Abstract
Cytokines play an important role in the immune system, and abnormalities in their production have been found in many human diseases. Interleukin-21 (IL-21), a type I cytokine produced by activated T cells, has diverse effects on the immune system, but its ability to induce production of other cytokines is not well delineated. Furthermore, the signaling pathway underlying its action is poorly understood. Here we have evaluated IL-21-induced cytokine production in human monocytes and U937 leukemia cells. We found that IL-21 induces upregulation of a variety of cytokines from multiple cytokine families. We also found that IL-21 triggers rapid activation of ERK1/2. Neutralizing antibody to the IL-21R prevented both IL-21-induced cytokine production and IL-21-induced activation of ERK1/2. Inhibition of ERK1/2 activity by the ERK-selective inhibitor U0126 reverses the ability of IL-21 to upregulate cytokine production, suggesting that IL-21-induced cytokine production is dependent on ERK1/2 activation.
Keywords: cytokines, ERK, IL-2, leukemia, monocytes
1. Introduction
Cytokines are essential for growth, survival, differentiation, and normal immune function. IL-21 is produced by CD4+-activated T cells, and has significant homology to members of the Type I cytokine family, i.e. IL-2, IL-4, and IL-15. In concert with CD3-specific antibody, it stimulates proliferation of CD4+ T cells (1). IL-21 promotes proliferation of CD8+ T cells in a synergistic manner with IL-7, and augments their antitumor activity (2–4). IL-21 promotes the growth and expansion of T cells, serves as an apoptotic agent in B cells, and inhibits dendritic cell maturation (5–9). In B cells, it promotes the production of IgG in cooperation with IL-4 (10), and induces their differentiation to plasma cells (11). IL-21 activates cytotoxic and antitumor activities of NK cells as well as induces their differentiation from progenitor cells (12, 13).
IL-21 has been reported to enhance IFN-γ and IL-13 production in NKT cells (14), and IL-17 production in T helper cells (15). Whether it induces the production of cytokines in the Type I family has not been thoroughly investigated, although at least one report (14) has indicated that IL-21 induces the production of IL-4. Here we utilized cytokine arrays and cytokine ELISA to investigate IL-21-induced cytokine production in human monocytes and U937 leukemia cells. We observed that stimulation of U937 leukemia cells and human monocytes with IL-21 resulted in both cytokine (GM-CSF, IL-1, IL-2, IL-7, IL-15, IFN-γ, and TGF-β) and chemokine (IL-8, RANTES, MIP-1α, Eotaxin, IP-10) production. In both cell types, IL-21 stimulated rapid activation of ERK1/2. Both cytokine production and ERK1/2 activation were completely inhibited by the use of an IL-21R neutralizing antibody. Inhibition of ERK activity with the ERK1/2-selective inhibitor U0126 led to significant reversal of IL-21-induced cytokine production, indicating the involvement of ERK1/2 in the induction of cytokine production by IL-21.
2. Materials and Methods
2.1 Leukemia Cells and Reagents
U937 leukemia cells (myeloid monocytic leukemia cell line) were purchased from American Type Culture Collection and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) containing 50 U per ml each of penicillin and streptomycin. The cultures were maintained in an incubator enriched with 5% CO2. FBS was obtained from Atlanta Biologicals (Atlanta, GA). Recombinant IL-21 was purchased from R & D Systems (Minneapolis, MN). According to the manufacturer, the cytokine was purified by sequential chromatography, and sterilized by filtration. The endotoxin level was less than 5.7 × 10− 2 pg /ml, as determined by the limulus amebocyte lysate (LAL) method. This method utilizes an aqueous extract of blood cells (amebocytes) from the horseshoe crab (Limulus polyphemus) which reacts with bacterial endotoxin, and can be measured with a chromogenic assay. Optimal dosage of IL-21 (50 ng/ml) used in this study was determined by preliminary dose response experiments conducted using U937 cells (data not shown). In those experiments, 50 ng/ml IL-21 was just as effective as 100 ng/ml IL-21 in activating protein tyrosine phosphorylation.
2.2 Isolation of Monocytes
Whole blood was obtained from anonymous healthy donors at the New York Blood Center (Long Island City, NY) and separated into fractions using Ficoll-Percoll gradient (GE Health Care, Piscataway, N.J.) centrifugation at 400 × g for 40 min at 20° C. White blood cells were harvested and suspended in RPMI 1640 medium supplemented with 10% FBS, 100 µg of streptomycin per ml, and 100 U of penicillin per ml. The white blood cells were layered onto 6-well plates and allowed to adhere at 37° C for 90 minutes. Nonadherent cells were then removed and adherent cells (monocytes) were incubated in complete medium.
2.3 Western Blotting
U937 cells or human monocytes (80 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and then either untreated or stimulated with IL-21 (50 ng/mL) over time up to 60 minutes. At the end of the period, 100 µg of lysate protein from each sample were resuspended in SDS-gel sample buffer, separated by SDS PAGE, and transferred to a nitrocellulose membrane. The membrane was immunoblotted in blocking buffer containing anti-phospho ERK1/2 antibody (1:1000 dilution) (Upstate, Charlotttesville, VA) for 1 hour and washed in Tris buffered saline with 0.1% Tween 20 (TBST) for 15 minutes at room temperature, with changes in buffer every 5 minutes. The membrane was exposed to horseradish peroxidase (HRP) goat anti-rabbit secondary antibody (Amersham, Piscataway, NJ) for 1 hour and developed with supersignal CL-HRP detection reagent (Pierce, Rockford, IL). To assess loading, the membrane was stripped by incubation in 0.1 N HCl for 5 minutes and re-probed for total ERK (1:1000 dilution) (Upstate) as a loading control. This experiment was also conducted using 100 ng/ml IL-21 (data not shown).
2.4 Measurement of ERK Activity
The enzymatic activity of ERK was measured using a kit from Assay Designs (Ann Arbor, MI). Briefly, U937 cells or human monocytes (40 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/ml) over time. In some experiments, cells were pretreated with a neutralizing antibody to the IL-21R (1 µg/ml) (Novus Biologicals, Littleton, CO) prior to stimulation with IL-21. The neutralizing antibody was developed against amino acids 65–80 and 505–521 of human IL-21R (16). The cells were then lysed using cell lysis buffer, and protein concentration was determined by using the Coomassie Plus Protein Assay Kit (Pierce, Rockford, IL). Ten micrograms of protein from each sample were used in the ERK activity assay. A positive control was performed using a Jurkat cell lysate stimulated with phorbol 12-myristate 13-acetate (PMA). A negative control was performed using assay buffer containing protease inhibitors and dimethyl sulfoxide (DMSO). Anti-phospho ERK IgG and HRP conjugated goat anti-rabbit IgG were used for detection. The absorbance, indicative of ERK1/2 activation, was measured at 450 nm with a microplate reader. These experiments were performed at least three times. This experiment was also conducted using 100 ng/ml IL-21 (data not shown).
2.5 Cytokine Antibody/Protein Arrays
U937 cells or human monocytes (30 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/ml) for 6 hours. In some experiments, cells were pre-incubated with 10 µM U0126, an ERK-1/2 selective inhibitor (Calbiochem, San Diego, CA), for 30 min prior to stimulation with IL-21. The cells were separated from the supernatant by centrifugation. The supernatants (2 ml) from each sample were incubated with the cytokine/chemokine antibody array (Panomics, Inc., Fremont, CA) for two hours before development, using streptavidin-HRP for detection as indicated in the kit.
2.6 Enzyme-linked immunosorbant assay (ELISA)
U937 cells and human monocytes (30 × 106) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/ml) for 2, 6, 12, or 24 hours. To determine whether ERK1/2 mediates the effects of IL-21 on cytokine production, the cells were pre-incubated with 10 µM U0126 for 30 min in some experiments prior to stimulation with IL-21. In order to ascertain that induction of cytokine production was the result of IL-21/IL-21R signaling, in some experiments cells were also pretreated with a neutralizing antibody to the IL-21R (1 µg/ml). The cells were separated from the supernatant by centrifugation. The levels of IFN-γ, IL-2, IL-15, RANTES, and IL-8 in the supernatants of monocytes and U937 cells were measured using ELISA kits purchased from RayBiotech (Norcross, GA). Reactions were measured at 450 nm using a microplate reader. For each assay, a standard curve was constructed and used to determine concentrations (pg/ml) of cytokine.
2.7 Statistics
Data are presented as means of three experiments ± standard deviation. Comparison between groups was analyzed by the Student’s t test or one-way or two-way ANOVA. Differences were considered to be significant at p < 0.05.
3. Results
3.1 IL-21 induces increase in cytokine and chemokine production
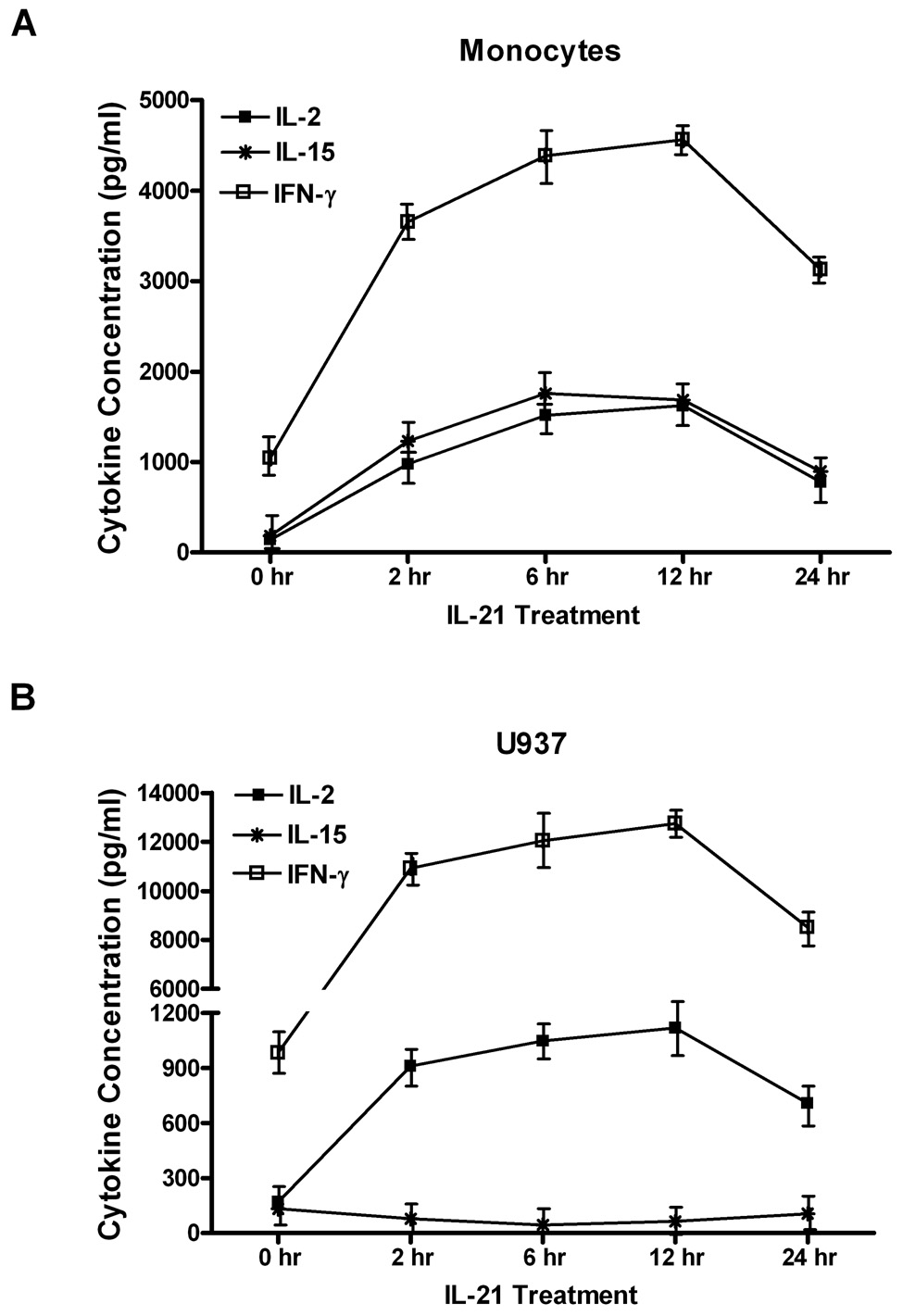
We employed ELISA-based cytokine assays to assess the levels of some cytokines (IL-2, IL-15, and IFN-γ) and chemokines (IL-8 and RANTES) in the cell supernatants and after stimulation with IL-21 for various times (Figure 1). Maximum levels of cytokine and chemokine production were found at 6 hours after treatment with IL-21. ELISA-based measurements showed that IL-2 levels in supernatants of monocytes increased from (200 ± 10 pg/ml) to (1400 ± 145 pg/ml) after 6 hr stimulation by IL-21. In U937 cells, IL-2 levels increased from (180 ± 13 pg/ml) to (900 ± 45 pg/ml) after stimulation with IL-21 for 6 hours (Figure 1A and 1B). In contrast, ELISA measurements showed that IL-21 induced differential induction of IL-15 production. In monocytes, IL-21 induced considerable IL-15 production, from (275 ± 13 pg/ml) to (1550 ± 74 pg/ml) in 6 hours. IL-21 stimulation led to a decline in IL-15 production from (152 ± 9 pg/ml) to (36 ± 5 pg/ml) in U937 leukemia cells (Figure 1A and 1B). IFN-γ levels increased in supernatants of monocytes by 133%, from (1180 ± 76 pg/ml) to (4200 ± 200 pg/ml) in response to IL-21. In U937 cells, IL-21 stimulation increases IFN-γ production from (1145 ± 53 pg/ml) to (8000 ± 110 pg/ml) in 6 hours (Figure 1A and 1B).
Figure 1. Time course analysis of IL-21-induced cytokine and chemokine production.
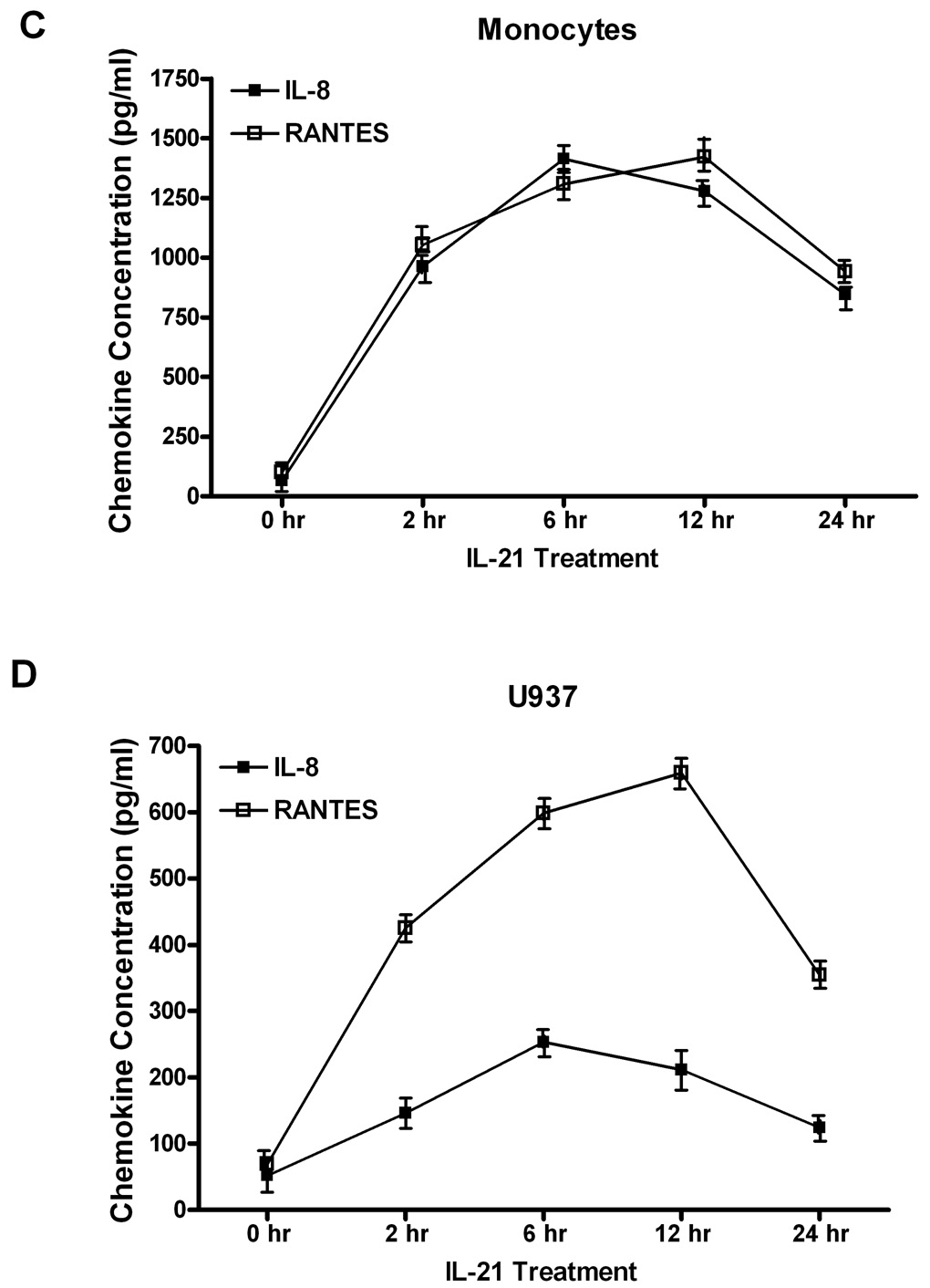
Human monocytes (1A, 1C) or U937 cells (1B, 1D) (30 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/mL) at 0, 2, 6, 12, and 24 hours. IL-2, IL-15, IFN-γ, IL-8, and RANTES concentrations were measured in the supernatants from each sample by ELISA. Data represents the mean ± SD of three experiments. (A) * Monocytes P < 0.05, (B) * U937 cells P < 0.05, (C) * Monocytes P < 0.001, (D) * U937 cells P < 0.05.
We also assessed the levels of chemokines in the supernatants of these cells after IL-21 stimulation by measuring the levels of IL-8 and RANTES. In monocytes, levels of IL-8 increased from (76 ± 6 pg/ml) to a maximum of (1170 ± 107 pg/ml) after IL-21 stimulation for 6 hours, while in U937 cells, it increased from (70 ± 9 pg/ml) to (1213 ± 76 pg/ml) after stimulation with IL-21 (Figure 1C and 1D). In monocytes, RANTES levels increased from (105 ± 15 pg/ml) to (1200 ± 82 pg/ml) after IL-21 stimulation. In U937 cells, RANTES levels increased from (87 ± 13 pg/ml) to (572 ± 67 pg/ml) after stimulation with IL-21 for 6 hours (Figure 1C and 1D).
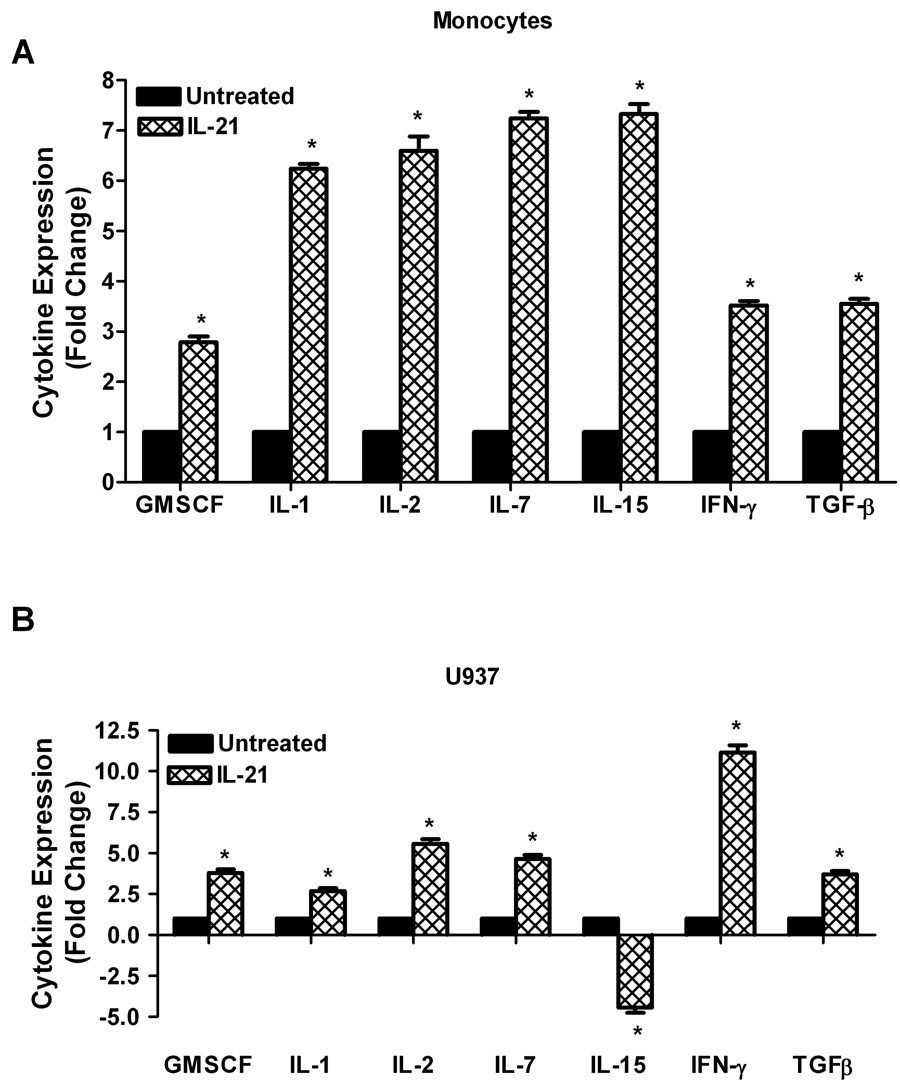
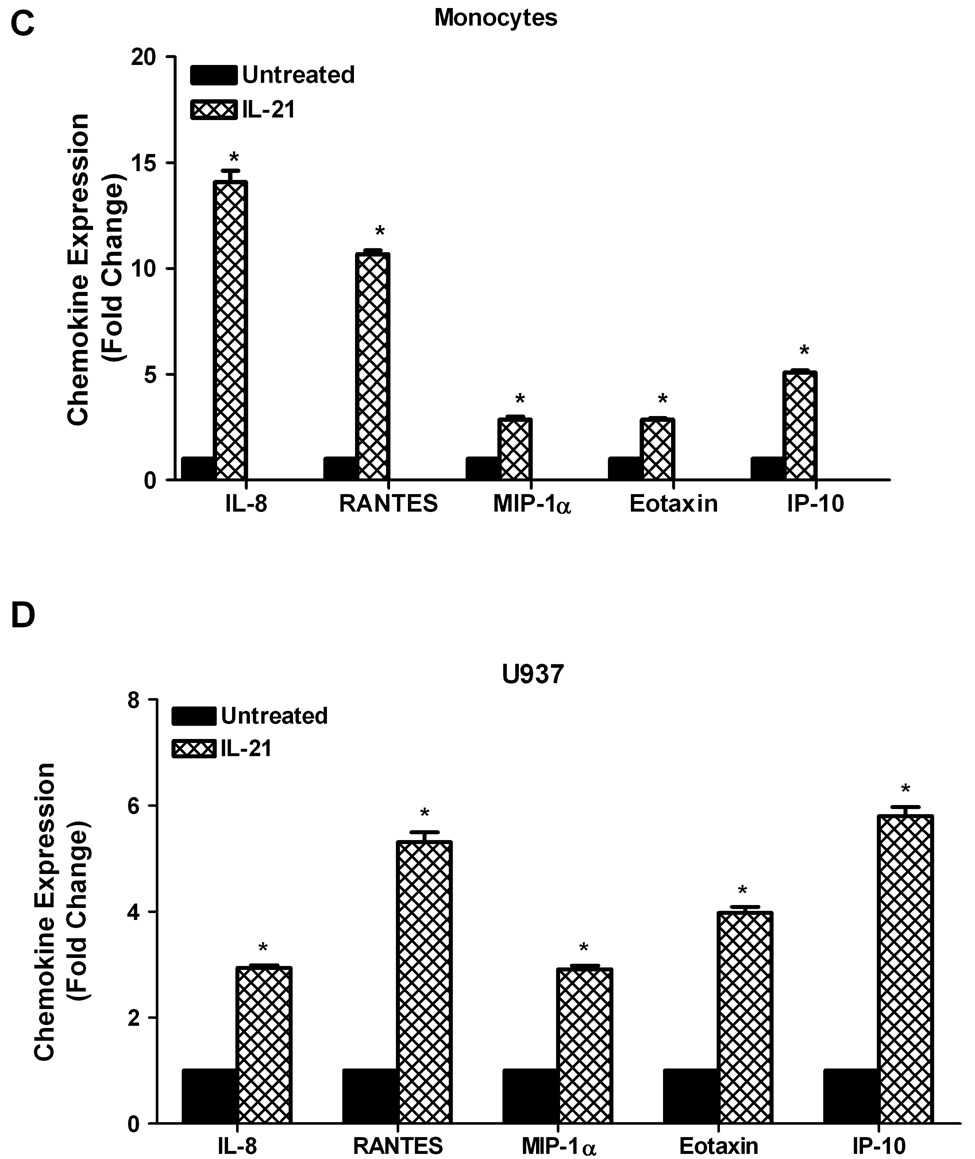
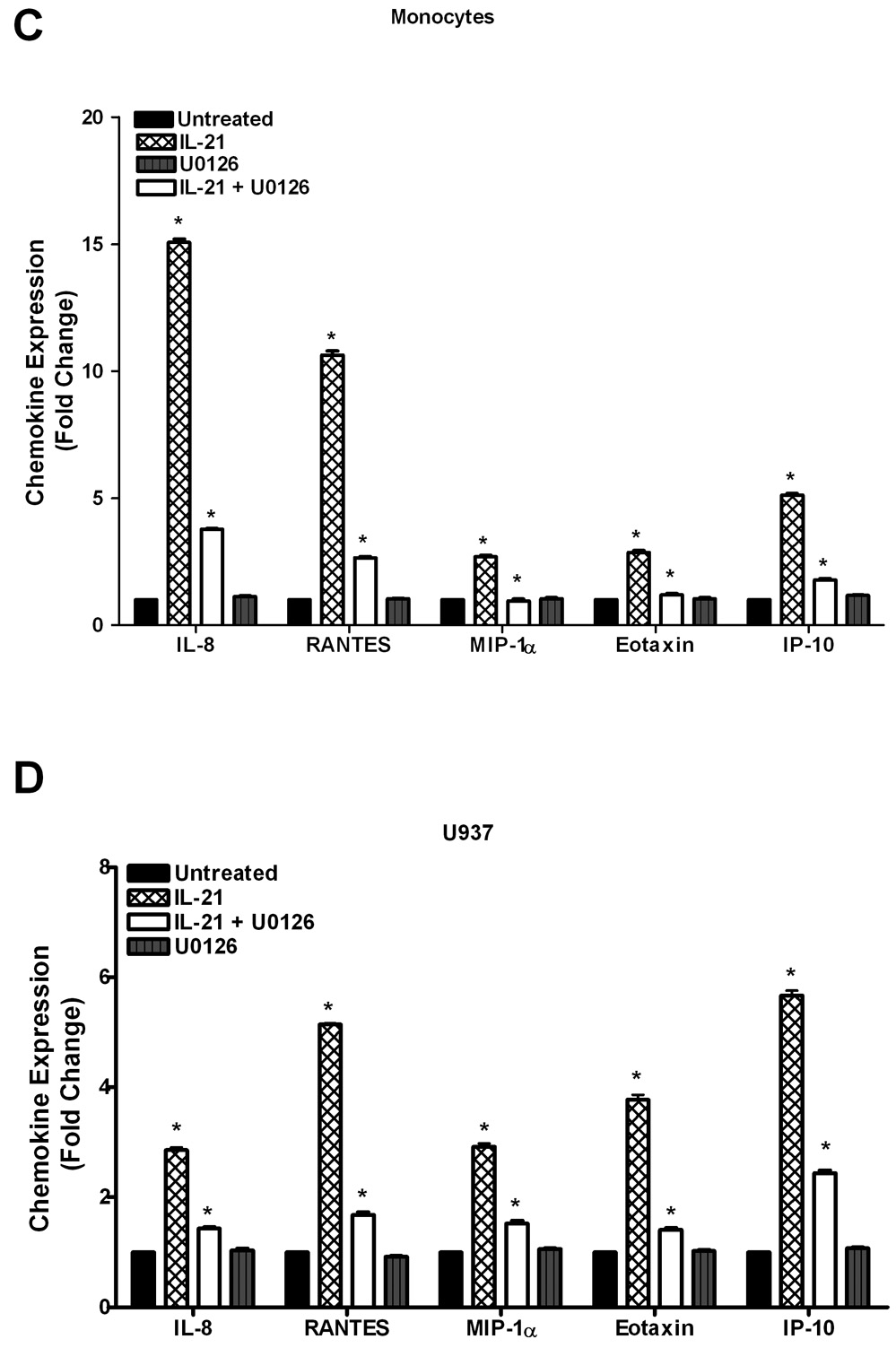
To validate the results in Figure 1, cytokine antibody/protein arrays (Panomics, Inc.) were used to investigate the effects of IL-21 on cytokine and chemokine production in human monocytes and U937 leukemia cells. As shown in Figure 2A, there were substantial increases in cytokine production in IL-21 treated monocytes. This stimulatory effect followed the order IL-7>IL-15>IL-2>IL-1>IFN-γ > TGF-β > GM-CSF. Similarly, expression of several cytokines was also significantly upregulated by IL-21 in U937 leukemia cells (Figure 2B); the stimulatory effect followed the order IFN-γ> IL-2>IL-7>GM-CSF>TGF-β> IL-1>IL-15. Secretion of several chemokines was also significantly upregulated by IL-21 in human monocytes in the following order; IL-8>RANTES>IP-10>MIP-1α> Eotaxin (Figure 2C), and in U937 cells in the following order; IP-10>RANTES>Eotaxin>IL-8>MIP-1α (Figure 2D). The cytokine/chemokine array results for IL-2, IL-15, IFN-γ, IL-8, and RANTES confirm the ELISA measurement levels of these cytokines.
Figure 2. IL-21 induces upregulation of cytokine and chemokine production.
Human monocytes (2A, 2C) or U937 cells (2B, 2D) (30 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/mL) for 6 hours. Supernatants from each sample were incubated with the cytokine/chemokine antibody array for two hours before development using streptavidin-HRP solution. Data represents the mean ± SD of three experiments. (A, C) * Monocytes P < 0.05, (B, D) * U937 P < 0.01.
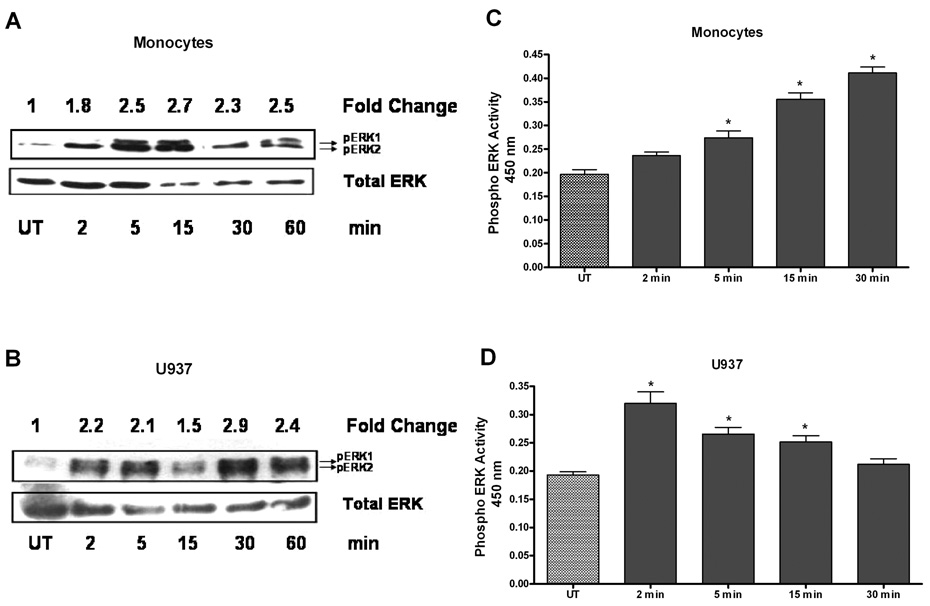
3.2 IL-21 induces rapid increases phosphorylation of ERK1/2
We were interested in the mechanism by which IL-21 induced cytokine/chemokine expression. In recent reports, ERK has been shown to play a role in cytokine regulation (17–19). To determine whether ERK1/2 mediated the induction of cytokine production by IL-21, we examined the effects of IL-21 on ERK activity. Western blot analyses show that IL-21 stimulated phosphorylation of ERK1/2 in human monocytes within 2 minutes and reached a plateau level of approximately 2.7-fold by 15 minutes (Figure 3A). In U937 cells, the phospho-ERK-1/2 level increased to 2.2 fold within 2 minutes and reached a plateau level of approximately 2.9-fold in 30 minutes (Figure 3B). Additional evidence for ERK1/2 activation by IL-21 was also provided by the use of ELISA in both monocytes and U937 cells. ELISA results show that ERK1/2 phosphoprotein levels increased in IL-21 treated U937 cells, and occurred rapidly in monocytes (Figure 3C and 3D). These results provide strong evidence that both ERK1 and 2 are activated by IL-21 in both monocytes and U937 leukemia cells. Taken together with the Western blot data, these results show that IL-21 induces activation of ERK-1 and ERK-2 in both monocytes and U937 cells.
Figure 3. IL-21 stimulates ERK phosphorylation.
Human monocytes (3A) or U937 cells (3B) (80 ×106) were incubated with orthovandate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/ml) over a time course. Total cell lysates (100 μg protein) were analyzed by SDS PAGE and immunoblotted using an anti-phospho-ERK1/2 Ab. The membranes were stripped and re-probed for total ERK as a loading control. The bands were scanned by Digital Image Analyzer and ERK1/2 phosphoprotein levels in each time point were normalized with total ERK levels. Fold changes were calculated by comparing the p-ERK1/2:ERK ratio in each time point with the ratio in the untreated samples. Each blot is a representative of repeated and reproducible experiments. Human monocytes (3C) or U937 cells (3D) (50 × 106) were incubated with orthovanadate (5mM) for 30 minutes, and untreated or stimulated with IL-21 (50 ng/ml) over a time course. An immunometric ELISA assay was used to monitor ERK activation. A positive control using a Jurkat cell lysate stimulated with PMA and negative control using assay buffer containing protease inhibitors and DMSO were performed to validity the assay (data not shown). Data represents mean ± SD of three experiments. (C) * Monocytes P < 0.001, (D) * U937 P < 0.001.
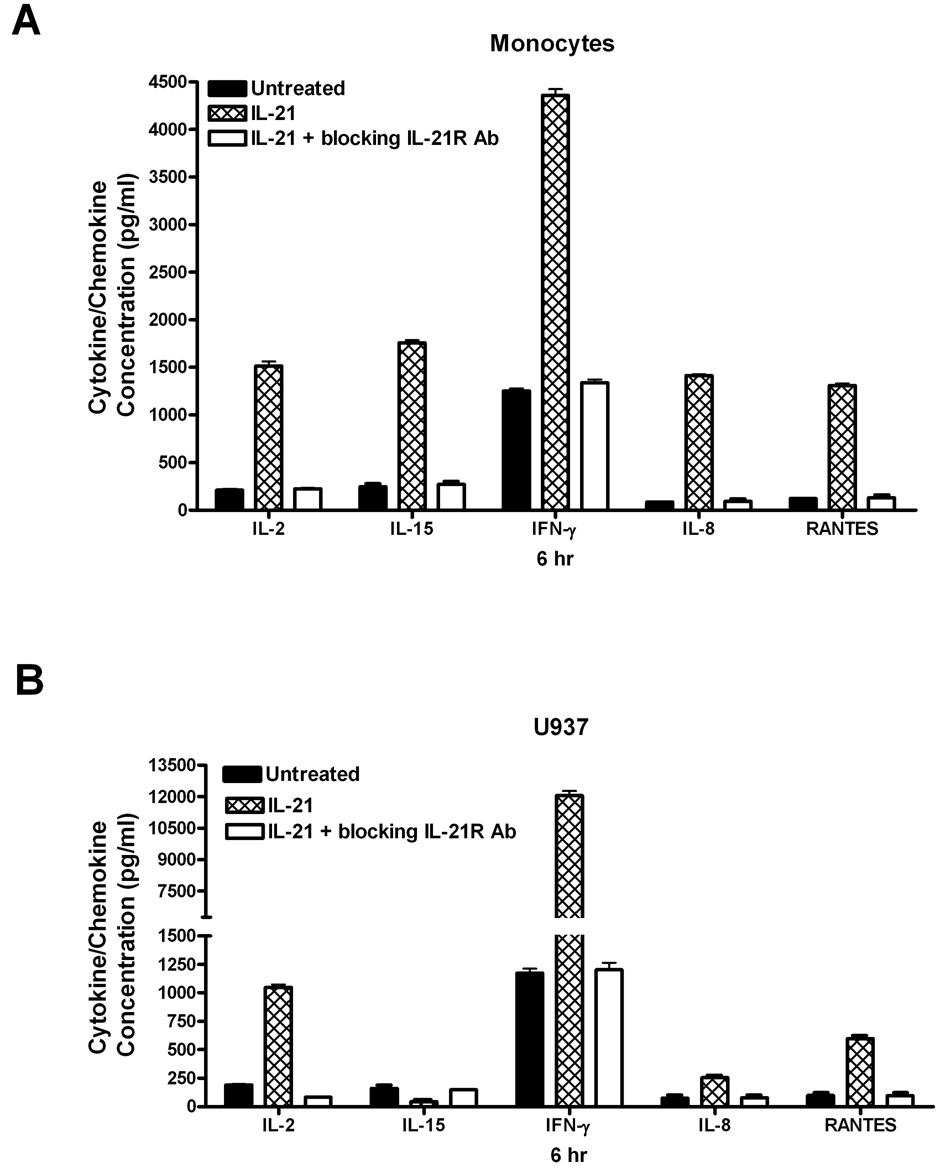
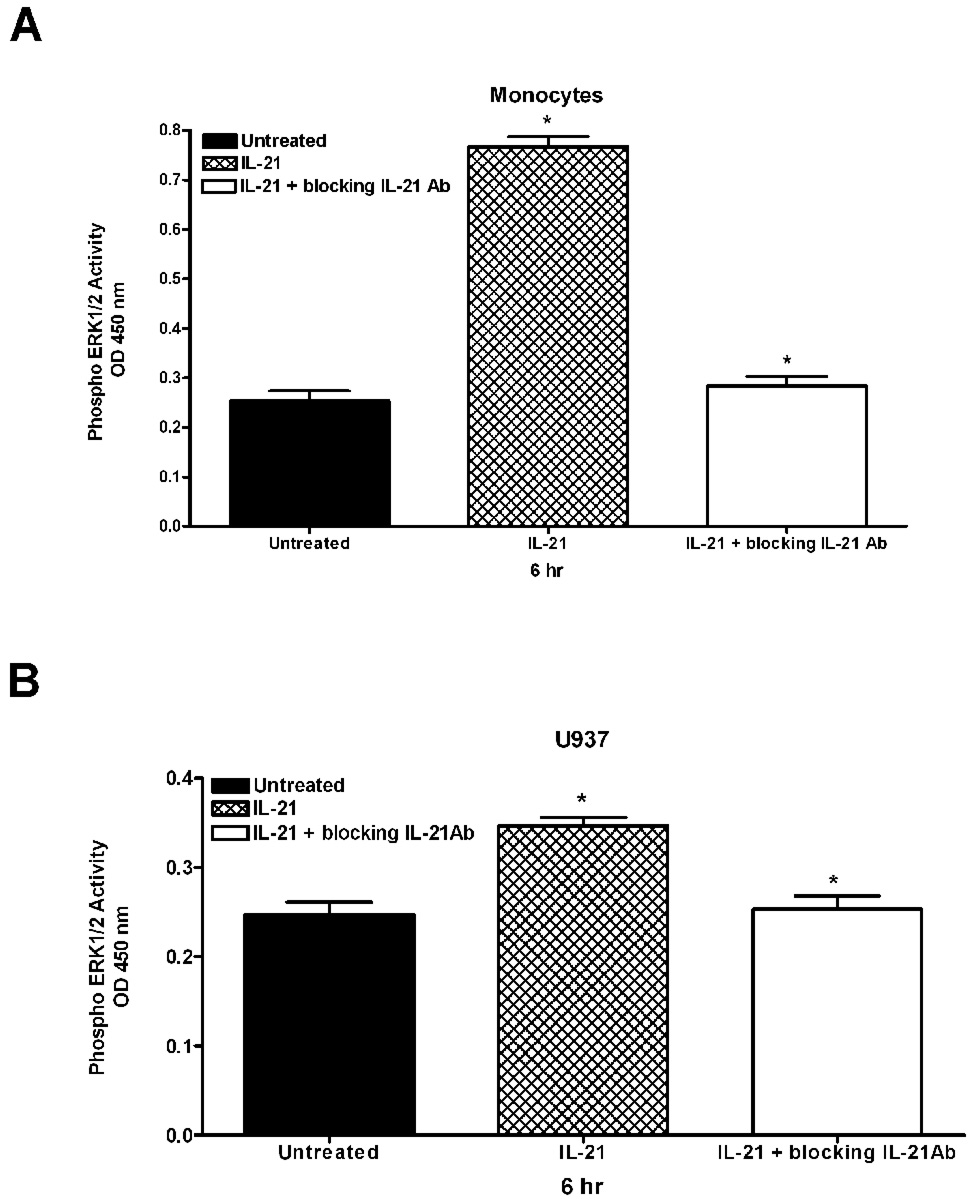
3.3 Neutralization of the IL-21R prevents IL-21 induction of ERK1/2 activation and cytokine/chemokine production
To specifically implicate IL-21 in the observed effects, cells were pretreated with a neutralizing IL-21R antibody before the addition of IL-21 in order to block the interaction of IL-21 with its receptor. As shown in Figure 4, pretreatment of cells with a neutralizing antibody to the IL-21R prior to stimulation with IL-21 completely reversed the stimulatory effect of IL-21 on cytokine and chemokine production. The inhibition of the stimulatory effect of IL-21 by the IL-21R neutralizing antibody suggests that the induction of cytokine production was the direct result of IL-21/IL-21R signaling. The data in Figure 5 also shows that neutralization of the IL-21R reverses the ability of IL-21 to stimulate ERK1/2 activation. This experiment was also conducted with an antibody to neutralize the IL-21 ligand, and similar results were found (data not shown). These data confirm that ERK1/2 plays a role in IL-21/IL-21R signaling.
Figure 4. IL-21 induction of cytokine and chemokine production is inhibited by neutralization of the IL-21R.
Human monocytes (4A) or U937 cells (4B) (30 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/mL) for 6 hours. In some experiments, cells were pretreated with an antibody to neutralize the IL-21R (1 µg/ml). IL-2, IL-15, IFN-γ, IL-8, and RANTES concentrations were measured in the supernatants from each sample by ELISA. Data represents the mean ± SD of three experiments. (A) * Monocytes P < 0.002 (B) * U937 cells P < 0.003
Figure 5. ERK phosphorylation inhibited by neutralization of the IL-21R.
Human monocytes (5A) or U937 cells (5B) (50 × 106) were incubated with orthovanadate (5mM) for 30 minutes, and untreated or stimulated with IL-21 (50 ng/ml) over a time course. In some experiments, cells were pretreated with an antibody to neutralize the IL-21R (1 µg/ml) or to neutralize the IL-21 ligand (3 µg/ml). An immunometric ELISA assay was used to monitor ERK activation. A positive control was performed using a Jurkat cell lysate stimulated with PMA. A negative control was performed using assay buffer containing protease inhibitors and DMSO. Data represents the mean ± SD of three experiments. (A) * Monocytes P < 0.001 (B) * U937 P < 0.001
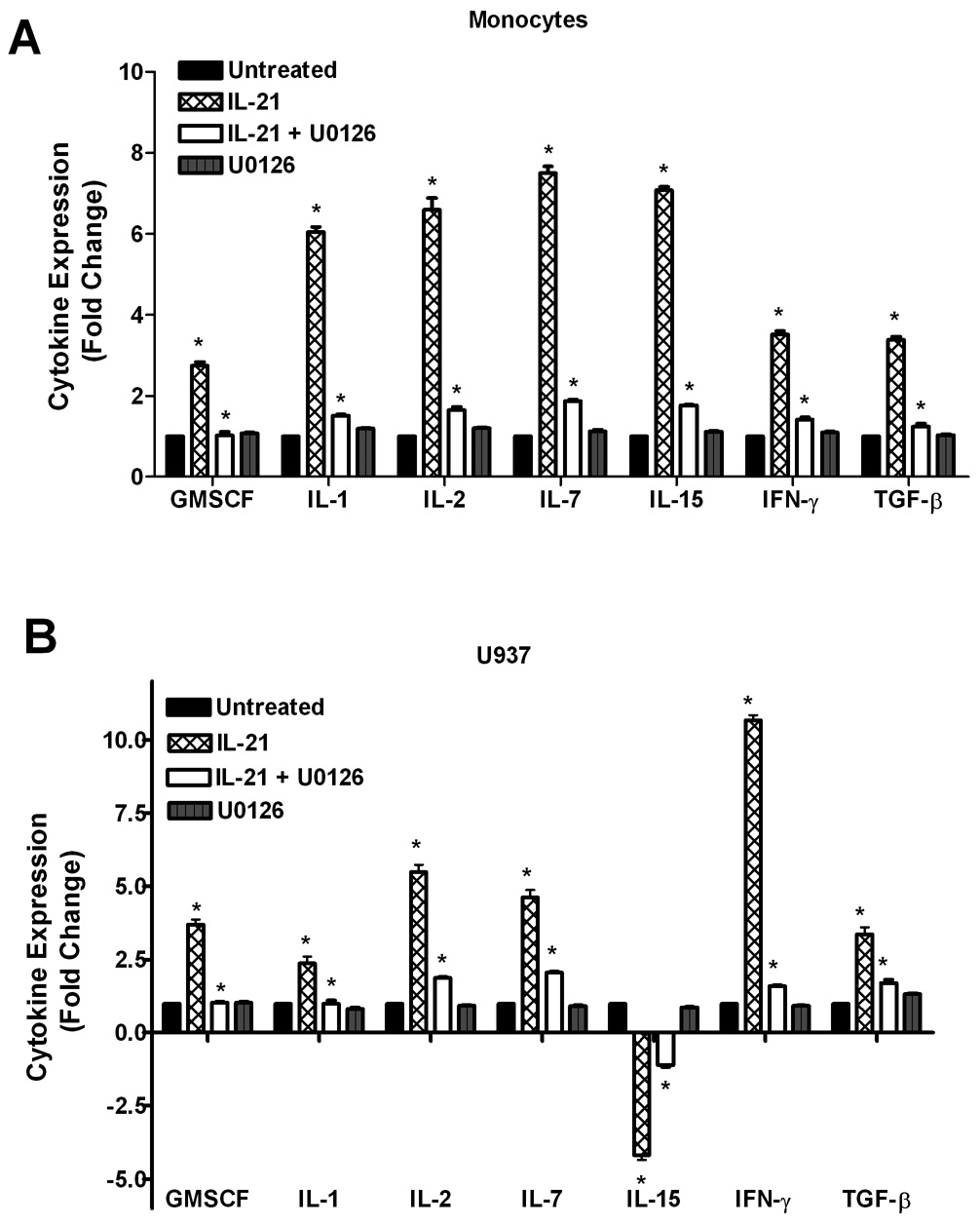
3.4 Inhibition of ERK1/2 activity reverses IL-21-induced cytokine and chemokine production
Pretreatment of cells with the ERK-1/2 inhibitor (U0126) prior to stimulation with IL-21 significantly reversed the stimulatory effect of IL-21 on cytokine/chemokine production (Figure 6). The inhibition of the stimulatory effect of IL-21 by U0126 suggests that ERK mediates partial regulation of IL-21-induced cytokine production. Even though IL-21 also upregulates chemokine production in monocytes and U937 leukemia cells, inhibition of ERK1/2 reversed the ability of IL-21 to stimulate chemokine production. This data indicates that ERK1/2 plays a role in the observed IL-21-induced effects.
Figure 6. U0126 inhibits IL-21-induced upregulation of cytokine and chemokine production.
Human monocytes (6A, 6C) or U937 cells (6B, 6D) (30 × 106 cells) were incubated with orthovanadate (5mM) for 30 minutes, and either untreated or stimulated with IL-21 (50 ng/mL) for 6 hours. In some of the experiments, the cells were pretreated with 10 µM U0126 (ERK-1/2 inhibitor) for 30 min prior to stimulation with IL-21. In some cases, the cells were treated with U0126 alone without IL-21. Supernatants from each sample were incubated with the cytokine/chemokine antibody array for two hours before development using streptavidin-HRP solution. Data represents the mean ± SD of three experiments. (A) * Monocytes P < 0.001, (B) * U937 P < 0.05, (C) * Monocytes P < 0.007, (D) * U937 P < 0.001.
4. Discussion
In this study, we have presented evidence that IL-21 significantly induced the production of several cytokines (GM-CSF, IL-1, IL-2, IL-7, IL-15, IFN-γ, and TGF-β) and chemokines (IL-8, RANTES, MIP-1α, Eotaxin, IP-10) in leukemia cells and human monocytes. We have also shown that IL-21 activates ERK1/2 in these cells. Both cytokine production and ERK1/2 activation are completely blocked by a neutralizing antibody to the IL-21R, suggesting that the effects of IL-21 reported here are mediated by IL-21/IL-21R signaling. We have also provided evidence that the inhibition of ERK1/2 activity by U0126 abrogates the IL-21-induced cytokine and chemokine production. However, this inhibition by U0126 does not completely ablate the cytokine production induced by IL-21, which suggests that other signaling pathway(s) may be involved. In a study by de Totero et al. (20), the authors observed IL-21-induced activation of the JAK/STAT pathway in chronic lymphocytic leukemia cells. Preliminary results in our laboratory also suggest the involvement of JAK2 in IL-21-induced cytokine production. Perhaps the JAK/STAT pathway may also be involved in IL-21-induced cytokine production, but its role requires more investigation.
Many if not all of the chemokines/cytokines studied here are important in the immune system. For example, IL-2 is effective in immunotherapy for many cancers, through the stimulation of immune response, activation of effector T cells, and increasing the cytotoxic activity of NK cells (21, 22). IL-15 also appears to be a likely candidate for immunotherapy in that it promotes NK cell maturation and increases the survival of memory CD8+ T cells (22). IL-21 stimulation of U937 leukemia cells also induced the production of TGF-β, a regulator of cell growth and differentiation in many cell types including regulatory T cells, which improves immune response and regulation in cancer patients (23, 24). GM-CSF, a colony stimulating factor, is a potent stimulator of specific cell-mediated cytotoxicity against autologous tumor targets and its presence in the microenvironment gives long-lasting anti-tumor immunity (25). IL-7 is known to be important for proliferation during certain stages of normal B-cell maturation (26). IFN-γ has been reported to exhibit negative regulatory effects on the growth of leukemic cells and to mediate the inhibitory actions of other cytokines (27). IP-10, interferon-gamma inducible protein 10, promotes natural killer and T-cell migration, and is a regulator of T-cell and bone marrow progenitor cell maturation (28, 29). By stimulating the production of these cytokines, IL-21 could influence immune response, NK cell maturation and cytotoxicity, and hematopoiesis.
Our data also show that IL-21 stimulation induced the production of IL-1, IL-8, MIP-1α, RANTES, and eotaxin. Both IL-1 and IL-8, pro-inflammatory cytokines, are involved in immune defense against infection (30, 31). MIP-1α, another pro-inflammatory cytokine, has the ability to promote growth inhibition to evade the development of hematological diseases (32). RANTES has been found to induce proliferation of NK cells and promote innate immune response (33). Interestingly, eotaxin has been reported to inhibit tumor growth (34). All of these findings suggest the potential role of IL-21 as a possible therapeutic agent for leukemia, and provide evidence in support of the notion that IL-21 could potentially alter immune response via cytokine regulation (35–38).
Abnormal cytokine signaling and production are hallmarks of leukemia, and contribute to aberrant growth, survival, and resistance to chemotheraphy. It remains possible that the upregulatory effect of IL-21 on cytokine production in U937 leukemia cells may be relevant to the mechanisms that underlie the possible therapies to treat B-cell malignancies, since many of the cytokines induced by IL-21 treatment are involved in inflammatory and growth responses.
In conclusion, IL-21 promotes cytokine production in both monocytes and leukemia cells. Here we show that in both leukemia cells and human monocytes, IL-21 stimulates rapid activation of ERK-1/2. The inhibition of ERK by an ERK1/2 inhibitor, U0126, leads to marked significant reversal of cytokine production by IL-21. These current findings implicate the involvement of ERK1/2 in the induction of cytokine production by IL-21, however, the data do not rule out the involvement of other signaling pathways such as the JAK/STAT pathway in this process.
Acknowledgments
This work was supported by research funds from NIGMS/SCORE, 5T32HL007735-13 and MMC-VICC U54 Cancer Health Disparities grant (U54 CA1408) to Dr. Samuel Evans Adunyah. Ms. C. Fuqua was supported in part by 5T32HL007735-13 and U54 Cancer Health Disparities grant (CA1408) from the National Institutes of Health. We thank Mr. Josiah J. Sampson III for his support and valuable discussions while this work was in progress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrish-Novak J, Dillon JR, Nelson A, Hammond A, Sprecher C, Gross JA. IL-21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 2.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–145. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Carlo E, Comes A, Orengo A, Rosso O, Meazza R, Musiani P, Colombo M, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 4.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparable evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 5.Mehta D, Wurster A, Whitters M, Young D, Collins M, Grusby M. IL-21 Induces the Apoptosis of Resting and Activated Primary B Cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 6.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 7.Asao H, Okuyama C, Kumaki S, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Sivakumar P, Foster D, Clegg C. IL-21 is a T-helper cytokine that regulates humoral immunity and cell-mediated anti-tumor responses. Immunology. 2004;112:177–182. doi: 10.1111/j.1365-2567.2004.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib T, Nelson A, Kaushansky K. IL-21: A novel IL-2 family lymphokine that modulates B, T, and NK cell responses. J Allergy Clin Immunol. 2004;112:1033–1045. doi: 10.1016/j.jaci.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher Z, Morse HC, III, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 12.Brady J, Hayakawa Y, Symth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Tschoi M, Sploski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- 14.Coquet J, Kyparissoudis K, Pellicci D, Besra G, Berzins S, Smyth M, Godfrey D. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 15.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by TH17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza CD, Evanson OA, Weiss DJ. Role of the MAPKERK pathway in regulation of cytokine expression by Mycobacterium avium subsp paratuberculosis–exposed bovine monocytes. Am J Vet Res. 2007;68:625–630. doi: 10.2460/ajvr.68.6.625. [DOI] [PubMed] [Google Scholar]
- 18.Stopak K, Chiu Y, Kropp J, Grant R, Greene WC. Distinct Patterns of Cytokine Regulation of APOBEC3G Expression and Activity in Primary Lymphocytes, Macrophages, and Dendritic Cells. J Biol Chem. 2007;282:3539–3546. doi: 10.1074/jbc.M610138200. [DOI] [PubMed] [Google Scholar]
- 19.Alexander JP, Acott TS. Involvement of the Erk-MAP Kinase Pathway in TNFα Regulation of Trabecular Matrix Metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003;44:164–169. doi: 10.1167/iovs.01-1201. [DOI] [PubMed] [Google Scholar]
- 20.deTotero D, Meazza R, Zupo S, Cutrona G, Matis S, Colombo M, Balleari E, Pierri I, Fabbi M, Capaia M, Azzarone B, Gobbi M, Ferrarini M, Ferrini S. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood. 2006;107:3708–3715. doi: 10.1182/blood-2005-09-3535. [DOI] [PubMed] [Google Scholar]
- 21.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 22.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4 + CD25− naive T cells to CD4 + CD25 + regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4 + CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 25.Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SR. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Platanias LC, Fish EN. Signaling pathways activated by interferons. Exp Hematol. 1999;27:1583–1592. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 28.Inoue Y, Tsushima H, Ando K, Sawayama Y, Sakai M, Yamasaki R, Matsuo E, Tsutsumi C, Imaizumi Y, Iwanaga M, Imanishi D, Taguchi J, Miyazaki Y, Tomonaga M. Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3alpha/CCL20 is induced by inflammatory cytokines. Exp Hematol. 2006;34:19–26. doi: 10.1016/j.exphem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): A novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 31.Xie K. Interleukin-8 and human cancer biology. Cytokine & Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 32.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 33.Makishima H, Ito T, Momose K, Nakazawa H, Shimodaira S, Kamijo Y, Nakazawa Y, Ichikawa N, Ueno M, Kobayashi H, Kitano K, Saito H, Kiyosawa K, Ishida F. Chemokine system and tissue infiltration in aggressive NK-cell leukemia. Leuk Res. 2007;9:1237–1245. doi: 10.1016/j.leukres.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 35.Okuma E, Inazawa Y, Saeki K, You A. Potential role of extracellular signal-regulated kinase but not p38 during myeloid differentiation of U937 cells stimulated by cytokines: augmentation of differentiation via prolonged activation of extracellular signal-regulated kinase. Exp Hematol. 2002;30:571–581. doi: 10.1016/s0301-472x(02)00801-9. [DOI] [PubMed] [Google Scholar]
- 36.Sachs L. The cellular and molecular environment in leukemia. Blood Cells. 1993;19:709–730. [PubMed] [Google Scholar]
- 37.Furukawa J, Hara I, Nagai H, Yao A, Oniki S, Fujisawa M. Interleukin-21 gene transfection into mouse bladder cancer cells results in tumor rejection through the cytotoxic T lymphocyte response. J Urol. 2006;17:1198–1203. doi: 10.1016/j.juro.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Curti BD. Immunomodulatory and antitumor effects of IL-21 in patients with renal cell carcinoma. Expert Rev. Anticancer Ther. 2006;6:905–909. doi: 10.1586/14737140.6.6.905. [DOI] [PubMed] [Google Scholar]