Abstract
The current vaccine for tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is an attenuated strain of Mycobacterium bovis bacillus Calmette-Guerin (BCG). BCG has proven to be effective in children, however, efficacy wanes in adulthood. Lactoferrin, a natural protein with immunomodulatory properties, is a potential adjuvant candidate to enhance efficacy of BCG. These studies define bovine lactoferrin as an enhancer of the BCG vaccine, functioning in part by modulating macrophage ability to present antigen and stimulate T-cells. BCG-infected bone marrow derived macrophages (BMMs) cultured with bovine lactoferrin increased the number of MHC II+ expressing cells. Addition of IFN-γ and lactoferrin to BCG-infected BMMs enhanced MHC II expressiona dna increased the ratio of CD86/CD80. Lactoferrin treated BCG-infected BMMs were able to stimulate an increase in IFN-γ production from presensitized CD3+ splenocytes. Together, these results demonstrate that bovine lactoferrin is capable of modulating BCG-infected macrophages to enhance T-cell stimulation through increased surface expression of antigen presentation and co-stimulatory molecules, which potentially explains the observed in vivo bovine lactoferrin enhancement of BCG vaccine efficacy to protect against virulent MTB infection.
Keywords: Lactoferrin, BCG, Vaccine, Adjuvant, Tuberculosis
1. Introduction
It is estimated that Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis (TB), currently infects one-third of the world's population and causes nearly 1.7 million deaths per year [1-3]. The existing vaccine is an attenuated strain of Mycobacterium bovis Bacillus Calmette-Guerin (BCG), which, unfortunately, has varying levels of reported efficacy (0–80%). While many novel TB vaccines have been developed [4,5], few have demonstrated protective efficacy that can surpass BCG, which remains the only vaccine approved for human use. Previous research indicates that addition of adjuvant components to the BCG vaccine significantly enhances immune responses to promote host protection against subsequent challenge with virulent MTB [6-9]. One such reported component is lactoferrin, an iron binding protein found primarily in mucosal secretions and secondary granules of neutrophils [10].
Lactoferrin possesses multiple biological functions, including bactericidal and bacteriostatic activities [11-15], anti-and pro-inflammatory responses [16], promotion of B- and T-cell maturation [17,18], and enhancement of the delayed type hypersensitivity (DTH) to defined antigens [19-21]. Mice immunized with BCG in the presence of bovine lactoferrin demonstrated increased host protection post-challenge with virulent MTB, as observed by decreasing organ bacterial load and pulmonary disease granulomatous pathology. The BCG/ lactoferrin immunized mice displayed increased presence of lymphocytes in granulomas and elevated production of IFN-γ from splenocytes reactive to BCG antigens [22,23]. Overall responses indicated that mice immunized with BCG admixed with bovine lactoferrin developed an enhanced mycobacterial antigen-specific T-cell response, suggesting that facilitation of efficacy of the BCG vaccine is through promotion of development of T-cell helper type 1 (TH1) immunity.
The TH1 phenotypic response is, in part, regulated by the presence of IL-12 produced primarily by macrophages and dendritic cells. The IL-12 assists in directed development of naïve CD4+ T-cells towards the TH1 subtype [24-26]. In addition, IL-12 functions as a co-stimulator for maximizing production of IFN-γ from TH1 cells and activates IFN-γ production from memory T-cells [27,28]. Lactoferrin has the potential to promote development of both TH1 and TH2 immune responses, depending on experimental conditions [29,30]. Towards its utility as an adjuvant, in vivo and in vitro studies indicate that lactoferrin can increase relative production of IL-12 while decreasing IL-10, a negative regulator of IL-12 [19,31-33]. Of critical importance, bovine lactoferrin added to murine macrophages infected with BCG enhanced the production of IL-12 relative to amounts of IL-10 [34].
Antigenic peptides presented by macrophages via the major histocompatibility complex molecules (MHC) I and II, along with co-stimulatory molecules such as CD80, CD86, and CD40, are essential in the T-cell activation process [35-37]. Recently, lactoferrin was shown to affect surface expression of CD40 on murine macrophages [38], suggesting that lactoferrin may modulate macrophage antigen presentation events to defined antigens. These studies will investigate the hypothesis that lactoferrin is capable of affecting surface expression of molecules on BMMs that are involved in antigen presentation (MHC II, CD80, and CD86), leading to enhanced macrophage stimulation of presensitized T-cell populations. Specifically, these experiments will address the mechanism of bovine lactoferrin to function as an adjuvant component to enhance efficacy of the BCG vaccine, promoting generation of specific immune responses, in vivo, and activation of existing specific responses that could assist in host protection against challenge with virulent MTB.
2. Materials and methods
2.1. Animals
Female C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) of 20–25 g initial body weight were used for in vitro macrophage development, and as a source of splenocytes. All in vivo experiments were conducted under approved guidelines of the animal welfare ethics committee at the University of Texas, Health Science Center at Houston, protocol HSCAWC-05-060.
2.2. Lactoferrin and BCG
Low endotoxin bovine milk lactoferrin (<0.2 EU/mg, <20% iron saturated, >95% purity) was provided by PharmaReview Corporation (Houston, TX). Endotoxin level was evaluated using the Limulus Amebocyte Lysate (BioWhittaker) according to the manufacturer's instructions. Mycobacterium bovis Bacillus Calmette-Guerin (BCG), Pasteur strain (TMC 1011, ATCC, Manassas, VA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37 °C for 2 weeks before use. BCG concentration was estimated using McFarland standards (Sigma) and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS). Plates were incubated at 37 °C for 3–4 weeks prior to enumeration of colonies.
2.3. Derivation and treatment of bone marrow derived macrophages
Bone marrow derived macrophages (BMMs) were differentiated as previously described [39]. Briefly, cells were isolated from C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) by flushing the femur with McCoy's medium supplemented with 100 μg/mL penicillin G (Sigma) and 50 μg/mL gentamycin sulfate (Sigma). Collected cells were treated with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ) and resulting cells were differentiated for 7 days at 1 × 106 cells/mL in McCoy's medium, supplemented with 2.2 g/L sodium bicarbonate, 10% FBS, and GM-CSF (10 ng/mL) (Cell Sciences, Canton, MA). At day 7, non-adherent cells were removed and adherent cells (estimated at 5 × 105 cells/well) rested overnight in DMEM complete medium (Dulbecco's modified Eagle's medium (Sigma) with 50 mg/L HEPES (Sigma), 50 mg/L l-arginine (Sigma), 2.2 g/L sodium bicarbonate (Sigma)) supplemented with 10% FBS (Sigma) at 37 °C with 5% CO2. The adherent cell population was >95% F4/80+ as determined by flow cytometric analysis. Rested macrophages were infected with BCG (MOI 1:1 or 10:1) with or without lactoferrin (100 μg/mL) for 72 h, supernatants were collected and stored at −20 °C for analysis by ELISA, and macrophages were isolated for surface marker staining and analyzed by fluorescence-activated cell sorting (FACS). Comparisons were made to control non-infected cells. To examine macrophage responses to IFN-γ stimulation, macrophages at 72 h post-infection were washed with 1× PBS and cultured with 10 ng/mL mouse recombinant IFN-γ (Cell Sciences). Supernatants and cells were collected at 72 h post-IFN-γ stimulation.
2.4. Macrophage stimulation of presensitized T-cells
Splenocytes from naïve and BCG (1 × 107 CFU/mouse) immunized mice were isolated as previously described [40]. CD3+ T-cells were purified by passing ACK treated splenocytes through a CD3+ enrichment column (R&D Systems, Minneapolis, MN), yielding a minimum of 90% CD3+ cells as determined by FACS analysis. CD4+ and CD8+ T-cells were purified from splenocytes, without ACK buffer treatment, resuspended in 2 mL of Macs buffer (0.5% BSA and 2 mM EDTA in 1× PBS), and incubated with CD4 or CD8 MicroBeads (Miltenyi Biotec) at 4 °C for 15 min. Labeled cells were washed once with Macs buffer, resuspended in 2 mL of Macs buffer, and separated by magnetic column. Recovered CD4+ and CD8+ were determined to be >95% positive. Purified CD3+(2 106 cells/mL per well), CD4+ (2 × 106 cells/mL per well), and CD8+(1 × 106 cells/mL per well) T-cells were resuspended in DMEM complete medium supplemented with 10% FBS, 0.005% (v/v) 2-mercaptoethanol (Gibco™, Invitrogen, Grand Island, NY), and antibiotics (100 μg/mL penicillin G and 50 μg/mL gentamycin sulfate) and subsequently overlaid onto prepared macrophages. Macrophage presenters were previously infected with BCG (1:1), BCG (10:1), or remained non-infected, and either treated with or without lactoferrin (100 μg/mL) for 72 h. Prior to co-culture with isolated T-cells, macrophage presenters were thoroughly washed with 1× PBS. Supernatants were collected at 72 h and cytokines analyzed by ELISA. For intracellular staining, splenocytes were restimulated with ConA (2 μg/mL) or PMA (10 ng/mL) and ionomycin (250 ng/mL) in the presence of 1 μL/well BD Golgi Plug (BD Biosciences, San Diego, CA) for 6 h and isolated for FACS analysis.
2.5. Examination of BCG proliferation
Effect of lactoferrin on BCG proliferation was performed for growth in broth culture or within macrophages. For broth culture, BCG, at log phase growth, was seeded (105 CFU/mL) into Dubos base with 10% supplement with increasing concentrations of bovine lactoferrin (0, 100, or 1000 μg/mL). For growth within cells, bone marrow derived macrophages (5 × 105 cells/mL per well) were infected with BCG at MOI 1:1 or 10:1, with or without increasing concentrations of lactoferrin (1, 10, or 100 μg/mL). At days 1, 3, and 5 post-infection, cells were lysed with 500 μL/well of 0.05% SDS, incubated at 37 °C, and then neutralized with an equal volume of 15% BSA. Aliquots of broth culture or cell lysates were serially diluted in 1× PBS and 100 μL plated onto 7H11 agar plates and incubated at 37 °C. CFU were enumerated as described above.
2.6. FACS analysis
Antibodies (1 μg/106 cells per 50 μL) in staining buffer (1% BSA in 1× PBS) were added to isolated cells following treatment on ice with Fc Block™ (CD16/32, BD Biosciences Pharmingen, San Diego, CA). Macrophages were incubated with anti-mouse F4/80-FITC (Cell Sciences), CD11c-PE, I-Ab-FITC, H-2kb-FITC, CD80-PE, CD86-PE, or CD40-PE (BD Biosciences Pharmingen) on ice for 30 min. Macrophages were washed with staining buffer and fixed with 4% paraformaldehyde (Fisher Scientific, Fair Lawn, NJ). For intracellular staining, splenocytes were block with Fc Block™ and stained with CD4-FITC or CD8-FITC on ice for 30 min. Intracellular staining was conducted after the cells were fixed with 4% paraformaldehyde on ice for 15 min and made permeable with BD Perm/Wash solution (BD Biosciences Pharmingen, San Diego, CA). Cells were further stained for IFN-γ-PE on ice for 1 h. Flow analysis was conducted using Coulter FlowCentre™ (EPICS XL-MCL). Graphs were generated with WinMDI 2.8 or GraphPad Prizm 4.
2.7. ELISA (enzyme linked immuno-sorbant assay)
Supernatants were assayed for cytokine production using the DuoSet ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. Supernatants were assayed for production of T-cell cytokines (IFN-γ, IL-2, and IL-4), proinflammatory mediators (TNF-α and IL-6), and T-cell mediators (IL-12p40 and IL-10). The lower limits of assay detection for all cytokines were between 15 and 32 pg/mL.
2.8. Statistics
All experiments were repeated, at least, in triplicate. CFU were enumerated from triplicate platings. ELISA analysis was averaged from triplicate wells. Changes in surface expression of CD11c, I-Ab, H-2kb, CD80, CD86, or CD40 were compared across repeated experiments with significance determined by paired t-test. Statistical analysis was carried out using one-way ANOVA, and differences were considered significant at p < 0.05.
3. Results
3.1. Lactoferrin increases of MHC II expression on BCG-infected macrophages
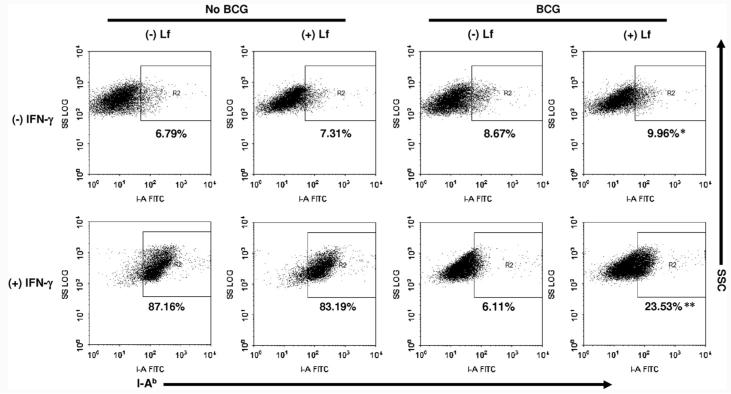
To address whether lactoferrin could mediate expression of presentation molecules on antigen presenting cells, BCG-infected bone marrow derived macrophages (BMMs) were examined for expression of MHC I (H-2kb) and MHC II (I-Ab) and co-stimulatory molecules (CD80, CD86, and CD40) when cultured with or without lactoferrin. Bone marrow derived macrophages remained non-infected or infected with BCG (MOI 10:1) in the presence or absence of lactoferrin (100 μg/mL). Expression of MHC II was low in non-activated BMMs (range for four experiments, 4.0–6.8%). Lactoferrin had only a slight, non-significant effect on non-activated BMM expression of MHC II (4.0–7.5%). Expression of MHC II on BCG-infected BMMs increased in the presence of lactoferrin (Fig. 1, top). Statistical analysis of results from four experiments found a modest but significant ( p < 0.05) increase of MHC II observed on BCG-infected BMMs that were cultured with lactoferrin. These results correlate well with previously published observations of MHC II expression on macrophages differentiated using an alternative method [41]. Lactoferrin had no effect on relative surface expression of MHC I, CD80, CD86, or CD40 on either BCG-infected or non-infected BMMs (data not shown).
Fig. 1.
Lactoferrin mediated change in surface expression of I-Ab (MHC II) in BCG-infected macrophages. Naïve bone marrow derived macrophages (BMMs), or BCG-infected BMMS (MOI 10:1) were cultured with or without lactoferrin (100 μg/mL), and stained for surface expression of I-Ab-(MHC II) after 72 h. Cells were cultured either alone (top) or stimulated with IFN-γ (10 ng/mL) (bottom). Positive events were gated against the isotype control; representative findings from duplicate readings from 4 repeated experiments were analyzed by paired t-test. *p < 0.05; **p < 0.001 relative to non-lactoferrin treated groups.
3.2. Lactoferrin reverses BCG-mediated suppression of MHC II expression in BMMs
Naïve (non-activated) BMMs express only low levels of presentation and co-stimulatory molecules unless activated. To study effects of lactoferrin on activated macrophages, experiments were repeated in the presence of IFN-γ. BMMs were cultured with or without BCG (MOI 10:1), with or without lactoferrin (100 μg/mL), and with recombinant IFN-γ (10 ng/mL). Cells were analyzed for expression of MHC I, MHC II, CD80, CD86, and CD40 after 72 h. The IFN-γ activated BMMs expressed high levels of MHC II (>80%) (Fig. 1, bottom); addition of lactoferrin had no observable effect on MHC II expression on these cells. BCG infection alone caused a dramatic decrease of MHC II+ expressing cells (p < 0.001) under IFN-γ stimulating conditions. The presence of lactoferrin during BCG infection allowed a significant proportion of BMMs to retain their expression of MHC II (p < 0.01). While the lactoferrin treated BCG-infected BMMs did not fully recover the level of expression found in IFN-γ stimulated uninfected BMMs, lactoferrin was clearly able to modulate the extent of BCG mediated MHC II down regulation.
An additional experiment was performed to evaluate events where stimulation of macrophages occurs post treatment of infection with lactoferrin. Macrophages were initially exposed to BCG and lactoferrin for 3 days. Cells were washed to remove extracellular BCG and lactoferrin, and then restimulated with 10 ng/mL of mouse recombinant IFN-γ. In this case, non-infected BMMs cultured with lactoferrin significantly (p = 0.034) increased the number of BMMs positive for MHC II expression compared to the non-treated cells (Fig. 2A). However, addition of IFN-γ to the lactoferrin treated BCG-infected BMMs did not induce significant change in expression of MHC II compared to the BCG alone infected BMMs. No significant changes were observed with expression of MHC I in all groups examined (data not shown).
Fig. 2.
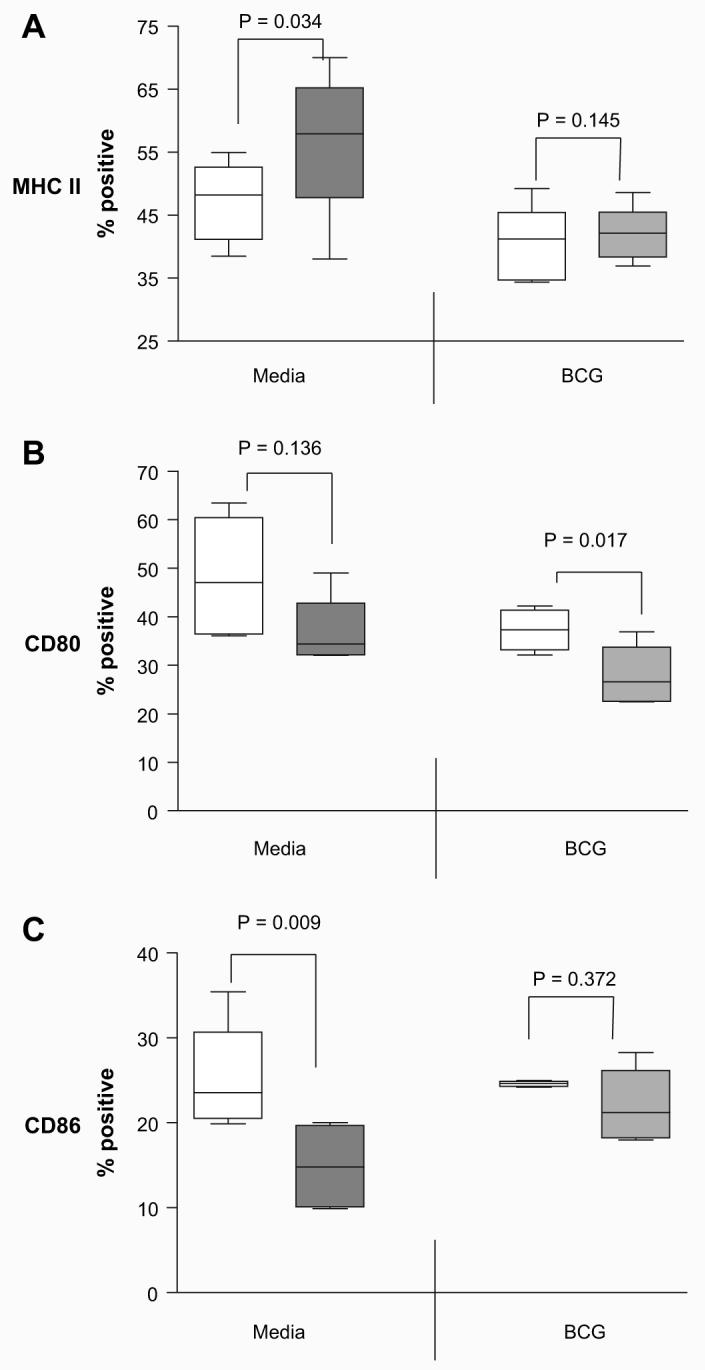
Effect of lactoferrin on presentation molecules expression on IFN-γ stimulated BCG-infected macrophages. Non-infected or BCG-infected BMMs were cultured with (shaded bars) or without lactoferrin (open bars) (100 μg/mL), and stimulated with 10 ng/mL mouse recombinant IFN-γ. After 72 h, cells were stained for surface expression of (A) I-Ab-(MHC II), (B) CD80, or (C) CD86. Positive events were gated against isotype controls. Percent positive cells from 4 or more repeat experiments were analyzed by paired t-test, with p value indicated.
3.3. Macrophages pre-exposed to BCG and lactoferrin enhance the CD86/80 ratio in response to IFN-γ stimulation
The expression of co-stimulatory molecules CD80 and CD86 on IFN-γ activated BMMs was also found to be differentially modulated by lactoferrin. There was a modest, but non-significant, trend of decrease in CD80 expression observed in non-infected BMM macrophages pre-treated with lactoferrin (range for 4 experiments, 32–49%) compared to those not treated with lactoferrin (36–63%). However, there was a significant (p < 0.05) decrease in CD80 expression on BCG-infected BMM pre-treated with lactoferrin (Fig. 2B).
Changes were also observed in CD86 expression which were unique from those seen for CD80 (Fig. 2C). In this case, there was a significant (p < 0.01) decrease in CD86 expression observed in non-infected macrophage pre-treated with lactoferrin (range for 4 experiments, 9–20%) compared to those not treated with lactoferrin (19–35%). However, there was no significant difference observed in CD86 expression from IFN-γ activated BCG-infected BMMs pre-treated with BCG/lactoferrin (17–28%) compared to BMMs infected with BCG only (23–25%). The relative ratio of CD86 to CD80 expression was further compared. BMM pre-treated with lactoferrin and infected with BCG showed a significant (p < 0.005) increase in the CD86:CD80 ratio (range for three experiments 0.65–0.93) when compared to non-infected lactoferrin pre-treated BMMs (0.31–0.55).
3.4. Increased IFN-γ production from BCG sensitized CD3+ splenocytes stimulated with lactoferrin treated presenters
The identified changes in MHC Class II expression and levels of CD80 and CD86 on the surface of infected cells indicate that lactoferrin may be capable of enhancing antigen presentation by BCG-infected BMMs. To examine this hypothesis, CD3+, CD4+, or CD8+ splenocytes were recovered from BCG immunized mice, and incubated with BMM presenting cells that were BCG-infected in the presence or absence of lactoferrin (100 μg/mL).
There was a modest, but significant (p < 0.001), increase in production of IFN-γ from CD3+ splenocytes overlaid onto BCG-infected BMMs treated with lactoferrin (Fig. 3). The sensitized CD3+ cells produced 116.0 ± 10.4 pg/mL of IFN-γ when lactoferrin was added, compared to only 59.3 ± 12.5 pg/mL in the non-lactoferrin treated group. When examined for response on highly infected presenting cells (MOI 10:1), the response remained significant, with 723.7 ± 9.3 pg/mL of 116.0 ± 10.4 pg/mL IFN-γ produced in combination with the lactoferrin treatment, compared to 656.3 ± 16.6 pg/mL with no lactoferrin added. The subpopulation of responding CD4+ lymphocytes was further examined by intracellular flow cytometric analysis (Fig 4). BCG-infected macrophages cultured with lactoferrin significantly increased the mean fluorescent intensity (MFI) of CD4+IFN-γ+ cells compared to macrophages infected with BCG only. There were no differences observed in the number of CD4+ IFN-γ+ cells stimulated by BCG-infected macrophages treated with or without lactoferrin. This suggests that macrophages infected with BCG in the presence of lactoferrin were able to increase IFN-g production from CD4+ splenocytes.
Fig. 3.
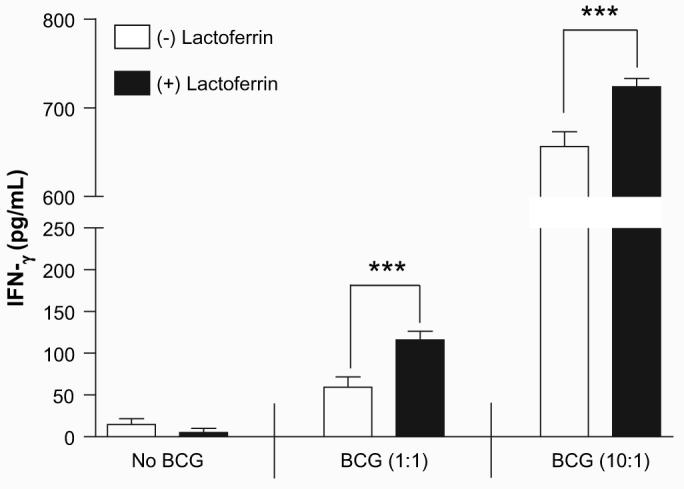
Elevated IFN-γ production from sensitized T-cells incubated with lactoferrin treated antigen presenting cells. CD3+ splenocytes isolated from mice previously immunized with BCG were incubated with BCG-infected bone marrow derived macrophages (MOI 1:1 or 10:1) in the presence or absence of lactoferrin (100 μg/mL). Supernatants were collected at 72 h and analyzed by ELISA for IFN-γ production. ***p < 0.001.
Fig. 4.
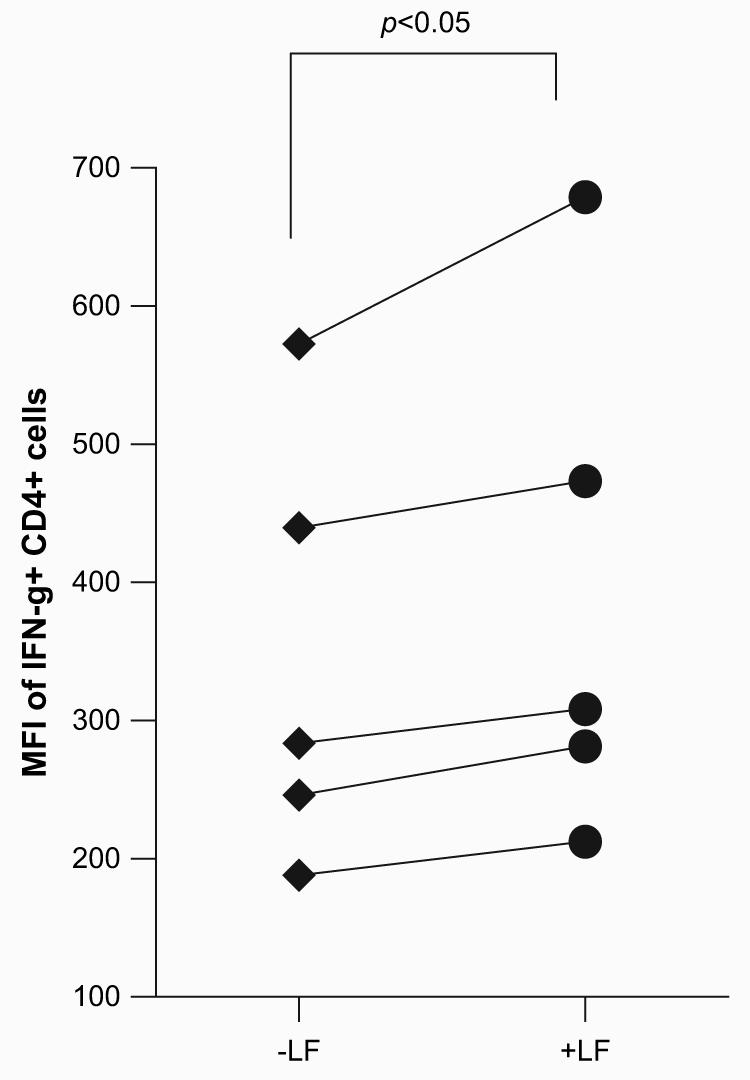
Lactoferrin enhanced BCG-infected macrophages stimulation of IFN-γ production from CD4+ splenocytes. CD3+ or CD4+ splenocytes were isolated from mice previously immunized with BCG. Bone marrow derived macrophages were infected with BCG in the presence or absence of lactoferrin (100 μg/mL) for 72 h prior to T-cells co-culturing. ConA (2 μg/mL) or PMA/ionomycin (10/250 ng/mL) restimulated cells were isolated and stained for CD4-FITC and IFNγ-PE. Matched samples are shown splenocytes from 5 individual mice, reflecting BMMs incubated with or without lactoferrin.
No significant differences were observed in production of IL-12, a macrophage cytokine that can mediate production of IFN-γ (Table 1). Analysis of proinflammatory cytokines, TNF-α and IL-6 production from CD3+ splenocytes incubated onto BCG-infected (MOI 10:1) macrophages in the presence of lactoferrin were significantly elevated compared to the non-lactoferrin group. Since both macrophages and CD3+ splenocytes are capable of producing TNF-α and IL-6, CD3+ splenocytes were further examined when incubated with similarly treated paraformaldehyde fixed macrophages. Similar increases in production of TNF-α (135 ± 9 pg/mL without lactoferrin vs. 239 ± 9 pg/mL with lactoferrin; p < 0.001) and IL-6 (17 ± 1 pg/mL without lactoferrin vs. 43 ± 5 pg/mL with lactoferrin; p < 0.001) were observed.
Table 1.
Lactoferrin mediation of pro-inflammatory cytokine production from sensitizedCD3+ cells cultured with BCG-infected macrophages
| No BCG | BCG (1:1) | BCG (10:1) | |
|---|---|---|---|
| IL-12(p40) | |||
| (−) Lf | 6 (5) | 51 (5) | 1176 (117) |
| (+) Lf | 5 (5) | 48 (12) | 1089 (43) |
| TNF-α | |||
| (−) Lf | 65 (55) | 45 (4) | 264 (26) |
| (+) Lf | 58 (19) | 55 (6) | 445 (44)* |
| IL-6 | |||
| (−) Lf | 17 (7) | 11 (9) | 91 (5) |
| (+) Lf | 7 (7) | 17 (2) | 148 (3)* |
CD3+ splenocytes isolated from mice previously immunized with BCG were incubated with non-infected bone marrow derived macrophages, or with BCG-infected BMM (MOI 1:1 or 10:1), in the presence or absence of lactoferrin (100 μg/mL). Secreted IL-12p40, TNF-α and IL-6 were and analyzed by ELISA. Average values of triplicate wells are given in pg/ml (with standard deviation)
p < 0.001.
3.5. No effect of lactoferrin on intracellular proliferation of BCG
The increased ability of lactoferrin treated BCG-infected BMMs to stimulate IFN-γ production from sensitized T-cells suggested greater proficiency in presenting BCG antigens. However, antigen loading onto MHC II involves active processing of the intracellular bacteria which may be directly explained by changes in BCG proliferation. Therefore, the effect of lactoferrin on BCG proliferation within BMMs was examined. BMMs were infected with BCG with or without lactoferrin (100 μg/mL), and with or without stimulation of IFN-γ. No differences were observed in BCG intracellular proliferation in BMMs treated with or without lactoferrin (Fig. 5A). Similarly, in BCG-infected BMMs activated with exogenous IFN-γ, no differences were observed between the lactoferrin and non-lactoferrin groups (Fig. 5B). Additionally, lactoferrin was examined for potential of direct anti-mycobacterial activity. No differences were observed in BCG proliferation when grown in culture with or without lactoferrin (Fig. 5C).
Fig. 5.
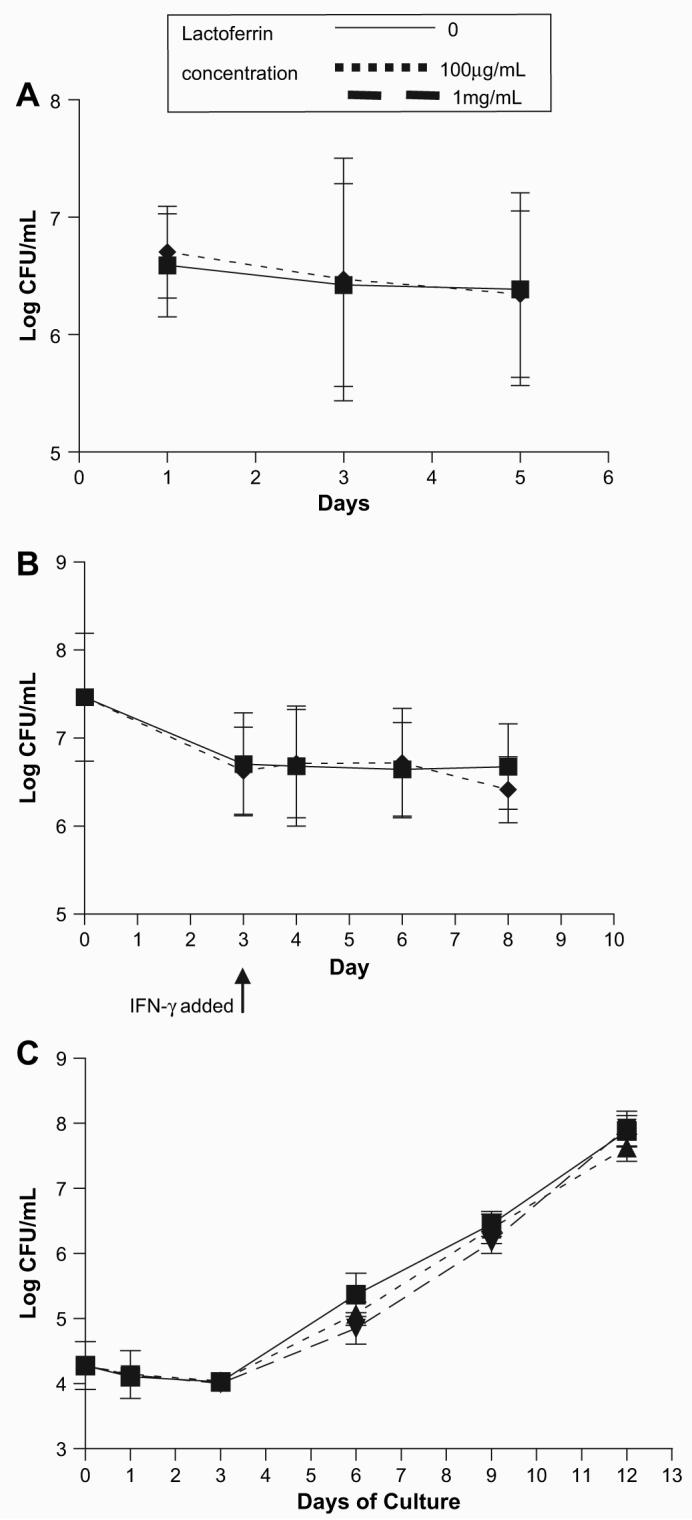
Lactoferrin does not affect extracellular or intracellular proliferation of BCG. The effect of lactoferrin growth on BCG was evaluated. (A) BMMs were infected with BCG with or without lactoferrin (100 μg/mL). (B) BMMs were infected with BCG with or without lactoferrin (100 μg/mL) for 72 h, and stimulated with IFN-μ (10 ng/mL). (C) BCG was grown on an orbital shaker in Dubos broth base with or without lactoferrin (100 μg/mL, 1 mg/mL). Serial dilutions of cell lysates or broth culture were plated onto 7H11 plates at indicated days post culture, and CFU were enumerated.
4. Discussion
The continuously falling efficacy of the BCG vaccine has generated intense research into development of novel vaccines to fight against TB, a disease that affects a third of the world's populations [1-4]. While examinations of newly developed attenuated virulent MTB and recombinant BCG strains have shown comparable efficacy with the current BCG vaccine, both involve use of live bacteria, with no comparative history of clinical safety [42-46]. The current BCG vaccine is the most widely administered vaccine in the world, and has a long established history of known benefits and risks [47]. Thus, the quickest and most likely route to improving TB vaccine for human use is through improvement of the current BCG. This strategy has already produced a vaccination regimen that is undergoing Phase II clinical trials by using an adenovirus containing Ag85 as a booster to the BCG vaccine [45].
The potential for use of naturally occurring lactoferrin as an adjuvant to augment immune function has been validated with in vivo studies that showed no toxic effects attributed to oral delivery of lactoferrin [48-50]. There are no published reports directly examining toxicity of injected bovine lactoferrin when utilized as an adjuvant, however, high concentrations (5–10 mg/mouse) of bovine lactoferrin are often delivered in experiments examining LPS and bacterial insults in murine models of sepsis [51-54] with no overt or reported toxicity. Previous studies demonstrated that addition of lactoferrin to the BCG vaccine increased host protection against subsequent virulent MTB challenge as observed by a decrease in organ bacterial load and a reduction in pulmonary disease pathology. This improvement in host response to disease correlated to an increase in development of a protective mycobacterial antigen-specific T-cell response, specially a TH1 response hallmarked by production of IFN-γ [22,23]. Thus, lactoferrin has the ability to modulate innate immune function during vaccination in a way that augments generation of long-lasting protective immunity.
It has long been recognized that lactoferrin exerts effects on a variety of leukocytes, including macrophages [55-57], the main host cell for both BCG and MTB [58,59]. Previous studies demonstrated that lactoferrin is capable of enhancing BCG-infected macrophage production of IL-12:IL-10 ratio [34], thus generating a cytokine environment that is favorable for promotion of TH1 immunity [24-26]. It was hypothesized that lactoferrin also enhanced efficacy of the BCG vaccine in vivo, in part, by modulating the ability of macrophages to present antigen and for subsequent stimulation of T-cells.
In this study, lactoferrin caused an increase in MHC II expression on BCG-infected bone marrow derived macrophages, suggesting an increase in the ability of infected cells to present antigen to lymphocytes. Resting BMMs express very low levels of MHC II on their surface, therefore, the effect of lactoferrin was also examined on IFN-γ activated BCG-infected BMMs. BCG infection of IFN-γ activated BMMs dramatically decreased MHC II+ BMMs, a trend that has been previously reported [60-62]. The presence of lactoferrin was able to reverse a significant percentage of the BCG induced down regulation of MHC II surface expression, again suggesting that lactoferrin enables activated macrophages to retain their ability to present antigen to T-cells. In the larger context of the diminished historical efficacy of the BCG vaccine, this ability of BCG to decrease MHC II expression in activated macrophages is hypothesized to be a natural immune evasion mechanism that limits development of an adequate immune response [63,64]. Lactoferrin is capable, in part, of reversing this inhibition.
In addition to examining the effect of lactoferrin on resting and stimulated BMMs, lactoferrin was also examined for its ability to modulate BMMs' responsiveness to exogenous IFN-γ. In the series of events that occur in vivo during vaccination, there is a lag time between when macrophages encounter BCG and lactoferrin, and the subsequent recruitment and activation of T-cells that would result in IFN-γ production to augment host intracellular events to control infection. The effect of BCG to downregulate expression of MHC II is indicative that the vaccine strain retains immune evasion mechanisms that are observed in the virulent MTB strains. Another hallmark of mycobacteria evasion mechanism involves attenuation of macrophage upregulation of MHC II in response to IFN-γ stimulation [65,66]. While lactoferrin can act synergistically with IFN-γ at the time of BCG infection, there were no changes in MHC II expression when macrophages were stimulated with IFN-γ 72 h after BCG infection. This strongly suggests a need for lactoferrin to be present at specific times relative to antigen delivery during the macrophage activation process. Although there was no change in MHC II in macrophage response to IFN-γ after previous exposure to BCG, these results indicate that lactoferrin is able to modulate response in a way to maximize T-cell stimulation, possibly through increasing the relative ratios of surface CD86 to CD80 expressed on cultured BCG-infected macrophages.
Both CD86 and CD80 are considered necessary co-stimulatory molecules for T-cell activation, functioning as secondary components involved in antigen presentation by MHC molecules. Significant disagreement exists in the literature on the possible differential functions of CD86 and CD80 [67,68]. Recently, support is given to indicate that they may preferentially bind different ligands on T-cells; CD86 may preferentially bind CD28, the ligand for T-cell activation, and CD80 may preferentially bind CTLA-4, a ligand to elicit T-cell anergy [69-71]. While lactoferrin cultured BCG-infected BMMs exhibit a total reduction in both molecules, there was a consistent increase of relative CD86:CD80 ratio in response to IFN-γ, suggesting that these macrophage populations may have a better potential to engage the CD28 ligand for activation of T-cells.
The effect of lactoferrin on BCG-infected BMMs suggested that it would increase the ability of macrophages to stimulate T-cells. Events relating to mycobacterial-specific antigen presentation were examined. BCG sensitized splenocytes were incubated with BCG-infected BMMs, cultured with or without lactoferrin. The lactoferrin treatment of BCG-infected BMMs enhanced production of IFN-γ from CD3+ splenocytes. In addition, CD4+ T-cells were shown to increase the amount of IFN-γ per cell in response to presented BCG antigens. Production of IL-12, which can indirectly influence IFN-g production [26,27,72], was not affected, suggesting that the increase in T-cell IFN-γ production was the result of direct cell to cell contact instead of promotion by the cytokine environment. Overall, the increase in T-cell IFN-γ production suggests that lactoferrin cultured BCG-infected BMMs can generate signals via surface molecules and cytokine expression which would aid in the development of in vivo TH1 immunity observed in previous studies [22,23].
Lactoferrin was originally defined as an iron binding protein that directly affects bacteria, functionally recognized as both bacteriostatic and bacteriocidal [11-13,73]. However, there was no direct bacteriostatic or bacteriocidal effect of lactoferrin on BCG proliferation. In addition, we also examined the effect of lactoferrin on macrophage intracellular control of BCG proliferation. One main mechanism for macrophage control of intracellular BCG involves production of NO [74,75], which can be stimulated by activation with IFN-γ[76]. Also, lactoferrin alone has been shown to increase macrophage NO production [77]. However, we observed no effect of lactoferrin on intracellular proliferation of BCG. Therefore, the enhanced antigenic stimulation of T-cells does not suggest an increase or mediation in intracellular processing mechanisms, which would result in more efficient antigen presentation. Indeed the studies presented here suggest that the effect of lactoferrin is through mechanisms other than mediation of macrophage direct intracellular killing events.
Receptors for lactoferrin have been identified on a variety of leukocytes, including macrophages [78]. A role for involvement utilizing c-type lectin receptors has also been identified; lactoferrin is able to prevent uptake of HIV by binding to DC-SIGN on human dendritic cells [79], and the effect of lactoferrin to enhance the DTH response in vivo can be blocked by addition of mannose [20]. Macrophage mannose receptor and the murine homolog of DC-SIGN (mSIGN-R1) are involved in binding and/or uptake of BCG and MTB [80-83], therefore addition of lactoferrin may influence this initial infection event. We have previously observed that BCG uptake is not hindered by the presence of lactoferrin [41]. We conclude that the effect of lactoferrin in the studies reported here demonstrates direct modulation of macrophage responses and not indirect interference of mechanical events regulating uptake of BCG. However, it is clear that there needs to be an awareness in trying to generalize immune modulatory potential of the lactoferrin molecule, as glycosylation pattern may be different depending on phenotypic cell source. Indeed, different glycoforms of human recombinant lactoferrin (sialylated and non-sialylated) expressed statistically different effects on in vitro secondary humoral immune responses [84].
These results demonstrate lactoferrin as a molecule capable of modulating macrophages to promote T-cell stimulation through upregulation of presentation and co-stimulatory molecules. Clearly macrophages play an important role in uptake, containment and control of BCG and MTB. This study is the first step in the examination of the mechanisms underlying lactoferrin effects as an adjuvant component. Efforts are now underway to extend this assessment to determine the action on dendritic cell populations, as those cells are critical in directing immune function of naïve lymphocyte populations [85] and for developing host immune responses to control MTB infection [86]. Overall, lactoferrin may present an important adjuvant to enhance efficacy of not only the BCG vaccine, but also other vaccines with poor immunogenic abilities to promote antigen-specific, cell mediated immunity.
Acknowledgements
The research was presented in part at the 8th International Conference on Lactoferrin, held in October, 2007, in Nice, France. The work was accomplished with support from NIH grants 1R41GM079810-01 and R42-AI051050-02.
References
- 1.Tuberculosis Fact Sheet No. 104. World Heath Organization; 2007. [Google Scholar]
- 2.Kaufmann SH. New issues in tuberculosis. Ann. Rheum. Dis. 2004;63(Suppl 2):ii50–ii56. doi: 10.1136/ard.2004.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin. Chest Med. 2005;26(2):167–182. doi: 10.1016/j.ccm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MJ. The tuberculosis vaccine challenge. Tuberculosis (Edinb.) 2005;85(12):7–12. doi: 10.1016/j.tube.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kamath AT, Fruth U, Brennan MJ, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine. 2005;23(29):3753–3761. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Aldwell FE, Baird MA, Fitzpatrick CE, McLellan AD, Cross ML, Lambeth MR, Buchan GS. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol. Cell Biol. 2005;83(5):549–553. doi: 10.1111/j.1440-1711.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 7.Aldwell FE, Brandt L, Fitzpatrick C, Orme IM. Mice fed lipid-encapsulated Mycobacterium bovis BCG are protected against aerosol challenge with Mycobacterium tuberculosis. Infect. Immun. 2005;73(3):1903–1905. doi: 10.1128/IAI.73.3.1903-1905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers MA, Wright DC, Brisker J, et al. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome) protects guinea pigs from lethal tuberculosis. Vaccine. 2004;22(8):1063–1071. doi: 10.1016/j.vaccine.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Haile M, Schroder U, Hamasur B, Pawlowski A, Jaxmar T, Kallenius G, Svenson SB. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine. 2004;22(11–12):1498–1508. doi: 10.1016/j.vaccine.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80(3):252–267. [PubMed] [Google Scholar]
- 11.Ellison RT, 3rd, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Invest. 1991;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellison RT, 3rd, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutteberg TJ, Rokke O, Andersen O, Jorgensen T. Early fall of circulating iron and rapid rise of lactoferrin in septicemia and endotoxemia: an early defence mechanism. Scand. J. Infect. Dis. 1989;21(6):709–715. doi: 10.3109/00365548909021701. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg ED. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 1984;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- 15.Zagulski T, Lipinski P, Zagulska A, Jarzabek Z. Antibacterial system generated by lactoferrin in mice in vivo is primarily a killing system. Int. J. Exp. Pathol. 1998;79(2):117–123. doi: 10.1046/j.1365-2613.1998.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005;62(22):2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhennin-Duthille I, Masson M, Damiens E, Fillebeen C, Spik G, Mazurier J. Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J. Cell. Biochem. 2000;79(4):583–593. [PubMed] [Google Scholar]
- 18.Zimecki M, Mazurier J, Spik G, Kapp JA. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology. 1995;86(1):122–127. [PMC free article] [PubMed] [Google Scholar]
- 19.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr., Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int. Immunopharmacol. 2002;2(4):475–486. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 20.Zimecki M, Kocieba M, Kruzel M. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology. 2002;205(1):120–131. doi: 10.1078/0171-2985-00115. [DOI] [PubMed] [Google Scholar]
- 21.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol. Lett. 2000;74(3):183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int. Immunopharmacol. 2005;5(3):591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25(3738):6730–6743. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur. J. Immunol. 1994;24(4):793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 26.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA. 1993;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 28.Murphy EE, Terres G, Macatonia SE, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J. Exp. Med. 1994;180(1):223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer R, Debbabi H, Dubarry M, Boyaka P, Tome D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem. Cell Biol. 2006;84(3):303–311. doi: 10.1139/o06-058. [DOI] [PubMed] [Google Scholar]
- 30.Zimecki M, Mazurier J, Spik G, Kapp JA. Lactoferrin inhibits proliferative response and cytokine production of TH1 but not TH2 cell lines. Arch. Immunol. Ther. Exp. (Warsz.) 1996;44(1):51–56. [PubMed] [Google Scholar]
- 31.Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals. 2004;17(3):231–234. doi: 10.1023/b:biom.0000027697.83706.32. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi H, Kurokawa M, Shin K, Teraguchi S, Tamura Y, Shiraki K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci. Biotechnol. Biochem. 2004;68(3):537–544. doi: 10.1271/bbb.68.537. [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi H, Takakura N, Yamauchi K, Tamura Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin. Vaccine Immunol. 2006;13(2):239–245. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med. Microbiol. Immunol. 2007;196(3):171–180. doi: 10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 37.Janeway J, Charles Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. fifth ed. Garland Publishing; New York: 2001. [Google Scholar]
- 38.Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell. Immunol. 2006;242(1):23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Connelly MA, Moulton RA, Smith AK, Lindsey DR, Sinha M, Wetsel RA, Jagannath C. Mycobacteria-primed macrophages and dendritic cells induce an up-regulation of complement C5a anaphylatoxin receptor (CD88) in CD3+ murine T cells. J. Leukoc. Biol. 2007;81(1):212–220. doi: 10.1189/jlb.1005582. [DOI] [PubMed] [Google Scholar]
- 40.Guidry TV, Hunter RL, Jr., Actor JK. CD3+ cells transfer the hyper-sensitive granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate in mice. Microbiology. 2006;152(Pt 12):3765–3775. doi: 10.1099/mic.0.29290-0. [DOI] [PubMed] [Google Scholar]
- 41.Wilk KM, Hwang SA, Actor JK. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postepy, Hig. Med. Dosw. (Online) 2007;61:277–282. [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty TM. Real world TB vaccines: clinical trials in TB-endemic regions. Vaccine. 2005;23(17–18):2109–2114. doi: 10.1016/j.vaccine.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 43.Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine. 2007;25(19):3742–3751. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 44.Langermans JA, Doherty TM, Vervenne RA, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23(21):2740–2750. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 45.Marris E. A dozen vaccine candidates bring shot of hope to TB epidemic. Nat. Med. 2007;13(3):274. doi: 10.1038/nm0307-274a. [DOI] [PubMed] [Google Scholar]
- 46.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 2004;72(10):6148–6150. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen P, Doherty TM. The success and failure of BCG – implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3(8):656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 48.Appel MJ, van Veen HA, Vietsch H, Salaheddine M, Nuijens JH, Ziere B, de Loos F. Sub-chronic (13-week) oral toxicity study in rats with recombinant human lactoferrin produced in the milk of transgenic cows. Food Chem. Toxicol. 2006;44(7):964–973. doi: 10.1016/j.fct.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Hayes TG, Falchook GF, Varadhachary GR, Smith DP, Davis LD, Dhingra HM, Hayes BP, Varadhachary A. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest. New Drugs. 2006;24(3):233–240. doi: 10.1007/s10637-005-3690-6. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi K, Toida T, Nishimura S, et al. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000;38(6):503–512. doi: 10.1016/s0278-6915(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 51.Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002;130(1):25–31. doi: 10.1046/j.1365-2249.2002.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimecki M, Artym J, Chodaczek G, Kocieba M, Kruzel ML. Protective effects of lactoferrin in Escherichia coli-induced bacteremia in mice: relationship to reduced serum TNF alpha level and increased turnover of neutrophils. Inflamm. Res. 2004;53(7):292–296. doi: 10.1007/s00011-004-1257-1. [DOI] [PubMed] [Google Scholar]
- 53.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24(1):33–44. doi: 10.1023/a:1006935908960. [DOI] [PubMed] [Google Scholar]
- 54.Artym J, Zimecki M, Kruzel ML. Enhanced clearance of Escherichia coli and Staphylococcus aureus in mice treated with cyclophosphamide and lactoferrin. Int. Immunopharmacol. 2004;4(9):1149–1157. doi: 10.1016/j.intimp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Birgens HS. The interaction of lactoferrin with human monocytes. Dan. Med. Bull. 1991;38(3):244–252. [PubMed] [Google Scholar]
- 56.Birgens HS. The monocytic receptor for lactoferrin and its involvement in lactoferrin-mediated iron transport. Adv. Exp. Med. Biol. 1994;357:99–109. doi: 10.1007/978-1-4615-2548-6_10. [DOI] [PubMed] [Google Scholar]
- 57.Britigan BE, Serody JS, Hayek MB, Charniga LM, Cohen MS. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J. Immunol. 1991;147(12):4271–4277. [PubMed] [Google Scholar]
- 58.Flynn JL, Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 59.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Adv. Exp. Med. Biol. 2003;531:241–247. doi: 10.1007/978-1-4615-0059-9_20. [DOI] [PubMed] [Google Scholar]
- 60.Fulton SA, Reba SM, Pai RK, Pennini M, Torres M, Harding CV, Boom WH. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 2004;72(4):2101–2110. doi: 10.1128/IAI.72.4.2101-2110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sendide K, Deghmane AE, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z. Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S.J. Immunol. 2005;175(8):5324–5332. doi: 10.4049/jimmunol.175.8.5324. [DOI] [PubMed] [Google Scholar]
- 62.Wojciechowski W, DeSanctis J, Skamene E, Radzioch D. Attenuation of MHC class II expression in macrophages infected with Mycobacterium bovis bacillus Calmettee–Guerin involves class II transactivator and depends on the Nramp1 gene. J. Immunol. 1999;163(5):2688–2696. [PubMed] [Google Scholar]
- 63.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260(5110):984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 64.Pieters J. Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 2001;3(3):249–255. doi: 10.1016/s1286-4579(01)01376-4. [DOI] [PubMed] [Google Scholar]
- 65.Hmama Z, Gabathuler R, Jefferies WA, de Jong G, Reiner NE. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J. Immunol. 1998;161(9):4882–4893. [PubMed] [Google Scholar]
- 66.Mohagheghpour N, Gammon D, van Vollenhoven A, Hornig Y, Bermudez LE, Young LS. Mycobacterium avium reduces expression of costimulatory/adhesion molecules by human monocytes. Cell. Immunol. 1997;176(1):82–91. doi: 10.1006/cimm.1996.1070. [DOI] [PubMed] [Google Scholar]
- 67.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 68.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 69.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21(3):401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol. Lett. 2006;104(1–2):70–75. doi: 10.1016/j.imlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 71.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6(6):223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao A, Avni O. Molecular aspects of T-cell differentiation. Br. Med. Bull. 2000;56(4):969–984. doi: 10.1258/0007142001903634. [DOI] [PubMed] [Google Scholar]
- 73.Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem. Cell. Biol. 2002;80(1):95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- 74.Rook GA, Steele J, Ainsworth M, Champion BR. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- 75.Hanano R, Kaufmann SH. Nitric oxide production and mycobacterial growth inhibition by murine alveolar macrophages: the sequence of rIFN-gamma stimulation and Mycobacterium bovis BCG infection determines macrophage activation. Immunol. Lett. 1995;45(1–2):23–27. doi: 10.1016/0165-2478(94)00193-u. [DOI] [PubMed] [Google Scholar]
- 76.Flesch I, Kaufmann SH. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 1987;138(12):4408–4413. [PubMed] [Google Scholar]
- 77.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem. Mol. Biol. Int. 1997;43(1):79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell. Mol. Life Sci. 2005;62(22):2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groot F, Geijtenbeek TB, Sanders RW, et al. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J. Virol. 2005;79(5) doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C, Etienne G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J. Lipid Res. 2005;46(3):475–483. doi: 10.1194/jlr.M400308-JLR200. [DOI] [PubMed] [Google Scholar]
- 81.Rivera-Marrero CA, Schuyler W, Roser S, Ritzenthaler JD, Newburn SA, Roman J. M. tuberculosis induction of matrix metallo-proteinase-9: the role of mannose and receptor-mediated mechanisms. Am, J. Physiol. Lung Cell. Mol. Physiol. 2002;282(3):L546–L555. doi: 10.1152/ajplung.00175.2001. [DOI] [PubMed] [Google Scholar]
- 82.Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell. Microbiol. 2005;7(2):157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 83.Koppel EA, Ludwig IS, Hernandez MS, et al. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209(1–2):117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Choi BK, Actor JK, Rios S, et al. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj. J. 2008 Mar 26; doi: 10.1007/s10719-008-9123-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 86.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J. Immunol. 2005;175(5):3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]