Abstract
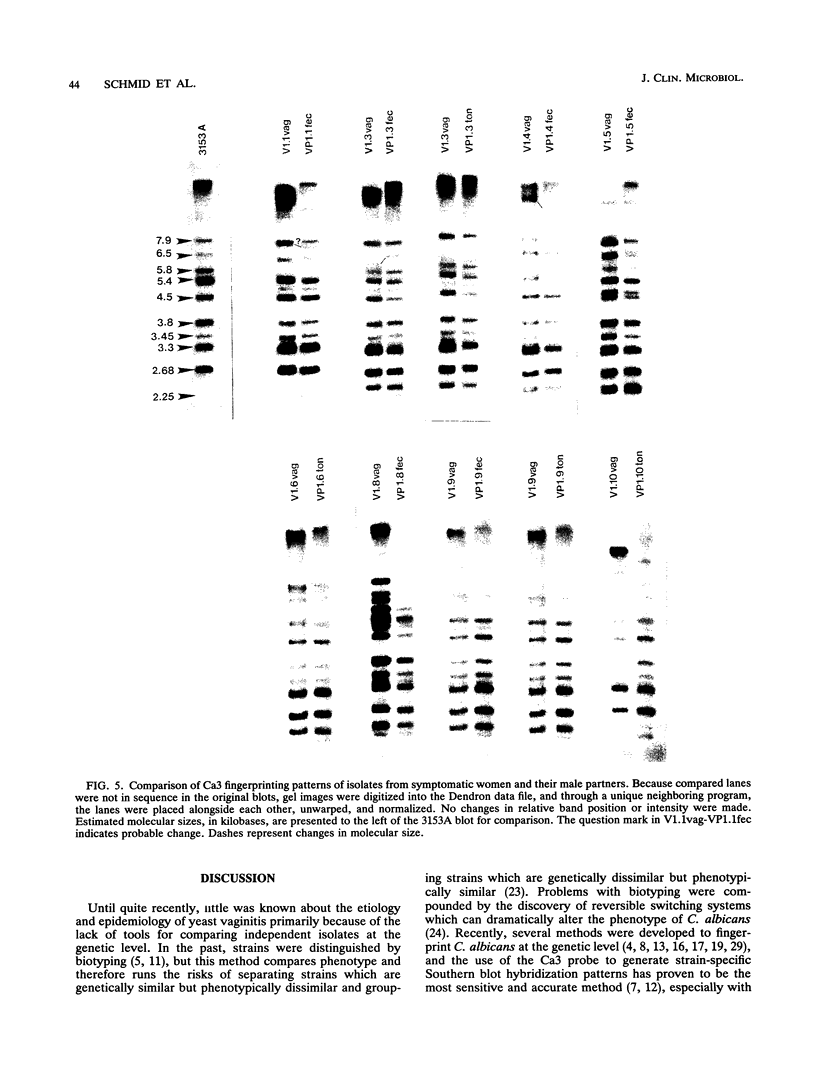
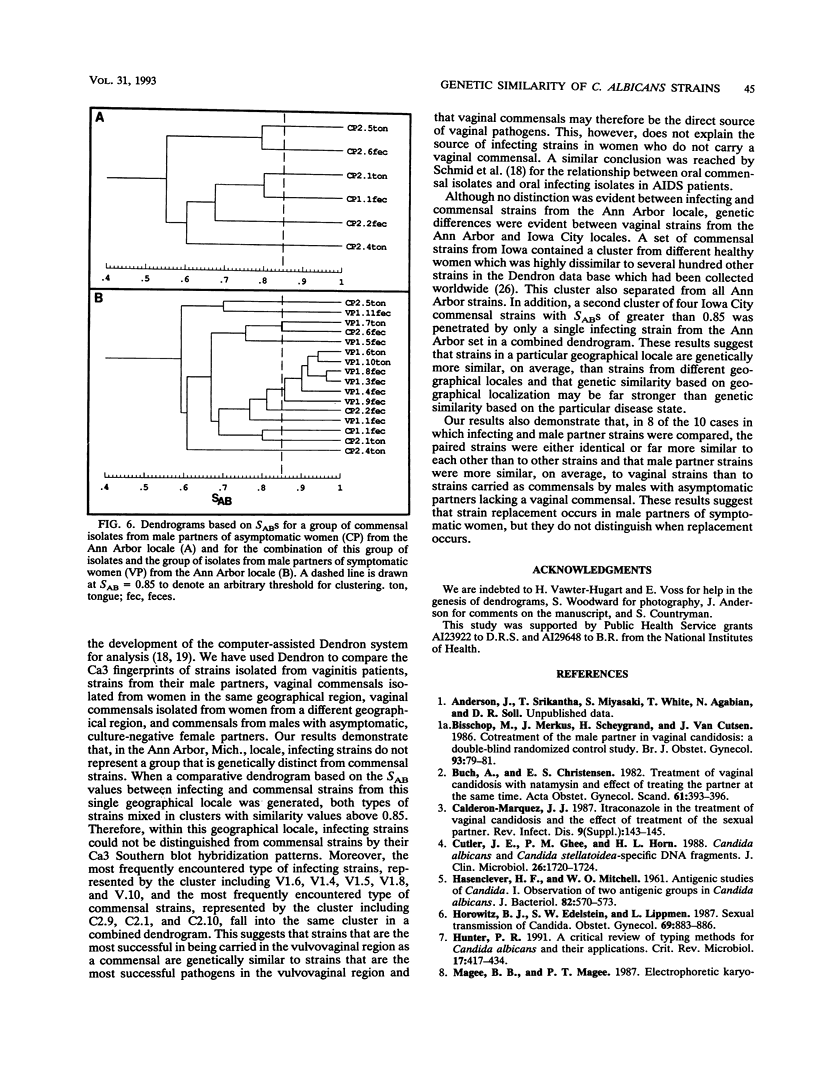
The moderately repetitive sequence Ca3 was used to fingerprint strains of Candida albicans isolated from vulvovaginal infections of 10 women and strains isolated from their male partners. The Dendron software package was then used to compare the DNA fingerprints of these strains with those of vaginal commensals from women from the same geographical locale, vaginal commensals from women from a different geographical locale, and commensals from male partners of asymptomatic women from the same geographical locale. The results demonstrate that, in the majority of cases (8 of 10), strains from symptomatic patients and their partners are either identical or more similar to each other than to other strains, infecting strains do not represent a group genetically distinguishable from vaginal commensal isolates from women from the same geographical locale, and both infecting strains and commensals from individuals in the test locale can be distinguished from commensals obtained in another geographical locale. The results also suggest that women with vaginal infections are responsible for strain replacement in their male partners.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisschop M. P., Merkus J. M., Scheygrond H., van Cutsem J. Co-treatment of the male partner in vaginal candidosis: a double-blind randomized control study. Br J Obstet Gynaecol. 1986 Jan;93(1):79–81. doi: 10.1111/j.1471-0528.1986.tb07818.x. [DOI] [PubMed] [Google Scholar]
- Buch A., Skytte Christensen E. Treatment of vaginal candidosis with natamycin and effect of treating the partner at the same time. Acta Obstet Gynecol Scand. 1982;61(5):393–396. doi: 10.3109/00016348209156578. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Glee P. M., Horn H. L. Candida albicans- and Candida stellatoidea-specific DNA fragment. J Clin Microbiol. 1988 Sep;26(9):1720–1724. doi: 10.1128/jcm.26.9.1720-1724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961 Oct;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B. J., Edelstein S. W., Lippman L. Sexual transmission of Candida. Obstet Gynecol. 1987 Jun;69(6):883–886. [PubMed] [Google Scholar]
- Hunter P. R. A critical review of typing methods for Candida albicans and their applications. Crit Rev Microbiol. 1991;17(6):417–434. doi: 10.3109/10408419109115206. [DOI] [PubMed] [Google Scholar]
- Miles M. R., Olsen L., Rogers A. Recurrent vaginal candidiasis. Importance of an intestinal reservoir. JAMA. 1977 Oct 24;238(17):1836–1837. doi: 10.1001/jama.238.17.1836. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Olivo P. D., McManus E. J., Riggsby W. S., Jones J. M. Mitochondrial DNA polymorphism in Candida albicans. J Infect Dis. 1987 Jul;156(1):214–215. doi: 10.1093/infdis/156.1.214. [DOI] [PubMed] [Google Scholar]
- Romero-Piffiguer M. D., Vucovich P. R., Riera C. M. Secretory IgA and secretory component in women affected by recidivant vaginal candidiasis. Mycopathologia. 1985 Sep;91(3):165–170. doi: 10.1007/BF00446295. [DOI] [PubMed] [Google Scholar]
- Sadhu C., McEachern M. J., Rustchenko-Bulgac E. P., Schmid J., Soll D. R., Hicks J. B. Telomeric and dispersed repeat sequences in Candida yeasts and their use in strain identification. J Bacteriol. 1991 Jan;173(2):842–850. doi: 10.1128/jb.173.2.842-850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Odds F. C., Wiselka M. J., Nicholson K. G., Soll D. R. Genetic similarity and maintenance of Candida albicans strains from a group of AIDS patients, demonstrated by DNA fingerprinting. J Clin Microbiol. 1992 Apr;30(4):935–941. doi: 10.1128/jcm.30.4.935-941.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Voss E., Soll D. R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990 Jun;28(6):1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985 Aug 1;152(7 Pt 2):924–935. doi: 10.1016/s0002-9378(85)80003-x. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Vulvovaginal candidiasis--what we do and do not know. Ann Intern Med. 1984 Sep;101(3):390–392. doi: 10.7326/0003-4819-101-3-390. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Schmid J., Hanna C., Mac K., Morrow B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991 Aug;29(8):1702–1710. doi: 10.1128/jcm.29.8.1702-1710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992 Apr;5(2):183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. E., Sheridan V. L., Magee B. B., Magee P. T. Use of rDNA restriction fragment length polymorphisms to differentiate strains of Candida albicans in women with vulvovaginal candidiasis. Diagn Microbiol Infect Dis. 1991 Nov-Dec;14(6):459–464. doi: 10.1016/0732-8893(91)90001-v. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]