Abstract
The purpose of this study was to examine the neurocognitive network for processing visual word forms in native Chinese speakers using functional magnetic resonance imaging (fMRI). In order to compare the processing of phonological and semantic representations, we developed parallel rhyming and meaning association judgment tasks that required explicit access and manipulation of these representations. Subjects showed activation in left inferior/middle frontal gyri, bilateral medial frontal gyri, bilateral middle occipital/fusiform gyri, and bilateral cerebella for both the rhyming and meaning tasks. A direct comparison of the tasks revealed that the rhyming task showed more activation in the posterior dorsal region of the inferior/middle frontal gyrus (BA 9/44) and in the inferior parietal lobule (BA 40). The meaning task showed more activation in the anterior ventral region of the inferior/middle frontal gyrus (BA 47) and in the superior/middle temporal gyrus (BA 22,21). These findings are consistent with previous studies in English that suggest specialization of inferior frontal regions for the access and manipulation of phonological vs. semantic representations, but also suggest that this specialization extends to the middle frontal gyrus for Chinese. These findings are also consistent with the suggestion that the left middle temporal gyrus is involved in representing semantic information and the left inferior parietal lobule is involved in mapping between orthographic and phonological representations.
Keywords: Chinese, Phonological, Semantic, Rhyming, Meaning, Inferior frontal gyrus, Middle frontal gyrus, Inferior parietal lobule, Middle temporal gyrus
1. Introduction
The nature of orthographic (spelling), phonological (sound), and semantic representations (meaning) has generated considerable interest in cross-linguistic research. In terms of orthography, about 95% of Chinese characters can be divided into sub-units that form complex visual-spatial patterns such as top-down, left to right, and outside to inside orientation (Li, 1993). Studies have demonstrated that visual-spatial properties such as shape, balance, closures, orientation, symmetry, or parallelism are used quickly enough to provide a perceptual basis for the orthographic processing of Chinese characters (Chen and Kao, 2002; Fu, 1993). In terms of semantics, many Chinese characters encode meaning by including a semantic radical. Semantic radicals can provide useful cues such as category information of the character (e.g. all characters indicating a metal element such as 铜 (/tong2/, copper), 银 (/yin2/, silver), 铁 (/tie3/, iron) have the semantic radical 金 (/jin1/, metal), and these radicals are important for character acquisition and recognition (Leck et al., 1995). In terms of phonology, Chinese orthography encodes no phonological information at the sub-syllabic level and every character is a monosyllable. Although most characters contain a phonetic radical, only about 25% of these radicals give fully consistent information about the pronunciation of the character. Although there is some controversy (Liu et al., 2002; Pollatsek et al., 2000; Spinks et al., 2000), researchers have speculated that phonological processing plays a less important role than semantics in visual identification of Chinese words or that phonology is activated only after semantics (Chen and Shu, 2001; Feng et al., 2001; Leck et al., 1995; Zhou, 1997).
English is different from Chinese in the nature of the representations and the mappings that are made between orthography, phonology, and semantics. In contrast to Chinese, English orthography has a serial left to right structure of letter strings. Some studies have shown that visual features such as envelope and initial or final positions of letters influence English word recognition (Rayner and Springer, 1986; Taft and Taft, 2002). In terms of semantics, English has a more arbitrary relationship between orthography and semantics than Chinese because, at a mono-morphemic level, English does not have robust cues to meaning. In other words, the relationship between orthography and semantics is arbitrary. For example, the words cat, cap, mat, and map are similar in orthography but this does not give a clue to their meaning. In terms of phonology, English has a more regular mapping between orthography and phonology than Chinese because about 75% of the words in English are fully consistent in this mapping (Ziegler et al., 1997). Compared to English, then, Chinese has a different orthographic system that has more clues to semantics but a less systematic relationship to phonology.
Lexical processing in English involves a distributed network predominantly in the left hemisphere. We have argued that orthographic representations include the fusiform gyrus, phonological representations include the superior temporal gyrus, semantic representations include the middle temporal gyrus, and mapping between these representational systems relies on posterior heteromodal areas in the inferior parietal cortex (Booth et al., 2001, 2002a,b, 2003a,b). Among other things, the inferior frontal gyrus appears to be involved in modulation of these posterior representational systems (Blumenfeld et al., in press). Researchers have proposed that the anterior ventral portion of the inferior frontal gyrus may be involved in semantic modulation, whereas the posterior dorsal portion of the inferior frontal gyrus may be involved in phonological modulation (Devlin et al., 2003; Poldrack et al., 1999).
Neuroimaging studies of Chinese language processing have used a variety of tasks including covert generation of semantically related words (Tan et al., 2000), semantic judgment (Chee et al., 2000), tone judgment (Klein et al., 2001), covert reading (Kuo et al., 2001), reading aloud (Tan et al., 2001a), real word likeness judgment (Chen et al., 2002), silent reading (Fu et al., 2002), semantic and syntactic plausibility judgment (Luke et al., 2002), covert naming (Kuo et al., 2003), rhyming (Tan et al., 2003), semantic priming (Chee et al., 2003), orthographic search and semantic classification (Ding et al., 2003), homophone and initial consonant judgment (Siok et al., 2003), homophone and visual-spatial judgment (Kuo et al., 2004), lexical decision to noun and verbs (Li et al., 2004), and masked word reading (Peng et al., 2004). Despite structural differences between the Chinese and English languages, several neuroimaging studies on Chinese-English bilinguals have demonstrated little language differences using different tasks such as verb generation (Pu et al., 2001), semantic decision (Xue et al., 2004), and cued word generation (Chee et al., 1999). This overall similarity suggests that the language operation may involve common brain mechanisms across languages that translate visual percepts into a set of sounds that convey meaning. Several studies have reported differences between Chinese and English, but some of these differences may be attributed to factors related to bilingual status of the subjects such as age of acquisition, exposure, and usage (Ding et al., 2003; Klein et al., 1999; Tan et al., 2001b).
A recent meta-analysis of 19 studies examining phonological processing of written word forms in Chinese and English showed that both languages exhibited activation in bilateral occipito-temporal regions including left fusiform gyrus (Tan et al., 2005a). Both languages also showed activation in left inferior parietal lobule but it was greater for Chinese. Only Chinese showed activation in left middle frontal gyrus and it was significantly greater than English. It was suggested that the middle frontal gyrus is involved in visual spatial analysis of characters and accessing whole word phonology, whereas the left inferior parietal lobule is involved in phonological short-term memory. Both languages also showed activation in left inferior frontal gyrus, but it was greater in English. Only English showed activation in left temporo-parietal region (including superior/middle temporal gyrus, supramarginal gyrus and inferior parietal lobule) and it was significantly greater than in Chinese. It was suggested that these areas are involved in the mapping between letters (graphemes) and sounds (phonemes) in English. This meta-analysis clearly shows that, at least for phonological processing, there are similarities as well as differences between Chinese and English.
Direct comparisons between tasks that tap into phonological and semantic processing are needed to determine whether brain regions are specialized for processing these representations. Although some studies have examined both phonological and semantic processing in Chinese (Tan et al., 2001b), to our knowledge only two studies have directly compared activation between tasks that differentially tap into these two representational systems. Peng et al. (2003) asked 7 participants to decide whether a presented character contained the vowel /a/ or to decide whether the meaning of a presented character was positive or not (Peng et al., 2003). They found that the semantic task showed greater activation than the phonological task in several brain regions including the left inferior frontal gyrus (BA 47), but that the phonological task did not show greater activation than the semantic task in any region. Dong et al. (2005) asked 12 participants to decide whether two successive words sounded the same (i.e. were homophones) or whether two successive words were associated in meaning (Dong et al., 2005). This study only reported a direct comparison of the semantic task to the phonological task that showed greater activation in the left (BA 47) and right (BA 45) inferior frontal gyrus. Both studies, therefore, support the role of the anterior ventral portion of the inferior frontal gyrus in semantic processing, but do not support the involvement of the posterior dorsal region of the inferior frontal gyrus in phonological processing. Moreover, both studies did not show any differences between the tasks in posterior regions of the language network including the middle temporal gyrus or the inferior parietal cortex. Perhaps the small number of subjects in the first study prevented them from finding reliable task differences due to lack of power or the phonological tasks in both studies were so simple that they were not sensitive to task differences in semantic vs. phonological processing.
In the current experiment, we examined the neural correlates of lexical processing in native Chinese speakers in two parallel word reading tasks-one that involved a rhyming judgment that tapped into phonological representations and the other that involved a meaning association judgment that tapped into semantic representations. In order to maximize the demand on phonological and semantic representations, we used word judgment tasks that involved the serial presentation of three words in each trial. Although semantic association judgment tasks have been used in Chinese word reading before, we chose to use a novel rhyming paradigm to increase the demands on phonological processing. If word processing in Chinese is similar to that previously reported for English (Booth et al., 2002a,b; Poldrack et al., 1999), we expected that the rhyming task requiring mapping from orthography to phonology would generate greater activation than the meaning task in the inferior parietal cortex and in the posterior dorsal region of the inferior frontal gyrus. We also expected that the meaning task requiring mapping between orthography and semantics would generate greater activation than the rhyming task in the middle temporal gyrus and in the anterior ventral region of the inferior frontal gyrus. Although this study does not directly compare Chinese to English speakers, it would be the first to show that specialization of phonological and semantic processing in Chinese is similar to previous reports of this specialization in English speakers.
2. Results
2.1. Behavioral performance
Table 1 presents error rates and reaction times on the lexical processing tasks in the scanner. We calculated task (rhyming, meaning) by condition (word, control) ANOVAs separately for error rates and reaction time. There were no significant task main effects or interactions between task and condition for either error rates or reaction time. There were significant main effects for condition showing that the word stimuli had higher error rates, F(1,51) = 4.63, P < 0.05, and slower reaction time, F(1,51) = 65.87, P < 0.001, than the control stimuli. t tests additionally showed that there were no significant differences between the rhyming and meaning task for the word pairs for either error rates, t(1, 25) = 0.76, P = 0.45, or reaction time, t(1, 25) = 0.58, P = 0.56. We also calculated t tests of O-P+ vs. O+P+ pairs for the rhyming task and of low vs. high association pairs for the meaning task. This showed that O-P+ pairs had higher error rates (M = 12.8%) and slower reaction times (M = 1146 ms) than O+P+ pairs (M = 3.1%, t(1, 25) = 2.32, P < 0.05; M = 1002 ms, t(1, 25) = 2.59, P < 0.05) and that low association pairs had higher error rates (M = 14.8%) than high association pairs (M = 3.6%, t(1, 25) = 2.74, P < 0.05). Reaction times were not significantly different for the low (M = 1159 ms) and high association pairs (M = 1054 ms, t(1, 25) = 1.71, P = 0.09).
Table 1.
Means and (standard deviations) for error rates (%) and reaction time (RT) on the rhyming and meaning lexical tasks and their respective controls
| Lexical |
Control |
|||
|---|---|---|---|---|
| % | RT | % | RT | |
| Rhyming | 5.3 (5.0) | 1140 (123) | 3.8 (3.6) | 839 (157) |
| Meaning | 6.7 (4.1) | 1171 (143) | 2.9 (5.0) | 824 (148) |
2.2. Brain activation patterns
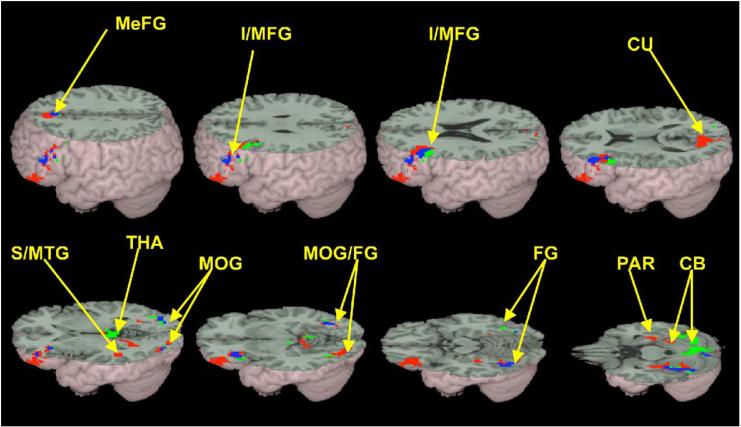
Sites with significant main effects of task (rhyming-control and meaning-control) are listed in Table 2 with significant differences between tasks listed in Table 3. Fig. 1 shows the main effects of task on brain activation. Subjects activated a neural network that was mostly lateralized to the left. In both meaning and rhyming tasks, areas of left inferior/middle frontal gyri, bilateral medial frontal gyri, bilateral middle occipital/fusiform gyri, and bilateral cerebella were activated. For the rhyming task, subjects additionally activated bilateral thalamus. For the meaning task, they additionally activated left superior/middle temporal gyrus, left cuneus, and right parahippocampus.
Table 2.
Brain activations for the rhyming and meaning tasks
| Task | Region | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Rhyming-control | Inferior frontal gyrus/Middle frontal gyrus | L | 9/44/45 | 4.39 | 230 | -51 | 24 | 21 |
| Inferior frontal gyrus | L | 47 | 4.3 | 56 | -36 | 27 | -9 | |
| Medial frontal gyrus | L + R | 8 | 4.06 | 44 | 0 | 21 | 48 | |
| Middle occipital gyrus/Fusiform gyrus/Cerebellum | L | 19/37/* | 4.43 | 140 | -42 | -60 | -21 | |
| Middle occipital gyrus/Fusiform Gyrus/Cerebellum | R | 19/37/* | 4.97 | 316 | 27 | -90 | 6 | |
| Thalamus | L + R | * | 4.05 | 73 | 0 | -33 | 0 | |
| Meaning-control | Inferior frontal gyrus/Middle frontal gyrus | L | 9/44/45/47 | 5.64 | 516 | -45 | 27 | 21 |
| Medial frontal gyrus | L + R | 9 | 4.30 | 97 | 0 | 33 | 39 | |
| Middle occipital gyrus/Fusiform gyrus/Cerebellum | L | 19/37/* | 5.14 | 298 | -36 | -54 | -21 | |
| Middle occipital gyrus/Fusiform gyrus | R | 19/37 | 5.81 | 87 | 27 | -90 | 6 | |
| Cuneus | L | 18/19 | 4.65 | 271 | -12 | -75 | 12 | |
| Superior temporal gyrus/Middle temporal gyrus | L | 22/21 | 4.65 | 47 | -48 | -39 | 3 | |
| Parahippocampus | R | 35 | 3.98 | 51 | 33 | -24 | -24 | |
| Cerebellum | R | * | 4.70 | 91 | 6 | -66 | -33 |
Note. H: left (L), right (R) hemispheres. BA: Brodmann's areas of activation. Voxels: number of voxels in cluster. For clusters with multiple anatomical locations, peak of activation is presented in bold.
Indicates no Brodmann area for that brain structure.
Table 3.
Greater activation for the rhyming compared to the meaning task and for the meaning task compared to the rhyming task
| Group | Regions | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Rhyming-meaning | Inferior parietal lobule/Superior parietal lobule | L | 7/40 | 4.03 | 48 | -18 | -66 | 54 |
| Postcentral gyrus/Inferior parietal lobule | L | 2/40 | 4.02 | 75 | -54 | -27 | 51 | |
| Inferior frontal gyrus/Middle frontal gyrus | L | 9/44 | 3.85 | 52 | -24 | -3 | 48 | |
| Meaning-rhyming | Superior temporal gyrus/Middle temporal gyrus | L | 22/21 | 3.94 | 47 | -39 | -57 | 18 |
| Inferior frontal gyrus/Middle frontal gyrus | L | 45/47 | 4.48 | 42 | -36 | 42 | -21 |
Note. See Table 2 note.
Fig. 1.
Brain activations for rhyming minus control (green) vs. meaning minus control (red). The overlap between the tasks is represented in blue. Rhyming minus control activated left inferior/middle frontal gyri (I/MFG), bilateral medial frontal gyri (MeFG), bilateral middle occipital gyri/fusiform gyri/cerebella (MOG/FG/CB), and bilateral thalamus (THA). Meaning minus control activated left inferior/middle frontal gyri (I/MFG), bilateral medial frontal gyri (MeFG), left middle occipital gyrus/fusiform gyrus/cerebellum (MOG/FG/CB), right middle occipital gyrus/fusiform gyrus (MOG/FG), left cuneus (CU), left superior/middle temporal gyri (S/MTG), right parahippocampus (PAR), and right cerebellum (CB).
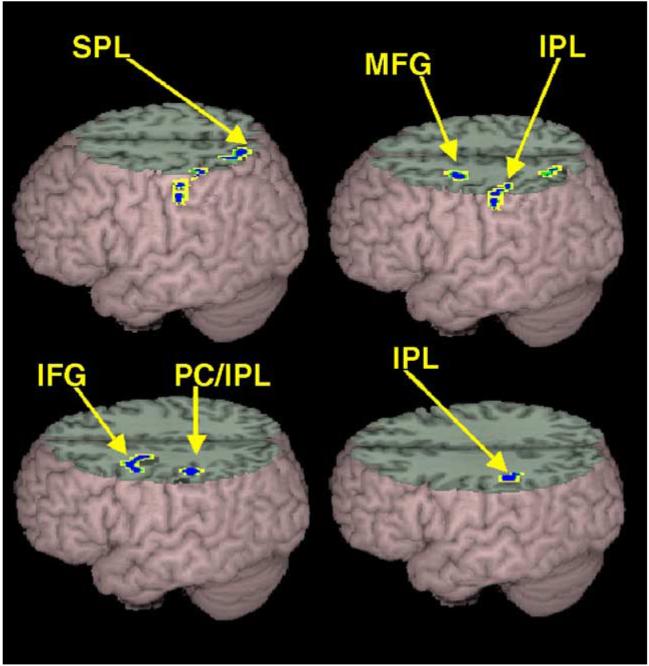
As illustrated in Fig. 2, direct comparisons between tasks revealed significantly greater activation for the rhyming than for meaning task in the posterior dorsal portion of left inferior/middle frontal gyrus (BA 9/44) and left inferior parietal lobule (BA 40). There was also greater activation in left superior parietal lobule and left postcentral gyrus. Because the superior/inferior parietal lobule showed activation by the rhyming task in the between-task comparison but not when compared to the control condition, we compared the control to word condition for the meaning and rhyming tasks to determine if the task difference in this region was due to less activation for the word condition compared to the control condition in the meaning task. This analysis showed large clusters of activation for the control condition in the meaning task in bilateral superior (z = 4.40, k = 164, x = 6, y = -69, z = 54; z = 5.01, k = 77, x = -9, y = -66, z = 54) and inferior parietal lobules (z = 5.04, k = 479, x = 42, y = -33, z = 42; z = 4.75, k = 411, x = -33, y = -48, z = 57). However, this analysis showed smaller clusters of activation for control condition for the rhyming task in right superior (z = 3.71, k = 53, x = 30, y = -54, z = 63) and bilateral inferior parietal lobules (z = 4.39, k = 141, x = 60, y = -33, z = 21; z = 3.58, k = 167, x = -51, y = -60, z = 39). Thus, superior/inferior parietal activation in the rhyming task in this region appears to be better characterized as greater activation for the control compared to the word condition in the meaning task.
Fig. 2.
Significantly greater activation for rhyming than for meaning task included left inferior/superior parietal lobule (I/SPL), left postcentral gyrus/inferior parietal lobule (PC/IPL), and left inferior/middle frontal gyrus (I/MFG).
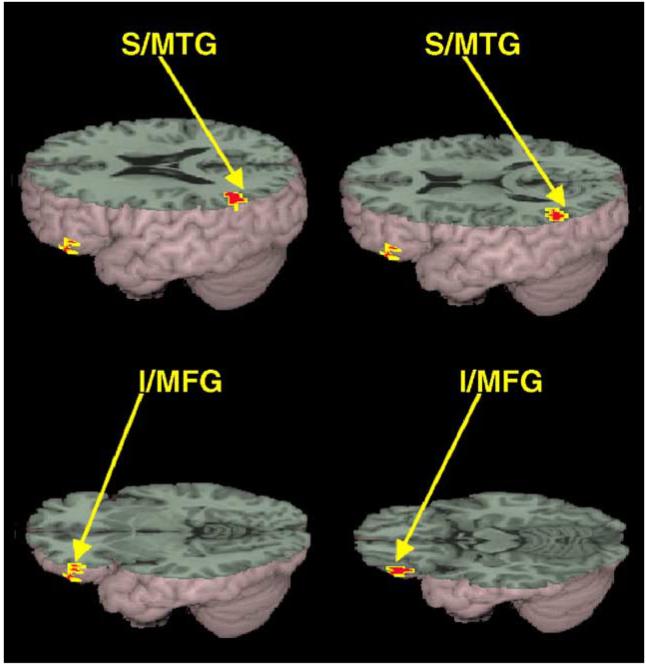
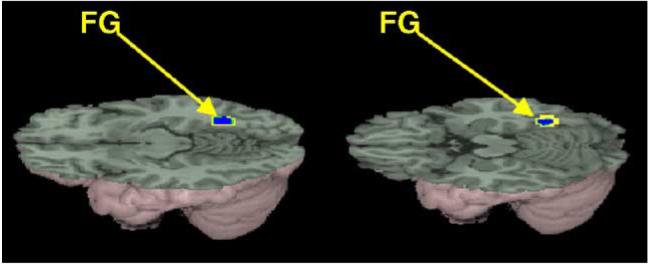
As illustrated in Fig. 3, significantly greater activation for the meaning than for the rhyming task was revealed in the anterior ventral portion of the left inferior frontal gyrus (BA 45/47) and left superior/middle temporal gyrus (BA 22/21). We also correlated signal intensity with accuracy separately for the rhyming and meaning task. Although there were no significant correlations involving the meaning task, greater accuracy was associated with less activation in the right fusiform gyrus (BA 37) in the rhyming task (see Fig. 4). There were no significant correlations of activation with age of starting to learn English or number of years exposed to English, but this lack of correlation could be due to the limited range of these measures or due to the measures not accurately reflecting English language ability. Unfortunately, we did not have standardized measures of reading skill in either Chinese or English.
Fig. 3.
Significantly greater activation for meaning than for the rhyming task included left superior/middle temporal gyrus (S/MTG), and left inferior/middle frontal gyrus (I/MFG).
Fig. 4.
Brain activations significantly correlated with performance. Activation in right fusiform gyrus (FG) decreases with increasing accuracy in the rhyming task.
3. Discussion
Both the meaning and rhyming judgment tasks produced activation in the left inferior/middle frontal gyri, bilateral medial frontal gyri, bilateral middle occipital/fusiform gyri, and bilateral cerebella (see Fig. 1). Because each lexical task was compared to a simple nonlinguistic baseline, a direct comparison between lexical tasks was necessary to examine brain regions specialized for semantic and phonological processing. This analysis revealed that the meaning task produced more activation than the rhyming task in the left superior/middle temporal gyrus and in the anterior ventral region of the inferior frontal gyrus (see Fig. 3). These results are consistent with previous studies in English that suggest that the middle temporal gyrus includes verbal semantic representations (Blumenfeld et al., in press; Booth et al., 2002b) and that the anterior ventral region of the inferior frontal gyrus is specialized for access and manipulation of these representations (Poldrack et al., 1999). A direct comparison between the tasks also revealed that the rhyming task produced more activation than the meaning task in the left inferior parietal lobule and in the posterior dorsal region of the inferior frontal gyrus (see Fig. 2). These results are consistent with previous studies in English that suggest that the posterior dorsal region of the inferior frontal gyrus is specialized for processing phonological representations (Poldrack et al., 1999) and with studies that suggest that the inferior parietal region may be involved in the mapping between orthography and phonology (Booth et al., 2002a, 2003a). Indeed, one of the clusters in the inferior parietal cortex found to be greater in the phonological than the semantic task in our study on Chinese is less than 1 cm from a cluster reported when directly comparing phonological vs. semantic processing in English (Devlin et al., 2003). Our results, therefore, show specialization for phonological and semantic processing, despite the fact that there may have been some cross-task interference because the rhyming and meaning tasks were administered in the same session.
Several previous studies using a variety of tasks report activation for Chinese in the middle frontal gyrus (Chee et al., 1999; Luke et al., 2002; Siok et al., 2003; Tan et al., 2000, 2001b, 2003). Some have argued that the middle frontal gyrus activation in Chinese is associated with the visual processing demanded by the complex shape of the characters and the access of syllable level phonology (Tan et al., 2000, 2001b). Consistent with these previous studies, we found activation in the middle frontal gyrus for both the rhyming and the meaning task compared to baseline (see Fig. 1). Perhaps, the most important contribution of the current research is the finding that the posterior dorsal portion of the middle frontal gyrus was more active in the rhyming compared to the meaning task and that the anterior ventral portion of the middle frontal gyrus was more active in the meaning compared to the rhyming task (see Figs. 2 and 3). This extends the hypothesis that the middle frontal gyrus is crucial for Chinese reading and shows that there is regional specialization of this area associated with semantic and phonological processing.
Although we suggest that the inferior parietal lobule is involved in mapping between orthography and phonology, other roles for this region have been proposed. Some have considered the left inferior parietal region as part of the phonological loop (Paulesu et al., 1993) and this area has also been implicated in sequencing of phonemes in cooperation with Broca's area which seems to be generally involved in sequencing operations (Gelfand and Bookheimer, 2003). Studies that have directly compared verbal and visual-spatial tasks also show that verbal working memory tasks predominantly activate the left inferior parietal regions whereas visual-spatial tasks predominantly activate the right parietal regions (Smith et al., 1996; Zurowski et al., 2002). Although error rates on our task were low, our experimental design involved a judgment of whether a final word matched with one of two previous words, so the demands on short-term memory may have been enhanced. However, both the rhyming and meaning task required the maintenance of three words in memory, so the short-term memory interpretation of activation in the left inferior parietal cortex for phonological processing must assume that the rhyming task requires greater memory resources. It is more plausible that some of the activation, e.g. inferior frontal gyrus, produced when each lexical task was compared to the visual control condition is attributable to short-term memory processes.
Several previous neuroimaging studies on Chinese have reported activation in the superior parietal lobule (Siok et al., 2003; Tan et al., 2000, 2001a,b). We did not observe activation in this region when comparing each task to its baseline. We did, however, find activation in the superior parietal lobule when comparing activation in the rhyming task to the semantic task. Studies using visual spatial tasks have consistently shown the involvement of the superior parietal region in visual search and spatial attention (Booth et al., 2003c; Gitelman et al., 1999). It may be that the rhyming task encouraged more visual spatial attention than the meaning task. In the rhyming task, 30% of word pairs were orthographically similar because they shared the same phonetic radical, so subjects may have relied on visually segmenting this radical and on using this information in their matching judgment (see Fig. 5). Evidence for this segmentation was that the orthographically dissimilar rhyming pairs (O-P+) had higher error rates and slower reaction times than the orthographically similar rhyming pairs (O+P+). In the meaning task, the separation of the phonetic radical would not aid in performance. All words also had semantic radicals that can provide cues to meaning, but a small proportion of associated pairs (only 5) had overlapping semantic radicals between the final word and its preceding word, and these radicals only provide general information. Consequently, visually segmenting the semantic radical from the character and making an association judgment based on the radical would not aid performance (see Fig. 6). In fact, the control condition involving a matching judgment to lines appeared to have greater demands on visual-spatial processing than the meaning judgment, as the control condition produced large clusters activation in the superior/inferior parietal region when compared to the word condition for this task.
Fig. 5.
Examples of Chinese stimuli used for the rhyming tasks with their English interpretation and their pronunciation in pinyin. Numbers for the pinyin translations indicate tone. There are four different tones including the high level tone (first tone), the rising tone (second tone), the falling rising tone (third tone) and the falling tone (fourth tone).
Fig. 6.
Examples of Chinese stimuli used for the meaning tasks with their English interpretation and their pronunciation in pinyin. See Fig. 5 caption.
We also showed that a decrease in the right fusiform gyrus activation was correlated with increasing accuracy on the rhyming task. Some have suggested that reading acquisition is initially a right hemisphere process relying more on global visual forms, with greater engagement of the left hemisphere with increasing skill as analytic mappings are made between letters/symbols and phonemes/syllables (Orton, 1937). Decreases in right fusiform gyrus and right superior temporal sulcus activation with increasing skill in children have been shown for an implicit reading task that involved determining whether there was an ascending letter in visually presented words (Turkeltaub et al., 2003). Learning studies in adults also show decreasing involvement of the right hemisphere on reading and language tasks as a function of skill. In a study of French adults who were learning to associate English auditory words with pictures, the investigators showed activation decreases with learning in right inferior frontal regions in participants who maintained their learning to a higher degree 2 months after the fMRI testing (Raboyeau et al., 2004). Similarly, learning to read mirror-reversed text is associated with decreases in occipital, temporal, and parietal regions in the right hemisphere and accompanied by increases of activation in left fusiform gyrus (Poldrack et al., 1998).
Many studies have examined the neural correlates of rhyming judgments in English using single letter rhyming (Paulesu et al., 1996; Pugh et al., 1996), nonword rhyming (Xu et al., 2001), and real word rhyming (Crosson et al., 1999); (Kareken et al., 2000); (Lurito et al., 2000) compared to various baselines including similarity judgment to Korean letters (Paulesu et al., 1996), case judgment (Pugh et al., 1996), line judgment (Kareken et al., 2000); (Lurito et al., 2000), letter judgment to consonant strings (Crosson et al., 1999), and color matching to letter strings (Xu et al., 2001). Although there are some contradictory findings, one of the most consistent activation sites appears to be left superior temporal gyrus. Effective connectivity studies in English also suggest that the lateral temporal cortex may be a convergence zone where phonological information is integrated to perform rhyming judgments (Bitan et al., 2005). The present study found that Chinese did not activate the superior temporal gyrus during rhyming judgments. Because Chinese characters map onto syllables and because the large percentage of homophones in Chinese provides unreliable cues to semantics, learning to read in Chinese may not be strongly associated with spoken language processing (Tan et al., 2005a). Although studies show that phonological awareness is related to reading skill in Chinese (Ho and Bryant, 1997; McBride-Chang and Kail, 2002; Siok and Fletcher, 2001), studies have shown that children's hand writing skills are more strongly associated with learning to read Chinese (Tan et al., 2005b).
Our results are consistent with previous studies that suggest some similarities in the neural correlates of Chinese and English language processing using a variety of different tasks (Chee et al., 1999; Klein et al., 1999; Pu et al., 2001; Xue et al., 2004). All of the subjects in our study had some experience with English (11 years on average). Perhaps the bilingual status of our subjects influenced the organization of the language network in their native language. Tan et al. (2003) reported that bilingual Chinese subjects process English using a similar network employed for processing Chinese, but which is different from the network that native English speakers use to process English (Tan et al., 2003). This suggests that one's native language shapes the acquisition of a second language. It is also plausible that the acquisition of a second language will shape the neural organization of ones native language. Although this idea has not been tested directly by comparing brain activation for the native language of monolinguals and bilinguals, behavioral evidence suggests that one's native language processing is affected by the acquisition of the second language. This influence has been shown most comprehensively for phonological awareness (Chen et al., 2004; Loizou et al., 2003; Oller et al., 1998). When comparing literate and illiterate adults, imaging evidence also shows that oral language processing is affected by the acquisition of written language (Castro-Caldas et al., 1998, 1999). These results suggest that perhaps some of the similarities in brain activation between languages may be due to the interactivity of the first and second language.
In conclusion, this study demonstrated specialization in the language network in Chinese for mapping of orthography to phonology as indexed by a rhyming judgment task and for mapping of orthography to semantics as indexed by an association judgment task. These results are consistent with English studies that suggest that the inferior parietal lobule and posterior dorsal region of the left inferior frontal gyrus are involved in phonological processing and the left middle temporal gyrus and anterior ventral region of the left inferior frontal gyrus are involved in semantic processing. It extends this finding to show that a similar specialization for phonological and semantic processing exists in the middle frontal gyrus for Chinese. However, further research needs to be conducted comparing Chinese monolinguals to bilinguals in order to determine whether the acquisition of English as a second language influences the specialization for phonological and semantic processing.
4. Experimental procedures
4.1. Participants
The participants were 13 right-handed native speakers of Chinese (M age = 22.3; 7 males) in Beijing. However, all the participants began learning English as a second language at about 12 years old (range: 11-13) and had about 11 years of English experience (range: 9-13). The following exclusionary criteria were used for all participants: learning disability, Attention Deficit Hyperactivity Disorder (ADHD), uncorrected visual impairment or significant hearing impairment, neurological disease or psychiatric disorders, medication affecting central nervous system processing, or significant head injury.
4.2. Rhyming and meaning tasks
The rhyming and meaning tasks required the participant to determine the relation of the final word with two previous words according to a predefined criterion. All words were presented visually. If there was a match, they pressed a button with their index finger of their right hand; if there was no match, they pressed a different button with their middle finger of their right hand. Applying different criteria for a match in these tasks (either rhyming or meaning) allowed us to measure the effects of conscious access to phonologic and semantic representations. Participants were encouraged to respond as quickly as possible without making errors.
4.2.1. Rhyming task
In the rhyming judgment task, participants determined whether the final word rhymed with either of the two preceding words. Stimuli were two character words with the final character (foot) consisting of two radicals. Most of the final characters had a left-right radical structure with less than 5 having an up-down radical structure. O+P+ pairs (30% of trials) had similar orthography and phonology in that they shared the same phonetic radical and same vowel phoneme. O-P+ pairs (30% of trials) had different orthography and similar phonology in that they had different phonetic radicals, but shared the same vowel phoneme. O-P- pairs (40% of trials) had different orthography and phonology in that they had different radicals and did not share the same vowel phoneme (they did not rhyme). All pairs had a different initial consonant phoneme. Refer to Fig. 5 for examples of rhyming stimuli. One of the three conditions included O-P+ pairs to make it so that the subjects could not just base their judgment only on the spelling of the word. Including words that rhymed but were spelled differently encouraged subjects to access phonology.
4.2.2. Meaning task
In the meaning judgment task, participants determined whether the final word was associated with either of the two preceding words. Thirty percent of the trials contained pairs with a high association, 30% contained pairs with a low association, and 40% contained pairs of words that were unrelated. A 7-point scale was used to assess the association between prime and target. Forty subjects in Beijing were asked to judge to what extent pairs of words were related. An average score across subjects below 4.2 was considered low association (M = 3.7), whereas an average score over 5.0 was considered high association (M = 5.5). Refer to Fig. 6 for examples of meaning stimuli. We included a manipulation of difficulty in the meaning task so that it would be comparable to the rhyming that included O-P+ pairs that were likely to be more difficult (Kramer and Donchin, 1987, 5040) and we wanted to equate difficulty across tasks.
4.2.3. Control conditions
The control conditions were designed to equate the experimental and control blocks in terms of response characteristics. The experimental set-up and timing (see below) for the control blocks were exactly the same as for the word blocks. For control blocks, the three stimuli were nonlinguistic symbols consisting of straight lines (e.g. / /, \ \, / \). Participants determined whether the third stimulus was the same as one of the first two stimuli.
4.2.4. Timing
Each word reading task lasted 9 min, consisting of 10 blocks of 54 s (including a 4-second introduction screen to each block). The 5 experimental blocks alternated with the 5 control blocks. Because the design of the study was blocked with random presentation of trial types, we could not contrast words of different orthographic similarity (O+P+, O-P+, O-P-) in the rhyming task or words of different association (high, low) in the meaning task. In each trial, three consecutive words were presented with each word presented for 800 ms followed by a 200 ms blank interval. A yellow fixation cross (+) appeared on the screen after the third stimulus was removed, indicating the need to make a response during the subsequent 2000 ms interval. Participants were told that they could respond before the yellow cross (+) appeared on the screen. Each trial lasted a total of 5000 ms and there were 10 trials in each block. During the scanning procedure, brief written instructions were given before each block for 4 s: ‘Rhyme’ for phonological, ‘Meaning’ for semantic, and ‘Lines’ for visual control. Each participant was scanned during each task and each task was in a separate run in the same session.
4.2.5. Stimulus characteristics
Several stimulus variables were controlled across tasks so that our effects of interest were not confounded by nuisance variables. First, all of the words contained two syllables. Second, the tasks consisted of words with similar written and spoken word frequency. Chinese written frequency was determined by a corpus (1.3 million words and 1.8 million characters) that covers almost all fields of human activity, such as politics, economy, philosophy, literature, biology, and medicine (Wang et al., 1985). Chinese spoken word frequency was determined by a corpus of 1.7 million characters that came from 374 persons living in Beijing with different age, gender, education level, and occupation (Lu, 1993). Third, the number of strokes was the same across tasks. Stroke is the smallest component of Chinese characters and is a measure of visual spatial complexity, i.e. it is the number of steps required to write a character. Refer to Table 4 for information on word frequency and strokes for the Chinese stimuli for the fMRI session. We confirmed that there were no significant main effects or interactions on these nuisance variables by calculating the ANOVAs including the following independent variables: 2 session (practice, test) by 2 task (rhyming, meaning), 3 condition (O+P+, O-P+, O-P- for the rhyming task and high association, low association, unrelated for the meaning task).
Table 4.
Means (standard deviations) for written and spoken word frequency and stroke count for the rhyming and meaning tasks during the fMRI session
| Written |
Spoken |
Strokes |
||||
|---|---|---|---|---|---|---|
| Rhyming | Meaning | Rhyming | Meaning | Rhyming | Meaning | |
| Target | 38.5 (63.4) | 32.6 (63.7) | 10.9 (18.8) | 17.6 (26.3) | 17.3 (4.6) | 16.9 (4.8) |
| Prime | 37.1 (51.8) | 31.6 (27.3) | 12.2 (24.6) | 21.1 (26.5) | 17.2 (4.2) | 16.0 (3.9) |
| Filler | 38.7 (47.0) | 33.9 (28.9) | 16.9 (45.4) | 22.4 (32.8) | 17.2 (3.4) | 15.3 (4.5) |
4.3. MRI procedure and data analysis
The participant practiced a full-length version of each experimental task before the fMRI scanning session. Different stimuli were used in the practice and fMRI sessions.
4.3.1. MRI acquisition
All images were acquired using a 2 T GE/Elscint Prestige. For the functional imaging studies, a susceptibility weighted single-shot EPI (echo planar imaging) method with BOLD (blood oxygenation level-dependent) was used with the following scan parameters: TE = 45 ms, flip angle = 90°, matrix size = 128 × 72, field of view = 37 × 21 cm, slice thickness = 5 mm, number of slices = 28; TR = 3000 ms. At the end of the functional imaging session, a high resolution, T1 weighted 3D image was acquired. The following scan parameters were used: TR = 25 ms, TE = 6 ms, flip angle = 28°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 2 mm, number of slices = 62.
4.3.2. Data preprocessing
Data analysis was performed using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). The functional images were realigned to the last functional volume in the scanning session. This step used affine transformations to estimate a set of 6 rigid-body transformation parameters for each image by using an iterative procedure to minimize the mean squared difference between each individual image to the reference image. All statistical analyses were conducted on these movement-corrected images (no participant had more than 2 mm of movement in any plane). Images were then segmented and the gray-white matter information was used to co-register the structural and functional images. The images for each individual were normalized to the Montreal Neurological Institute (MNI) average template (12 linear affine parameters for brain size and position, 8 nonlinear iterations, and 2 × 2 × 2 nonlinear basis functions for subtle morphological differences).
4.4. Statistical analyses
Statistical analyses were calculated on the smoothed data (7 mm isotropic Gaussian kernel) using a delayed boxcar design with a 6 s delay from onset of the block in order to account for the lag in hemo-dynamic response. Statistics were also calculated with a high pass filter equal to 2 cycles of the experimental and control conditions (216 s) in order to remove signal drift, cardiac and respiratory effects, and other low frequency artifacts. We used global normalization to scale the mean of each scan to a common value in order to correct for whole brain differences over time. Parameter estimate images were calculated for the 13 subjects across the entire brain. Using random effect statistics, we calculated contrasts comparing the experimental to control conditions separately for the 2 word judgment tasks (rhyming and meaning) and also directly between the two tasks using the control as a baseline for each contrast. Using the random effects approach, we also examined the correlation of behavioral performance with signal intensity at the voxel level by entering accuracy as a covariate of interest. This was done for positive and negative correlations and separately for the rhyming and meaning task. All reported areas of activation were significant using P < 0.05 corrected at the cluster level for multiple comparisons.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to James R. Booth and by the PANDENG Project (95-special-09) of China to Peng Danling.
REFERENCES
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. doi: 10.1016/j.bandl.2005.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TR, et al. The development of specialized brain systems in reading and oral-language. Child. Neuropsychol. 2001;7(3):119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Modality independence of word comprehension. Hum. Brain Mapp. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. The relation between brain activation and lexical performance. Hum. Brain Mapp. 2003a;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Choy J, Gitelman DR, et al. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. J. Neurolinguist. 2003b;16:383–405. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Trommer BL, Davenport N, et al. Neural development of selective attention and response inhibition. NeuroImage. 2003c;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain: learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121(6):1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Miranda PC, Carmo I, Reis A, Leote F, Ribeiro C, et al. Influence of learning to read and write on the morphology of the corpus callosum. Eur. J. Neurol. 1999;6(1):23–28. doi: 10.1046/j.1468-1331.1999.610023.x. [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan EW, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. J. Neurosci. 1999;19(8):3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, et al. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: evidence from fmri. NeuroImage. 2000;12(4):392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chee MW, Soon CS, Lee HL. Common and segregated neuronal networks for different languages revealed using functional magnetic resonance adaptation. J. Cogn. Neurosci. 2003;15(1):85–97. doi: 10.1162/089892903321107846. [DOI] [PubMed] [Google Scholar]
- Chen X, Kao HSR. Visual-spatial properties and orthographic processing of Chinese characters. In: Kao HSR, editor. Cognitive Neuroscience Studies of the Chinese Language. Hong Kong Univ. Press; Aberdeen, Hong Kong: 2002. pp. 175–194. [Google Scholar]
- Chen H-C, Shu H. Lexical activation during the recognition of Chinese characters: evidence against early phonological activation. Psychon. Bull. Rev. 2001;8(3):511–518. doi: 10.3758/bf03196186. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM. Testing for dual brain processing routes in reading: a direct contrast of Chinese character and pinyin reading using fmri. 2002;14(7):1088–1098. doi: 10.1162/089892902320474535. [DOI] [PubMed] [Google Scholar]
- Chen X, Anderson RC, Li W, Hao M, Wu X, Shu H, et al. Phonological awareness of bilingual and monolingual Chinese children. J. Educ. Psychol. 2004;96(1):142–151. [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13(2):171–187. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Ding G, Perry C, Peng D, Ma L, Li D, Xu S, et al. Neural mechanisms underlying semantic and orthographic processing in Chinese-English bilinguals. NeuroReport. 2003;14(12):1557–1562. doi: 10.1097/00001756-200308260-00003. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nakamura K, Okada T, Hanakawa T, Fukuyama H, Mazziotta JC, et al. Neural mechanisms underlying the processing of Chinese words: an fmri study. Neurosci. Res. 2005;52:139–145. doi: 10.1016/j.neures.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Feng G, Miller K, Shu H, Zhang H, Miller K.k.u.e. Rowed to recovery: the use of phonological and orthographic information in reading Chinese and English. J. Exper. Psychol. Learn., Mem., Cogn. 2001;27(4):1079–1100. doi: 10.1037//0278-7393.27.4.1079. [DOI] [PubMed] [Google Scholar]
- Fu S. The structure and construction of Chinese characters. In: Chen Y, editor. Computational Analysis of Modern Chinese Characters. Shanghai Educational Press; Shanghai: 1993. pp. 108–169. [Google Scholar]
- Fu S, Chen Y, Smith S, Iversen S, Matthews PM. Effects of word form on brain processing of written Chinese. NeuroImage. 2002;17(3):1538–1548. doi: 10.1006/nimg.2002.1155. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38(5):831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on strigent behavioral and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Ho CS, Bryant P. Phonological skills are important in learning to read Chinese. Dev. Psychol. 1997;33(6):946–951. doi: 10.1037//0012-1649.33.6.946. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychol. Behav. Neurol. 2000;13(4):264–270. [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Zhao V, Nikelski J. Cerebral organization in bilinguals: a pet study of Chinese-English verb generation. NeuroReport. 1999;10:2841–2846. doi: 10.1097/00001756-199909090-00026. [DOI] [PubMed] [Google Scholar]
- Klein D, Zatorre RJ, Milner B, Zhao V. A cross-linguistic pet study of tone perception in mandarin Chinese and English speakers. NeuroImage. 2001;13(4):646–653. doi: 10.1006/nimg.2000.0738. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Donchin E. Brain potentials as indices of orthographic and phonological interaction during word matching. J. Exper. Psychol., Learn., Mem., Cogn. 1987;13(1):76–86. doi: 10.1037//0278-7393.13.1.76. [DOI] [PubMed] [Google Scholar]
- Kuo W-J, Yeh T-C, Cuann J-R, Wu T-T, Ho L-T, Hung D, et al. A left-lateralized network for reading Chinese words: a 3 t fmri study. NeuroReport. 2001;12(18):3997–4001. doi: 10.1097/00001756-200112210-00029. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu YT, Chou CC, Ho LT, et al. Frequency effects of Chinese character processing in the brain: an event-related fmri study. NeuroImage. 2003;18(3):720–730. doi: 10.1016/s1053-8119(03)00015-6. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, et al. Orthographic and phonological processing of Chinese characters: an fmri study. NeuroImage. 2004;21(4):1721–1731. doi: 10.1016/j.neuroimage.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Leck KJ, Weekes BK, Chen MJ. Visual and phonological pathways to the lexicon: evidence from Chinese readers. Mem. Cogn. 1995;23:468–476. doi: 10.3758/bf03197248. [DOI] [PubMed] [Google Scholar]
- Li D. A Study of Chinese Characters. Peking Univ. Press; Beijing: 1993. [Google Scholar]
- Li P, Jin Z, Tan LH. Neural representations of nouns and verbs in Chinese: an fmri study. NeuroImage. 2004;21(4):1533–1541. doi: 10.1016/j.neuroimage.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Liu W, Inhoff AW, Ye Y, Wu C. Use of parafoveally visible characters during the reading of Chinese sentences. J. Exp. Psychol. Hum. Percept. Perform. 2002;28(5):1213–1227. doi: 10.1037//0096-1523.28.5.1213. [DOI] [PubMed] [Google Scholar]
- Loizou M, Stuart M, Stuart M.m.s.i.a.u. Phonological awareness in monolingual and bilingual English and Greek five-year-olds. J. Res. Read. 2003;26(1):3–18. [Google Scholar]
- Lu B. Modern Beijing Spoken Chinese Corpus. Beijing Language Univ. Language Education Institute; Beijing: 1993. [Google Scholar]
- Luke KK, Liu HL, Wai YY, Wan YL, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Hum. Brain Mapp. 2002;16:133–145. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP. Comparison of rhyming and word generation with fmri. Hum. Brain Mapp. 2000;10(3):99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride-Chang C, Kail RV. Cross-cultural similarities in the predictors of reading acquisition. Child Dev. 2002;73(5):1392–1407. doi: 10.1111/1467-8624.00479. [DOI] [PubMed] [Google Scholar]
- Oller D, Cobo-Lewis AB, Eilers RE. Phonological translation in bilingual and monolingual children. Appl. Psycholinguist. 1998;19(2):259–278. [Google Scholar]
- Orton ST. Reading, Writing and Speech Problems in Children and Selected Papers; Paper presented at the The International Dyslexia Association; Baltimore. 1937. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, et al. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Peng DL, Xu SY, Ding GS, Li EZ, Liu Y. Brain mechanism for the phonological and semantic processing of Chinese character. Chin. J. Neurosci. 2003;19:287–291. [Google Scholar]
- Peng D.-l., Ding G.-s., Perry C, Xu D, Jin Z, Luo Q, et al. Fmri evidence for the automatic phonological activation of briefly presented words. Cogn. Brain Res. 2004;20(2):156–164. doi: 10.1016/j.cogbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JD. The neural basis of visual skill learning: an fmri study of mirror reading. Cereb. Cortex. 1998;8(1):1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Tan LH, Rayner K. The role of phonological codes in integrating information across saccadic eye movements in Chinese character identification. J. Exp. Psychol. Hum. Percept. Perform. 2000;26(2):607–633. doi: 10.1037//0096-1523.26.2.607. [DOI] [PubMed] [Google Scholar]
- Pu Y, Liu HL, Spinks JA, Mahankali S, Xiong J, Feng CM, et al. Cerebral hemodynamic response in Chinese (first) and English (second) language processing revealed by event-related functional mri. Magn. Reson. Imaging. 2001;19:643–647. doi: 10.1016/s0730-725x(01)00379-4. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Raboyeau G, Marie N, Balduyck S, Gros H, Demonet JF, Cardebat D. Lexical learning of the English language: a pet study in healthy French subjects. NeuroImage. 2004;22(4):1808–1818. doi: 10.1016/j.neuroimage.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Rayner K, Springer CJ. Graphemic and semantic similarity effects in the picture-word interference. Br. J. Psychol. 1986;77:207–222. doi: 10.1111/j.2044-8295.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Siok WT, Fletcher P. The role of phonological awareness and visual-orthographic skills in Chinese reading acquisition. Dev. Psychol. 2001;37(6):886–899. [PubMed] [Google Scholar]
- Siok WT, Jin Z, Fletcher P, Tan LH. Distinct brain regions associated with syllable and phoneme. Hum. Brain Mapp. 2003;18(3):201–207. doi: 10.1002/hbm.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using pet. Cereb. Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Spinks JA, Liu Y, Perfetti CA, Tan LH. Reading Chinese characters for meaning: the role of phonological information. Cognition. 2000;76(1):B1–B11. doi: 10.1016/s0010-0277(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Taft M, Taft M.M.T.u.e.a. Orthographic processing of polysyllabic words by native and nonnative English speakers. Brain Lang. 2002;81(13):532–544. doi: 10.1006/brln.2001.2545. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Gao J-H, Liu H-L, Perfetti CA, Xiong J, et al. Brain activation in the processing of Chinese characters and words: a functional mri study. Hum. Brain Mapp. 2000;10(1):16–27. doi: 10.1002/(SICI)1097-0193(200005)10:1<16::AID-HBM30>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Feng C-M, Fox PT, Gao J-H. An fmri study with written Chinese. NeuroReport: Int. J. Rapid Commun. Res. Neurosci. 2001a;12(1):83–88. doi: 10.1097/00001756-200101220-00024. [DOI] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. NeuroImage. 2001b;13(5):836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, et al. Neural systems of second language reading are shaped by native language. Hum. Brain Mapp. 2003;18(3):158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Karl L, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum. Brain Mapp. 2005a;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Eden GF, Perfetti CA, Siok WT. Reading depends on writing, in Chinese. Proc. Natl. Acad. Sci. U. S. A. 2005b;102:8781–8785. doi: 10.1073/pnas.0503523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Garaeu L, Flowers DL, Zefirro TA, Eden G. Development of the neural mechanisms for reading. Nat. Neurosci. 2003;6(6):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wang H, Chang RB, Li YS. Modern Chinese Frequency Dictionary. Beijing Language Univ. Press; Beijing: 1985. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb. Cortex. 2001;11(3):267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Zhang L, Wang Y. An fmri study with semantic access in low proficiency second language learners. NeuroReport. 2004;15(5):791–796. doi: 10.1097/00001756-200404090-00010. [DOI] [PubMed] [Google Scholar]
- Zhou XL. Phonology plays a limited role in semantic activation. In: Peng DL, Shu and H, Chen XC, editors. Cognitive Research on the Chinese Language. Shandong Educational Publishing; Shandong: 1997. [Google Scholar]
- Ziegler JC, Stone GO, Jacobs AM. What is the pronunciation for -ough and the spelling for /u/? A database for computing feedforward and feedback consistency in English. Behav. Res. Meth. Instrum. Comput. 1997;29(4):600–618. [Google Scholar]
- Zurowski B, Gostomzyk J, Gron G, Weller R, Schirrmeister H, Neumeier B, et al. Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. NeuroImage. 2002;15(1):45–57. doi: 10.1006/nimg.2001.0968. [DOI] [PubMed] [Google Scholar]