Abstract
Cardiovascular disease (CVD) exceeds infection and cancer as the leading cause of death. In the USA alone, approximately a million individuals suffer an acute myocardial infarction (AMI) annually. As the prevalence of CVD risk factors (e.g. hypertension, obesity and type 2 diabetes) rises, CVD is increasing in younger individuals. Fortunately, existing therapies have improved post-AMI mortality, but in turn have increased the prevalence of post-AMI heart failure (HF). Approximately half-a-million new HF cases are diagnosed each year in the USA. In the next 25 years, up to 15% of the population over the age of 65 in the USA is projected to have HF. Therapeutic interventions that prevent/reverse atherosclerosis, prevent post-AMI HF and halt the progressive functional deterioration once HF occurs are all needed. Cell therapy – either via exogenous delivery or by endogenous mobilization of cells – may be able to do so, in part, by improving the body’s capacity for repair. To date, primarily bone marrow- or blood-derived cells have been utilized after AMI to prevent left ventricular dysfunction, and skeletal myoblasts have been transplanted into failing myocardium. Preclinical studies are directed at prevention/reversal of atherosclerosis with bone marrow precursors, and ultimately at replacing failing heart with a cell-based bioartificial construct.
Keywords: acute myocardial infarction, bioartificial, cell therapy, endogenous repair, heart failure, stem cells
Introduction
For the first time, cardiovascular disease (CVD), exceeds infection and cancer as the leading cause of death throughout most of the world [1]. Although treatments for atherosclerosis, hypercholesterolaemia, hypertension, type 2 diabetes, acute myocardial infarction (AMI) and post-AMI left ventricular (LV) remodelling have improved mortality to some extent [2,3], CVD still accounts for one in every 2.8 deaths in the United States, translating into approximately 2.5 million deaths each year [1]. Furthermore, the incidence of CVD at a younger (30–50 years old) age is rising [4] as the prevalence of risk factors for CVD (e.g. hypertension, obesity and type 2 diabetes) increases [3,5,6]. As ‘baby-boomers’ age, the number of people over 65 years of age in the USA is expected to double, with nearly 15% of this population projected to develop HF because of ageing, CVD and type 2 diabetes [1].
The increase in patients surviving AMI has lead to a concomitant increase in the prevalence of post-AMI heart failure (HF) – with at least a third of patients manifesting HF symptoms within a year [7]. Currently, this increase is attributed both to limited efficacy of pharmacological agents at reducing LV remodelling and HF exacerbations [8–11], and to underutilization of these drugs in general practice, which has precluded translating the successes of research into clinical reality [12,13]. Finally, post-AMI HF patients are surviving longer, in part because of a wider use of implantable cardioverter defibrillators [14].
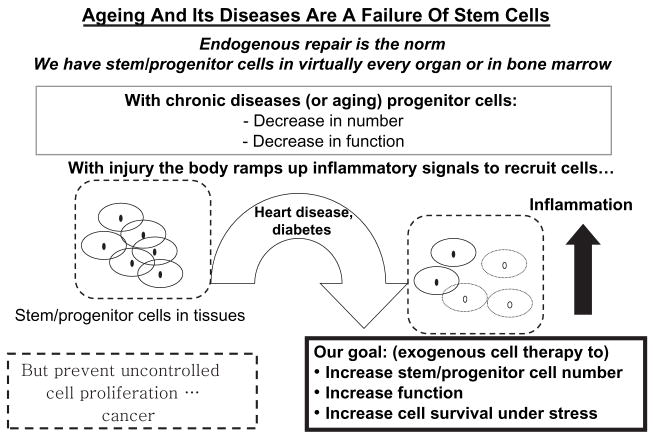
Ageing population, increased CVD risk factor prevalence, disease onset at an earlier age, more patients surviving AMI but progressing to HF, and more patients living with HF have led to an ever-growing demand for treatments that reverse atherosclerotic disease in the coronary and peripheral beds, prevent remodelling after acute myocardial injury and halt the progressive loss of cardiac function in chronically failing myocardium. With the awareness that both tissues and bone marrow contain undifferentiated immature ‘stem’ cells normally used to replenish body tissues throughout life (figure 1), and that these cells can be harvested and delivered to sites of injury, a new therapeutic option has emerged: the transplantation of stem or progenitor cells (PCs) for functional repair or even regeneration of vasculature, acutely injured and/or failing myocardium or previously irreversibly damaged heart – giving hope for cell-mediated prevention, treatment and possibly even cure of CVD.
Fig. 1.
Ageing and chronic disease represent a failure of stem cell-mediated endogenous repair, providing a rationale for cell therapy.
Chronic Disease Represents a Failure of Endogenous Repair: The Basis for Cell Therapy
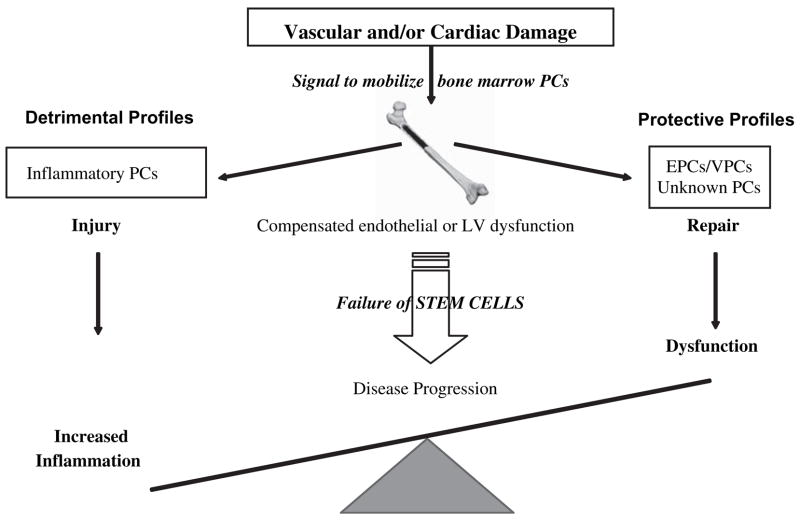
Maintenance of tissues and organs in the face of a continuous workload is an ongoing process throughout much of our lives. This repair process is mediated, in part, by reserve (stem or progenitor) cells found within most organs and/or in bone marrow (figure 1). There is growing evidence that with ageing, the number and/or functional capacity of these reparative stem cells decreases [15] reducing capacity for repair. Stated differently, as an individual ages and an injury occurs, ineffective repair allows disease progression. We have hypothesized that tissue integrity represents a balance between injury and repair (figure 2). If this is true on multiple levels in the pathogenesis of disease, then progression of disease – including vascular and cardiac syndromes –can be viewed as abnormal/inefficient cellular repair following insult.
Fig. 2.
We view disease as a process of ongoing endogenous repair that ultimately fails as either the number or quality of ‘protective’ cells decreases or the number of ‘detrimental’ cells predominates. Thus, to identify, diagnose and attenuate disease process the focus should be on the balance of cell profiles that leads to repair vs. progression of disease.
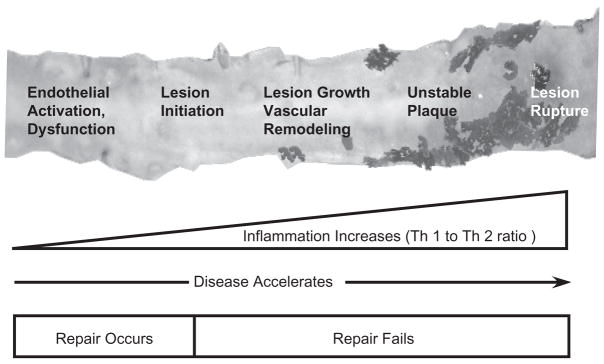
Although this schematic (figure 2) depicts vascular/cardiac muscle injury, the idea may well be applicable more ubiquitously to other diseases. As tissue damage (e.g. atherosclerosis) occurs, pro-inflammatory (Th1-type) cytokines are produced that serve as an inducement to mobilization of reparative PCs to the site of injury via multiple signalling loops [16]. If sufficient and appropriate cell recruitment occurs, repair ensues, inflammation is extinguished and tissue integrity is, at least partially, restored [16,17]. However, in the face of decreased PC availability with ageing, endothelial or myocardial injury – reviewed by us elsewhere [18] – the demand for PCs outstrips the capacity of the body to respond with sufficient numbers of PCs, resulting in a failure of intrinsic tissue repair. This failure of ‘endogenous repair’ is accompanied by a secondary repair process (often resulting in scarring), a failure of tissue integrity and a sustained pro-inflammatory response, which in turn exacerbate injury permitting disease progression. Taken together, these ideas based on animal and preliminary human data begin to suggest that ageing and chronic disease (atherosclerosis, type 2 diabetes, etc.) may, at their simplest, represent a failure of PC-mediated repair (figure 3). Thus, the concept of exogenous cell therapy is based on the assumption that supplying appropriate PCs can overcome this pre-existing failure of repair, reduce inflammation and restore tissue integrity and tip the balance between injury and repair towards repair (figure 2).
Fig. 3.
Schematic representation of relationships between failure of endogenous repair and atherosclerosis progression.
Locating a source of appropriate cells in an ageing or diseased individual becomes a challenge. Currently, the two most prevalent autologous cell sources are bone marrow and tissues – although peripheral and umbilical cord blood are more frequently being evaluated. Tissues that have served as a source of cells for cardiovascular repair in animals include skeletal muscle, heart and fat. From bone marrow, several cell populations [e.g. mononuclear cells and subsets thereof: CD34+ cells, endothelial progenitor cells (EPCs) and mesenchymal (MSCs)/stromal cells] have been used to treat the continuum of CVD. Either skeletal myoblasts (SKMBs) or bone marrow-derived cells have been used in patients with HF or after AMI [19]. Thus, translating research from bench to bedside allows thinking about cell therapy as suitable for prevention, treatment and possibly, a cure for CVD.
Cell Therapy as Prevention of CVD
The use of specific PC populations to treat the heart before an acute injury or fulminant HF is based on their newly described role as mediators of ongoing endogenous repair in the vessel wall and/or myocardial tissue. For example, cardiovascular risk factors, surrogate imaging endpoints of atherosclerosis (i.e. carotid intima-media thickness) and major adverse cardiac events (MACE) have all been related to circulating EPCs – defined blood (or bone marrow) cells expressing combinations of CD133 (AC133), CD34, VEGF-R2, CD31 and occasionally CD14. Patients with reduced EPC levels have elevated Framingham risk scores, increased intima-media thickness and a shorter survival (higher MACE) following a first AMI. Similarly, a loss in the number of circulating EPCs [15,20–22] and a defect in their ability to migrate (i.e. functional capacity) were shown in patients with increased risk for CVD, including AMI [23–25].
Atherosclerosis represents a continuous process of injury, inflammation, responses to injury and remodelling of the vascular wall in order to maintain the integrity of the circulation and organ perfusion [26]. Insights into the basic mechanisms involved in atherogenesis (figure 3) indicate that endothelial dysfunction represents a key early step in the development of atherosclerosis, and sets a fertile ground for plaque progression, leading to complications [27,28]. It stands to reason then that the endothelium would represent a reasonable target for ongoing vascular repair, when failure occurs. Endothelial dysfunction is characterized by a reduction of the bioavailability of vasodilators, in particular nitric oxide. Furthermore, endothelial dysfunction is characterized by a pro-inflammatory and procoagulatory milieu that remains such throughout all stages of atherogenesis [28]. Recent observations demonstrated that endothelial dysfunction may take place because of the reduction in the numbers of circulating endothelial PCs required for reparative maintenance thus supporting the hypothesis that atherosclerosis may be initiated by a loss of these reparative PCs or alteration of their function [15,18,29]. Therefore, the identification of specific changes in the PC populations during the endothelial dysfunction may enhance our understanding of the mechanism of atherosclerosis – from the standpoint of failure of endogenous repair [18]. An ongoing study (at our institution in collaboration with Dr. Amir Lerman, Mayo Clinic, Rochester, MN, USA) evaluating PC profiles in patients with varying degrees of endothelial dysfunction is expected to advance our knowledge in this area.
Cell therapy has primarily been used clinically to treat patients after AMI or the onset of HF. We propose that PC-based assessment of endogenous repair may provide a unique opportunity for early diagnosis and timely prevention of consequences of atherosclerosis. Likewise, early cell-based interventions might help reduce the risk of progression to clinical CVD.
Cells as Treatment of CVD
Atherosclerosis
In an animal model of atherosclerosis (ApoE−/− mice), we showed that disease progression is tied to obsolescence of endogenous PCs that normally repair and rejuvenate the arteries, and that chronic treatment with bone marrow-derived PCs from young non-atherosclerotic mice prevents atherosclerosis progression in recipients despite persistent hypercholesterolaemia [17]. In contrast, treatment with bone marrow cells from older mice with atherosclerosis is much less effective. Donor cells engraft on recipient arteries in areas at risk for atherosclerotic injury and are associated with less overall plaque burden [17].
In more recent studies in ApoE−/− mice, we have described sex-based differences in this capacity for endogenous or cell-mediated repair [16]. After chronic delivery of bone marrow mononuclear cells (BM-MNCs) in a sex-matched or sex-mismatched fashion, only female cells infused into male ApoE−/− recipients significantly decreased plaque burden. This reparative response correlated with an increase in three main progenitor populations (CD34+, p = 0.02; CD45+, p = 0.0001; AC133+/CD34+, p = 0.001) in the bone marrow of recipients, but not with total serum cholesterol. The plaque burden reduction was associated with increased Th1-type (pro-inflammatory), Th2-type (anti-inflammatory) and hematopoietic/regulatory cytokines. Increased hematopoietic/regulatory cytokine, such as granulocyte colony-stimulating factor (G-CSF) – that mobilizes PCs from bone marrow to peripheral blood via Th2-type cytokine mechanisms, highly correlated with plaque attenuation (r = −0.86, p = 0.0004). Hematopoietic/regulatory cytokines IL-15 and IL-8 were clustered with G-CSF [30]. This observation demonstrated that cell-mediated repair requires a degree of inflammation to recruit appropriate reparative PCs out of the bone marrow, and to allow for the digestion of plaque lesions (presumably by macrophages) and for the engraftment of EPCs to generate a new endothelium. Therefore, it is clear that hematopoietic/regulatory and Th2-type cytokines have roles in the reparative process that have not been previously appreciated. In addition, shutting down the inflammatory process in the early phase by inhibition of Th1-type cytokines (either by direct or by non-specific antagonism) may not result in the recruitment of appropriate cells necessary for vascular repair.
Of note, female bone marrow cells secreted approximately 4 times more G-CSF than did male cells, and the levels of endogenous G-CSF in female ApoE−/− mice were twice as high compared to male mice; none of these differences correlated with oestriol levels [16]. These sex-based differences in PC-mediated capacity for vascular repair may not only begin to explain why CVD occurs earlier in men (as repair fails) but may have implications for cell therapy clinical trials in that female cells far outperformed male cells. Further exploration of sex-based differences in the PCs present in bone marrow and peripheral blood and in the capacity for PC-mediated disease prevention and treatment is warranted.
Acute Myocardial Infarction
The idea of using a bone marrow aspirate – a ‘cocktail’ of the cells – to accelerate cardiac repair after AMI has gained wide enthusiasm among clinical investigators. The easy translatability of protocols from haematology to cardiology and an intracoronary administration of bone marrow via a catheter have come to represent an attractive therapeutic modality. Today, more than 1000 patients have been treated with bone marrow cells in phase I and II trials. We have reviewed these individual trials elsewhere [19] but the results are summarized here, as are the lessons to be learned.
Trials performed to date have focused on the use of BM-MNCs, EPCs, MSCs and other cells throughout the continuum of CVD – from advanced coronary atherosclerosis to end-stage HF, with the most patients treated following AMI. Three recent meta-analyses suggest that BM-MNCs provide statistically significant yet small (in clinical terms) benefit when administered post-AMI [31–33]. However, on close examination of individual studies, the outcomes are discrepant. Whether the discrepancies represent differences in disease context, patient population, cell type and dose or some other factors remains to be resolved. In other words, 7 years after the initiation of the first study we still have as many (or more) questions as answers.
Discrepancies notwithstanding, the overall data on the use of BM-MNCs in atherosclerotic disease (including AMI) are encouraging. Although only a small number of patients with advanced atherosclerosis (no AMI or HF) have been studied [34,35], BM-MNCs substantially reduced anginal episodes per week, to an extent greater than ranolazine, a new anti-anginal agent [36]. The improvement in symptomatology with BM-MNCs correlated with increased myocardial perfusion. Unfortunately, BM-MNCs in reperfused and/or stented AMI were not as beneficial. Although a reduction in infarction size was observed, no functional improvement occurred [37]. This lack of effect might have occurred because of prompt restoration of coronary flow that deemed cell-based repair unnecessary. When reperfusion and stenting were not uniformly applied, BM-MNCs and other cells (AC133+ EPCs and MSCs) improved myocardial viability, wall motion, coronary flow and left ventricular ejection fraction (LVEF). However, several patients showed either restenosis or de novo lesions after AC133+ EPCs [38]. It is possible that the success of BM-MNCs lies in the administration of unfractionated cells, permitting cell–cell and cell–tissue interactions in vivo that are otherwise absent when an isolated cell population is administered. In other words, unfractionated BM-MNCs have both mature and immature progenitors, and it is likely that a combination of these cells may be the best choice, although a unique population cardiac progenitors, newly discovered by us [39], has not been clinically tested yet.
The REPAIR-AMI [40] study highlighted translation of small, in clinical terms, effects of cell therapy into a meaningful reduction of events in long-term follow-up. The administration of approximately 236 × 106 BM-MNCs in AMI patients resulted in a higher event-free survival at 1 year vs. placebo. This was the first randomized phase II study showing that exogenous BM-MNCs do in fact participate in tissue repair and can withstand the rigorous test of clinically driven endpoints. However, there needs to be a sufficient degree of tissue injury for the cells to show efficacy: those patients that had a baseline LVEF ≤ 48.5% benefited the most from cell therapy, and those above this cut-off showed no benefit. Ongoing trials will shed light on the importance of timing and dose to the outcome of BM-MNC therapy in AMI. A phase III trial investigating event reduction with BM-MNCs is also underway.
Heart Failure
Despite years of efforts to reduce the progression of the pathophysiological process of remodelling, up to 50% of post-AMI patients still manifest symptomatic HF by year seven [7]. Thus, there is a strong unmet need to develop therapies to improve quality of life in HF patients while reducing hospitalizations and ultimately – mortality. Because cells in a failing myocardium lose their contractile function due to ongoing ischaemia and apoptosis, replacing these cells with new ones is a therapeutic option that makes sense to both physicians and patients. Having begun in 1998 with the demonstration of LV functional improvement and antiremodelling effects post-SKMB transplantation in a rabbit model [41], the data on approximately 250 patients that received SKMBs has been published. We have reviewed individual trials elsewhere [42]. In our review, we highlighted a relationship between the contractile impairment at baseline and the LV functional improvement by SKMBs. Specifically, in patients with baseline LVEF < 25%, the mean follow-up LVEF was 32%. We saw similar results in patients with baseline LVEF between 25–<30%: the mean improvement was approximately 7–8%. The only exception was a study by Chachquez et al., where mean LVEF improved from 28 to 52% in 14 ± 5 months [43]. In patients with LVEF between 30 and 40%, myocardial contractility at follow-up registered 9–19% higher. Finally, patients with mildly abnormal contractility (LVEF > 40%), experienced no significant benefit. Therefore, it is likely that the patients with severe myocardial function impairment (and a larger portion of scarred LV) may not experience extensive benefits compared to patients with a lesser myocardial damage (i.e. LVEF between 30 and 40%). In other words, residual myocardial viability may be required for a successful outcome of SKMB therapy in HF, and the low viability conditions may not harbour the environment required for cell engraftment and survival. Despite varying benefits, SKMB administration significantly improved patients‘ HF symptoms – overall by approximately one NYHA functional class – albeit the number of controlled studies is small.
The field of SKMB cell therapy has had its first randomized placebo-controlled study (The Myoblast Autologous Grafting in Ischemic Cardiomyopathy Trial) published this year [44]. Briefly, it was an international study of 97 HF patients with LVEF ranging from 15 to 35% comparing safety and efficacy of low dose (400 × 106) or high-dose (800 × 106) autologous expanded for 3 weeks SKMBs vs. placebo. The patients had a history of AMI and at least two contiguous segments with severely compromised function. SKMBs were delivered into the myocardium after bypass grafting using 30 injections. The primary endpoints were a change in LVEF at 6 months and LV segmental function recovery. The primary safety endpoints were 30-day and 6-month MACE and arrhythmia rates.
Despite no differences in the primary outcomes vs. placebo, this study demonstrated several significant points. First, a multicentre trial of SKMB therapy with cell expansion (and preserving cell viability) is feasible. Secondly, multiple cell injections into the LV guided by echocardiography in settings with multiple operators are feasible. Finally, the high-dose SKMB group did exhibit a significant reduction of LV end-systolic and end-diastolic volumes vs. placebo indicating that cell-based treatment exerts antiremodelling effects of probable long-term importance.
Even though SKMB investigations in HF preceded studies of BM-MNCs in AMI, the progress of SKMB-based therapy has not been equally rapid. Looking at the state of SKMB therapy, there are some unanswered safety and efficacy questions. Similar to BM-MNCs, the transplantation of autologous SKMBs may have comparable limitations with regards to cell functionality. In other words, much like with AMI, we do not fully understand the inflammatory milieu of chronic HF. Nor do we appreciate the array of cytokines/chemokines that SKMBs secrete. The role of inflammation in CVD is well-established: it drives plaque progression forward leading to AMI [28]. But the studies beyond that point in HF are limited to measurements of single cytokines and effects their pharmacological antagonism [45]. Borrowing from our atherosclerosis work discussed earlier in this review, we propose that the interaction of the inflammatory milieu of the patient with that of the cells may determine the safety and efficacy of cell therapy.
The issue of arrhythmogensis shown in several SKMB therapy trials [42], has decreased the enthusiasm of clinical investigators and has tampered with the progress of studies. Co-administration of amiodarone dramatically reduced the incidence of arrhythmias after SKMB transplantation but has not restored the enthusiasm because of safety issues associated with long-term amiodarone use [19]. We have demonstrated that the location of cell placement (in relevance to the centre and the periphery of the scar), rather than the cells themselves, may be a critical determinant of safety and efficacy of SKMBs [46]. Specifically, a direct injection into the scar centre resulted in a worse LV performance and in pro-remodelling effects (increased LV end-diastolic and end-systolic volumes) in a rabbit model. On the contrary, peripheral administration improved those parameters showing an antiremodelling benefit. Holter monitoring data also related to the location of cell placement showed a higher prevalence of ventricular arrhythmias was registered in animals that received SKMBs into the scar centre vs. the periphery. These data strongly suggest that the famous real estate slogan ‘location, location, location’ may be in fact applicable to cell therapy with SKMBs in HF.
BM-MNCs have been investigated in HF patients, although to a lesser extent than SKMBs [42]. The prominent BM-MNC trials in AMI – TOPCARE-HF and BOOST-2 – have continued to gain insight into BM-MNC efficacy when HF pathophysiology predominates. Albeit in a very small number of patients, Silva et al. [47] showed that targeted placement of BM-MNCs into the ischaemic zones resulted in delisting of several patients from transplantation because of increased exercise tolerance. This outcome has hinted at possible beneficial effects on BM-MNCs in HF, when placement is targeted in the myocardium [48]. Given the reduced number and migratory capacity of and the deficits in EPC quantity seen in patients with advanced CVD and HF [20], it will be interesting to see if BM-MNCs are capable of improving LV function or if the HF milieu only allows for ischaemia-resistant cells. So far, unlike in SKMB trials, symptomatic and functional improvements in HF patients treated with BM-MNCs occurred without adverse electrical events, although concerns have been raised by animal data [49]. Large trials will definitively answer these questions.
Lessons Learned and Outstanding Questions
Cell therapy trials to date have raised as many questions as it has answered. In both AMI and in HF studies, the lessons learned fall into three major groups: (i) nonstandardized protocols have brought discrepant results in similar patient cohorts; (ii) technical details of cell processing can affect outcome; (iii) autologous cells have inherent limitations because of the impact of age and disease on the availability and functional proficiency.
As large-scale multicentre trials proceed, standardization of inclusion and exclusion criteria will largely be resolved. However, ‘million dollar’ questions remain. What are the right endpoints for cell therapy trials? Can we move towards more biologically relevant endpoints recognizing that we deal with cells that are biological organisms and not chemical substances? Are clinical endpoints superior to surrogate markers? As the field matures, these answers will be forthcoming. We are, likewise, yet to learn whether delivery route impacts the outcome. A possibility of intravenous (IV) administration of bone marrow cells is attractive for two reasons. First, it takes interventional procedures out of the therapeutic process making patients who may not be eligible for a catheterization laboratory experience suitable for cell therapy. Secondly, IV cell administration may act on cytokines/chemokines, which in turn play a role in both engraftment of exogenous cells and in the egress of endogenous stem/PCs out of the bone marrow differently than cell delivered right into the myocardium. We have recently obtained preliminary data on the biodistribution of IV administered CD34+ cells that support this supposition. Specifically, we observed an initial cell homing into the liver, the lung and the kidney and a delayed migration into the heart and into the bone marrow. Even though work remains to be done to understand the time course of the cytokine/chemokine milieu in AMI and HF and the ways it is modified by various cell types, the interactions of bone marrow (and other) cells (i.e. ‘cytokine factories’) with the inflammatory milieu inherent to the disease may be the primary determinant of both safety and efficacy of cell therapy. At this point, our understanding of these interactions is extremely rudimentary. Centralized collection and storage of blood samples in cell therapy trials should shed light on many of these questions and move us closer to begin to deciphering the mechanisms of endogenous repair and its augmentation by bone marrow cells. The new knowledge will allows us to match cell types, timing of administration and clinical patient characteristics to enjoy the full potential of stem/PCs.
Cardiac Stem Cells: Better Cells or a New Way Forward?
Recently, undifferentiated cell populations have been isolated from neonatal and acutely infarcted failing hearts by expression of c-kit, MRD-1, isl-1 or sca-1 stem cell markers and by lack of expression of haematopoetic markers [50–52]. Interestingly, the number of some of these cells was increased after AMI, but was very low in failing hearts suggesting a role of these cells in ongoing repair, which becomes insufficient in HF [53]. Recently, we have isolated SSEA-1+ uncommitted cardiac progenitor cells (UPCs) in neonatal and adult rat hearts, which can be expanded in vitro and differentiated down myocyte, smooth muscle and endothelial cell pathways [39]. To date, the methods for harvest and expansion of all cardiac PC populations are limited. We have shown that we can expand UPCs in vitro over several weeks to quantities sufficient for cardiac repair [39]. The biology of UPCs and their capacity for repair present interest. It is possible that the future of cardiac repair may involve endogenous mobilization and/or delivery of these cells.
Cells as Cure
Can a Cell-Based Bioartificial Heart Provide New Hope for Patients?
The primary approach in the treatment of AMI and HF is restoration of LV function. This goal can be achieved utilizing pharmacological approaches directed at the preload, afterload, and inhibition of signalling that governs the decline of cardiac function. Transplantation of cells into the heart has a potential of reaching that goal by directly replacing the native cells that have undergone necrosis and apoptosis. However, cell replacement must be efficient, that is, the new cells must engraft in sufficient numbers and regenerate the myocardium lost by transforming into a scar.
Another approach – folds of magnitude more ambitious – would be to bioengineer a functional cardiac tissue. The field of cardiac bioengineering has been growing over the past 10 years. Most studies have used a scaffold (e.g. hydrogel, porous, fibrous or other) seeded with cells and cultivated prior to being used as a cardiac patch. In principle, a patch can be an on-the-shelf product or created on demand and applied on scar tissue. Then, as the cells generate contractile force in vivo, the fibrotic area would no longer be dyskinetic and possess a risk for aneurysm development. The most well-known approach of engineering cardiac patches is growing cardiomyocytes in a collagen gel and then subjecting the cells to cyclic mechanical stretch to induce maturation [54,55].
Realizing one of the major limitations in cardiac tissue engineering – the difficulty in producing a perfusable 3-D construct – we have undertaken a different approach. Because nature engineered a scaffold that serves as a basis of a heart, we hypothesized that we would be able to wash out the cellular components of the myocardium to obtain a 3-D matrix. Then, because the major vascular conduits should remain in place, we would be able to recellularize the decellularized myocardial matrix to recapitulate the developmental process. Finally, as the construct matures, the recellularized LV wall would be capable of contractile force with reasonable synchronicity.
We have been able to successfully achieve all these goals and have recently published the characterization of the bioartificial heart construct. Briefly, Ott et al. [56], completely removed cellular structures (<3% of deoxyribonucleic acid remaining) out of the heart using perfusion decellularization with a detergent combination. The cell removal process did not significantly decrease the glycosaminoglycans in the myocardial matrix. Stress-strain testing was similar between decellularized rat heart and cadaveric matrices. This is most likely a critical piece, as the matrix is likely to retain the majority of the cues required for recellularization of the myocardium. In addition, the decellularized matrix had a significantly higher tangential modulus strain than fibrin (a material used by others as a scaffold). The perfusability of the matrix was proven by (i) mercox resin casts showing structural integrity from the major coronary vessels to fourth/fifth order capillaries and (ii) heterotopically transplanting the decellularized construct and obtaining angiograms showing essentially normal blood inflow. Recellularization of this matrix was performed by infection of neonatal cells into the matrix in a bioreactor. The construct matured over time, and by days 8–10, reasonable contractions of the recellularized LV segments were recorded. Histological characterization of the recellularized construct showed live cells expressing myosin heavy chain protein, von Willebrandt factor, CD31+ cells, connexin43 and other markers. Therefore, we have moved one step closer to a creation of autologous organ and constructed a tool to test hypotheses relevant to developmental biology, disease pathophysiology and cell therapy. By doing so, we have, to a reasonable degree, overcome several major limitations of tissue engineering.
Obviously, time will determine which of the currently developed approaches will translate into a clinical product and will serve patients across a wide CVD spectrum. Cardiac patches hold a promise for a narrow aspect of myocardial repair, while decellularization-recellularization approach seems to be translatable to other organs that are often impacted by CVD – the kidney and the liver, and possibly to the pancreas and the lungs.
Summary
Cell therapy has made a significant amount of progress in the past decade. The early clinical phase I and first phase II trials brought promising results but also offered lessons to be learned going forward. To do so successfully, it is imperative that we acquire a deeper level of mechanistic understanding of cell-mediated repair throughout the CVD continuum. We propose that the interactions among the inflammatory milieu of each of the pathophysiological state from coronary atherosclerosis to end-stage HF and the cell delivered into those settings may define most of the mechanistic targets. We must more precisely define the patient subgroups and optimize the timing of cell administration that would bring the most benefits to the patients in terms of sustained antiremodelling effects and symptomatic relief.
Along with cell therapy, cardiovascular tissue engineering is now the new frontier in the future treatment of CVD. Although the successes are very preliminary, partial and whole bioartificial organ constructs may become a reality and offer a wide range of solutions in the future. Going forward, translating the more recent methods, such as perfusion decellularization–recellularization, to the bio-organogenesis of other organs impacted by CVD (kidney, lungs, liver) is of crucial importance.
Even though we have decades worth of work ahead of us, we cannot forget why we are devoted to finding potential new therapies. Year after year, it remains the number one killer, taking away lives every minute. As the population ages, the unmet need for therapies to make an impact on cardiovascular morbidity and mortality increases. Achieving that difficult goal will require coordinated efforts between bench and bedside research and continuous funding support.
Acknowledgments
This work has been supported in part by the NIH/NHLBI award to Dr. Doris A. Taylor (R01-063346), the Minnesota partnership for Biotechnology and Medical Genomics award to Dr. Taylor and by funding from the Center of Cardiovascular Repair, University of Minnesota. Dr. Taylor is Medtronic Bakken Chair in Cardiovascular Repair at the University of Minnesota.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 4.Juonala M, Viikari JS, Rasanen L, et al. Young adults with family history of coronary heart disease have increased arterial vulnerability to metabolic risk factors: the cardiovascular risk in Young Finns Study. Arterioscl Thromb Vasc Biol. 2006;26:1376–1382. doi: 10.1161/01.ATV.0000222012.56447.00. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM. Can reducing peaks prevent type 2 diabetes: implication from recent diabetes prevention trials. Int J Clin Pract Suppl. 2002;129:33–39. [PubMed] [Google Scholar]
- 7.Miller LW, Missov ED. Epidemiology of heart failure. Cardiol Clin. 2001;19:547–555. doi: 10.1016/s0733-8651(05)70242-3. [DOI] [PubMed] [Google Scholar]
- 8.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez AF, Velazquez EJ, Solomon SD, et al. Left ventricular assessment in myocardial infarction: the VALIANT registry. Arch Intern Med. 2005;165:2162–2169. doi: 10.1001/archinte.165.18.2162. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN. Lessons learned from the valsartan- heart failure trial (Val-HeFT): angiotensin receptor blockers in heart failure. Am J Cardiol. 2002;90:992–993. doi: 10.1016/s0002-9149(02)02667-x. [DOI] [PubMed] [Google Scholar]
- 11.Torp-Pedersen C, Poole-Wilson PA, Swedberg K, et al. Effects of metoprolol and carvedilol on cause-specific mortality and morbidity in patients with chronic heart failure – COMET. Am Heart J. 2005;149:370–376. doi: 10.1016/j.ahj.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 13.Lenzen MJ, Boersma E, Reimer WJ, et al. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: a report from the Euro Heart Survey on Heart Failure. Eur Heart J. 2005;26:2706–2713. doi: 10.1093/eurheartj/ehi499. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 15.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 16.Nelson WD, Zenovich AG, Ott HC, et al. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ Res. 2007;101:1319–1327. doi: 10.1161/CIRCRESAHA.107.155564. [DOI] [PubMed] [Google Scholar]
- 17.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 18.Zenovich AG, Taylor DA. Atherosclerosis as a disease of failed endogenous repair. Front Biosci. 2008;13:3621–3636. doi: 10.2741/2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zenovich AG, Davis BH, Taylor DA. Comparison of intracardiac cell transplantation: autologous skeletal myoblasts versus bone marrow cells. Handb Exp Pharmacol. 2007;180:117–165. doi: 10.1007/978-3-540-68976-8_6. [DOI] [PubMed] [Google Scholar]
- 20.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 22.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 23.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 24.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher KA, Liu Z, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 29.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 30.Zenovich AG, Panoskaltsis-Mortari A, Caron GJ, et al. Sex-based differences in vascular repair with bone marrow cell therapy: relevance of regulatory and Th2-type cytokines. Transpl Proc. 2008;40:641–643. doi: 10.1016/j.transproceed.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Hristov M, Heussen N, Schober A, Weber C. Intra-coronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med. 2006;10:727–733. doi: 10.1111/j.1582-4934.2006.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 33.Lipinski MJ, Biondi-Zoccai G, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Tse HF, Thambar S, Kwong YL, et al. Safety of catheter-based intramyocardial autologous bone marrow cells implantation for therapeutic angiogenesis. Am J Cardiol. 2006;98:60–62. doi: 10.1016/j.amjcard.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 35.Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 36.Chaitman B. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 37.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 38.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 39.Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a souce for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 40.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 41.Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 42.Taylor DA, Zenovich AG. Cell therapy for left ventricular remodeling. Curr Heart Fail Rep. 2007;4:3–10. doi: 10.1007/s11897-007-0019-0. [DOI] [PubMed] [Google Scholar]
- 43.Chachques JC, Herreros J, Trainini J, et al. Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int J Cardiol. 2004;95(Suppl 1):S29–S33. doi: 10.1016/s0167-5273(04)90009-5. [DOI] [PubMed] [Google Scholar]
- 44.Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 45.Yan AT, Yan RT, Liu PP. Pharmacotherapy for chronic heart failure: evidence from recent clinical trials. Ann Intern Med. 2005;142:132–145. doi: 10.7326/0003-4819-142-2-200501180-00013. [DOI] [PubMed] [Google Scholar]
- 46.McCue JD, Swingen C, Feldberg T, et al. The real estate of myoblast cardiac transplantation: negative remodeling is associated with location. J Heart Lung Transpl. 2008;27:116–123. doi: 10.1016/j.healun.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva G, Perin E, Dohmann H, et al. Catheter-based transendocardial delivery of autologous bone-marrow-derived mononuclear cells in patients listed for heart transplantation. Tex Heart Inst J. 2004;31:214–219. [PMC free article] [PubMed] [Google Scholar]
- 48.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 49.Fukushima S, Varela-Carver A, Coppen SR, et al. Direct Intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–2261. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 50.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 52.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann WH, Didie M, Wasmeier GH, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106(12 Suppl 1):I151–I157. [PubMed] [Google Scholar]
- 55.Eschenhagen T, Didie M, Munzel F, et al. 3D engineered heart tissue for replacement therapy. Basic Res Cardiol. 2002;97(Suppl 1):I146–I152. doi: 10.1007/s003950200043. [DOI] [PubMed] [Google Scholar]
- 56.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]