Summary
Repeated exposure to cocaine causes sensitized behavioral responses and increased dendritic spines on medium spiny neurons of the nucleus accumbens (NAc). We find that cocaine regulates myocyte enhancer factor 2 (MEF2) transcription factors to control these two processes in vivo. Cocaine suppresses striatal MEF2 activity in part through a novel mechanism involving cAMP, the regulator of calmodulin signaling (RCS), and calcineurin. We show that reducing MEF2 activity in the NAc in vivo is required for the cocaine-induced increases in dendritic spine density. Surprisingly, we find that increasing MEF2 activity in the NAc, which blocks the cocaine-induced increase in dendritic spine density, enhances sensitized behavioral responses to cocaine. Together, our findings implicate MEF2 as a key regulator of structural synapse plasticity and sensitized responses to cocaine, and suggest that reducing MEF2 activity (and increasing spine density) in NAc may be a compensatory mechanism to limit long-lasting maladaptive behavioral responses to cocaine.
Introduction
A major clinical challenge for effective treatment of drug addiction is its persistence even after long periods of drug abstinence. One of the longest-lasting neural correlates observed, across several animal models of addiction, is an increase in dendritic spine density on medium-sized spiny neurons (MSNs) in the nucleus accumbens (NAc) (Robinson and Kolb, 2004). Dendritic spines in the NAc are the primary sites of excitatory synapses from prefrontal cortex and other glutamatergic inputs. The necks of these dendritic spines receive dopaminergic inputs from the ventral tegmental area (Hyman et al., 2006). Therefore, altering the density of NAc MSN dendritic spines could have dramatic effects on the information processing from several upstream limbic structures and ultimately addiction-related behaviors. Although several groups have documented that repeated cocaine exposure increases NAc spine density (Lee et al., 2006; Li et al., 2003; Norrholm et al., 2003; Robinson et al., 2001; Robinson and Kolb, 1999a), the precise molecular mechanisms that control this process have remained elusive. Moreover, the cocaine-induced increase in NAc spine density has been hypothesized to contribute to the long-lasting behavioral sensitization that occurs after repeated cocaine exposure (Robinson and Kolb, 1999), but direct evidence concerning the functional relationship between these two processes is lacking.
A recent study revealed that the cocaine-induced increase in NAc spine density is most stable in the D1 dopamine receptor-expressing neurons (Lee et al., 2006), suggesting that D1 receptor signaling plays a major role in long-lasting stabilization of altered spine density. Interestingly, the D1 receptor-expressing neurons correlated with the same population of NAc neurons that express high levels of the stable transcription factor, ΔFosB, which has been shown to play important roles in addiction-related behaviors (Colby et al., 2003; Hiroi et al., 1997; Kelz et al., 1999). A key ΔFosB gene target in the NAc is Cdk5 (cyclin-dependent kinase 5). Chronic cocaine increases the levels and activity of Cdk5 in the NAc (Bibb et al., 2001), and chemical inhibition of Cdk5 activity in this region blocks the cocaine-induced increase in dendritic spine density (Norrholm et al., 2003). These observations led to the hypothesis that ΔFosB accumulation upregulates Cdk5, which presumably then phosphorylates key substrates to facilitate the increase in dendritic spine density.
One group of Cdk5 substrates in the brain is the MEF2 (myocyte enhancer factor 2) family of transcription factors (Gong et al., 2003). MEF2 proteins (MEF2A-D) are expressed in unique but overlapping patterns throughout the developing and adult brain (McKinsey et al., 2002; Shalizi and Bonni, 2005). They bind to DNA as hetero- and homodimers and recruit co-activators, such as p300, or co-repressors, such as class II histone deacetylases (HDACs), to regulate target gene expression. MEF2 proteins play essential roles in cardiac and skeletal muscle development, but their role in the nervous system has remained largely unknown. In addition to its initially discovered role as an activity-dependent pro-survival factor in cultured cerebellar granule neurons (Mao et al., 1999), more recent work has shown that MEF2 regulates excitatory synapses (Flavell et al., 2006; Shalizi et al., 2007; Shalizi et al., 2006). In cultured hippocampal neurons, MEF2 activity negatively regulates excitatory synapse density in part by promoting activity-dependent synapse elimination (Flavell et al., 2006). MEF2 activity in hippocampal neurons is stimulated by glutamatergic synaptic activity, which stimulates Ca2+ influx via L-type voltage-sensitive Ca2+ channels (LT-VSCCs) and activation of Ca2+/calmodulin (Ca2+/CaM)-dependent signaling pathways. Ca2+/CaM then stimulates the protein phosphatase, calcineurin (also referred to as protein phosphatase 2B) to dephosphorylate MEF2 proteins at a number of sites, including the inhibitory Cdk5 sites on MEF2A and MEF2D (Ser408 and Ser444, respectively), to promote MEF2 activation (Flavell et al., 2006; Gong et al., 2003; Mao and Wiedmann, 1999). Therefore, MEF2 activity appears to be regulated in part by the balance of protein kinase (Cdk5 phosphorylation of Ser408/444) and protein phosphatase (calcineurin dephosphorylation of Ser408/444) activities toward the conserved Cdk5 site. Since chronic cocaine exposure increases Cdk5 activity, glutamatergic transmission, and synapse density in the NAc, we investigated the potential involvement of MEF2 in the long-lasting cocaine-induced structural changes in dendritic spine density.
In this study, we present evidence that cocaine exposure suppresses striatal MEF2 activity in part through cAMP-dependent inhibition of calcineurin activity. We show that reduction of NAc MEF2 activity is required for cocaine-induced increases in dendritic spine density. Surprisingly, we find that expressing a hyperactive version of MEF2 in the NAc, which blocks the cocaine-induced spine density increase, enhances behavioral responses to cocaine, whereas reduction of endogenous MEF2 proteins attenuates these behaviors. Finally, using a genome-wide ChIP-chip analysis of MEF2 binding in the NAc and gene microarray analysis of cocaine regulated genes, we identify a number of MEF2 gene targets, including a catalytic subunit of PI3-kinase, which may play important roles in regulating structural and behavioral plasticity in the NAc after cocaine exposure. Together, our findings reveal a novel role for MEF2 transcription factors in regulating aspects of addiction biology through which we observe an important disconnect between dendritic spine density in the NAc and addiction-related behaviors.
Results
MEF2 Transcription Factors in the NAc
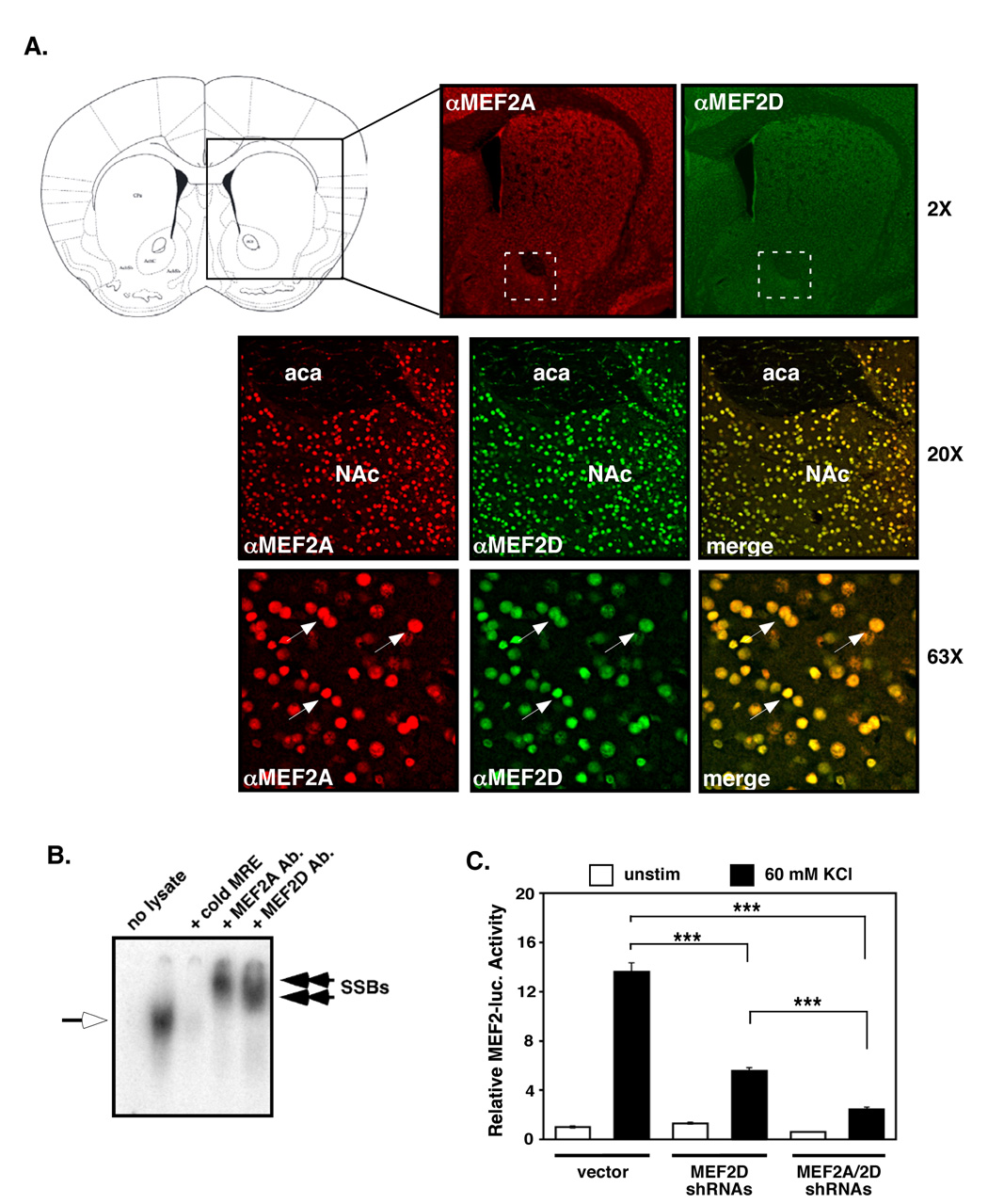
To test the role of MEF2-dependent transcription in cocaine-induced NAc dendritic spine plasticity, we first analyzed the expression of MEF2 proteins in the adult striatum. We observed strong, nuclear MEF2A and MEF2D immunostaining throughout the striatum, including the NAc (Fig. 1A, and Supplemental Figs. S1). MEF2A and MEF2D proteins appear to account for most of the MEF2 DNA binding activity in the NAc, since NAc extracts incubated with either anti-MEF2A- or anti–MEF2D-specific antibodies (see Supplemental Figs. S2B and S2C) show completely “supershifted” consensus MEF2 response element (MRE)-binding activity in electrophoretic mobility shift assays (EMSA) (Fig. 1B). This also suggests that MEF2A and MEF2D bind to DNA as heterodimers in the striatum. Moreover, expression of short-hairpin RNAs (shRNAs) against both MEF2A and MEF2D in cultured striatal neurons reduces the ability of K+ depolarization to induce endogenous MEF2-dependent transcription by ~90% (Fig. 1C), further indicating that MEF2A and MEF2D account for most of the depolarization-dependent MEF2 activity in the NAc.
Figure 1. MEF2A and MEF2D are highly expressed in the adult striatum.
(A) Immunohistochemistry for MEF2A and MEF2D demonstrate strong nuclear staining throughout the adult striatum. MEF2A and MEF2D co-localize in the nucleus of most striatal neurons. (B) Electrophoretic mobility shift assays (EMSA) were performed on 10 µg of NAc lysate and 32P-labeled MRE duplex oligos. The shifted MRE band (open arrow) was competed away with excess, unlabelled MRE. Pre-incubation with anti-MEF2A or anti-MEF2D antibodies results in supershifted MRE bands (SSBs, closed arrows) that migrate more slowly in the native gel. (C) Cultured striatal neurons transfected with an MRE-luciferase reporter plasmid were co-transfected with either plasmids expressing MEF2A and MEF2D specific shRNAs or vector alone. Reduction of MEF2A and MEF2D significantly reduces both basal and membrane depolarization (60 mM KCl)-induced MEF2 activity (***p<0.001, n=9, three independent experiments).
Chronic Cocaine Exposure Increases MEF2 Phosphorylation at its Inhibitory Cdk5 Site
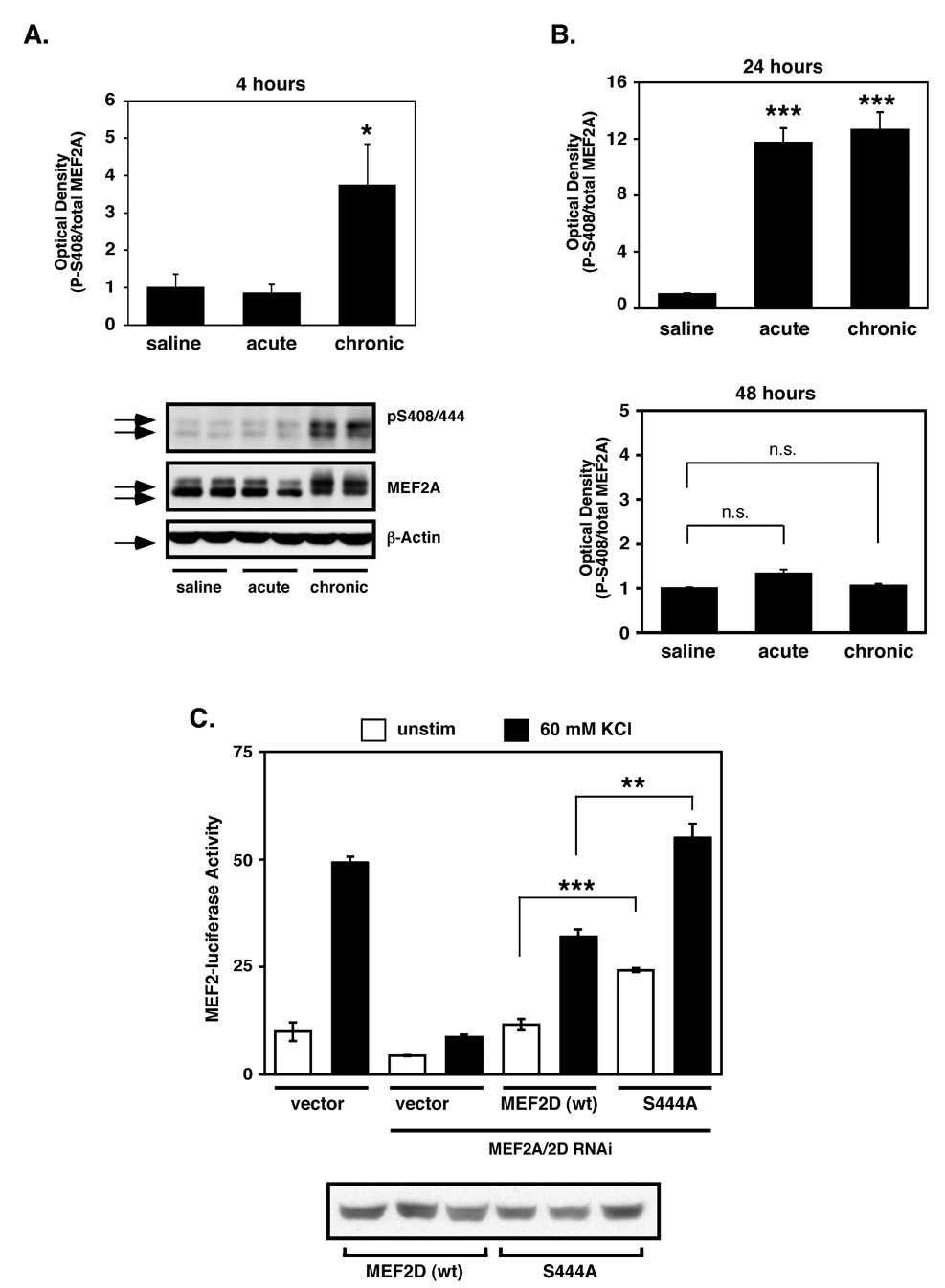
Since chronic cocaine administration increases Cdk5 activity in the NAc (Bibb et al., 2001), and Cdk5 phosphorylates MEF2 to suppress its activity in cultured cerebellar granular neurons (Gong et al., 2003), we hypothesized that MEF2 activity in the NAc might be attenuated by cocaine treatment. To test this idea, we injected adult rats daily for 7 days with saline (chronic saline) or cocaine (chronic cocaine), with acute cocaine-treated animals receiving 6 days of saline followed by a single cocaine injection on day 7. The animals were then analyzed 4, 24 or 48 hours after the last injection, and the striatum and cerebellum were isolated for western blot analysis with a phosphorylation site-specific antibody to P-Ser408/444 (MEF2A/2D) (Flavell et al., 2006). At 4 hours post-injection, we observed a robust increase in MEF2 P-Ser408/444 levels in striatal lysates of rats treated with chronic cocaine compared to those treated with saline or acute cocaine (Fig. 2A). The chronic cocaine-induced hyperphosphorylation of MEF2 in the striatum persisted at 24 hours after chronic cocaine exposure (Fig. 2B). Similarly, we observed a strong increase in P-MEF2 after a single acute injection of cocaine at 24 hours, suggesting that there is a delayed upregulation of P-MEF2 that is not yet observed at 4 hours after an acute injection. Moreover, these findings suggest that the increase of P-MEF2 at 4 hours after chronic cocaine represents a maintained level of MEF2 phosphorylation observed after the previous cocaine exposure. However, the inhibitory P-MEF2 levels returned to baseline conditions 48hrs after an acute or chronic course of cocaine (Fig. 2B), suggesting that active, repeated drug exposure is needed to maintain suppression of MEF2 activity. In contrast to the striatum, neither acute nor chronic cocaine injections significantly altered MEF2 P-S408/444 levels in the cerebellum at any time point examined (Supplemental Fig. S4A and data not shown). Moreover, using an RNAi-based protein replacement assay (Flavell et al., 2006) in cultured striatal neurons, we found that MEF2 phosphorylation site mutants (i.e. S408A (MEF2A) or S444A (MEF2D)) were hyperactive compared to wild-type MEF2 (Fig. 2C and Supplemental Figure S3A), suggesting that endogenous striatal MEF2 activity is normally regulated by phosphorylation at these Cdk5 sites. Taken together, these data suggest that cocaine-induced MEF2 phosphorylation at Ser408/444 in striatum, in vivo, suppresses its transcriptional activity.
Figure 2. Chronic cocaine upregulates inhibitory MEF2 phosphorylation at Ser408/444.
(A) Western blots using a MEF2A/2D phospho-S408/444-specific antibody demonstrate that chronic cocaine administration significantly increases MEF2 P-S408/444 phosphorylation in striatum 4 hours after the last injection (*p<0.05, n=3). (B) Quantification of MEF2 P-S408/444 western blotting at 24 hours after the last injection reveal that both acute and chronic cocaine significantly increase striatal P-MEF2 levels (***p<0.001, n=4–5). P-MEF2 levels returns to control levels by 48 hours after the final dose (p>0.05, n=4–5). (C) RNAi-based protein replacement assays comparing wild-type and S444A MEF2D activity. Expression of MEF2D S444A results in significantly elevated basal and KCl-induced MEF2-dependent transcription in MEF2-luciferase assays (***p<0.001 and ** p<0.01, respectively, n=3) (top). Similar effects are observed for the MEF2A phospho-mutant (S408A) (Supplemental Fig. S3A). Anti-MEF2D western blots of HEK-293T total cell lysates of cultures transfected with equal amounts of expression plasmids (bottom).
MEF2 Regulates Dendritic Spine Density in the NAc
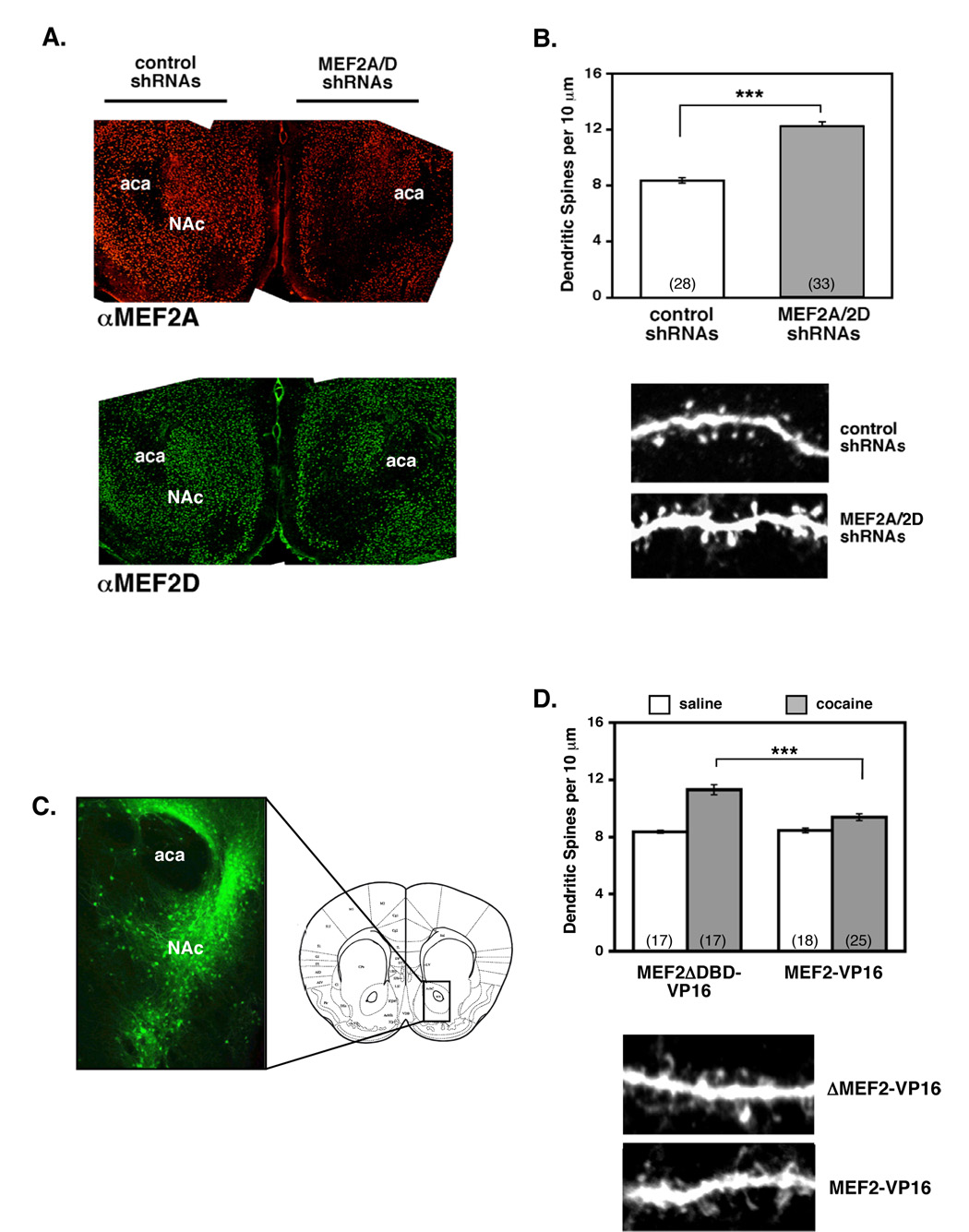
Since MEF2 activity regulates dendritic spine density and excitatory synapses in cultured hippocampal neurons (Flavell et al., 2006), we hypothesized that the cocaine-induced decrease in MEF2 activity in the striatum may contribute to the increased density of dendritic spines seen after chronic cocaine exposure. To test this hypothesis, we generated RNAi-expressing adeno-associated viruses (AAVs) to reduce MEF2 levels in the NAc in vivo. As negative controls, we generated similar AAV-shRNA viruses that expressed MEF2 shRNA with point mutations that prevented their recognition of endogenous MEF2A/2D mRNAs. All AAV-shRNA constructs co-expressed EGFP or mCherry, which allowed us to clearly visualize dendritic spines after immunostaining. We co-injected MEF2A and MEF2D shRNA viruses into the NAc on one side of the brain and co-injected the mutant shRNA control viruses into the contralateral NAc. By 2–3 weeks post-injection, when transgene expression is maximal, we observed a strong, sustained reduction of MEF2A and MEF2D protein in the NAc (Fig. 3A and Supplemental Fig. S6A). In MEF2 shRNA- or mutMEF2 shRNA-infected neurons, we observed no evidence of neuronal apoptosis (by nuclear or dendritic morphology or TUNEL staining, data not shown), suggesting that MEF2 activity is not required for NAc MSN survival in vivo.
Figure 3. Cocaine regulation of MEF2 activity is necessary and sufficient to regulate NAc dendritic spine density in vivo.
(A) Representative images of MEF2A (top) and MEF2D (bottom) immunostaining 28 days after stereotactic delivery of control AAV-shRNAs (left) or AAV-shRNAs against MEF2A/2D into the NAc. Coronal sections through striatum reveal dramatic knockdown of both MEF2A and MEF2D within the NAc (right). (B) RNAi-mediated reduction of MEF2A and MEF2D in the NAc significantly increases dendritic spine density in saline-treated mice (8.35+/−0.20 vs. 12.24+/−0.33, ***p<0.001). Representative confocal scans of NAc medium spiny neurons infected with either control or MEF2A/2D shRNAs (bottom). Dendritic spines were counted manually from confocal z-stacks of optically sectioned secondary and tertiary dendrites. Data represent spine density analysis from 4 saline-injected mice (4 weeks). (C) Representative image of AAV-MEF2-VP16 infection in the NAc 19 days after stereotactic delivery of virus. The bicistronically co-expressed GFP was visualized by immunohistochemistry. (D) Expression of MEF2-VP16 in the NAc significantly blocked cocaine-induced increases in dendritic spine density. Repeated cocaine injections (4 weeks at 20 mg/kg) induced a significant increase in NAc dendritic spine density compared to chronic saline in mice infected with the control MEF2ΔDBD-VP16 virus in their NAc (11.31 +/−0.34 vs. 8.36 +/−0.11; cocaine vs. saline, ***p<0.001). Expression of constitutively-active MEF2 (MEF2-VP16) significantly blocked the cocaine-induced increase in dendritic spine density in the NAc (11.31 +/−0.34 vs. 9.37 +/−0.24; control cocaine vs. MEF2-VP16 cocaine, ***p<0.001), but did not affect basal NAc dendritic spine density in saline-treated mice (8.36 +/−0.11 vs. 8.46 +/−0.17; control saline vs. MEF2-V16 saline, p>0.05). Representative confocal scans of NAc medium spiny neurons infected with either AAVs expressing control MEF2ΔDBD-VP16 or wild-type MEF2-VP16 (bottom). Data represent spine density analysis from 4 mice per condition (cocaine vs. saline).
Using an established cocaine injection protocol to induce dendritic spines in the NAc (Norrholm et al., 2003), we injected mice once daily with cocaine or saline for 4 weeks before analyzing NAc MSN dendritic spine density. We analyzed dendritic spine density of GFP-positive MSNs using serial optical sections (z-stacks) gathered by laser scanning confocal microscopy, and spine density was manually determined by two independent investigators (blind to the conditions). For this study, detectable dendritic shaft protrusions were counted as dendritic spines; they were not analyzed for dendritic spine sub-type or spine length/volume. We found that in both saline- and cocaine-injected mice, MEF2A/2D shRNA-expressing neurons had significantly higher NAc MSN dendritic spine density than the control neurons (Fig. 3B; Supplemental Fig. S6C). These results indicate that reducing MEF2 activity in NAc is sufficient to increase MSN dendritic spine density, and reveal an important role for MEF2 in regulating basal dendritic spine density in the NAc in vivo.
We next tested whether suppression of MEF2 activity by cocaine is required for the cocaine-induced increase in NAc MSN dendritic spine density. To this end, we produced AAVs that co-express EGFP together with a constitutively-active form of MEF2 (MEF2-VP16) or a DNA binding-deficient negative control (AAV-MEF2ΔDBD-VP16). MEF2-VP16 is a fusion between the MEF2 DNA binding and dimerization domains and the basal transcription activation domain of the viral transcription factor VP16 (Black et al., 1996). In cultured striatal neurons, MEF2-VP16 increased MEF2-dependent transcription, whereas MEF2DBD-VP16 or vector control had no effect (Supplemental Fig. S7). Similar to the MEF2 RNAi experiments described above, we then injected either the AAV-MEF2-VP16 or the control AAV-MEF2ΔDBD-VP16 virus into the NAc on opposite sides of the brain. After a three-week recovery and expression period, we injected the mice daily with either saline or cocaine for 4 weeks before analyzing NAc spine density of GFP-expressing neurons. Compared to the saline-injected mice, we found that cocaine significantly increased the spine density of NAc neurons infected by the control MEF2ΔDBD-VP16 virus (Fig. 3D). In contrast, expression of constitutively-active MEF2 in the NAc blocked the ability of chronic cocaine to increase NAc MSN spine density (Fig. 3D). Therefore, these combined observations indicate that reduction of MEF2 activity is required to increase NAc dendritic spine density in vivo, and suggest that chronic cocaine exposure regulates NAc spine density in large part by reducing MEF2 activity.
MEF2 Activity in the NAc Modulates Behavioral Responses to Cocaine
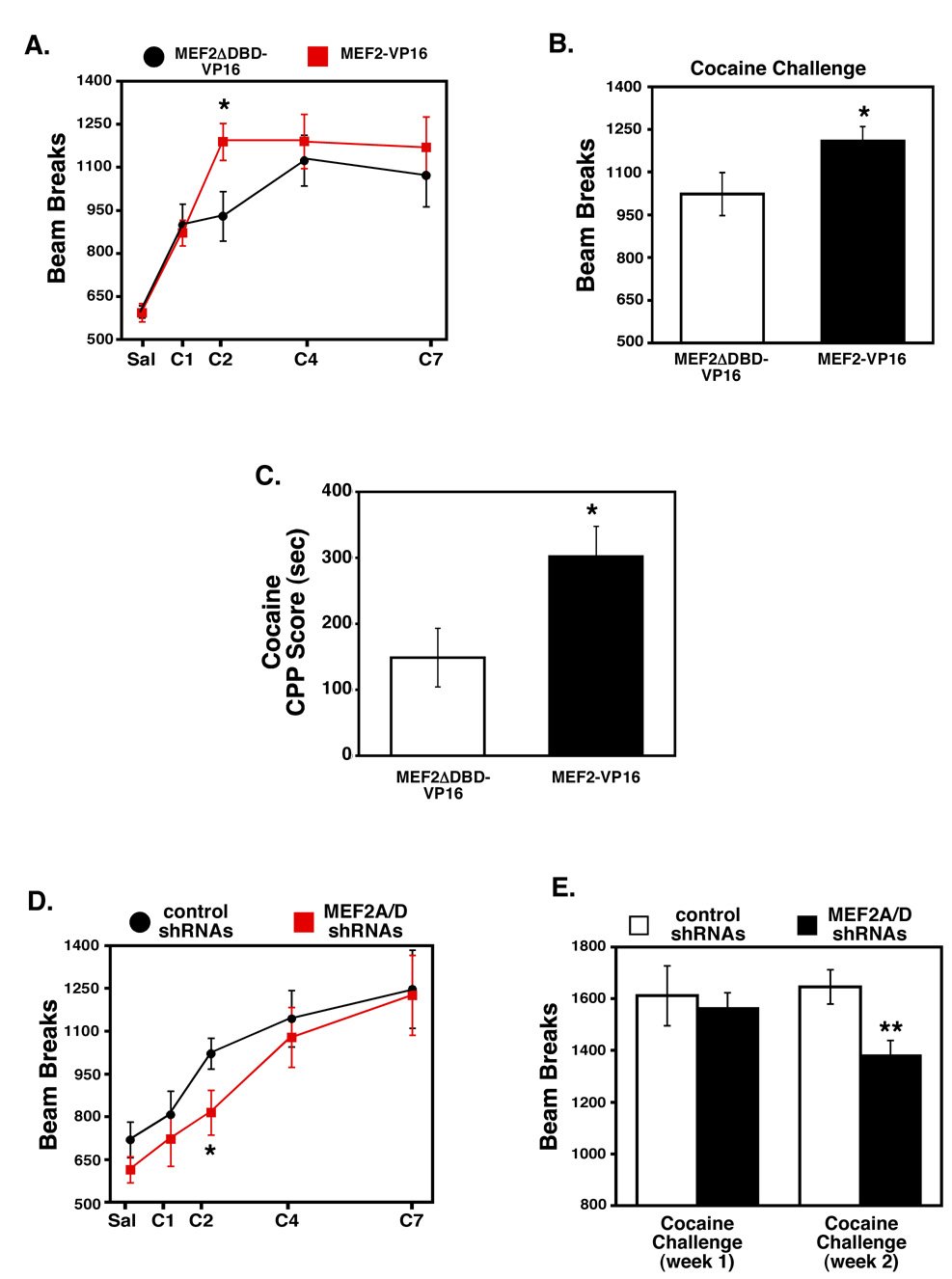
Regulation of MEF2 activity has a potent effect on NAc dendritic spine density; therefore, we sought to test the functional relationship between MEF2-dependent NAc spine plasticity and sensitized behavioral responses to cocaine in vivo. We hypothesized that bilateral expression of MEF2-VP16, which blocked the cocaine-induced spine increase in the NAc, might reduce cocaine-induced behaviors. Contrary to this hypothesis, however, we found that NAc expression of constitutively-active MEF2 enhanced locomotor responses to cocaine (Figs. 4A–C). Specifically, mice expressing MEF2-VP16 in the NAc responded normally to an initial injection of cocaine, but demonstrated significantly higher locomotor responses to cocaine on day 2 compared to control mice (Fig. 4A), and trended higher than the control mice for the duration of the injections. After one week of withdrawal, the MEF2-VP16-expressing mice remained significantly more sensitive to a cocaine challenge dose than the control mice (Fig. 4B). Consistent with the sensitizing effect of MEF2-VP16 expression in the NAc on cocaine-induced locomotor behaviors, MEF2-VP16 expression also sensitizes mice to the rewarding effects of cocaine as measured by conditioned place preference. That is, mice overexpressing MEF2-VP16 in their NAc spend significantly more time in a cocaine-paired vs. a saline-paired environment (Fig. 4C). These findings suggest that enhanced MEF2 activity in the NAc, a condition that blocks cocaine-induced spine increases, promotes acquisition and maintenance of cocaine-induced locomotor sensitization as well as strengthens the rewarding effects of the drug.
Figure 4. MEF2 activity in the NAc modulates behavioral responses to cocaine.
(A) Viral-mediated expression of MEF2-VP16 in the NAc significantly increases sensitivity to repeated cocaine administration. Mice expressing MEF2-VP16 in the NAc have normal locomotor response to saline and the first cocaine injection (15 mg/kg), but are significantly more sensitive to the subsequent dose compared to the MEF2-VP16 DNA binding mutant control (MEF2ΔDBD-VP16) (*p<0.05, n=9–10, Student's t-test on day 2). (B) Mice expressing MEF2-VP16 in their NAc remain significantly more sensitive to a challenge dose of cocaine (15 mg/kg) after one week of withdrawal (*p<0.05, n=9–10, Student's t-test). (C) Mice expressing MEF2-VP16 in the NAc spend more time in a cocaine-paired (8 mg/kg) environment as measured by conditioned place preference (*p<0.05, n = 13, Student's t-test). (D) Viral-mediated knockdown of MEF2A/2D in the NAc significantly reduces sensitivity to repeated cocaine administration. Mice expressing shRNAs against MEF2A/2D in the NAc have normal locomotor responses to saline and the first cocaine injection (15 mg/kg) but are significantly more sensitive to the subsequent dose compared to mice expressing control shRNAs (*p<0.05, n=9–11, Student's t-test on day 2). (E) Mice expressing shRNAs against MEF2A/2D in the NAc show significantly less cocaine-induced locomotor activity in response to a challenge dose (15 mg/kg) given two weeks after the acquisition of cocaine sensitization (**p<0.01, n=9–11, Student's t-test). RNAi-mediated reduction of MEF2A/2D in the NAc has only a slight trend towards reducing locomotor responses to a challenge dose of cocaine (15 mg/kg) one week after acquisition of cocaine sensitization (p>0.05, n=9–11).
As described earlier, cocaine induces the hyperphosphorylation of MEF2 in the NAc, which presumably suppresses its transcriptional activity. To explore the behavioral consequences of suppressing MEF2 activity in the NAc, we co-infected this region of adult mice bilaterally with AAV-MEF2A/2D shRNAs or mutant shRNA control viruses and tested their locomotor responses to repeated cocaine injections. Reducing MEF2 levels in the NAc, which was sufficient to increase dendritic spine density, both delayed the acquisition of cocaine-induced locomotor sensitization and attenuated sensitized behavioral responses to a cocaine challenge dose after two weeks of withdrawal (Fig. 4D and 4E). These observations are in agreement with the opposite behavioral responses observed in mice with increased NAc MEF2 activity (Fig. 4A–B).
In sum, these data reveal that MEF2 activity modulates the acquisition and maintenance of sensitized behavioral responses to cocaine. Unexpectedly, we find that enhanced MEF2 activity in the NAc, a condition that blocks the cocaine-induced dendritic spine density increase, actually enhances sensitized behavioral responses to cocaine. These findings suggest that the cocaine-induced increase in spine density is not required for locomotor sensitization and reward learning, but may instead represent a compensatory process to limit maladaptive behavioral changes.
Dopamine D1 Receptor Signaling Inhibits MEF2 Activity in Striatal Neurons
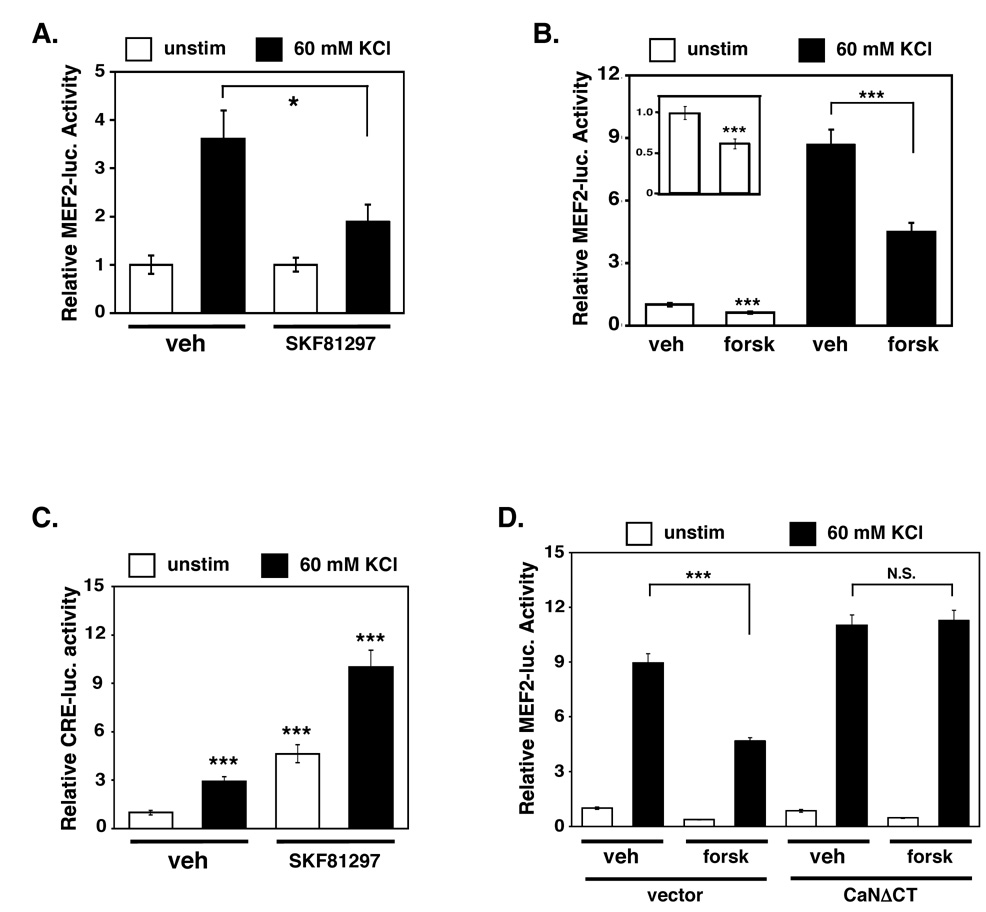
Since cocaine exposure upregulates an inhibitory phosphorylation event on MEF2 in the striatum in vivo, we sought to explore molecular signaling events by which cocaine might regulate MEF2 activity in the striatum. To this end, we cultured dissociated striatal neurons and transfected them with a MEF2-luciferase reporter plasmid (MEF2-luc) in order to measure endogenous MEF2 activity. Since cocaine exposure has been associated with increased glutamatergic and dopaminergic neurotransmission in the striatum, we studied whether these stimuli regulate MEF2-dependent transcription in striatal neurons. We found that membrane depolarization (60 mM KCl), which, like glutamate, stimulates calcium influx, dramatically increased MEF2-dependent transcription (Fig. 5A). This activation of MEF2 required calcium influx through L-type voltage sensitive calcium channels (LT-VSCCs) and calcineurin phosphatase activity (Supplemental Figs. S5A and S5B). While the stimulation of dopamine D1 receptors (10 µM SKF81297) alone had little effect on basal MEF2 activity, dopamine D1 receptor stimulation significantly attenuated the activation of MEF2 upon membrane depolarization (KCl) (Fig. 5A). To test whether the suppression of MEF2 activity by dopamine D1 receptor signaling was due to elevation of cAMP, we treated the cultures with the adenylyl cyclase-activator, forskolin (10 µM), and observed a similar reduction of calcium-induced MEF2 activity (Fig. 5B). This suggests that activated dopamine D1 receptors increase cAMP levels, which in turn antagonize calcium-dependent activation of MEF2 in striatal neurons.
Figure 5. Dopamine D1 receptor signaling and cAMP reduces calcium-dependent activation of MEF2 in cultured striatal neurons.
(A) Dopamine D1 receptor stimulation (SKF81297, 10 µM) significantly reduces calcium-dependent activation of MEF2-luciferase activity in cultured striatal neurons (*p<0.05; n=15, five independent experiments, Student's t-test). (B) Forskolin (forsk) treatment (10 µM) of cultured striatal neurons significantly attenuates basal and KCl-induced MRE-luciferase activity. The inset shows the effect of forskolin on basal MRE-luciferase activity over a smaller scale (***p<0.001; n=21, seven independent experiments, Student's t-test). (C) Membrane depolarization (60 mM KCl) of cultured striatal neurons significantly increases CRE-luciferase activity. Treatment with dopamine D1 receptor agonist (SKF81297, 10 µM) significantly increases basal and KCl-induced CREB activity in cultured striatal neurons (***p<0.001; n=9, three independent experiments). (D) Constitutively-active calcineurin (CaNΔCT) blocks the inhibitory effect of forskolin on KCl-induced MEF2 activity (***p<0.001 or N.S. (p>0.05); n=6, two independent experiments, Student's t-test).
As a point of comparison, we also analyzed endogenous CREB activity using a CRE (cAMP response element)-luciferase reporter plasmid. As expected, CREB activity is significantly increased in our striatal cultures by either the dopamine D1 receptor agonist or upon membrane depolarization (Fig. 5C). Unlike MEF2, however, combining membrane depolarization (KCl) with dopamine D1 receptor activation synergistically activated CREB-dependent transcription (Fig. 5C). These findings suggest that calcium signaling and dopamine signaling cooperate to activate CREB activity in striatal neurons while combining to reduce activation of MEF2, which is consistent with recent findings in hippocampal neurons (Belfield et al., 2006). Interestingly, several studies suggest that cocaine-induced CREB activity serves to limit behavioral responses to cocaine (Carlezon et al., 1998; Dong et al., 2006). Therefore, dopamine signaling may differentially regulate CREB and MEF2 activities to limit sensitized behavioral responses to cocaine.
Dopamine and Calcium Signaling Regulates MEF2 Activity through Calcineurin and RCS
Since membrane depolarization stimulates MEF2 activity in striatal cultures through a calcineurin-dependent mechanism (Supplemental Fig. S5B), we speculated that dopamine signaling might regulate MEF2 activity by reducing calcineurin activity. To test this possibility, we transfected striatal neurons with constitutively-active, Ca2+/CaM-independent calcineurin (CaNΔCT) and measured the effect of cAMP signaling on calcium-dependent MEF2 activation. We observed that expression of CaNΔCT blocked the forskolin-dependent inhibition of MEF2 activity (Fig. 5D), which indicates that cAMP signaling attenuates MEF2 in part by reducing Ca2+/CaM-calcineurin activity.
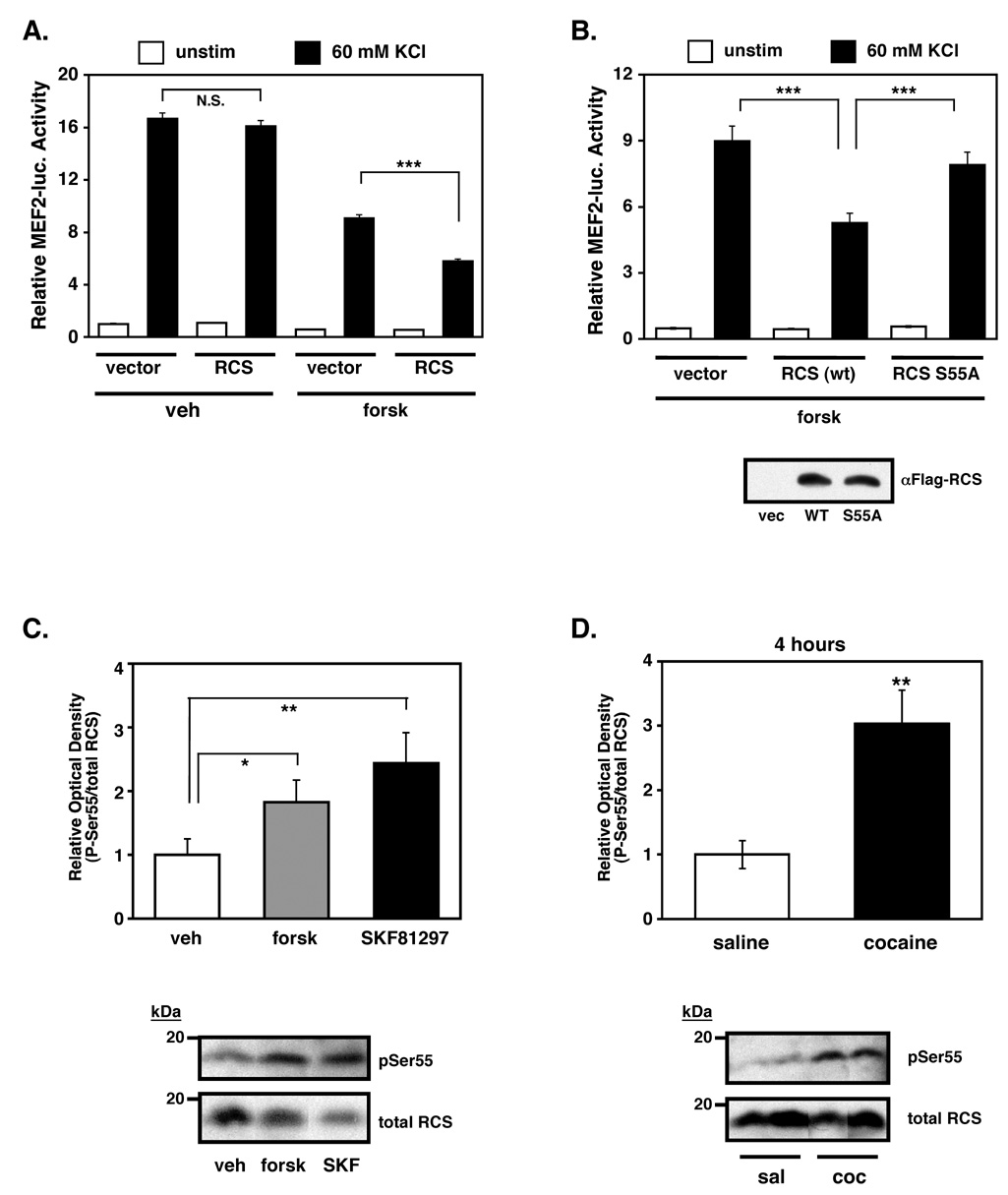
Recently, the regulator of calmodulin signaling (RCS) was shown to negatively regulate calcineurin activity in striatal neurons (Rakhilin et al., 2004). Activation of the dopamine D1 receptor stimulates PKA-dependent phosphorylation of RCS at Ser55, which induces direct interaction of phospho-RCS with Ca2+/CaM and competitive inhibition of calcineurin activity (Rakhilin et al., 2004). To test whether RCS regulates MEF2 activity, we expressed RCS in striatal neurons and found that in the presence of forskolin, RCS inhibited calcium-stimulated MEF2 activity compared to the effect of forskolin alone (Fig. 6A, right). Notably, RCS had no effect on calcium-inducible MEF2 activity in the absence of forskolin (Fig. 6A, left), suggesting that RCS phosphorylation by PKA is necessary to inhibit MEF2 activity. Indeed, the cAMP-dependent inhibition of MEF2 activity by RCS required phosphorylation at Ser55 (PKA site) since a non-phosphorylatable RCS mutant (S55A) failed to suppress MEF2 activity in the presence of forskolin (Fig. 6B). Importantly, the inhibition of MEF2 activity by cAMP/RCS is partial, and might therefore indicate that P-RCS levels are limited and/or that additional cAMP-insensitive mechanisms regulate calcineurin/MEF2 activity.
Figure 6. RCS mediates cAMP-dependent suppression of MEF2 activity.
(A) Overexpression of RCS significantly enhances forskolin-induced inhibition of MEF2 activity. Cultured striatal neurons were transfected with a Flag-tagged RCS expression plasmid or vector and stimulated with either vehicle or forskolin (10 µM). Overexpression of RCS did not alter KCl-induced MRE-luciferase activity, but significantly potentiated the repressive effects of forskolin on KCl-induced activity (***p<0.001, n=12, four independent experiments, Student's t-test). (B) Phosphorylation of RCS at its protein kinase A (PKA) site is necessary for forskolin-induced inhibition of MEF2 activity. Cultured striatal neurons were transfected with either wild-type RCS or a point mutant of RCS (S55A) that cannot be activated by PKA. Neurons were then treated with forskolin alone or forskolin + 60 mM KCl. The enhanced suppression of MEF2 by wild-type RCS expression is not observed by expression of RCS S55A (***p<0.001, n=6, two independent experiments, Student's t-test). Anti-Flag western blots showing equal expression of wild-type RCS and mutant RCS in HEK-293T cell lysates transfected with equal amounts of the respective plasmids (bottom). (C) Adult striatal slices treated with forskolin (50 µM) or SKF81297 (10 µM) for 10 minutes significantly increased P-Ser55 RCS levels (*p<0.05 and **p<0.01, respectively, Student's t-test). (D) Repeated cocaine administration (7 days of daily IP injections of 20 mg/kg cocaine) significantly increased RCS Ser55 phosphorylation in the striatum at 4 hours after the last injection (cocaine vs. saline, **p<0.01, n=6, Student's t-test).
To determine if the cAMP/RCS signaling pathway might regulate MEF2 in the adult striatum in vivo, we generated phosphorylation site-specific antibodies to P-Ser55 RCS (Supplemental Fig. S8A). We found that treatment of acute, adult striatal slices with either forskolin or SKF81297 increased levels of P-RCS (Fig. 6C). Similarly, we analyzed striatal lysates from rats treated repeatedly with saline or cocaine for one week by western blotting. Similar to P-MEF2, chronic cocaine exposure increased P-RCS levels at 4 hours and 24 hours after the last injection (Fig. 6D and Supplemental Fig. S8B). Together, these findings suggest that cocaine regulates MEF2 activity in the striatum through activation of a dopamine- and cAMP-dependent signaling cascade that functions, at least in part, by RCS-dependent suppression of calcineurin activity.
Genome-wide Analysis of MEF2 Promoter Binding Identifies Novel Targets That Regulate Structural Plasticity
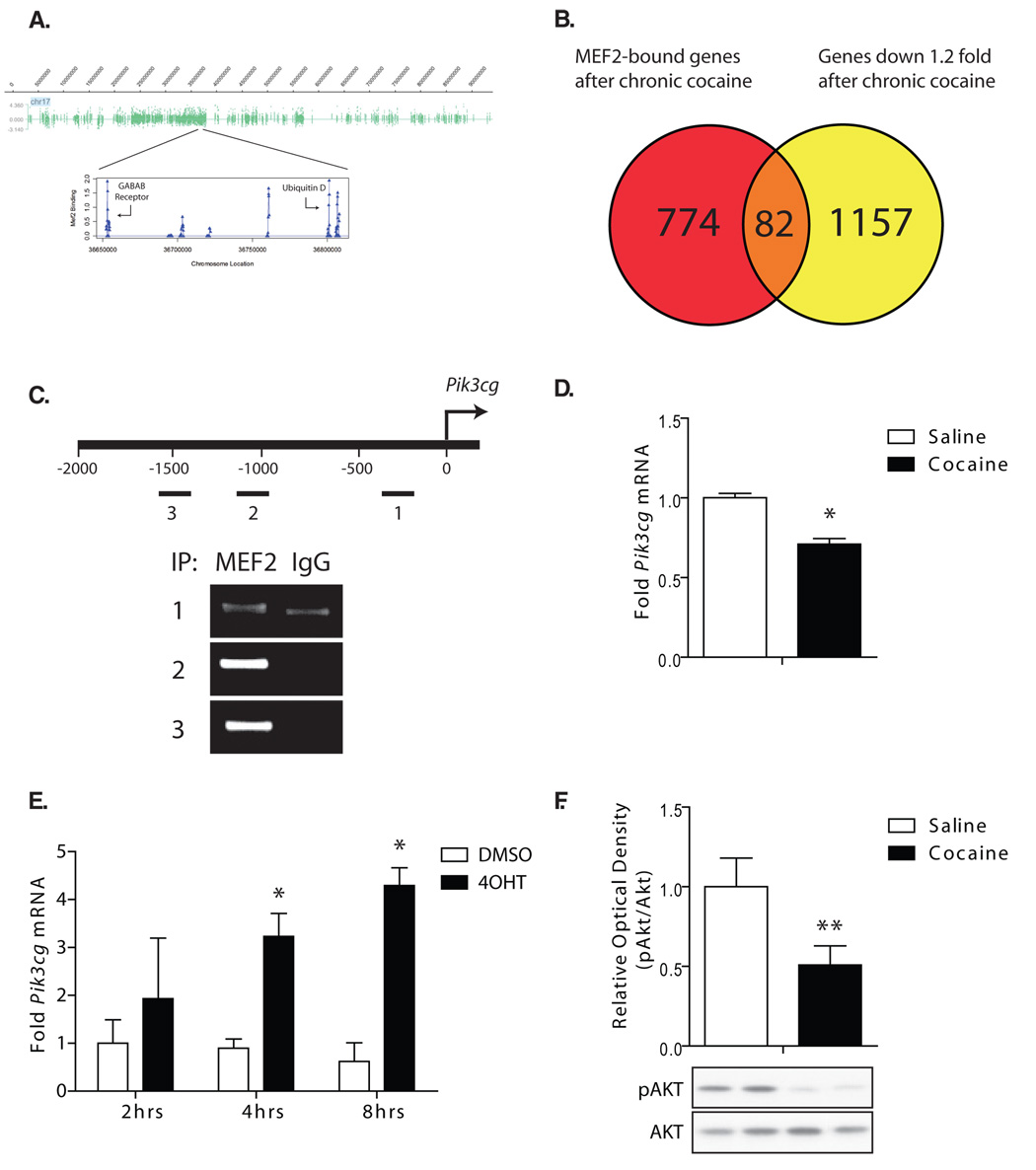
To identify MEF2 gene targets in vivo that ultimately mediate MEF2’s effects on dendritic spine plasticity and behavioral responses to cocaine, we isolated NAc from chronic cocaine-treated mice and performed chromatin immunoprecipitation (ChIP) using a MEF2A antibody or IgG control. Immunoprecipitated DNA was then amplified and hybridized to genome-wide promoter arrays to identify potential MEF2 target genes. An example of the genome-wide MEF2-binding data is shown for chromosome 17 (Fig. 7A). We found that in cocaine-treated mice, MEF2 significantly binds to the promoters of ~900 genes in the NAc at a significance level of P < 0.0001.
Figure 7. ChIP-chip analysis of MEF2 in the NAc reveals potential target genes involved in dendritic plasticity and cocaine sensitization.
(A) MEF2 binding in the NAc of chronic cocaine-treated mice is displayed (log2 ratio) along chromosome 17. One region is magnified to display the MEF2 target genes, GABAB receptor and the ubiquitin protein, Ubd. (B) Venn diagram illustrating the overlap between the genes on which MEF2 is significantly enriched and the genes whose expression is downregulated >1.2 fold by chronic cocaine in the nucleus accumbens. (C) The location of 3 MEF2-response element (MRE)-like regions (labeled 1–3) are shown on the gene promoter of pik3cg, a gene both significantly bound by MEF2 and downregulated >1.2 fold by cocaine. Semi-quantitative ChIP demonstrates enrichment of MEF2 at each of these regions over the IgG control. (D) Confirmation that Pik3cg mRNA is significantly downregulated by cocaine in an independent set of mice (Student's t-test, *P < 0.05, n=6). (E) PC12 cells transfected with a plasmid expressing MEF2-VP16-ERtm, which allows the inducible expression of MEF2-VP16 upon addition of 4-hydroxytomaxifen (4OHT), were treated with 4OHT or DMSO for 2hrs, 4hrs, or 8hrs, and Pik3cg mRNA levels were quantified by qRT-PCR. Activation of MEF2-VP16 expression significantly upregulated Pik3cg mRNA at 4hrs and 8hrs (*p<0.05, n=3, Student's t-test) compared to its DMSO control. (F) Chronic cocaine (7 days × 20 mg/kg daily IP injections) significantly reduced Akt phosphorylation (Ser473) in the NAc at 4 hours after the final injection (**p<0.01, n=8–11, Students t-test).
To explore the ascribed cellular processes that these MEF2-bound genes regulate, we performed Ingenuity pathway analysis on the significant MEF2-bound genes. We identified several highly-enriched signaling pathways and cellular processes, including actin cytoskeleton signaling and cAMP signaling, which are known to have pronounced effects on dendritic structural plasticity and/or behavioral responses to cocaine (Supplemental Fig. S9A). For example, MEF2 is bound to the proximal promoters of the Wiskott-Aldrich syndrome proteins, N-WASP (Wasl), WAVE3 (Wasf3) and Profilin 1 (Pfn1) (Supplemental Fig S9B and S9C), which have all been shown to regulate F-actin cytoskeletal remodeling in dendritic spines (Ackermann and Matus, 2003; Irie and Yamaguchi, 2002; Pilpel and Segal, 2005; Wegner et al., 2008).
Since chronic cocaine exposure appears to reduce MEF2 activity in the striatum, we compared MEF2-bound genes from our ChIP-chip arrays to a list of genes that are downregulated by chronic cocaine (24 hours after the last injection) as determined from gene expression microarrays performed with the NAc of cocaine-treated vs. saline-treated mice (Renthal et al., 2007). We identified a subset of 82 MEF2-bound genes whose mRNA expression was downregulated >1.2 fold twenty-four hours after repeated cocaine administration (Fig. 7B). From this group of cocaine-regulated and MEF2-bound genes, we focused our attention on one, the PI3-kinase gamma catalytic subunit gene, pik3cg. PI3-kinase activity has been shown to be important for the expression of sensitized locomotor responses to repeated cocaine (Izzo et al., 2002), so we speculated that regulation of pik3cg could have an important impact on behavioral responses to cocaine. Using qRT-PCR, we confirmed that chronic cocaine exposure significantly downregulates pik3cg mRNA in the NAc in an independent cohort of mice (Fig 7D). Using quantitative ChIP on NAc samples from chronic cocaine-treated mice, we found that MEF2 DNA binding to the pik3cg promoter was highly enriched at 3 near-consensus MEF2 response elements (MREs) (Fig. 7C), suggesting that it could significantly regulate the expression of this gene. Consistent with this idea, PC12 cells transfected with a 4-hydroxytamoxifin (4OHT)-inducible MEF2-VP16 construct (MEF2-VP16-ERtm (Flavell et al., 2006)) show significant increases in Pik3cg mRNA after 4OHT treatment (Fig. 7E).
Since MEF2 regulates pik3cg transcription, and chronic cocaine reduces both MEF2 activity and pik3cg levels, we speculated that repeated cocaine exposure might lower PI3-kinase activity in the NAc. Consistent with this idea, we found that chronic cocaine exposure significantly reduced the phosphorylation of Akt, a key PI3-kinase substrate, in the NAc (Fig 7E). Since PI3-kinase activity is important for facilitating expression of cocaine locomotor sensitization (Izzo et al., 2002), then the role of MEF2 in modulating the sensitized responses to cocaine might be due in part to regulation of this important signaling pathway. Interestingly, chronic morphine has been shown to reduce P-Akt levels in the ventral tegmental area (Russo et al., 2007), further supporting an important role for Akt signaling in drug addiction. Together, these data provide the first genome-wide analysis of MEF2 target genes in brain, and describe how a novel MEF2 target gene, pik3cg, might regulate important aspects of the complex biological events that underlie sensitized behavioral responses to drugs of abuse.
Discussion
In this study, we find that repeated cocaine exposure regulates MEF2 transcription factors to control aspects of long-lasting synaptic and behavioral plasticity. Our findings suggest that chronic cocaine exposure reduces MEF2-dependent transcription to promote increased MSN dendritic spine density in the NAc. Surprisingly, this MEF2-controlled increase in dendritic spine density is associated with reduced behavioral sensitivity to cocaine, suggesting that the strong correlation between increases in NAc spine density and sensitized behavioral responses to cocaine may be functionally uncoupled processes. Our findings suggest that repeated cocaine exposure suppresses MEF2 activity in part by a cAMP/RCS-dependent reduction in calcineurin activity, which regulates MEF2 phosphorylation at the inhibitory Cdk5 sites, P-Ser408/444 (Fig. 8). This inhibition of MEF2 through increased P-Ser408/444 levels is also likely influenced by the previously reported up-regulation of Cdk5 levels and activity in the striatum after chronic cocaine exposure (Bibb et al., 2001). Finally, by combining in vivo MEF2 ChIP on genome-wide promoter arrays and gene expression microarrays, we identified a number of putative MEF2-target genes that likely contribute to aspects of cocaine-induced dendritic spine and behavioral plasticity in the NAc.
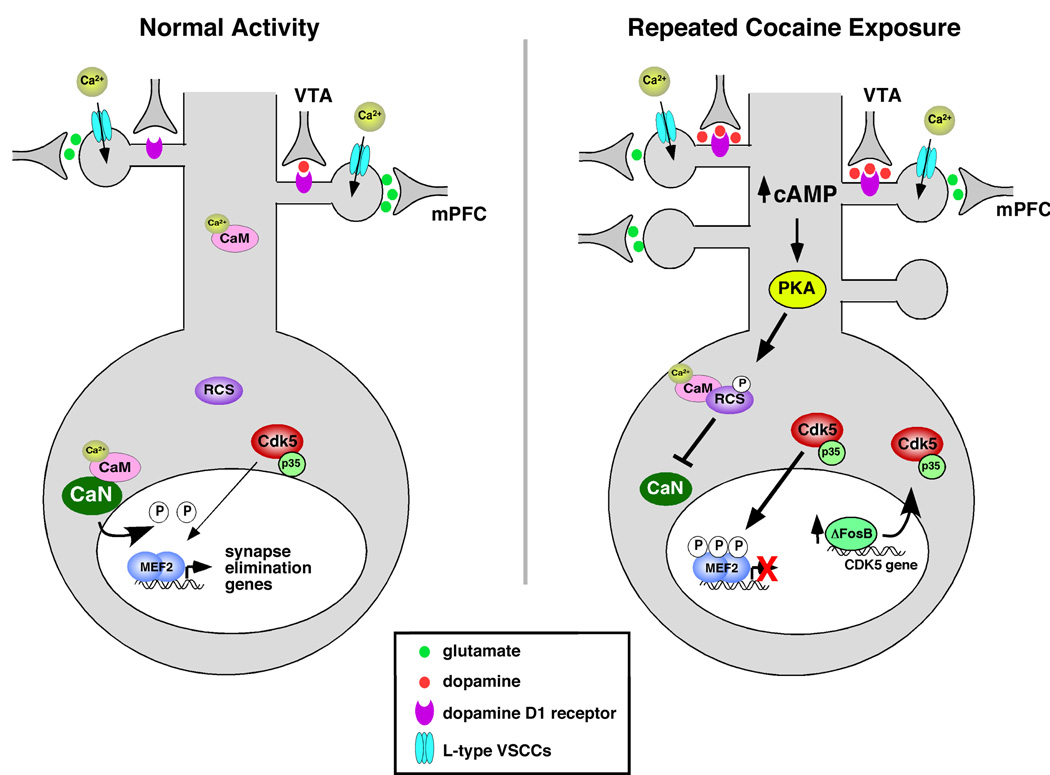
Figure 8.
Model for how cocaine regulates MEF2 in the NAc to alter dendritic spine density and behavioral responses.
Cocaine administration increases the inhibitory Ser408/444 phosphorylation of MEF2A/2D in striatum with a complex time course. A single dose of cocaine is sufficient to induce MEF2 phosphorylation, but is only detected at 24 hours after the first injection. Repeated, daily cocaine injections appear to maintain the levels of MEF2 P-Ser408/444, as it is detected at 4 hours and 24 hours after the last cocaine injection (7-day injection regimen). However, in the absence of reinforcing cocaine injections, we observe that P-S408/444 levels return to baseline by 48 hours after the last injection in the 7-day injection regimen. This indicates that MEF2 inhibitory phosphorylation does not persist in the striatum for as long as the cocaine-induced spine changes that MEF2 activity controls. Moreover, it also suggests that suppression of MEF2 activity by cocaine likely regulates the initiation and maintenance of spine density changes observed during and shortly after chronic cocaine exposure, but may not play a significant role in the maintenance of those spine changes during extended withdrawal periods. In the future, it will be important to explore the regulation and potential role of MEF2 in the maintenance phase of spine plasticity during withdrawal.
Several recent reports have demonstrated an important role for Cdk5 activity in regulating chronic cocaine-induced spine and behavioral plasticity (Benavides et al., 2007; Bibb et al., 2001; Norrholm et al., 2003; Taylor et al., 2007). We show here that MEF2 potently regulates NAc spine plasticity and, as a key target of Cdk5, may mediate some of its downstream effects on synapse and network plasticity. There are a number of interesting parallels between our MEF2 findings and reports in the literature regarding the function of Cdk5 activity in cocaine-induced spine plasticity and behavioral responses (Norrholm et al., 2003; Taylor et al., 2007). Specifically, chronic infusion of roscovitine into the NAc region, which we find increases MEF2 activity in striatal neurons (Supplemental Fig. S3B), blocks the chronic cocaine-induced increase in NAc MSN dendritic spine density (Norrholm et al., 2003). Similarly, we find that increasing MEF2 activity in the NAc (via MEF2-VP16 expression) also blocks the cocaine-induced increase in dendritic spine density (Fig. 3D), suggesting that downstream inhibition of MEF2 by Cdk5 is a key component of roscovitine’s effect on spine plasticity in the NAc. Another interesting parallel between the Cdk5 and MEF2 studies are the recent findings that daily roscovitine injections into the NAc (Taylor et al., 2007) or conditional Cdk5 gene deletion in the NAc (Benavides et al., 2007) enhanced locomotor sensitization to repeated cocaine treatments. Consistent with these observations, we find that enhanced MEF2 activity in the NAc (via MEF2-VP16 expression) increased the locomotor responses to repeated cocaine injections (Fig. 4A–B). Together these studies provide evidence that experimental manipulations that block cocaine-induced increases in NAc dendritic spine density enhance sensitized behavioral responses to cocaine, suggesting that spine density increases are not required for locomotor sensitization and might instead be functioning to antagonize the process of sensitization. It is perhaps not surprising that spine increases are not required for behavioral sensitization since chronic morphine exposure, which elicits long-lasting locomotor sensitization, actually decreases NAc dendritic spine density (Robinson and Kolb, 1999b). In our study, we cannot directly determine whether lower spine density actually causes increased cocaine sensitivity, per se, because the proximal manipulation in our study is MEF2 – not the spines themselves. It is possible that MEF2 could function through independent mechanisms to control cocaine behavior and dendritic spine density. Nevertheless, one can conclude from this and previous reports that cocaine-induced increases in NAc dendritic spine density does not appear to be required for behavioral sensitization to cocaine. This functional relationship between cocaine-induced dendritic spines and behavioral responses suggests that the additional NAc dendritic spines may contribute to homeostatic adaptations that counteract the neural plasticity processes that cause sensitized behavioral responses to cocaine, or that MEF2-regulated spine plasticity might represent an independent process that does not significantly impact sensitized behavioral responses to cocaine.
How does regulation of MEF2-dependent transcription control NAc synapse density? In cultured hippocampal neurons, MEF2-dependent transcription is induced by glutamatergic synaptic activity to promote elimination of existing excitatory synapses (Flavell et al., 2006). As such, it is possible that suppression of MEF2 activity by cocaine in the NAc increases dendritic spine density by reducing the elimination rate of existing synapses rather than increasing the formation rate of new synapses. NAc neurons may therefore rely upon MEF2-dependent transcription to control homeostatic synaptic plasticity after repeated cocaine exposure (Turrigiano, 2007). Indeed, intrinsic neuronal excitability is reduced in the NAc of chronic cocaine-treated animals (Hu et al., 2004), and glutamatergic inputs to the NAc may be decreased after extended drug taking as a result of a decreased mPFC function (hypofrontality), which has been observed in both animal models of addiction and in human brain imaging studies of drug addicts (Jentsch and Taylor, 1999; Volkow et al., 2003). Therefore, it is interesting to speculate that increased NAc dendritic spine density may function homeostatically to compensate for reduced NAc excitability, and ultimately limit an animal’s sensitivity to the cocaine.
Cocaine regulation of MEF2-dependent transcription ultimately mediates structural and behavioral changes in the NAc through the altered expression of downstream target genes. We utilized genome-wide ChIP-chip technology to identify nearly 900 gene promoters in cocaine-treated NAc tissue where MEF2A binding is enriched. Many of the target genes we identified cluster to cellular functions that regulate structural plasticity, such as F-actin remodeling. The MEF2 target genes that encode the proteins, N-WASP, WAVE3, and profilin 1, are all known to potently regulate cytoskeletal remodeling as well as dendritic spine density (Irie and Yamaguchi, 2002; Pilpel and Segal, 2005; Wegner et al., 2008). Importantly, dysregulation of actin polymerization with latrunculin A or a LIM-kinase peptide antagonist in the NAc promoted cocaine reinstatement behaviors in self-administering rats (Toda et al., 2006). Therefore, MEF2 gene targets that regulate actin remodeling may play important roles in sensitized cocaine responses, perhaps independent of the spine changes observed after chronic cocaine exposure.
Another functional group of MEF2-bound genes in the NAc clustered to the PI3-kinase/Akt signaling pathway (Supplemental Fig. S9A). Interestingly, a chemical inhibitor of PI3-kinase delivered ICV (intracerebroventricular) significantly blocked the expression of cocaine locomotor sensitization (Izzo et al., 2002), suggesting that MEF2-dependent regulation of this signaling pathway may play an important role in locomotor sensitization. Since cocaine suppresses MEF2 activity in the NAc, it was notable that a catalytic subunit of PI3-kinase was one of 82 MEF2 target genes downregulated by chronic cocaine exposure (Fig. 7C–D). Pik3cg expression is also potently regulated by MEF2 activity in culture, suggesting that the cocaine-induced suppression of MEF2 activity directly contributes to the downregulation of this gene in the NAc. Consistent with a reduction in Pik3cg mRNA in the NAc after chronic cocaine exposure, we also observed a significant reduction in the phosphorylation state of Akt (Ser473), a key substrate of PI3-kinase activity, in the NAc after chronic cocaine. Moreover, RNAi-based reduction of MEF2 levels in the NAc, which would be expected to reduce Pik3cg expression and PI3-kinase activity, reduces behavioral sensitivity to cocaine much like the infusion of a PI3-kinase inhibitor.
In this study, we found that cocaine administration regulates MEF2-dependent gene transcription in the NAc to control dendritic spine plasticity and behavioral responses to cocaine. We show here that chronic cocaine reduces MEF2 activity through a novel signaling mechanism involving Cdk5, calcineurin and RCS. We find that reducing MEF2 activity in NAc in vivo is required for cocaine-induced increases in dendritic spine density. Our findings also suggest that behavioral sensitization to cocaine is functionally uncoupled from these cocaine-induced increases in dendritic spine density. Taken together, these observations implicate a new transcription factor in the molecular mechanisms controlling cocaine-induced structural and behavioral plasticity and could ultimately lead to the development of improved treatments for drug addiction.
Experimental Procedures
Plasmids
3X MRE-luciferase, pcDNA3-MEF2-VP16, pSuper-MEF2A(1234), pSuper-MEF2D(479), pcDNA3-MEFA (rat; wild-type and RNAi-resistant (RiR), pcDNA3-MEF2D (rat; wild-type and RiR), pcDNA3-rMEF2A S408A (RiR), and pcDNA3-rMEF2D S444A (RiR) were described previously (Flavell et al., 2006). To generate pcDNA3-Flag-RCS, we used PCR amplification from reverse transcriptase reactions of purified rat NAc mRNAs. The degenerate PCR primers incorporated unique BamHI (5′) and NotI (3′) sites, and the coding sequence for the M2 Flag epitope tag in frame with the second amino acid of RCS. The PCR fragment was subcloned into pcDNA3, and the insert region was confirmed by sequencing.
Dissociated Striatal cultures
Embryonic striatal neurons (E18/19) were cultured from Long Evans rats (Charles River Labs) as described previously (Lindsay, 1998) with modifications. Details can be found in the supplemental online materials.
Luciferase Assays in Primary Neurons
Dissociated striatal neurons were transfected at 8 days in culture using calcium phosphate as described previously (Flavell et al., 2006), then stimulated and harvested for dual luciferase activity (Promega). Details can be found in the supplemental online materials.
Animals
Adult male C57BL/6 mice (Jackson Laboratory) and adult male Sprague-Dawley rats (Harlen) were housed on a 12-hr light-dark cycle with access to food and water ad libitum. All procedures were in accordance with the Institutional Animal Care and Use (IACUC) guidelines.
Western Blots of in vivo samples and Immunohistochemistry
Details can be found in the supplemental online materials.
Generation of P-RCS antibodies
Rabbits were injected with a synthesized P-RCS peptide encompassing RCS amino acids 50–60 where position S55 was phosphorylated (Covance). The injected peptide was conjugated to KLH via an N-terminal lysine residue.
Electrophoretic Mobility Shift Assay (EMSA)
Details can be found in the supplemental online materials.
Slice Pharmacology and Recombinant Adeno-associated Viruses
Details can be found in the supplemental online materials.
Stereotactic Surgery
Details can be found in the supplemental online materials.
Dendritic Spine Analysis
Mice were unilaterally infused into their NAc with AAVs expressing MEF2A and MEF2D shRNAs or control shRNA viruses. For MEF2 overexpression studies, mice were unilaterally infused with AAVs expressing MEF2-VP16 or its respective control virus. 19 days post-op, mice were given daily cocaine (30 mg/kg) or saline 5 days/week for 4 weeks (20 total injections) and sacrificed 24 hrs after the last dose. GFP-labeled neurons within the NAc core and shell regions were imaged at high resolution using a 100X oil immersion lens on a Zeiss LSM 510 confocal microscope. PMT assignment, pinhole sizes and contrast values were kept constant across different confocal sessions. Confocal stacks consisted of 31–178 sections at 0.23 m in thickness imaged with a Z-step of 0.1 µm. Images were taken at 1024 × 1024 pixel resolution to cover the entire Z dimension of the labeled neurons. Lengths of dendritic segments were measured using NIH ImageJ software. Spine densities were quantified by counting the number of spines along 30- to 100-µm segments of secondary dendrites (2–3 dendrite segments/neuron). Spine densities were expressed as spines per 10 µm. Only spines appearing continuous with their parent dendrite shaft in maximum-intensity z-projection were used for quantitative analysis. Mean spine densities were analyzed by pair-wise comparisons using the Student’s t-test.
Cocaine-induced Locomotor Sensitization
At the same time each day, mice were injected with saline or cocaine (IP) and were placed in standard plastic cages similar to their home cages for two hours. These cages were inside the Photobeam Activity System (San Diego Instruments, San Diego, CA), where five photobeams measured the mouse’s locomotor activity in 5-minute bins. Mice received saline injections on day 1–3 to habituate them to the novel environment. The locomotor activity for their final saline day is displayed. On days 4–10 mice received cocaine injections (15 mg/kg). Challenge doses of cocaine (15 mg/kg) occurred on day 17 (1 week of withdrawal) and on day 24 (2 weeks of withdrawal; RNAi experiment only) of the experiment. Day 1 of the experiment was 21 days after viral delivery, a time point at which high levels of expression were verified. For each day, the sum of the first 30 minutes (MEF2-VP16) or 45 minutes (shRNA) of locomotor activity after injection is displayed.
Conditioned Place Preference
18–21 days following stereotactic delivery of AAV-MEF2VP16 or its control virus into the NAc, mice were conditioned to cocaine in a standard 3 chamber conditioned place preference box (gray side, middle, and striped side). Using an unbiased 6-day paradigm, mice were pretested on day 1 to balance pre-existing side bias. On days 2 and 4, mice received a saline injection and were confined to the appropriate chamber. On days 3 and 5, mice received a cocaine injection (8 mg/kg) and were confined to the opposite chamber. On the final day, mice were placed again in the middle chamber with free access to all chambers and the time spent on each side was quantified. Data are expressed as time spent on the cocaine-paired side minus the time spent on the saline-paired side (CPP Score).
RNA Isolation and Reverse Transcription PCR
Bilateral 14 gauge punches of rat nucleus accumbens were rapidly dissected and frozen at −80°C. Punches were thawed in TriZol (Invitrogen), homogenized, and processed according to the manufacturer’s protocol. Total RNA was reverse-transcribed using Superscript III (Invitrogen) and random hexamers.
ChIP-chip analysis
Details can be found in the supplemental online materials.
Supplementary Material
Acknowledgments
The authors would like to thank Kole Roybal, Phuong Tran, Marjorie Centeno, Duy Ngo, Scott Edwards and Sumana Chakravarty for technical assistance, and Mike Greenberg and Steve Flavell (Children’s Hospital Boston) for generously sharing various reagents. The authors also thank Quincey LaPlant for experimental samples and Diane Lagace for technical advice. We acknowledge the generous support of the Whitehall Foundation (C.W.C.) and grants from the National Institute on Drug Abuse and National Institute of Mental Health (E.J.N., P.G., and A.C.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers: All microarrays have been deposited in the GEO database under the accession GSEXXXX
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Belfield JL, Whittaker C, Cader MZ, Chawla S. Differential effects of Ca2+ and cAMP on transcription mediated by MEF2D and cAMP-response element-binding protein in hippocampal neurons. J Biol Chem. 2006;281:27724–27732. doi: 10.1074/jbc.M601485200. [DOI] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Black BL, Ligon KL, Zhang Y, Olson EN. Cooperative transcriptional activation by the neurogenic basic helix-loop-helix protein MASH1 and members of the myocyte enhancer factor-2 (MEF2) family. J Biol Chem. 1996;271:26659–26663. doi: 10.1074/jbc.271.43.26659. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Basu S, White FJ. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J Neurophysiol. 2004;92:1597–1607. doi: 10.1152/jn.00217.2004. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr., Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Robinson TE, Bhatnagar S. Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res. 2003;960:42–47. doi: 10.1016/s0006-8993(02)03752-6. [DOI] [PubMed] [Google Scholar]
- Lindsay RVaRM. Rat Striatal Neurons in Low-Density, Serum-Free Culture. In: Goslin GBaK., editor. Culturing Nerve Cells. London: The MIT Press; 1998. pp. 371–393. [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Mao Z, Wiedmann M. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J Biol Chem. 1999;274:31102–31107. doi: 10.1074/jbc.274.43.31102. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Rapid WAVE dynamics in dendritic spines of cultured hippocampal neurons is mediated by actin polymerization. J Neurochem. 2005;95:1401–1410. doi: 10.1111/j.1471-4159.2005.03467.x. [DOI] [PubMed] [Google Scholar]
- Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC, Surmeier DJ, Greengard P. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999a;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999b;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Supp l:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Shalizi A, Bilimoria PM, Stegmuller J, Gaudilliere B, Yang Y, Shuai K, Bonni A. PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J Neurosci. 2007;27:10037–10046. doi: 10.1523/JNEUROSCI.0361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Shalizi AK, Bonni A. Brawn for Brains: The Role of MEF2 Proteins in the Developing Nervous System. Curr Top Dev Biol. 2005;69:239–266. doi: 10.1016/S0070-2153(05)69009-6. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-WASP and the Arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008 doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.