Abstract
Protein-zero related (PZR) is an IgV-type immunoreceptor with two immunoreceptor tyrosine based inhibitory motifs (ITIMs). PZR interacts with Src homology 2 domain containing tyrosine phosphatase (SHP-2) via its tyrosine phosphorylated ITIMs, for which c-Src is a putative kinase. Towards elucidating PZR function in endothelial cells (ECs), we cloned PZR from bovine aortic endothelial cells (BAECs) and characterized it. Mature bovine PZR had 94.8% and 92.7% sequence identity with canine and human proteins, respectively, and the two ITIM sequences were conserved among higher vertebrates. PZR was expressed in many cell types and was localized to cell contacts and intracellular granules in BAECs and mesothelioma (REN) cells. Co-immunoprecipitation revealed that PZR, Grb-2-associated binder-1 (Gab1) and platelet endothelial cell adhesion molecule-1 (PECAM-1) were three major SHP-2 binding proteins in BAECs. H2O2 enhanced PZR tyrosine phosphorylation and PZR/SHP-2 interaction in ECs in a dose- and time-dependent manner. To see if tyrosine kinases other than Src are also capable of phosphorylating PZR, we co-transfected HEK293 cells with PZR and one of several tyrosine kinases and found that c-Src, c-Fyn, c-Lyn, Csk and c-Abl but not c-Fes phosphorylated PZR and increased PZR/SHP-2 interaction. These results suggest that PZR is a cell adhesion protein which may be involved in SHP-2-dependent signaling at interendothelial cell contacts.
Keywords: protein zero related (PZR), endothelial cells, tyrosine phosphorylation, ITIM, SHP-2, cell contacts
INTRODUCTION
Immunoreceptor tyrosine based inhibitory motifs (ITIM) receptors are involved in various outside-in signaling through an interaction between tyrosine phosphorylated ITIMs and Src homology 2 (SH2)-domain containing tyrosine phosphatase-1 and -2 (SHP-1 and SHP-2), and regulate cellular activities such as adhesion, migration, immune response, and platelet aggregation (Newman 1999; Ravetch and Lanier 2000; Billadeau and Leibson 2002; Gibbins 2002). For example, platelet endothelial cell adhesion molecule-1 (PECAM-1) is an ITIM-containing receptor, expressed in endothelial and haematopoietic cells (Newman and Newman 2003). Its extracellular domain consists of six IgC-type domains, followed by a single transmembrane domain, and a short C-terminal cytoplasmic domain that contains two ITIMs (Newman et al. 1990). The PECAM-1 ITIM is tyrosine phosphorylated when endothelial cells (ECs) are mechanically stimulated and binds to SHP-2, and these events are thought to initiate certain mechanoresponses in ECs (Osawa et al. 2002; Tai et al. 2005; Fujiwara 2006). Gab1 is another protein with two ITIMs and is tyrosine phosphorylated in mechanically stimulated 3T3-L1 adipocytes (Janez et al. 2000) and ECs (Jin et al. 2005), and tyrosine phosphorylated Gab1 binds to SHP-2 (Dixit et al. 2005). Both PECAM-1 (Maas et al. 2003) and Gab-1 (Holgado-Madruga and Wong 2003) are tyrosine phosphorylated in cells treated with reactive oxygen species (ROS) and associate with SHP-2. It has been reported that both PECAM-1 and Gab1 are phosphorylated by Src family tyrosine kinases (SFKs) (Daub et al. 1997; Cao et al. 1998) and Fer/Fes kinases (Kogata et al. 2003).
Like PECAM-1, protein-zero related (PZR) is an immunoreceptor with two ITIMs and interacts with SHP-2 when its ITIMs are tyrosine phosphorylated (Zhao and Zhao 1998, 2000; Xu et al. 2002). PP1, a Src family kinase inhibitor, inhibits PZR phosphorylation and c-Src has been suggested to phosphorylate PZR (Zhao et al. 2002; Zhao et al. 2003). There are three alternatively spliced isoforms designated as PZR, PZRa and PZRb, and both PZRa and PZRb lack ITIMs (Zannettino et al. 2003; Zhao and Zhao 2003). PZR shares significant sequence homology with Myelin Protein Zero (MPZ) (Zhao and Zhao 1998), a transmembrane glycoprotein with a single IgV-type domain and a single ITIM-like motif in its extracellular and cytoplasmic domain, respectively (Lemke and Axel 1985). MPZ is the major structural protein of myelin and plays a key role in myelination and maintenance of the myelin structure (Eichberg 2002). A crystal structure analysis of its extracellular domain suggests tetramer formation in a cis manner within the plasma membrane of Schwann cells, and trans-interaction of tetramers is thought to establish tight adhesion between overlapping plasma membranes needed to form the myelin structure (Shapiro et al. 1996). Owing to its structural similarity to MPZ, PZR may also be involved in cell adhesion. Although the normal ligand for PZR is unknown, it binds avidly to concanavalin A (Con A) (Zhao et al. 2002).
Because PZR is expressed in ECs and because PECAM-1 and PZR are immunoreceptors with a similar cytoplasmic domain structure, we wished to study the function of PZR in ECs. Here we report our initial findings on PZR in bovine aortic endothelial cells (BAECs). Although some data similar to those we present here have been reported in experiments using tissues and cultured non-ECs, we describe these confirmatory data together with novel results as a part of the PZR biology in ECs. PZR was clones form BAECs and antibodies and nucleotide probes specific for bovine cells were made. To gain insights into PZR functions, we studied its tyrosine phosphorylation by ROS, interaction with SHP-2, and putative kinase. PZR was localized to cell contacts by immunofluorescence microscopy, which is consistent with the idea that it may play a role in cell-cell adhesion. Our phylogenetic analysis of type I transmembrane proteins with a single IgV extracellular domain showed that PZR was a member of a cell-cell adhesion protein family.
MATERIALS AND METHODS
Cloning of Bovine PZR
Total RNA was extracted from BAECs using an RNeasy Mini Kit (Qiagen, Valencia, CA). RT-PCR was performed using a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) and PfuUltra Hotstart DNA Polymerase (Stratagene, La Jolla, CA). Bovine PZR cDNA containing the entire cording region was generated using following primers based on Bos tauros EST sequences (primer: 5-TCGCCGATACCGGCGACGACGAGT-3 and 5-CTCCCTCTATGACTAAACATGTGGCTTCTCC-3; EST Genbank Acc. No.: BP111735 and AV615364). Amplified PCR products were subcloned into the pCR-BluntII-TOPO vector (Invitrogen). Sequencing was performed by the Functional Genomic Core Facility of the University of Rochester School of Medicine and Dentistry using an ABI Prism 3100 Genetic Analyzer and BigDye Terminator v3.1 Cycle Sequencing kits (Applied Biosystems, Forster City, CA). DNA sequencing was performed on 4 independent cDNA clones.
Construction of Expression Vectors
cDNA was amplified by PCR and subcloned into the pcDNA3.1(+) vector (BD Clonetech, San Jose, CA) (pcDNA3.1-PZR). The human c-Src, c-FynB, c-LynA, Csk, and c-Abl and bovine c-Fes cDNAs were amplified by RT-PCR using total RNA from HeLa cells or BAECs as a template (Genbank Acc. No. NM_005417, NM_002037, BC075001, NM_004383, AH005332 and NM_001032300). All cDNAs were sequenced as above. The c-Src, c-FynB, and Csk cDNAs were subcloned into the pcDNA3.1(+) vector (pcDNA3.1-Src, pcDNA3.1-FynB, pcDNA3.1-Csk). The c-LynA and c-Fes cDNAs were subcloned into the pcDNA3.1(−) vector (BD Clonetech) (pcDNA3.1-LynA, pcDNA3.1-Fes). The c-Abl cDNA was subcloned into the p3XFLAG-myc-CMV-24 vector (Sigma-Aldrich, Saint Louis, MI) (p3XFLAG-Abl).
Antibodies
The cDNA fragment corresponding to the cytoplasmic domain of bovine PZR was amplified by PCR and subcloned into the pGEX-6P1 vector (Amasham Pharmacia Biotech, Piscataway, NJ). The PZR cytoplasmic domain (pGEX-bPZRcyto) was expressed in bacteria, BL21-CodonPlus (DE3)-RIL strain (Stratagene), and the PZR fragment was purified using Glutathione Sepharose 4B beads (Amasham Pharmacia Biotech) followed by on-column cleavage using PreScission Protease (Amasham Pharmacia Biotech) according to the manufacturer’s protocol. Rabbits were immunized with the bovine PZR fragment and antiserum collected. Specific antibodies against PZR were affinity-purified using a HiTrap-NHS column (Amasham Pharmacia Biotech) conjugated with the cytoplasmic domain of bovine PZR.
Following monoclonal and polyclonal antibodies were purchased: mouse anti-protein phospho-tyrosine (4G10) and mouse anti-cortactin (4F11) (Upstate, Charlottesville, VA); mouse anti-β-catenin and mouse anti-SHP-2 (BD Transduction Laboratories, San Jose, CA); goat anti-PECAM-1, rabbit anti-SHP-2, and rabbit anti-Gab1 (Santa Cruz Biotechnology, Santa Cruz, CA). Rabbit anti-PECAM-1 (Osawa et al. 2002) was also used in some experiments. Following fluorescently tagged antibodies were purchased: IRDye680-goat anti-rabbit IgG, IRDye800-rabbit anti-goat IgG, IRDye800-donkey anti-rabbit IgG, and IRDye800-goat anti-mouse IgG (Rockland Immunochemicals, Gilbertsville, PA); Alexa488-goat anti-rabbit IgG, and Alexa546-goat anti-mouse IgG (Molecular Probes, Eugene, OR).
Cells and Cell Treatments
BAECs were obtained from Cambrex (East Rutherford, NJ) and cultured as described previously (Osawa et al. 2002). Human umbilical vein endothelial cells (HUVECs) were obtained from Cascade Biologics (Portland, OR) and cultured using Medium 2000 supplemented with Low Serum Growth Supplement (LSGS). Human adenocarcinoma (HeLa), human embryonic kidney (HEK293T/N19), mouse brain endothelial (bEnd.3) and mouse embryonic fibroblast (NIH3T3) cells were obtained from ATCC (Manassas, VA) and cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin G and 100 μg/ml streptomycin. Human mesothelioma REN and I-45 cells were kindly provided by Dr. W. Roy Smythe (M. D. Anderson Cancer Center, The University of Texas) (Mohiuddin et al. 2002) and cultured using RPMI medium supplemented with 10% FBS, 100 U/ml penicillin G and 100 μg/ml streptomycin.
For pervanadate treatment, confluent BAECs were serum starved for 4 hr and incubated in DMEM containing 1 mM pervanadate for 5 min. Peroxovanadate stock solution (100 mM) was generated by mixing an equal amount of 200mM H2O2 and 200mM sodium orthovanadate and incubating the mixture for 15 min at room temperature. For hydrogen peroxide treatment, BAECs were serum starved for 4 hr and incubated in DMEM containing H2O2 for various time periods.
Transfection of Expression Plasmids and siRNAs
For co-transfection of expression plasmids, HEK293 cells cultured in a 6-cm dish for 24 hr were co-transfected with 1.5 μg of pcDNA3.1-Src, pcDNA3.1-FynB, pcDNA3.1-LynB, pcDNA3.1-Csk, p3XFLAG-Abl, pcDNA3.1-Fes or pcDNA3.1(−) (mock) and 1.5 μg of pcDNA3.1-PZR or pcDNA3.1(+) (mock) using LipofectAMINE PLUS Reagent (Invitrogen) according to the manufacturer’s instructions. Cells were used 36 hr after transfection. An siRNA duplex against bovine PZR was based on the nucleotide sequence from 160 to 178 of the bovine PZR mRNA open reading frame: 5′-GGGAAGCUGACGUGCAAGU-dTdT-3′ (Fig. 1A) and was manufactured by Integrated DNA Technologies (Coralville, IA). BAECs cultured in a 6-cm dish for 24 hr were transfected with 2.5 nM siRNA using Lipofectamine 2000 Reagent (Invitrogen) by the manufacturer’s protocol. Cells were used 48 hr after transfection.
FIG. 1.
Predicted cDNA and amino acid sequences of bovine PZR. (A) The entire coding sequence (upper) and the corresponding amino acid sequence (lower) are shown. The signal sequence (thin line), the transmembrane domain (dotted line), two cysteine residues for the disulfate bond within the IgV domain (white boxed C), putative N-glycosylation sites (black boxed N), and two ITIMs (thick line) are indicated. (B) Alignment of the bovine PZR amino acid sequence with those of other species (GenBank Accession No. canine, XP_537211; human, NP_003944; murine, BAE41132; chicken ESTs, XP_423036 and XP_416652). Double dots indicate the same residues as in the bovine sequence. Note that the two ITIM sequences (thick line) and the position of the two cysteines (boxed) for the S-S bond are conserved. The signal sequence (thin line), putative N-glycosylation sites (black boxed N), and the transmembrane domain (dotted line) are indicated.
Immunoprecipitation and Immunoblotting
Cells were washed with cold PBS and solubilized in SDS-PAGE sample buffer (62.5 mM Tris-HCl, 2% SDS, pH 6.8). Protein concentrations were determined using the BCA microprotein assay kit (Pierce, Rockford, IL), appropriately adjusted, and 2-mercaptoethanol (ME) added to the samples to make the final concentration of 2%. Samples were subjected to SDS-PAGE and transferred to nitrocellulose filters. After blocking with Tris buffered saline (TBS; 0.15 M NaCl, 50 mM Tris-HCl; pH 7.4) containing 3% BSA and 3% non-fat milk, filters were incubated with TBS-3% BSA containing primary antibodies for 2 hr at room temperature. Immunoreactive bands were visualized using the IRDye680 or IRDye800 conjugated secondary antibodies and an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE) using a high sensitivity setting in order to detect all labeled bands. When required, filters were stripped using a solution containing 62.5 mM Tris-HCl, 2% SDS, and 2% 2-ME (pH 6.8) for 30 min at 60°C and re-blotted.
For immunoprecipitation, cells were washed with cold PBS and lysed at 4°C in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 0.1 mM Pefabloc, 1 μg/ml aprotinin, 1 mM E-64, 1 μg/ml leupeptin, 1 μg/ml pepstatin). After centrifugation at 20,000 g for 30 min at 4°C, cell lysates were precleared by incubating it with protein A-Sepharose (Amersham Pharmacia Biotech) for 1 hr at 4°C and then further incubated with beads carrying appropriate antibodies for 8 hr at 4°C. The beads were washed with the lysis buffer 5 times, and bound proteins were analyzed by SDS-PAGE and immunoblotting. All experiments were repeated at least 3 times, and when appropriate, data were analyzed using Student’s t test. P values of less than 0.05 were considered significant. All data are expressed as mean ± S.E.M.
Immunocytochemistry
Cells were fixed with methanol at −20°C for 20 min and then rehydrated with PBS at room temperature. They were blocked with 5% BSA in PBS and treated with appropriately diluted primary antibodies for 1 hr at room temperature, followed by washes and incubation with secondary antibodies for 30 min at room temperature. Images were observed using an Olympus BX51 epifluorescence microscope (Tokyo, Japan) equipped with a SPOT RT Color (Model 2.2.1) CCD digital camera and a SPOT Imaging Software (Diagnostic instruments, Sterling Heights, MI).
Phylogenetic Analysis
A phylogenetic tree of type I transmembrane proteins with a single extracellular IgV domain was constructed by the neighbor-joining method. Sequence data were obtained for following proteins: bovine PZR (Q32PI9), human PZR (O95297), bovine epithelial V-like antigen (Q5EAB0), human epithelial V-like antigen (O60487), human AAQ88989 protein (also known as myelin protein zero-like protein 3 precursor (MPZL3)) (Q6UWV2), bovine MPZL3 (A5D7C3), bovine MPZ (P10522), human MPZ (P25189), human voltage gated sodium channel β1-subunit (Q07699), human voltage gated sodium channel β2-subunit (O60939), human voltage gated sodium channel β3-subunit (Q9NY72), human voltage gated sodium channel β4-subunit (Q81WT1), human programmed death 1 (Q15116), human CD28 (P10747), human inducible T-cell co-stimulator (Q9Y6W8), human cytotoxic T-lymphocyte protein 4 (P16410), human B- and T-lymphocyte attenuator (Q7Z6A9), human CD8 α chain 1 (P01732), human CD8 β chain 1 (P10966), human Thy-1 (P04216), and human CD47 (Q08722).
RESULTS
Molecular Cloning of Bovine PZR
We cloned bovine PZR cDNA from the total RNA of BAECs based on bovine ESTs (EST Genbank Acc. No.: BP111735 and AV615364). Through RT-PCR and sequencing, the entire coding region was determined (Fig. 1A). This sequence was deposited in the Genbank sequence database under Accession No. DQ375557. Bovine PZR encoded 269 (232 for mature form) amino acids, and the calculated molecular weight (MW) of immature and mature proteins were 29,314 Da and 25,732 Da, respectively (Fig. 1A). A multiple amino acid sequence alignment of bovine, canine, human, murine, and chicken PZR revealed that the amino acid sequence of PZR was highly conserved (Fig. 1B). For example, bovine PZR had 94.8% sequence identity with canine PZR followed by human (92.7%), murine (81.5%), and chicken (68.5%). Note that cysteines in the immunoglobulin-like domain (Fig. 1B, white boxed), two out of three potential N-linked glycosylation sites (Fig. 1B, black bold), and the two ITIMs (Fig. 1B, bold underline) are conserved among these species.
Expression of PZR and SHP-2 Binding Proteins in ECs
When BAEC total lysates were immunoblotted with affinity purified anti-PZR, a broad major band (40–45 kDa) with some minor bands above and below was detected under the reducing condition (Fig. 2A). This MW was much higher than the calculated MW (25,732 Da), suggesting that PZR might be highly glycosylated. Using this anti-PZR in immunoblotting, we first studied PZR expression in several cultured cells and found that it was abundantly expressed in various types of cells while PECAM-1 expression was limited to ECs (Fig. 2B). As shown in this figure, we used three types of cultured ECs from different species (human, bovine, and mouse) and also from different types of blood vessels (arteries, veins, and microvessels). Although they are in vitro ECs, all of them expressed PZR. In addition, PZR expression was not limited to cells of any specific origin. Our results are consistent with the earlier report that showed a high level of PZR expression in haematopoietic cells and various organs (Xu et al. 2002). Apparent MWs were slightly different among PZRs from different cell types, presumably due to different levels of glycosylation. Although tyrosine phosphorylation-dependent association of PZR with SHP-2 has been reported in other cell types, we wished to know how extensive this association was in ECs. We treated BAECs with or without 1 mM pervanadate, due to which tyrosine phosphorylated proteins should accumulate in the cells, lysed cells, and from the lysates, immunoprecipitated SHP-2 or PZR. Anti-phosphotyrosine (4G10) immunoblots revealed four major tyrosine phosphorylated bands in the anti-SHP-2 immunoprecipitates (Fig. 2C, upper left). From the MW of each band and their ability to bind SHP-2, they were thought to be PECAM-1 (135 kDa), Gab1 (110–120 kDa), SHP-2 itself (65 kDa), and PZR (40–45 kDa). To confirm this, we immunoblotted the immunoprecipitates with specific antibodies against the suspected proteins and found that the four bands were from the top, indeed PECAM-1, Gab1, SHP-2 and PZR (Fig. 2C, upper). When immunoprecipitation was done using anti-PZR, SHP-2 was co-immunoprecipitated in a tyrosine phosphorylation-dependent manner (Fig. 2C, lower). These results confirm that PZR is a major SHP-2 binding protein (Xu et al. 2002) and as such it may play some important roles in SHP-2-mediated signaling in ECs.
FIG. 2.
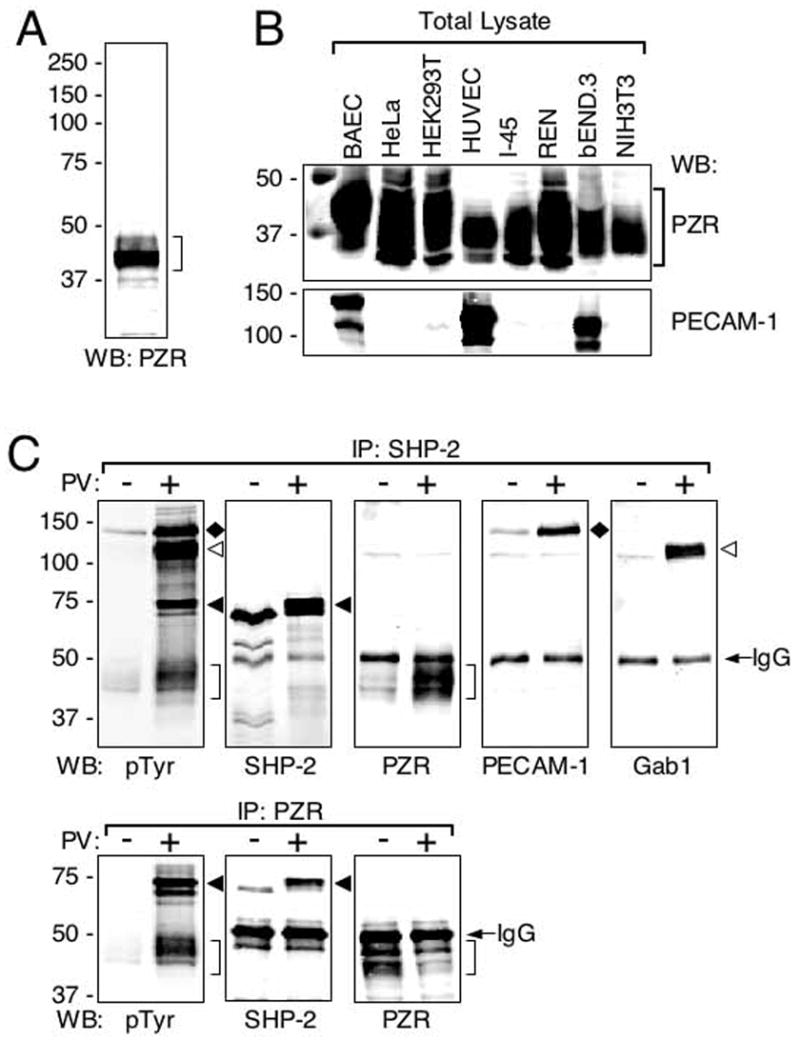
PZR is a major SHP-2 binding protein in ECs. (A) An anti-PZR immunoblot against a BAEC lysate. As it has been reported by others, anti-PZR immunoreactivity was found within a wide zone around 40 kDa. (B) Expression of PZR (upper) and PECAM-1 (lower) in cultured cells. Total cell lysates were immunoblotted with anti-PZR or anti-PECAM-1. While PECAM-1 expression was limited to ECs, PZR was expressed by all the cell types tested: BAEC (endothelial), HeLa cells (epithelial), HEK293T (epithelial), HUVEC (endothelial), I-45 (mesothelial), REN (mesothelial), bEND.3 (endothelial), and NIH3T3 (fibroblastic). (C) BAECs were treated with (+) or without (−) 1 mM pervanadate (PV) for 5 min and cell lysates were made. The lysates were mixed with anti-SHP-2 (upper) or anti-PZR (bottom) and immunoprecipitates analyzed by immunoblotting with anti-phosphotyrosine (pTyr), anti-SHP-2 (SHP-2), anti-PZR (PZR), anti-PECAM-1 (PECAM-1), or anti-Gab1 (Gab1). Immunoreactive bands corresponding to PECAM-1 (135kDa, black diamonds), Gab1 (110-120kDa, white arrowheads), SHP-2 (65kDa, black arrowheads), PZR (40–45kDa, brackets) and IgG heavy chain (IgG) are indicated.
Hydrogen Peroxide-induced Tyrosine Phosphorylation And SHP-2 Binding
ECs are known to respond to ROS, such as hydrogen peroxide (Lounsbury 2000). Both PECAM-1 (Maas et al. 2003) and Gab1 (Holgado-Madruga and Wong 2003), which are other major SHP-2 binding proteins, have been shown to be tyrosine phosphorylated by H2O2. To see if H2O2 also induced tyrosine phosphorylation of PZR, BAECs were treated with increasing concentrations of H2O2 for various time periods and cell lysates made. PZR was immunoprecipitated and its tyrosine phosphorylation was analyzed as a function of H2O2 concentration (Fig. 3A) or treatment time (Fig. 3B). The data show the dose- and time-dependent PZR tyrosine phosphorylation by H2O2. Furthermore, SHP-2 binding was also stimulated by H2O2 as PZR phosphorylation levels increased (Fig. 3B; graph). Since ROS is a potent activator of NRPTKs, PZR could be phosphorylated by one or more of these kinases.
FIG. 3.
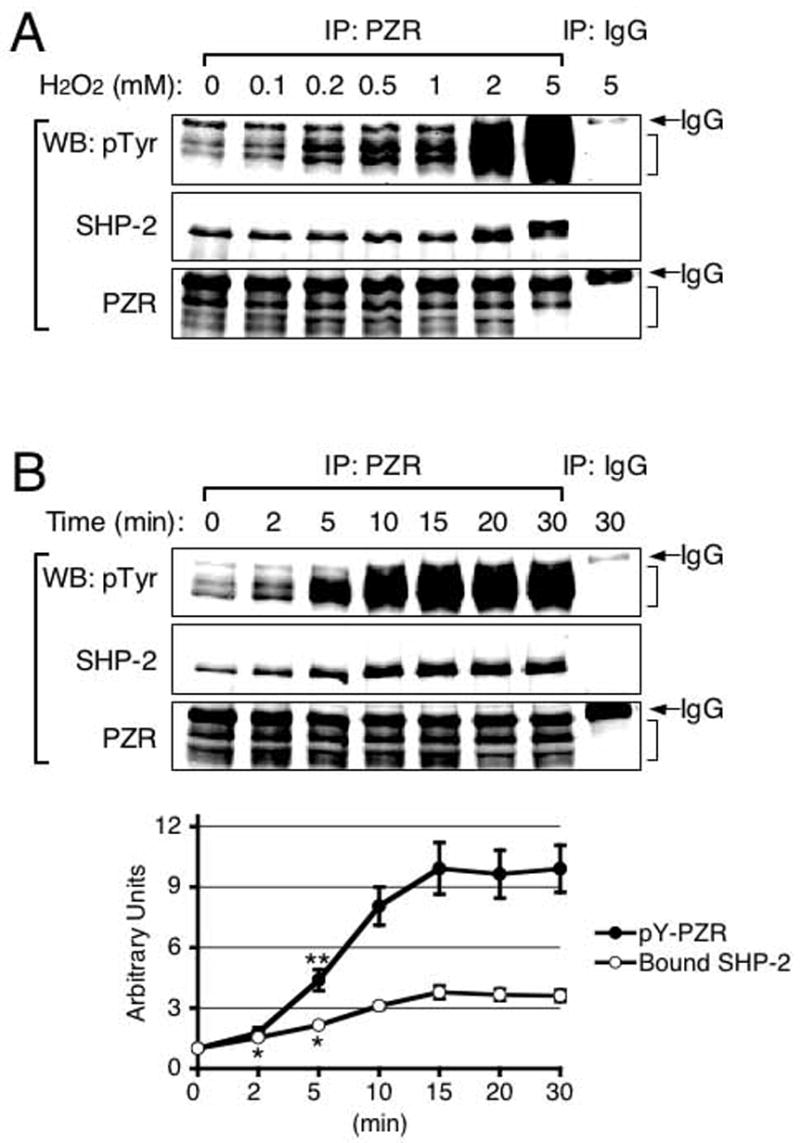
Hydrogen peroxide-induced PZR tyrosine phosphorylation and PZR/SHP-2 interaction. (A) BAECs were treated with various concentrations of H2O2 for 10 min. Cell lysates were made and mixed with anti-PZR. Immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine (pTyr)), anti-SHP-2 (SHP-2) or anti-PZR (PZR). Brackets and IgG indicate the PZR region and IgG heavy chain, respectively. (B) BAECs were treated with 2 mM H2O2 for 0 – 30 min, cell lysates made, and immunoprecipitation performed using anti-PZR. Immunoprecipitates were immunoblotted with anti-phosphotyrosine (pTyr), anti-SHP-2 (SHP-2), or anti-PZR (PZR). Brackets and IgG indicate the PZR region and IgG heavy chain, respectively. Relative amounts of tyrosine phosphorylated PZR (pY-PZR) and bound SHP-2 were calculated and shown in a graph. Each data point represents means ± S.E.M. (n = 3; *, P < 0.03; **, P < 0.01 vs. 0 min).
SFKs, Csk and Abl But Not Fes Phosphorylate PZR
Although Src has been shown to phosphorylate PZR, it may not be the only kinase capable of phosphorylating PZR. To investigate if NRPTKs other than Src could be also involved in PZR phosphorylation, we co-transfected HEK293T/N17 cells with bovine PZR and various NRPTKs including human c-Src, human c-FynB, human c-LynA, human Csk, human cAbl, and bovine c-Fes. PZR was immunoprecipitated from lysates of the co-transfected cells and probed with anti-phosphotyrosine and anti-SHP-2. Because cortactin is a known substrate for c-Src and Fer/Fes family kinases (Lounsbury et al. 2000), its phosphorylation was used as a positive control. Constitutive PZR tyrosine phosphorylation was observed in cells overexpressing c-Src, Csk and c-Abl. Other SFK members, c-FynB and c-LynA also phosphorylated PZR. c-Fes failed to phosphorylate PZR although it did phosphorylate cortactin. SHP-2 was co-immunoprecipitated with PZR in a tyrosine phosphorylation-dependent manner. These studies identified Fyn, Lyn, Csk and c-Abl as novel putative PZR kinases and suggest potential roles of SFKs, Csk, and c-Abl in PZR function.
Subcellular Localization of PZR
We studied subcellular localization of PZR in BAECs. To show staining specificity, we used BAECs transfected with a control or a PZR siRNA duplex. Immunoblots showed that siRNA down-regulated PZR expression by >95% (Fig. 5A, left). Anti-PZR staining in BAECs treated with control siRNA with a scrambled sequence was seen at cell contacts that were identified by anti-β-catenin and intracellular granules (Fig. 5A, middle, Fig. 5B) while in cells treated with PZR siRNA, these staining patterns were greatly diminished (Fig. 5A, right). REN cells showed more distinct localization at cell contacts than BAECs (Fig. 5C). Note that PZR is not localized at the free edge of cells and lamellipodia, suggesting its role in the formation of cell contacts (Fig. 5C). The integrity of interendothelial contacts did not appear to be affected by the PZR siRNA treatment. This is probably due to the presence of other cell adhesion molecules such as VE-cadherin and PECAM-1.
FIG. 5.
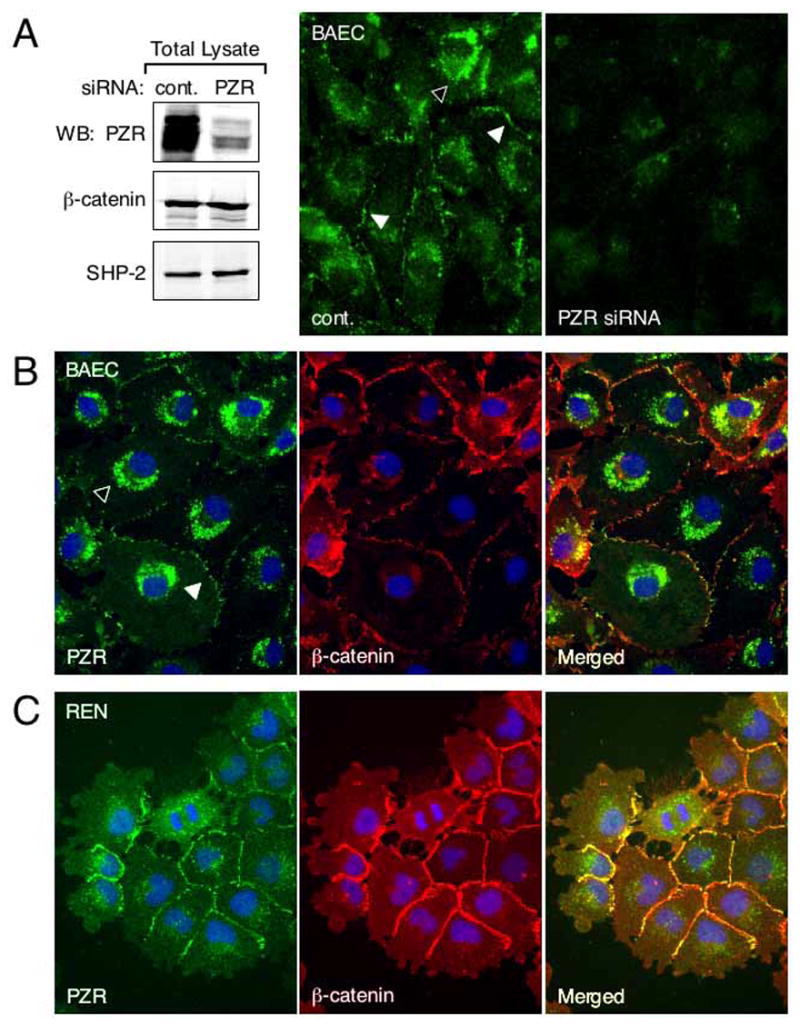
Subcellular localization of PZR. (A) BAECs were transfected with control or PZR siRNA duplex. Immunoblots show reduced PZR expression in siRNA treated cells. The expression of β-catenin and SHP-2 was not downregulated in these cells. When ECs transfected with control siRNA (cont.) were immunofluorescently stained with anti-PZR, cell contacts (white arrowheads) and some cytoplasmic granules (black arrowhead) were stained. This staining pattern was reduced in cells treated with PZR siRNA. (B) and (C) BAECs and REN cells were fixed and triple-stained with anti-PZR (green), anti-β-catenin (red), and DAPI for nuclear identification (blue). PZR was localized to cell-cell contacts (white arrowhead in BAECs) and intracellular granules (black arrowhead in BAECs). In merged images, yellow cell border staining is seen.
An earlier study reported that PZR had some role in fibronectin-dependent cell motility (Zannettino et al. 2003). Using BAECs treated with siRNA, we performed the “wound healing” assay of confluent monolayers and found repeatedly that the wound closure was not affected by the reduced PZR expression (data not shown). In addition, the random walk behavior exhibited by sparsely cultured BAECs on various extracellular matrices including fibronectin was not affected by reduced expression of PDZ (data not shown).
Phylogenetic Analyses
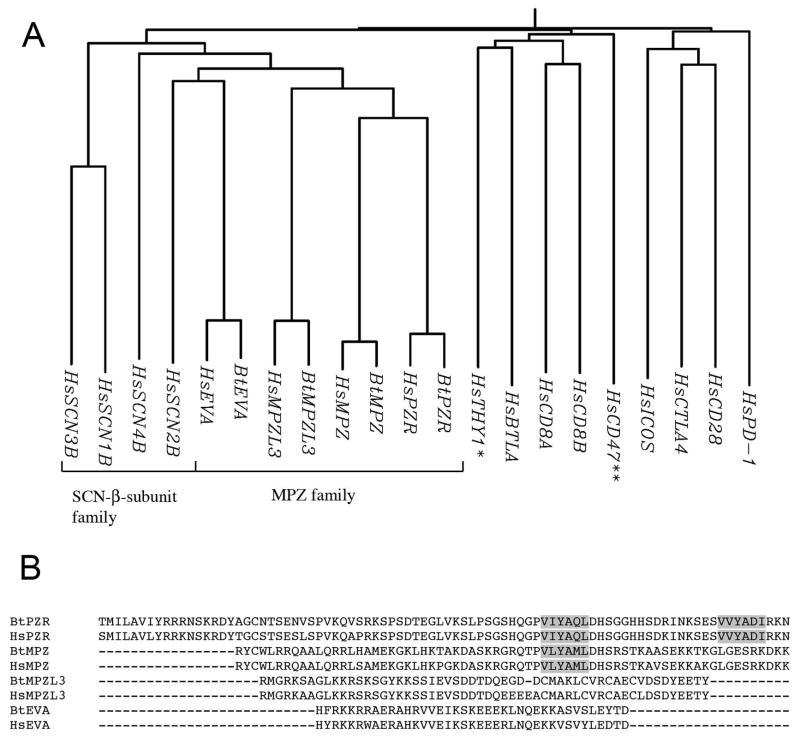
There is a number of type I transmembrane proteins with a single IgV domain. We constructed a phylogenetic tree of these proteins based on their amino acid sequence obtained from a data base. As shown in Fig. 6, PZR is a member of the MPZ family, and epithelial V-like antigen (EVA) and MPZL3 are other members of the family. MPZ, EVA and MPZL3 are known or suggested cell-cell adhesion molecules (Filbin et al. 1990; 1993; Guttinger et al. 1998). Thus, this analysis strongly implicates PZR as a cell-cell adhesion protein that belongs to the MPZ family of cell adhesion proteins. This is consistent with the intercellular localization of PZR observed in BAECs and REN cells. In addition, voltage gated sodium channel subunits β1 and β2, members of the SCN β-subunit family which is closely related to the MPZ family, have been shown to mediate cell-cell adhesion via trans-homophilic interaction (Malhotra et al. 2000). In fact, most of the proteins listed on the left half of the tree form trans-homophilic association while no homophilic association has been reported for those on the right half of the tree (proteins not grouped as a family), further supporting that PZR is a cell-cell adhesion protein. A considerable similarity exists between the cytoplasmic portions of MPZ and PZR (Fig. 6B). MPZ has one ITIM, and the amino acid sequence around this ITIM is near identical to the N-terminal ITIM of PZR. No sequence similarities are found in the cytoplasmic domain of PZR, EVA, and MPZL3. These analyses suggest that PZR and MPZ are indeed closely related proteins and are involved in cell-cell adhesion.
FIG. 6.
Phylogenetic tree indicating the relationship between PZR and other single IgV-type membrane proteins. (A) A phylogenetic tree based on the entire sequences of proteins was constructed by the neighbor-joining method. Two families are indicated. PZR is a member of the MPZ family of proteins whose other members include MPZ, MPZL3 protein and EVA. BtPZR, bovine PZR; HsPZR, human PZR; BtEVA, bovine EVA; HsEVA, human EVA; BtMPZL3, bovine MPZ-like protein 3; HsMPZL3, human MPZ-like protein 3; BtMPZ, bovine MPZ; HsMPZ, human MPZ; HsSCN1B, human voltage gated sodium channel subunit beta-1; HsSCN2B, human voltage gated sodium channel subunit beta-2; HsSCN3B, human voltage gated sodium channel subunit beta-3; HsSCN4B, human voltage gated sodium channel subunit beta-4; HsPD-1, human programmed cell death protein 1; HsCD28, human CD28; HsICOS, human inducible T-cell co-stimulator; HsCTLA4, human cytotoxic T-lymphocyte protein 4; HsBTLA, human B- and T-lymphocyte attenuator; HsCD8A, human CD8 α chain; HsCD8B, human CD8 β chain; HsThy1, human Thy-1; HsCD47, human CD47. The tree contains two proteins that are not type I membrane protein. Thy-1 (*) has no transmembrane domain and CD47 (**) is a multi-pass membrane protein. (B) Sequence alignment of the cytoplasmic region of the proteins in the MPZ family. ITIMs are indicated by grey shading.
DISCUSSION
PZR is a highly conserved protein and is expressed in many cell types, suggesting its role in some basic cell function(s). In its cytoplasmic domain, PZR has two ITIM motifs. Each ITIM contains a tyrosine residue that can be phosphorylated and SHP-2 is known to interact with phosphorylated ITIMs. This interaction presumably activates certain SHP-2-dependent signaling and other events in cells. Because little is known about PZR’s function in ECs and because its phosphorylation may be critical for its function, we initiated a series of studies that relate to phosphorylation of PZR. By overexpressing various NRPTKs, we showed that PZR was phosphorylated by SFKs (Src, FynB, and LynA), Csk, and Abl but not Fes. These studies suggest that Fyn, Lyn, Csk and Abl are possible kinases for PZR. It is known that H2O2 activates tyrosine kinase including Src (Barchowsky et al. 1995), Lyn (Kharbanda et al. 1994) and Abl (Sun et al. 2000), and indeed, when we treated ECs with H2O2, both tyrosine phosphorylation of PZR and subsequent interaction with SHP-2 occurred in a robust manner. Thus, the results from the NRPTK overexpression and the H2O2 studies are mutually consistent.
This study as well as many other earlier studies showed that PECAM-1 and Gab1 were other major SHP-2 binding proteins with two ITIMs in ECs. SFKs (Cao et al. 1998), Csk (Cao et al. 1998), and Fer and Fes kinase (Kogata et al. 2003) have been reported to phosphorylate PECAM-1 while SFKs (Daub et al. 1997) and Fer and Fes (Kogata et al. 2003) phosphorylate Gab1. Although the three major SHP-2 binding proteins in ECs share a common set of tyrosine kinases for their phosphorylation, each appears to have its unique kinase partners. For example, the Fer/Fes family NRPTKs do not phosphorylate PZR although they phosphorylate PECAM-1 and Gab1. These results suggest that the three SHP-2 interacting proteins are differently regulated in ECs although they may also have common mechanisms for activation and regulation.
Our phylogenetic analysis of the type I transmembrane proteins with one IgV domain revealed that PZR was most closely related to MPZ, suggesting that these proteins have similar functions. Because MPZ is a homophilic cell adhesion protein responsible for the formation of the myelin structure, PZR may also have a role in cell adhesion. Indeed, the phylogenetic analysis places PZR in the MPZ family and all the other proteins in this family (MPZ, EVA, and MPZL3) are known or proposed cell adhesion proteins. In addition, voltage-gated sodium channel subunits β1 and β2 are also shown to be cell adhesion proteins (Malhotra et al. 2000). Based on these phylogenetic data, we propose that PZR is a cell adhesion protein. In support of this conclusion are our localization data, especially the results of REN cells that show PZR localization limited to the cell contacts (i.e. not localized to the free border of cells) argue strongly for this conclusion.
Anti-PZR also stained granules of the perinuclear region. This staining pattern is not due to non-specific staining because our siRNA control largely abolished this labeling. Interestingly, anti-MPZ also stains vesicles in the areas of the Golgi complex and cell-cell contacts (Trapp et al. 1981). Thus, these closely related proteins show similar localization. The staining associated with the Golgi area suggests that PZR and MPZ are continuously synthesized and thus are turned over. It is interesting to note that PZR in any cell types has never appeared as a well-defined single band in our immunoblots. Because our anti-PZR was made against the cytoplasmic region of the molecule, it should recognize both fully and partially glycosylated PZR, and this could be the reason for less well-defined appearance of anti-PZR immunoblotted bands.
Many unanswered questions remain. For example, although Con A has been shown to bind PZR, its natural ligand or ligands remain unknown. It is highly likely that similar to other members of the MPZ family, PZR interacts homophilically with another PZR, but this has to be experimentally demonstrated. In addition, it is not known how the state of phosphorylation or SHP-2 binding is related to these possible extracellular events and cellular signaling. Identifying cellular function that depends on PZR is critical. In this regard, an earlier study that showed PZR’s role in fibronectin-dependent cell migration (Zannettino et al. 2003) is important. However, our repeated attempts to observe any effects of reduced PZR expression on BAEC motility were unsuccessful. It appears that PZR function on cell motility is complex because overexpression of this protein enhances cell motility while downregulated expression of this protein did not affect cell movements. These are some of the important questions that need to be investigated in future studies in order to elucidate PZR functions in ECs.
FIG. 4.
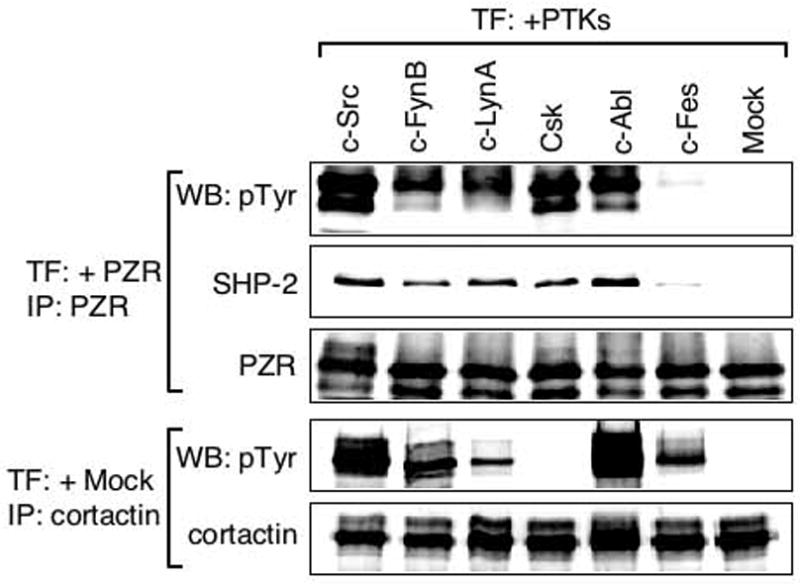
PZR phosphorylation by SFKs, Csk, and c-Abl but not c-Fes. HEK293T/N17 cells were co-transfected with bovine PZR and one of several NRPTKs (human c-Src, human c-FynB, human c-LynA, human Csk, human c-Abl, bovine c-Fes). Lysates of co-transfected cells were mixed with anti-PZR or anti-cortactin and the immunoprecipitates were immunoblotted with anti-phosphotyrosine (pTyr), anti-SHP-2 (SHP-2), anti-PZR (PZR), or anti-cortactin. PZR was tyrosine phosphorylated by c-Src, Csk and c-Abl. Other SFK members, c-FynB and c-LynA also phosphorylated PZR while c-Fes did not. The data indicate that SHP-2 interaction with PZR depends on the state of PZR tyrosine phosphorylation.
Acknowledgments
We thank Dr. W. Roy Smythe (M. D. Anderson Cancer Center, The University of Texas) for REN and I-45 cells. This work was supported in part by a grant from NIH (HL69041).
References
- Barchowsky A, Munro SR, Morana SJ, Vincenti MP, Treadwell M. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am J Physiol. 1995;269:L829–836. doi: 10.1152/ajplung.1995.269.6.L829. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest. 2002;109:161–168. doi: 10.1172/JCI14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao MY, Huber M, Beauchemin B, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- Daub D, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res. 2005;97:1236–1244. doi: 10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- Eichberg J. Myelin P0: new knowledge and new roles. Neurochem Res. 2002;27:1331–1340. doi: 10.1023/a:1021619631869. [DOI] [PubMed] [Google Scholar]
- Filbin MT, Tennekoon GI. Homophilic adhesion of the myelin P0 protein requires glycosylation of both molecules in the homophilic pair. J Cell Biol. 1993;122:451–459. doi: 10.1083/jcb.122.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT, Walsh FS, Trapp BD, Pizzey JA, Tennekoon GI. Role of myelin P0 protein as a homophilic adhesion molecule. Nature. 1990;344:871–872. doi: 10.1038/344871a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Internal Med. 2006;259:373–380. doi: 10.1111/j.1365-2796.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- Gibbins JM. The negative regulation of platelet function: extending the role of the ITIM. Trends Cardiovasc Med. 2002;12:213–219. doi: 10.1016/s1050-1738(02)00164-0. [DOI] [PubMed] [Google Scholar]
- Guttinger M, Sutti F, Panigada M, Porcellini S, Merati B, Mariani M, Teesalu T, Consalez GG, Frassi F. Epithelial V-like antigen (EVA), a novel member of the immunoglobulin superfamily, expressed in embryonic epithelia with a potential role as homotypic adhesion molecule in thymus histogenesis. J Cell Biol. 1998;141:1061–1071. doi: 10.1083/jcb.141.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga M, Wong AJ. Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol Cell Biol. 2003;23:4471–4484. doi: 10.1128/MCB.23.13.4471-4484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janez A, Worrall DS, Imamura T, Sharma PM, Olefsky JM. The osmotic shock-induced glucose transport pathway in 3T3-L1 adipocytes is mediated by gab-1 and requires Gab-1-associated phosphatidylinositol 3-kinase activity for full activation. J Biol Chem. 2000;275:26870–26876. doi: 10.1074/jbc.M001654200. [DOI] [PubMed] [Google Scholar]
- Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Yuan ZM, Rubin E, Weichselbaum R, Kufe D. Activation of Src-like p56/p53lyn tyrosine kinase by ionizing radiation. J Biol Chem. 1994;269:20739–20743. [PubMed] [Google Scholar]
- Kogata N, Masuda M, Kamioka Y, Yamagishi A, Endo A, Okada M, Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol Biol Cell. 2003;14:3553–3564. doi: 10.1091/mbc.E03-02-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985;40:501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med. 2000;28:1362–1369. doi: 10.1016/s0891-5849(00)00222-7. [DOI] [PubMed] [Google Scholar]
- Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible PECAM-1 tyrosine phosphorylation and SHP-2 binding. Am J Physiol Heart Circ Physiol. 2003;285:H2336–2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Mohiuddin L, Cao X, Ozvaran MK, Zumstein L, Chada S, Smythe WR. Phosphatase and tensin analog gene overexpression engenders cellular death in human malignant mesothelioma cells via inhibition of AKT phosphorylation. Ann Surg Oncol. 2002;9:310–316. doi: 10.1007/BF02573071. [DOI] [PubMed] [Google Scholar]
- Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 1999;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron. 1996;17:435–449. doi: 10.1016/s0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem. 2000;275:17237–17240. doi: 10.1074/jbc.C000099200. [DOI] [PubMed] [Google Scholar]
- Tai IK, Sheng Q, Pan S, Jin EG, Berk BC. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem. 2005;280:29620–29624. doi: 10.1074/jbc.M501243200. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Itoyama Y, Sternberger NH, Quarles RH, Webster H. Immunocytochemical localization of P0 protein in Golgi complex membranes and myelin of developing rat Schwann cells. J Cell Biol. 1981;90:1–6. doi: 10.1083/jcb.90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Xu MJ, Zhao R, Guerrah A, Zeng F, Zhao ZJ. Tyrosine phosphatases SHP-1 and SHP-2 are associated with distinct tyrosine-phosphorylated proteins. Exp Cell Res. 2002;272:75–83. doi: 10.1006/excr.2001.5397. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Roubelakis M, Welldon KJ, Jackson DE, Simmons PJ, Bendall LJ, Henniker A, Harrison KL, Niutta S, Bradstock KF, Watt SM. Novel mesenchymal and haematopoietic cell isoforms of the SHP-2 docking receptor, PZR: identification, molecular cloning and effects on cell migration. Biochem J. 2003;370:537–549. doi: 10.1042/BJ20020935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Fu X, Teng L, Li Q, Zhao ZJ. Blocking the function of tyrosine phosphatase SHP-2 by targeting its Src homology 2 domains. J Biol Chem. 2003;278:42893–42898. doi: 10.1074/jbc.M306136200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Guerrah A, Tang H, Zhao ZJ. Cell surface glycoprotein PZR is a major mediator of concanavalin A-induced cell signaling. J Biol Chem. 2002;277:7882–7888. doi: 10.1074/jbc.M111914200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Zhao ZJ. Dissecting the interaction of SHP-2 with PZR, an immunoglobulin family protein containing immunoreceptor tyrosine-based inhibitory motifs. J Biol Chem. 2000;275:5453–5459. doi: 10.1074/jbc.275.8.5453. [DOI] [PubMed] [Google Scholar]
- Zhao R, Zhao ZJ. Identification of a variant form of PZR lacking immunoreceptor tyrosine-based inhibitory motifs. Biochem Biophys Res Commun. 2003;303:1028–1033. doi: 10.1016/s0006-291x(03)00484-4. [DOI] [PubMed] [Google Scholar]
- Zhao ZJ, Zhao R. Purification and cloning of PZR, a binding protein and putative physiological substrate of tyrosine phosphatase SHP-2. J Biol Chem. 1998;273:29367–29372. doi: 10.1074/jbc.273.45.29367. [DOI] [PubMed] [Google Scholar]