .90 – .98 | 0.94** | 0.90 – 0.98 |
| ODD/CD | --- | --- | 0.95 | 0.88 – 1.02 | --- | --- | 1.11+ | 0.99 – 1.25 |
| Anxiety | --- | --- | 1.31*** | 1.21 – 1.41 | --- | --- | 1.11 | 0.97 – 1.26 |
| Dep/Dys | --- | --- | 0.99 | 0.88– 1.10 | --- | --- | 1.33*** | 1.14 – 1.55 |
Note: p < .10,
p <= .05,
p < .01,
p < .001;
MR – Mean Ratio; ADHD – Attention Deficit/Hyperactivity Disorder; IN – Inattentive; HI – Hyperactive Impulsive; ODD – Oppositional Defiant Disorder; CD – Conduct Disorder
Daytime Sleepiness
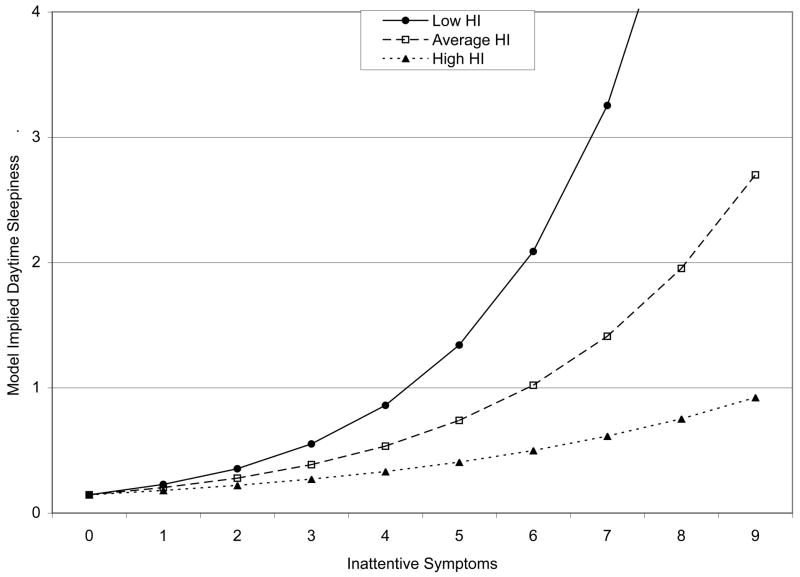
Finally, we tested whether there was an association between ADHD symptom counts and problems with daytime sleepiness. The results are summarized in Table 4. There was a significant interaction between IN and HI symptom counts (MR = 0.94, p = .004). Moreover, there was a trend for longer sleep duration to be associated with more daytime sleepiness (MR = 1.24, p = .07). The interaction between IN and HI symptoms remained significant, and the magnitude of the effect was largely unchanged (MR = 0.94, p = .007), even after controlling for ODD/CD, anxiety, and depression/dysthymia symptom counts. As shown in Figure 2, higher levels of IN symptoms were associated with higher levels of daytime sleepiness; moreover, the strength of this association was stronger at lower, relative to higher, levels of HI symptoms. Higher levels of depressive symptoms were also associated with more daytime sleepiness (MR = 1.33, p = .0003). The trend for longer sleep duration to be associated with greater daytime sleepiness remained (MR = 1.21, p = .07), and males were also reported to exhibit more daytime sleepiness (MR = 1.80, p = .02).
Figure 2.
Model-Implied Relationship Between Inattentive Symptoms and Daytime Sleepiness at Three Levels of Hyperactive-Impulsive (HI) Symptoms.
Discussion
Although there has been interest in the association between ADHD and sleep problems for nearly four decades, it has only been relatively recently that researchers have attended to the common methodological shortcomings of earlier studies, including improved measurement of both ADHD and sleep outcomes, as well as the incorporation of an appropriate range of covariates (especially psychiatric comorbidity and medication status). The results of this study demonstrated that although there were consistent bivariate associations between hyperactive-impulsive symptomatology and parasomnias, dyssominas, and sleep assistance, even in the presence of demographic and sleep duration covariates, these effects were all attenuated to non-significance once ODD/CD, anxiety, and depressive symptom counts were included as additional covariates. Anxiety symptom counts were the only consistent psychiatric predictor of parasomnias, dyssominas, and the need for sleep assistance. These results underscore the importance of attending to comorbidity when examining the association between ADHD and sleep outcomes, and highlight the strong association between anxiety symptomatology and sleep problems in young children.4, 6
In contrast, there was consistent evidence that elevated levels of ADHD inattentive symptomatology were related to daytime sleepiness, particularly at lower levels of ADHD hyperactive-impulsive symptomatology. This effect persisted (and was largely unchanged in magnitude) in the presence of all other psychiatric covariates, although depressive symptomatology also emerged as a significant predictor. This finding is consistent with previous work showing that ADHD youth had longer reaction times and were more likely to fall asleep during an objective assessment of daytime sleepiness (i.e., the multiple sleep latency test [MSLT]) relative to their age and sex matched controls.13, 25
Daytime sleepiness has substantial overlap with the construct of slow cognitive tempo (SCT). SCT has been proposed as a dimension of behavior/cognition that has the potential to better characterize ADHD Inattentive type youth than do current DSM-IV symptoms.26–28 Indeed, many of the items that are used to define SCT (e.g., child appears sluggish, daydreams or often seems ‘in a fog’) are synonyms for the behavioral indicators of daytime sleepiness. From this perspective, the association between ADHD symptoms (especially high levels of inattentive symptomatology in conjunction with low levels of hyperactive-impulsive symptomatology) and daytime sleepiness may be better conceptualized as additional evidence for the overlap between ADHD and SCT, rather than evidence for an association between ADHD and a “sleep problem” per se. Current neurophysiologic models of ADHD that emphasize “bottom-up” processes, including arousal systems, may be particularly relevant for understanding the observed associations between ADHD, SCT, and daytime sleepiness.29, 30 Indeed, although the dominant model for conceptualizing the brain basis of ADHD has involved the prefrontal cortex, for some ADHD youth a more appropriate model may involve the brain stem/cerebellum. Finally, rather than conceptualizing the association between inattentive symptoms and sleep problems with respect to SCT, an alternative sleep-based explanation may suggest that inattention, daytime sleepiness, and SCT are all manifestations of problems involving circadian organization.
This study had at least four weakness. First, although ADHD symptomatology was conceptualized as a predictor of sleep problems, given our reliance on a cross sectional design, these results only demonstrate a within time association. As longitudinal data become available for this sample, we will be in a better position to understand the (bi)directional associations between ADHD and sleep problems across development. Second, although we asked about a variety of sleep problems, we did not measure movement during sleep or sleep-disordered breathing, both of which have been associated with ADHD in the past.2 A related limitation is our sole reliance on parental reports of their children’s sleeping behaviors. It is unclear if the association between inattention and daytime sleepiness would be observed had we used an objective indicator of child daytime sleepiness (e.g., performance on the multiple sleep latency test). Third, because none of the children in our sample met diagnostic criteria for ADHD Inattentive type, we studied the association between symptom counts and sleep outcomes. As such, our results may not generalize to diagnosed youth. Fourth, although we excluded two participants who were reported to take psychostimulants, we did not exclude other youth taking other medications or having other medical conditions that may be related to attention or sleep problems. Fifth, we relied on an empirical strategy for grouping sleep problems; however, the resulting composite variables may not conform well to current neurophysiologic models of sleep.
On the other hand, we note several strengths of the study: We relied on a community-derived (pediatric) sample which permits greater generalization of results than many of the previous studies in this area that relied on psychiatric clinic samples. By studying the association between ADHD and sleep problems in preschool-aged children, the threat of medication use as a potential confounder variable was avoided (we excluded 2, out of the 307 total, children whose parents reported current stimulant use). Moreover, the relatively uniform age of participants reduced the developmental variation in sleep problems that occurs from early through middle childhood. Finally, our use of the PAPA, which is the only psychiatric interview for use with young children that has demonstrated test retest reliability, provides added confidence in our measurement of psychiatric behaviors and sleep problems in young children.
With the exception of an association between daytime sleepiness and inattentive behaviors, ADHD symptomatology was not uniquely associated with parasomnias, dyssyomnias, and sleep assistance, at least not in early childhood. In contrast, anxiety symptoms were consistently associated with these sleep problems. These results underscore the importance of both distinguishing inattentive from hyperactive-impulsive symptomatology and taking into account children’s developmental stage when examining associations between ADHD and sleep problems. Clinicians working with families of young children who present with either anxiety symptomatology or sleep problems should be aware of their joint association and consider their joint treatment. Conversely, when children are being evaluated for ADHD, the presence of sleep disturbances should not be considered as being “just another manifestation of ADHD.” Rather, clinicians should be cued to make a careful exploration for the presence of anxiety disorders. This study also adds to a growing body of evidence that elevated ADHD inattentive symptomatology co-occurs with low alertness (SCT). We are unaware of other studies that have demonstrated this association in early childhood. Although elevated levels of inattentive symptomatology in early childhood do not appear to result in impairment in functioning, it is likely that these behaviors will impair functioning once children transition into formal schooling with the associated attentional demands of the classroom environment. The preschool period may represent an important opportunity for early intervention of problems involving inattention and low alertness.
Acknowledgments
This research was supported by National Institute of Mental Health grant MH63670 to Dr. Angold.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acadf Child Adolesc Psychiatry. 1998;37(6):637–646. doi: 10.1097/00004583-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Cortese S, Mid EK, Yateman N, Mouren MC, Lecendreux M. Sleep and alertness in children with attention-deficit/hyperactivity disorder: A systematic review of the literature. Sleep. 2006;29(4):504–511. [PubMed] [Google Scholar]
- 3.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20(12):1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 4.Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R. Sleep problems in children with attention-deficit/hyperactivity disorder: Impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 5.LeBourgeois MK, Avis K, Mixon M, Olmi J, Harsh J. Snoring, sleep quality, and sleepiness across attention-deficit/hyperactivity disorder subtypes. Sleep. 2004;27(3):520–525. [PubMed] [Google Scholar]
- 6.Mick E, Biederman J, Jetton J, Faraone SV. Sleep disturbances associated with attention deficit hyperactivity disorder: The impact of psychiatric comorbidity and pharmacotherapy. J Child Adolesc Psychopharmacol. 2000;10(3):223–231. doi: 10.1089/10445460050167331. [DOI] [PubMed] [Google Scholar]
- 7.Owens J, Maxim R, Nobile C, McGuinn M, Msall M. Parental and self-report of sleep in children with Attention-Deficit/Hyperactivity Disorder. Arch Pediatr Adolesc Med. 2000;154:549–555. doi: 10.1001/archpedi.154.6.549. [DOI] [PubMed] [Google Scholar]
- 8.Marcotte AC, Thacher PV, Butters M, Bortz J, Acebo C, Carskadon MA. Parental report of sleep problems in children with attentional and learning disorders. Dev Behav Pediatr. 1998;19:178–186. doi: 10.1097/00004703-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Costello EJ, Janiszewski S. Who gets treated? Factors associated with referral in children with psychiatric disorders. Acta Psychiatrica Scandinavica. 1990;81(6):523–529. doi: 10.1111/j.1600-0447.1990.tb05492.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodman SH, Lahey BB, Fielding B, Dulcan M, et al. Representativeness of clinical samples of youths with mental disorders: A preliminary population-based study. J Abnorm Psychol. 1997;106:3–14. doi: 10.1037//0021-843x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Archbold KH, Pituch KJ, Panabi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- 12.Brown TE, McMullen WJ. Attention deficit disorders and sleep/arousal disturbance. Ann N Y Acad Sci. 2001;931:271–286. doi: 10.1111/j.1749-6632.2001.tb05784.x. [DOI] [PubMed] [Google Scholar]
- 13.Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41(6):803–812. [PubMed] [Google Scholar]
- 14.Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: Implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clin Psychol Sci Pract. 2001;8(4):463–488. [Google Scholar]
- 16.Egger HL, Angold A. The Preschool Age Psychiatric Assessment (PAPA): A structured parent interview for diagnosing psychiatric disorders in preschool children. In: DelCarmen-Wiggins R, Carter A, editors. Handbook of Infant and Toddler Mental Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 17.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 18.Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006;47(3–4):313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 19.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles: An Integrated System of Multi-informant Assessment. Burlington, VT: University of Vermont Department of Psychiatry; 2000. [Google Scholar]
- 20.Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Task Force on Research Diagnostic Criteria: Infancy and Preschool. Research diagnostic criteria for infants and preschool children: The process and empirical support. J Am Acad Child Adolesc Psychiatry. 2003;42:1504–1512. doi: 10.1097/01.chi.0000091504.46853.0a. [DOI] [PubMed] [Google Scholar]
- 22.Luby J, Heffelfinger A, Mrakotsky C, et al. The clinical picture of depression in preschool children. J Am Acad Child Adolesc Psychiatry. 2003;42:340–348. doi: 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Angold A, Costello EJ. The relative diagnostic utility of child and parent reports of oppositional defiant behaviors. Int J Methods Psychiatr Res. 1996;6(4):253–259. [Google Scholar]
- 24.Owens JA. The ADHD and sleep conundrum: A review. J Dev Behav Pediatr. 2005;26(4):312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261–266. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- 26.McBurnett K, Pfiffner LJ, Frick PJ. Symptom properties as a function of ADHD type: An argument for continued study of sluggish cognitive tempo. J Abnorm Child Psychol. 2001;29(3):207–213. doi: 10.1023/a:1010377530749. [DOI] [PubMed] [Google Scholar]
- 27.Todd RD, Rasmussen ER, Wood C, Levy F, Hay DA. Should sluggish cognitive tempo symptoms be included in the diagnosis of attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2004;43(5):588–597. doi: 10.1097/00004583-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Milich R, Balentine AC, Lynam DR. ADHD combined types and ADHD predominately inattentive type are distinct and unrelated disorders. Clin Psychol Sci Pract. 2001;8(4):463–488. [Google Scholar]
- 29.Sergeant JA. The cognitive-energetic model: an empirical approach to Attention-Deficit Hyperactivity Disorder. Neurosci Biobehav Rev. 2000;24:7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 30.Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and bottom of ADHD: a neuropsychological perspective. Neurosci Biobehav Rev. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]