Abstract
Objective
The aim of this study was to examine the endothelial distribution and activity of selected PKC isoforms in coronary vessels with respect to their functional impact on endothelial permeability under the experimental conditions relevant to diabetes.
Methods and Results
En face immunohistochemistry demonstrated a significant increase of PKCβII and decrease of PKCδ expression in coronary arterial endothelium of Zucker diabetic rats. To test whether changes in PKC expression alter endothelial barrier properties, we measured the transcellular electric resistance in human coronary microvascular endothelial monolayers and found that either PKCβII overexpression or PKCδ inhibition disrupted the cell–cell adhesive barrier. Three-dimensional fluorescence microscopy revealed that hyperpermeability was caused by altered PKC activity in association with distinct translocation of PKCβII to the cell–cell junction and PKCδ localization to the cytosol. Further analyses in fractionated endothelial lysates confirmed the differential redistribution of these isozymes. Additionally, FRET analysis of PKC subcellular dynamics demonstrated a high PKCβII activity at the cell surface and junction, whereas PKCδ activity is concentrated in intracellular membrane organelles.
Conclusion
Taken together, these data suggest that PKCβII and PKCδ counter-regulate coronary endothelial barrier properties by targeting distinctive subcellular sites. Imbalanced PKCβII/PKCδ expression and activity may contribute to endothelial hyperpermeability and coronary dysfunction in diabetes.
Keywords: diabetes, inflammation, permeability, protein kinase, FRET
Protein kinase C (PKC) is a family of serine/threonine kinases consisting of at least 10 isoforms, including the classical PKCs (α, βI, βII, and γ), which bind to and are activated by diacylglycerol and Ca2+, the novel isoforms (δ, ε, η, and θ) capable of binding to diacylglycerol but independent of Ca2+, and the atypical class (ζ and ι) insensitive to either diacylglycerol or Ca2+. Most of these isozymes are regulated at 3 levels. First, autophosphorylation is required for them to become catalytically competent. Second, binding to cofactors, such as diacylglycerol or its mimetic phorbol esters, induces translocation from the cytosol to plasma membrane, where the enzymes undergo conformational changes enabling catalytic activation. Finally, direct interaction with substrate proteins determines site-specific kinase activities.1
As endogenous stress sensors, PKCs mediate diverse cellular responses to physical forces, chemical agents, and molecular signals that are elaborated under different physiological or pathological conditions. Altered expression or enzymatic activity of PKCs have been linked to circulatory disturbance in coronary artery disease, arthrosclerosis, hypertension, myocardial ischemia reperfusion, and circulatory shock. Recently, evidence is emerging that PKCs contribute to the pathogenesis of diabetic vascular inflammation characterized by endothelial barrier dysfunction and increased filtration of plasma proteins or inflammatory cells into the vascular wall and surrounding tissues.2–4 We and others have shown elevated PKC activity5,6 and expression7 in the heart of diabetic rats and pigs, concomitant with coronary venular hyperpermeability.6 Consistently, PKC inhibitors delay the progression of microvascular leakage in the retina and kidney in diabetic patients.8–10 Although the overall importance of PKCs in inflammation is well recognized, the specific contribution of individual isozymes to vascular permeability and the underlying mechanisms remain poorly understood. Tremendous controversy exists regarding PKC isoform-specific effects on endothelial barrier function.11,12
The aim of this study was to test the hypothesis that PKCβII and PKCδ play a counter-regulatory role in the control of endothelial barrier properties and altered expression or activity of these isozymes shifts this equilibrium leading to hyperpermeability. The expression of PKCβII and PKCδ was quantitatively assessed in the native endothelium of intact coronary microvessels from the Zucker fatty rat, a model of type II diabetes with cardiovascular pathology comparable to humans.13 To test whether altered isozyme expression or activity affects endothelial permeability, we overexpressed PKCβII in human coronary microvascular endothelial cells (HCMECs) and measured the transcellular electric resistance (TER) as an indicator of barrier function reflecting the tightness of cell–cell and cell–matrix adhesions. The mechanism by which PKC isozymes confer opposing barrier effects was examined using 3D microscopy in conjunction with immunoblotting. Furthermore, a modified fluorescent resonance energy transfer (FRET) assay was performed to measure isoform-selective substrate phosphorylation at specific subcellular locations of living endothelial cells in real-time.
Materials and Methods
Animals
Age-matched (15 to 17 weeks) male Zucker lean and fatty rats were used according to the protocol approved by the IACUC in compliance with the NIH guidelines. Animal were anesthetized with Urethane (1.75g/kg) and their hearts excised and coronary arteries dissected as previously described.14
Cell Culture and Experiments
HCMECs obtained from Cambrex (East Rutherford, NJ) were transfected with pcDNA as described in the supplement.
Transendothelial Resistance
The endothelial barrier property was determined by measuring TER as described previously15,16 (see supplemental materials, available online at http://atvb.ahajournals.org, for details).
Fluorescence Microscopy
En face coronary arteries were prepared as previously described.14,17 Samples were viewed with a Zeiss Axiovert 200 M microscope equipped with an Apotome system allowing optical sectioning (see supplemental Figures I & II).
FRET
Cells overexpressing PKCβII and transfected with mpCKAR were incubated in an on-stage incubator at 37°C, pH7.4 and imaged with a Zeiss Axiovert 200 M mounted with a dual-view module (Photometric) controlled by MetaFluor 7.0 (Molecular Device) for simultaneous acquisition of CFP and FRET emissions. CFP/FRET images and values were computed and created with MetaFluor Analyst and displayed as pseudocolor images (see supplemental materials for details).
Statistical Analysis
For mean fluorescence intensity (FI) per cells, data were analyzed with unpaired t test with Welch corrections. For all other data sets, statistical analysis was performed using ANOVA followed by Bonferroni’s multiple comparisons.
Results
Increased PKCβII and Decreased PKCδ Expression in Diabetic Endothelium
At the time of experiment, the diabetic fatty rats had significantly higher body weight and blood glucose than their lean controls (705±5g versus 410±5g, P<0.001, and 6.4±0.2 mmol/L versus 4.3±0.1 mmol/L, P<0.001, respectively). The diabetic status of the fatty rats was further supported by glucose intolerance as their blood sugar levels remained elevated (10.55±0.2 mmol/L) 2 hours after an oral glucose challenge. The controls showed a rapid recovery with blood glucose returning to the normal level within 2 hours (4.73±0.9 mmol/L).
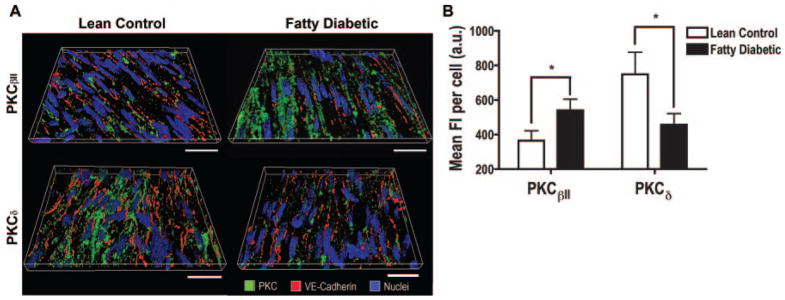
En face immunohistochemistry demonstrated significant expression of PKCβII and PKCδ in the endothelium of coronary arteries. 3D reconstructions of the optical sections revealed a more intensive labeling of PKCβII on the endothelium of diabetic coronary vessels than that of controls (Figure 1A, top panels). In contrast, less PKCδ was observed in the diabetic endothelium compared to controls (Figure 1A, bottom panels). Further quantitative analyses show that diabetes caused a 1.5-fold increase in PKCβII (P<0.05) and 1.6-fold decrease in PKCδ (P<0.05) expression (Figure 1B).
Figure 1.
Increased PKCβII and decreased PKCδ expression in diabetic coronary endothelium. A, 3D reconstruction of en face coronary arteries from Zucker fatty diabetic rats (right) and their lean controls (left) (section depth=6.16 μm, scale bar= 20 μm). B, Mean fluorescence intensity (FI) in individual endothelial cells (n=35 to 56 cells/3 rats/group, *P<0.05).
PKCβII Overexpression and PKCδ Inhibition Increase Endothelial Permeability
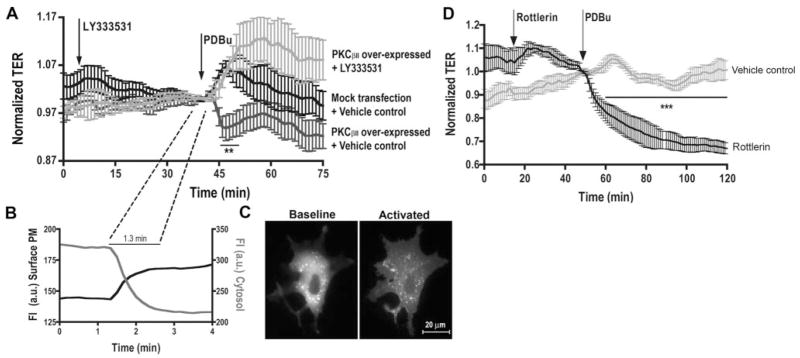
The transendothelial electric resistance was measured in coronary microvascular endothelial monolayers overexpressing PKCβII and treated with PKC activators or inhibitors. PDBu (Phorbol-12,13-dibutyrate) caused a rapid increase in TER peaking at ≈10 minutes gradually recovering over 20 minutes (Figure 2A). In PKCβII-expressing cells, PDBu produced a significant reduction in TER, which was prevented by pretreatment with the PKCβ-selective inhibitor LY333531, indicating a barrier-weakening effect of the β isozyme. The TER response of PKCβII-expressing cells occurred in an average course of 1.3 minutes (Figure 2A and 2B). This delayed onset corresponded to the time required for PKCβII to completely translocate from the cytosol to cell surface membrane, as indicated by the dynamic changes of RFP-PKCβII intensity in these locations (Figure 2B and 2C). In different groups, treatment with the PKCδ inhibitor Rottlerin induced a decrease in TER on PDBu stimulation, indicating a barrier-tightening effect of this isozyme (Figure 2D). Together, the data suggest an opposing role for PKCβII and PKCδ in regulating coronary endothelial permeability.
Figure 2.
TER across HCMECs. A, LY333531 prevents PDBu-induced changes in barrier function during PKCβII overexpression (n=6, **P<0.05 vs vehicle). B, RFP-PKCβII translocation indicated by inverse changes in membrane versus cytosol FI. C, RFP-PKCβII distribution before and after activation. D, Rottlerin pretreatment enhances PDBu-induced TER decrease (n=4, ***P<0.001 vs vehicle).
PKCβII Translocates to Cell–Cell Junctions but PKCδ Is Confined to the Perinuclear Region
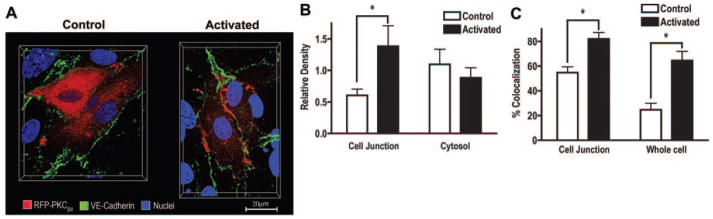
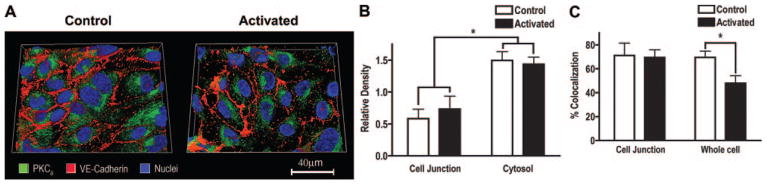
To test whether the opposing barrier modulatory effects were attributed to different targeting sites, we examined the subcellular compartmentalization of PKCβII (Figure 3) and PKCδ (Figure 4) in HCMECs. Representative 3D images of cells that were transfected with RFP-PKCβII (Figure 3A) or immunolabeled for PKCδ (Figure 4A) and colabeled with VE-cadherin and Hoechst are presented. On activation, PKCβII translocated to the cell membrane with preferential distribution at cell–cell junctions (determined by VE-cadherin), whereas PKCδ was localized in the perinuclear area and remained confined to the cytosol after activation. Further analyses revealed a 2.3-fold increase of PKCβII density at junctions on activation (P<0.05; Figure 3B). In contrast, PKCδ density was significantly higher in the cytosol (intracellular area distal from VE-cadherin) than at the junction (P<0.05), and its labeling pattern remained unchanged after activation (Figure 4B). Additionally, colocalization between PKCβII and VE-cadherin was relatively higher in junctions compared to across the whole cell (55% in junction versus 25% in cell), and both the junction and whole cell showed an increased colocalization after PDBu (from 55% to 82% in junction, P<0.05, from 25% to 65% in cell, P<0.05; Figure 3C). In contrast, the overall colocalization between PKCδ and VE-cadherin decreased on PDBu (from 70% to 48%, P<0.05), and no significant change was observed at junctions (Figure 4C). The distinctive subcellular redistribution of the two isozymes was confirmed in a Western blot analysis of fractionated HCMEC lysates (see supplemental Figure III).
Figure 3.
PKCβII preferentially translocates to cell–cell junctions. A, 3D images showing subcellular distribution of PKCβII relative to VE-cadherin in nonactivated and PDBu-activated HCMECs (image depth=10.15μm). B, PKCβII density (labeled pixels) at the junction and cytosol before and after activation. C, Subcellular colocalization between PKCβII and VE-cadherin (B and C, n=8, *P<0.05).
Figure 4.
PKCδ is localized to perinuclear cytosol. A, 3D images showing subcellular distribution of PKCδ relative to VE-cadherin in nonactivated and PDBu-activated HCMECs (image depth=8.4μ m). B, PKCδ density (labeled pixels) at the junction and cytosol before and after activation. C, Subcellular colocalization between PKCδ and VE-cadherin (B and C, n=14 to 16, *P<0.05).
Basal PKC Activity Measured With FRET
FRET was performed in HCMECs expressing PKCβII and a genetically encoded C kinase activity reporter (CKAR), a fluorescent probe that has recently been characterized as a sensitive indicator of PKC-substrate phosphorylation in nonendothelial cells.18 The probe has been modified (mpCKAR) for enhanced plasma membrane-targeting capability (for more details see supplemental Figure IV). We compared the level of PKC-substrate phosphorylation in the cell–cell junction (JCT), surface plasma membrane (SPM), and intracellular plasma membrane (IPM). PDBu caused a small but consistent and reproducible increase in phosphorylation, measured as the average CFP/FRET increase over time. The overall CFP/FRET under basal conditions was significantly higher at JCT (P<0.05) and IPM (P<0.01) than at SPM, suggesting compartmentalization of basal PKC activity (see supplemental Figure V).
Isoform-Specific PKC Activity at Different Subcellular Sites
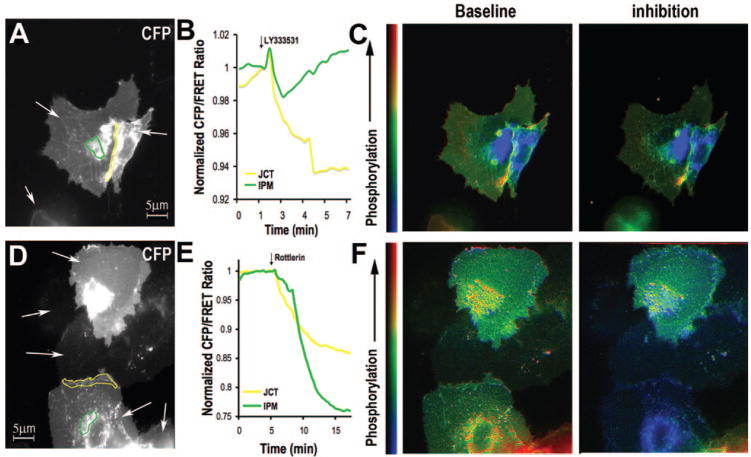
FRET was measured in HCMECs expressing PKCβII in the presence of isozyme-selective inhibitors (Figure 5A and 5D show representative CFP signals). We focused on the JCT and IPM as these subcellular areas had the highest basal PKC activity. LY333531 caused a rapid decrease of CFP/FRET in both regions, with the largest drop occurring at the junction (Figure 5B, representative of 4 to 10 cells from ≥3 experiments). In comparison, Rottlerin induced a slower CFP/FRET decrease but of higher amplitude, especially at IPM (Figure 5E, representative of 4 to 10 cells from ≥3 experiments). Corresponding pseudocolor images of CFP/FRET are shown in Figure 5C and 5F. The inhibitory effect of Rottlerin was greater than that of LY333531 in IPM (P<0.01), but there was no significant difference between the 2 inhibitors in JCT and SPM. The largest reduction in CFP/FRET occurred at IPM on adding Rottlerin, indicating that this area is most sensitive to the δ inhibitor. A significant difference between Rottlerin and LY333531 was observed at IPM and SPM during PKC inhibition (P<0.05). In cells stimulated with PDBu, pretreatment with LY333531 blunted the PDBu response mostly in SPM and JCT but pretreatment with Rottlerin blunted the PDBu response mostly in IPM (based on observations made in 3 to 10 cells from ≥3 experiments, data not shown).
Figure 5.
Effects of LY333531 (A–C) and Rottlerin (D–F) on PKC activity at JCT (yellow) and IPM (green) (A&D). White arrows indicate mpCKAR-expressing cells. B and E, Greater FRET response at JCT on LY333531 and at IPM on Rottlerin inhibition. C and F, Pseudo-color images of CFP/FRET before and after PKCβII or PKCδ inhibition.
Discussion
We examined the expression and activity of selected PKC isoforms in the coronary vascular endothelium with respect to their roles in regulating permeability under stimulatory conditions relevant to type II diabetes. The results yield several lines of evidence supporting PKC isoform-dependent endothelial responses. First, we observed increased PKCβII and decreased PKCδ expression on the endothelium of diabetic coronary vessels. Second, overexpressing PKCβII produced a barrier disruptive effect during endothelial stimulation, an effect comparable to pharmacological inhibition of PKCδ, indicating a counteracting role of PKCβII versus PKCδ in modulating endothelial barrier resistance. Third, the opposing functions of these isozymes correlated with their distinctive subcellular localizations, as PKCβII translocated to the junction leading to cell– cell adhesive barrier dysfunction, whereas PKCδ was confined to the cytosol conferring barrier protection. Further, we developed an endothelial-based FRET assay for real-time quantification of PKC signaling. In line with 3D microscopy and immunoblotting, the FRET data demonstrated a high basal phosphorylation activity of PKCβII in cell junctions, whereas PKCδ was most prominent in intracellular compartments. Taking the current data together with our previous findings from isolated coronary vessels,6,7 we suggest that PKCβII and PKCδ may serve as counter-regulators of endothelial permeability by phosphorylating junctional and cytosolic proteins. In disease states such as diabetes, altered expression or activity of individual isozymes compromise the barrier homeostasis, leading to vascular hyperpermeability. To the best of our knowledge, this is the first report of isoform-selective, subcellular site-specific PKC regulation of coronary endothelial barrier function.
PKC Signaling and Endothelial Permeability
The endothelium forms an effective barrier that controls the passage of blood fluid, proteins, and cells from the circulation into the vessel wall and surrounding tissues. A variety of pathophysiological factors can alter the barrier integrity causing abnormal transendothelial flux and intimal accumulation of blood components.4,19,20 Phosphorylation of inter-cellular junctional structures is a key molecular event underlying vascular leakage in inflammation. For certain molecules known to participate in cell contraction or adhesion and thus affect permeability (eg, myosin light chain, catenins, nitric oxide synthase, and MAP kinases16,21–23), serine or threonine phosphorylation are required for their activation and downstream targeting. As potent serine/threonine kinases, PKCs are capable of signal transduction in the endothelium that ultimately affects the barrier property.24 In particular, some isoforms (PKCα and β) have been linked to microvascular leakage during inflammatory stimulations,6,12,25–27 whereas others (PKCδ) have been shown to protect barrier function.28,29 While these observations support the basis of our hypothesis that classical and novel PKCs exert opposing effects on the endothelial barrier, the current study provides a direct comparative analysis for isozyme-dependent permeability regulation in the coronary vascular endothelium.
PKCβII overexpression did not alter the basal barrier resistance but significantly reduced resistance on endothelial activation, and the response was blunted in the presence of PKCβ inhibitors, suggesting that the β isoform does not interfere the development of cell–cell adhesions but plays an active role in mediating the hyperpermeability response to stimulation. In contrast, PKCδ appears to be a barrier protector, as inhibition of this isoform produced a large decrease in barrier resistance. Additionally, we observed a small TER increase in nontransfected cells after treatment with PDBu, a diacylglycerol-mimetic phorbol ester known to activate both the classical and novel PKCs. This supports a net increase in barrier resistance during overall activation of the kinases, albeit the controversy regarding the permeability effect of phorbol esters.11,30,31 Taking the data together, we propose that in endothelial cells under normal nonstimulated conditions, PKCδ activity outweighs PKCβII so that activation of the both results in a tightened barrier. This balance may be shifted in pathological (diabetic) conditions where the βII isoform is significantly upregulated, rendering a PKCβ-dominant response manifested as hyperpermeability.
PKC Isozyme Subcellular Localization
All PKCs share extensive homology in their catalytic domains with each displaying low substrate selectivity32; yet, they exert pleiotropic effects by dispersing to different subcellular compartments. The availability of an activated isozyme at a particular location is an important determinant of its specific activity, and the subcellular distribution of individual isozymes may dictate their distinct biological functions. Our data showed that PKC isozyme localization correlated with substrate phosphorylation. On endothelial stimulation, PKCβII translocated from the cytosol to the cortical membrane colocalizing with VE-cadherin. The FRET analysis confirms compartmentalized kinase activities and increased phosphorylation at cell–cell junctions preferentially driven by PKCβII The redistribution may have a functional implication as it poises the activated isoform to junctions for direct targeting and phosphorylating substrates involved in cell-cell adhesions. In support of this hypothesis, we have previously reported that phosphorylation of junctional molecules contributes to paracellular hyperpermeability.16,21 Other studies also show PKC-dependent disruption of junctional integrity coupled with altered VE-cadherin structure33,34 or VE-cadherin/β-catenin association.35
In contrast to the βisoform, PKCδ was concentrated in the cytosol and did not change its location on activation. The distribution of PKCδ at cell–cell contacts remained low, without significant colocalization with VE-cadherin. While the intracellular localization and nuclear translocation of the novel PKCs have been observed in other cell types,36,37 this study provides evidence for prominent distribution of PKCδ in the cytoplasm with minimal junctional association in the coronary vascular endothelium. The primary intracellular location of this isoform may contribute to its barrier protective function in that it enables close interactions with cytoskeletal elements that stabilize the contractile machinery and maintain cell–cell adhesions. Alternatively, PKCδ can phosphorylate focal adhesion proteins28,29 leading to strengthened cell–matrix attachment and enhanced barrier properties. In addition, the isozyme may interact with other intracellular signaling molecules or scaffold proteins, which in turn modulate permeability. Indeed, there is report that PKCδ-dependent enhancement of barrier function correlates with RhoA GTPase activity and focal contact formation.38
FRET and PKC Subcellular Activity
We validated a FRET approach for quantification of PKC-substrate phosphorylation at specified subcellular locations of living endothelial cells in real-time. Similar FRET reporters for different kinases have been used in established or immortalized cell lines.18,39–41 However, only limited attempts have been made with primary cell cultures inherently resistant to transfection, such as endothelial cells.42,43 The modified mpCKAR provided an optimal spatial-temporal resolution at cell–cell junctions and perinuclear vesicles where the probe expressed at high levels.
The FRET data showed significant substrate phosphorylation in PKCβII overexpressing cells. This high basal activity may explain why further activation by PDBu only caused a small change in CFP/FRET, whereas inhibition by LY333531 or Rottlerin produced more dramatic responses. Because the measurement was based on CFP/FRET ratio normalized to its membrane content, the phosphorylation levels indicate compartmentalized kinase activities. Consistent with their distributions, PKCβII-driven phosphorylation was prominent at the junction, whereas PKCδ-driven phosphorylation primarily occurred in the cytosol. In addition, we observed significant PKCδ activity in cytosolic membranous vesicles constantly shuffling around the nucleus. Although the nature of such organelles remains to be identified, similar responses have been observed in COS-7 cells.44 In endothelial cells, these organelles may serve as molecular chaperones for signaling, or they interact with the cytoskeleton that poises the cell to a conformation supporting cell–cell adhesions. Intracellular organelle trafficking may direct PKCδ to its target for phosphorylation.
Implication in Diabetes
Endothelial hyperpermeability is a hallmark of functional angiopathy in early stage of diabetes before the onset of pathological lesion, atherosclerosis, hypertension, and coronary artery disease.45 PKC signaling is recognized as a central pathway leading to circulatory disturbance. Animal studies show that PKC inhibitors normalize endothelial function and delay the progress of diabetic microvascular complications.8,9 The development of effective therapies, however, has met with limited success, partially because of the incomplete understanding of the diabetic effects on specific PKC isoforms in different vascular beds.2 In this regard, a unique aspect of this study is that the en face immnohistochemistry provides direct in vivo evidence for PKC isoform changes in diabetic coronary endothelium. The increased PKCβII expression is consistent with our genomic analysis showing upregulated PKCβII gene in the diabetic myocardium.7 In diabetic pigs, PKCβII activation occurred in heart microvessels concomitantly with coronary venular hyperpermeability, and the injurious effect was reversed by selective inhibition of PKCβ.6 Based on the data that PKCβII and PKCδ counter-regulate barrier resistance and they show opposite expression patterns in diabetic coronary vessels, we postulate that their imbalanced expression and activity may contribute to endothelial dysfunction under diabetic conditions. Whether such changes are specific to the coronary system remains to be determined. While the current and our previous studies highlight coronary vessels as a prominent target of diabetic injury, others have shown alterations of PKCs and vascular leak in the retina and kidney during diabetes.8–10 We also observed similar changes in the microvasculature of other tissues such as the mesentery of Zucker diabetic rats (data not shown). However, tremendous heterogeneity exists across the vasculature, where macrovascular and microvascular endothelia display different barrier properties, and gender/sex is an important factor contributing to differential susceptibility of coronary vessels to diabetic injury.19 Further characterization and comparison of the PKC-dependent permeability response in specific vascular beds represent an interesting area of future investigation.
In summary, we report that in coronary microvascular endothelium, the basal activity of PKCβII is relatively high at the cell–cell junction, and this isoform preferentially translocates to the cell membrane on endothelial stimulation. In contrast, the distribution and activity of PKCδ are concentrated in intracellular compartments. Functionally, PKCβII and PKCδ exert opposite effects on endothelial barrier resistance, corresponding to their distinctive subcellular localization and substrate phosphorylation activity. We suggest that this counter-regulatory balance may be impaired in diabetes, where significant upregulation of PKCβII occurs concomitantly with downregulation of PKCδ in coronary vessels, contributing to increased vascular permeability.
Supplementary Material
Acknowledgments
We thank Drs Alexandra Newton and Roger Tsien from the University of California, San Diego for providing the CKAR and RFP-PKCβII
Sources of Funding
This study was supported by the NIH HL084542, HL061507, HL070752, and HL073324.
Footnotes
Disclosures
None.
References
- 1.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 2.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659 – 673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 4.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2003;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 5.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacyl-glycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan SY, Ustinova EE, Wu MH, Tinsley JH, Xu W, Korompai FL, Taulman AC. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ Res. 2000;87:412–417. doi: 10.1161/01.res.87.5.412. [DOI] [PubMed] [Google Scholar]
- 7.Guo M, Wu MH, Korompai F, Yuan SY. Upregulation of PKC genes and isozymes in cardiovascular tissues during early stages of experimental diabetes. Physiol Genomics. 2003;12:139–146. doi: 10.1152/physiolgenomics.00125.2002. [DOI] [PubMed] [Google Scholar]
- 8.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 10.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 11.Bogatcheva NV, Verin AD, Wang P, Birukova AA, Birukov KG, Mirzopoyazova T, Adyshev DM, Chiang ET, Crow MT, Garcia JG. Phorbol esters increase MLC phosphorylation and actin remodeling in bovine lung endothelium without increased contraction. Am J Physiol Lung Cell Mol Physiol. 2003;285:L415–L426. doi: 10.1152/ajplung.00364.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ferro T, Neumann P, Gertzberg N, Clements R, Johnson A. Protein kinase C-alpha mediates endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1107–L1117. doi: 10.1152/ajplung.2000.278.6.L1107. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7:307–317. doi: 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreault N, Scriven DR, Moore ED. Characterisation of glucose transporters in the intact coronary artery endothelium in rats: GLUT-2 upregulated by long-term hyperglycaemia. Diabetologia. 2004;47:2081–2092. doi: 10.1007/s00125-004-1583-4. [DOI] [PubMed] [Google Scholar]
- 15.Breslin JW, Sun H, Xu W, Rodarte C, Moy AB, Wu MH, Yuan SY. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol. 2006;290:H741–H750. doi: 10.1152/ajpheart.00238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tinsley JH, Breslin JW, Teasdale NR, Yuan SY. PKC-dependent, burn-induced adherens junction reorganization and barrier dysfunction in pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L217–L223. doi: 10.1152/ajplung.00248.2004. [DOI] [PubMed] [Google Scholar]
- 17.Gaudreault N, Scriven DR, Laher I, Moore ED. Subcellular characterization of glucose uptake in coronary endothelial cells. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol. 2007;293:H1196–H1205. doi: 10.1152/ajpheart.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayhan WG, Sharpe GM. Superoxide dismutase restores impaired histamine-induced increases in venular macromolecular efflux during diabetes mellitus. Microcirculation. 1998;5:211–218. [PubMed] [Google Scholar]
- 21.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem. 1999;274:24930–24934. doi: 10.1074/jbc.274.35.24930. [DOI] [PubMed] [Google Scholar]
- 22.Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- 23.Wu MH, Yuan SY, Granger HJ. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. J Physiol. 2005;563:95–104. doi: 10.1113/jphysiol.2004.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez MM, Kim DD, Duran WN. Protein kinase C modulates microvascular permeability through nitric oxide synthase. Am J Physiol. 1996;271:H1702–H1705. doi: 10.1152/ajpheart.1996.271.4.H1702. [DOI] [PubMed] [Google Scholar]
- 25.Hempel A, Lindschau C, Maasch C, Mahn M, Bychkov R, Noll T, Luft FC, Haller H. Calcium antagonists ameliorate ischemia-induced endothelial cell permeability by inhibiting protein kinase C. Circulation. 1999;99:2523–2529. doi: 10.1161/01.cir.99.19.2523. [DOI] [PubMed] [Google Scholar]
- 26.Nagpala PG, Malik AB, Vuong PT, Lum H. Protein kinase C beta 1 overexpression augments phorbol ester-induced increase in endothelial permeability. J Cell Physiol. 1996;166:249–255. doi: 10.1002/(SICI)1097-4652(199602)166:2<249::AID-JCP2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Vuong PT, Malik AB, Nagpala PG, Lum H. Protein kinase C beta modulates thrombin-induced Ca2+ signaling and endothelial permeability increase. J Cell Physiol. 1998;175:379–387. doi: 10.1002/(SICI)1097-4652(199806)175:3<379::AID-JCP16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Harrington EO, Brunelle JL, Shannon CJ, Kim ES, Mennella K, Rounds S. Role of protein kinase C isoforms in rat epididymal microvascular endothelial barrier function. Am J Respir Cell Mol Biol. 2003;28:626–636. doi: 10.1165/rcmb.2002-0085OC. [DOI] [PubMed] [Google Scholar]
- 29.Klinger JR, Murray JD, Casserly B, Alvarez DF, King JA, An SS, Choudhary G, Owusu-Sarfo AN, Warburton R, Harrington EO. Rottlerin causes pulmonary edema in vivo: a possible role for PKC{delta} J Appl Physiol. 2007;103:2084–2094. doi: 10.1152/japplphysiol.00695.2007. [DOI] [PubMed] [Google Scholar]
- 30.Moy AB, Blackwell K, Wang N, Haxhinasto K, Kasiske MK, Bodmer J, Reyes G, English A. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol. 2004;287:L153–L167. doi: 10.1152/ajplung.00292.2003. [DOI] [PubMed] [Google Scholar]
- 31.Tinsley JH, Teasdale NR, Yuan SY. Involvement of PKCdelta and PKD in pulmonary microvascular endothelial cell hyperpermeability. Am J Physiol Cell Physiol. 2004;286:C105–C111. doi: 10.1152/ajpcell.00340.2003. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol. 2001;533:433–445. doi: 10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waschke J, Golenhofen N, Kurzchalia TV, Drenckhahn D. Protein kinase C-mediated endothelial barrier regulation is caveolin-1-dependent. Histochem Cell Biol. 2006;126:17–26. doi: 10.1007/s00418-005-0140-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Pampou S, Fujikawa K, Varticovski L. Opposing effect of angiopoietin-1 on VEGF-mediated disruption of endothelial cell-cell interactions requires activation of PKC beta. J Cell Physiol. 2004;198:53–61. doi: 10.1002/jcp.10386. [DOI] [PubMed] [Google Scholar]
- 36.Giorgione JR, Lin JH, McCammon JA, Newton AC. Increased membrane affinity of the C1 domain of protein kinase Cdelta compensates for the lack of involvement of its C2 domain in membrane recruitment. J Biol Chem. 2006;281:1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heit I, Wieser RJ, Herget T, Faust D, Borchert-Stuhltrager M, Oesch F, Dietrich C. Involvement of protein kinase Cdelta in contact-dependent inhibition of growth in human and murine fibroblasts. Oncogene. 2001;20:5143–5154. doi: 10.1038/sj.onc.1204657. [DOI] [PubMed] [Google Scholar]
- 38.Harrington EO, Shannon CJ, Morin N, Rowlett H, Murphy C, Lu Q. PKCdelta regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Exp Cell Res. 2005;308:407–421. doi: 10.1016/j.yexcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. J Biol Chem. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ting AY, Kain KH, Klemke RL, Tsien RY. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci U S A. 2001;98:15003–15008. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isshiki M, Mutoh A, Fujita T. Subcortical Ca2+ waves sneaking under the plasma membrane in endothelial cells. Circ Res. 2004;95:e11–e21. doi: 10.1161/01.RES.0000138447.81133.98. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 44.Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem. 2006;281:30947–30956. doi: 10.1074/jbc.M603741200. [DOI] [PubMed] [Google Scholar]
- 45.Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, Wu MH. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007;14:363–373. doi: 10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.