Abstract
Objectives
To investigate the effect of interleukin-1β (IL-1β) on osteogenic protein-1 (OP-1) signaling in human adult articular chondrocytes. We examined two major receptor-activated Smad (R-Smad) signaling pathways, the BMP and MAPK, in the model of IL-1β-induced cartilage degeneration.
Methods
Ankle chondrocytes from 26 normal human donors were cultured in high density monolayers in a serum-free media. Effect of IL-1β on BMP receptors was studied by RT-PCR and flow cytometry. Phosphorylation of R-Smads was tested in cells treated with IL-1β (10ng/ml), OP-1 (100ng/ml) or combined IL-1β and OP-1. Cell lysates were analyzed by Western blots with polyclonal antibodies against two R-Smads phosphorylated sites (BMP and MAPK) or total, non-phosphorylated R-Smad as a control. To identify through which MAPK IL-1β activates linker region, chondrocytes were pre-incubated with specific MAPK inhibitors (PD98059 for MAP/ERK; SP600125 for JNK; and SB203580 for p38).
Results
We found that IL-1β reduced the number of ALK-2 and ALK-3 receptors, inhibited Smad1 and Smad6 expression, delayed and prematurely terminated the onset of OP-1 mediated R-Smad phosphorylation and affected nuclear translocation of R-Smad/Smad4 complexes. We discovered that the alternative phosphorylation of R-Smad in the linker region via the MAPK pathway (primarily p38 and JNK) could be a possible mechanism through which IL-1β offsets OP-1 signaling and responses to OP-1. Conversely, OP-1 was found to directly inhibit phosphorylation of p38.
Conclusions
Our findings describe new mechanisms of the cross-talk between OP-1 and IL-1β in chondrocytes. The study also identifies potential targets for therapeutic interventions in the treatment of cartilage degenerative processes.
Keywords: Osteogenic protein-1, interleukin-1β, chondrocyte signaling, receptors, smads, MAP kinases
Introduction
The leading cause of cartilage degeneration is the loss of balance between anabolic and catabolic processes (1) Bone morphogenetic proteins (BMPs) act as important anabolic regulators of matrix production and repair (2). BMP-7 or osteogenic protein-1 (OP-1) stimulates matrix synthesis (3,4) and counteracts cartilage degradation induced by catabolic mediators (5-7). Although endogenous OP-1 was identified in adult human cartilage, its production was significantly reduced with cartilage aging and degeneration (8-10) suggesting that insufficient levels of OP-1 may contribute to or predispose the development of osteoarthritis (OA).
OP-1 signals through type I (ALK-2, 3, and 6; BMPR-I) and type II (BMPR-II) transmembrane serine/threonine kinase receptors (11,12). BMPR-I activates the intracellular mediators R-Smads and thus determines signaling specificity (11,13). After phosphorylation, R-Smads (Smad1/5/8) form heteromeric complexes with Smad4, which then translocate to the nucleus (14) and regulate transcriptional responses (15). OP-1 signaling is controlled by inhibitory Smad6 (in a negative feedback loop), which binds either to BMPR-I preventing its binding to R-Smads (16) or directly to the activated R-Smads, thus competing with the latter for the formation of complexes with Smad4 (17). R-Smads have two conserved globular domains, the MH1 and MH2 [18], connected by a linker region. Activated BMPR-I phosphorylates R-Smads in the conserved SSXS motif at C-terminal of MH2 domain (14). R-Smads could also undergo phosphorylation by mitogen-activated protein kinases (MAPK) within the four PXSP motifs in the linker region (19). Whereas phosphorylation by BMP receptors promotes nuclear translocation and transcriptional activity of R-Smads, phosphorylation by MAPK has the opposite effect causing their cytoplasmic localization and inhibition of transcriptional activity (20).
MAPK, extracellular signal regulated kinase (ERK-1 & ERK-2), Jun NH2-terminal kinases (JNK) and p38 (21,22), are expressed in chondrocytes and are involved in signaling of many growth factors and cytokines, including IL-1β (23).
IL-1β, the most studied catabolic cytokine in cartilage, activates matrix degrading enzymes (24,), suppresses matrix synthesis (25), inhibits chondrocyte proliferation (26), and stimulates production of various pro-inflammatory mediators (27). On the other hand, IL-1β induced endogenous BMP-2 and OP-1 in normal and OA cartilage (28,29).
The objectives of this study were to investigate the mechanisms of the IL-1β effects on OP-1 signaling in human adult articular chondrocytes and to determine the role of the two major Smad signaling pathways, the BMP- and MAPK-mediated, in the regulation of OP-1 responses in the model of IL-1β-induced cartilage degeneration. Our findings indicate that IL-1β affected all key steps in OP-1 signaling and induced alternative phosphorylation of R-Smad via the MAPK pathway (primarily p38 and to a lesser extent JNK), which resulted in reduced responsiveness to OP-1. Conversely, inhibition of p38 restored responsiveness to OP-1. In addition, an inhibitory effect of OP-1 on the IL-1β mediated phosphorylation of p38 was identified suggesting a novel mechanism for the anti-catabolic activity of OP-1 in chondrocytes.
Materials and Methods
Tissue Acquisition and Cell Culture
Normal human ankle (talar) articular cartilage obtained from tissue donors (n=26) within 48hrs of death through the Gift of Hope Organ & Tissue Donor Network (Elmhurst, IL) was enzymatically digested with pronase (Calbiochem, San Diego, CA) followed by Collagenase P (Roche, Indianapolis, IN). Cells were plated in high density monolayers (2×106 cells/well in 12 wells culture plate) for 48hrs in 1/1 ratio of Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 medium (DMEM/F-12) supplemented with 10% fetal bovine serum (FBS) and antibiotics (Invitrogen Life Technologies, Carlsbad, CA). A careful attention was given to maintaining high density culture and to controlling chondrocytic phenotype; cells retained round shape and displayed no morphological changes.
Chondrocyte stimulation with IL-1β and OP-1
Chondrocytes were pre-cultured in 10% FBS for 48hrs to adjust to culture conditions. The media were then replaced with serum-free media containing “mini”-ITS (“mini”-dose [5nM] insulin, 2μg/ml transferrin, 2ng/ml selenous acid), 25μg/ml ascorbic acid, and BSA/linoleic acid at 420/2.1 μg/ml (BD Biosciences, Franklin Lakes, NJ). IL-1β (R&D Systems, Minneapolis, MN) was used at 10ng/ml; human recombinant OP-1 (Stryker Biotech, Hopkinton, MA) was used at 100ng/ml. For gene expression and metabolic studies, chondrocytes were cultured for 3 days and were divided into 4 groups: 1) media control; 2) IL-1β control: day1, IL-1β treatment; days 2&3, media only; 3) OP-1 control: day1, media only; days 2&3, OP-1 treatment; and 4) combined treatment: day1, IL-1β; days 2&3, OP-1. Every experiment was repeated at least 3-5 times.
Proteoglycan (PG) synthesis
At the end of treatments, the cells were labeled with 5μCi/ml 35S-sulfate (Perkin Elmer, Boston, MA) for 4hrs. The amount of 35S-labeled PGs was analyzed by the alcian blue (Bio-Rad, Hercules, CA) precipitation method (30); the values were normalized to the DNA content detected by Hoescht dye. All measurements were done in triplicate for each donor sample.
RNA isolation and Reverse Transcription (RT)-PCR
RNA (200ng) isolated from cultured chondrocytes with the RNeasy mini kit according to the manufacturer’s instruction (Qiagen, Valencia, CA) was converted into cDNA using the Omniscript RT kit (Qiagen). Polymerase chain reaction (PCR) was performed with specific primer pairs for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), OP-1, aggrecan, ALK-2, -3, -6, BMPR-II, Smad1, 4, 5 and 6 (primer sequences, annealing temperature, and product size are provided in supplemental material). For all experiments, optimal conditions were determined and the tests reactions were performed in the linear range for the PCR amplification. PCR products were separated by 1% agarose gels and stained with ethidium bromide. Densities of each band were measured using the Fluor-S MultiImager (Bio-Rad, Hercules, CA) with attached software program to yield semi-quantitative assessment and were normalized to the densities of the corresponding GAPDH band to control for sample variability.
Flow cytometry of BMP receptors
After 48-hour culture in serum, cells were serum-starved overnight to reach the basal metabolic level before treatment with IL-1β for 24-, 48- and 72hrs with daily changes of the media. After the treatment, chondrocytes were collected using 1X cell detachment buffer (catalog #C5789, Sigma-Aldrich, St. Louis, MO), and incubated with primary antibodies for ALK-2, ALK-3, ALK-6 (rabbit polyclonal anti-human antibodies, Santa Cruz Biotechnology Inc; Santa Cruz, CA) or BMPR-II (mouse monoclonal anti-human antibody; R&D Systems, Minneapolis, MN) for 1hr at 4C°. Fluorescein isothiocyanate (FITC)-conjugated rabbit or mouse IgG secondary antibodies (MP Biomedical, Solon, OH) were added for 45min at 4°C in the dark. Cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry (FACS Calipur) using CellQuestPro software (both from Becton Dickinson, Franklin Lakes, NJ). For each sample, 10,000 events were analyzed. The mean fluorescence intensity (MFI) of the positive cell population was used to quantify the number of cells expressing BMP receptors on their surface.
Western blots analyses for phosphorylated and non-phosphorylated R-Smad
After chondrocytes reached confluency, they were serum-starved overnight to decrease the basal levels of phosphorylation. Cells were treated with either OP-1 or IL-1β for up to 4hrs. For the combined IL-1β and OP-1 treatment, chondrocytes were pre-treated with IL-1β for 30min prior to the replacement with fresh media contained OP-1 which was kept in culture from 5min to 4hrs. The same treatment schema was used for translocation experiments. The culture control contained only DMEM/Ham’s F-12 medium. After stimulation, the media were removed and the cells were washed with ice-cold PBS containing 0.1mM Na3VO4. Cell lysates were prepared with cell lysis buffer (20mM Tris/HCl, pH 7.5; 150mM NaCl, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1mM PMSF and 1μg/ml each of aprotinin, leupeptin and pepstatin). The samples were equalized for total protein (bicinchoninic acid kit; Pierce, Rockford, IL), separated by 10% SDS/PAGE and immunoblotted with antibodies against phosphorylated Smad1/5/8, BMP-specific site (called P-Smad throughout the paper), and control non-phosphorylated antibodies to total Smad1, 5, or 8 (called total R-Smad). All antibodies were rabbit polyclonal and were obtained from Cell Signaling (Beverly, MA). Immunoreactivity was determined with enhanced chemiluminescence (ECL, Amersham Biosciences).
Specificity of the PSSA antibody
The antibody against the phosphorylated Smad1 linker region (named PSSA) was custom-made and was developed in rabbits using a synthetic peptide Ac-HSPNSSYPN(pS)PC (a 186-196 amino acid sequence within the linker region). PSSA recognizes a phosphorylated serine residue within PXSP motif which is the same in all three Smad1/5/8 (Quality Controlled Biochemical, BioSource). The specificity of the antibody was tested on a slot blot where a nitrocellulose membrane was coated with 600ng/ml of the peptide and serial dilutions of either the PSSA antibody or the PSSA antibody pre-absorbed overnight at 4°C to the synthetic peptide (1:2 ratio) were applied to each dot on the membrane. The membrane was blocked with 5% non-fat dry milk followed by incubation with horseradish peroxidase conjugated rabbit IgG and developed with the Enhanced Chemiluminescent kit (Amersham Life Sciences, Poole, UK).
Smad1 linker region phosphorylation and its inhibition with specific MAPK inhibitors
Chondrocytes were pre-incubated with MAPK inhibitors for 1h (20μM of PD98059 for MAP/ERK kinase MEK; 20μM of SP600125 for JNK; and 20μM of SB203580 for p38; all inhibitors were purchased from Calbiochem [San Diego, CA]) before the addition of IL-1β, fibroblastic growth factor (FGF) (100ng/ml), OP-1 or an equal volume of vehicle for controls for 1h. After stimulation with growth factors or cytokines, cell lysates were prepared as described above and immunoblotted with PSSA antibody and an antibody recognizing total non-phosphorylated Smad1, 5, or 8.
Nuclear translocation for Smad4
Smad4 nuclear translocation was tested as previously described (31). Briefly, after treatment, the cells were fixed with 1% paraformaldehyde, permeabilized in 0.2% Triton X-100, extensively washed with PBS, and non-specific signal was blocked with normal Goat serum. After multiple washes the chondrocytes were incubated overnight at 4°C with rabbit anti-human Smad4 polyclonal antibody (Imgenex, San Diego, CA) followed by incubation with Rhodamine red conjugated goat anti-rabbit IgG (Pierce, Rockford, IL). In addition, as a nuclear counterstain, all cells were incubated with DAPI (Molecular Probes, Inc.). The nuclear image was visualized with a UV filter; the cellular fluorescent stain was visualized with a green filter using a Nikon Eclipse E600 microscope connected to a PC running MetaMorph Imaging series 6.1. Both images were overlaid with MetaView imaging software (Universal Imaging Corp.). The percentage of cells containing fluorescently labeled Smad4 antibody in the nucleus was determined by scoring 100 cells at each time point and in each treatment group.
Statistical analysis
Paired or unpaired t-tests were used for statistical analysis. When necessary, ANOVA (analysis of variance) with the Bonferroni test was used as a post hoc test for comparing different groups. The level of significance was set at a P<0.05.
Results
Effect of IL-1β on OP-1 responses
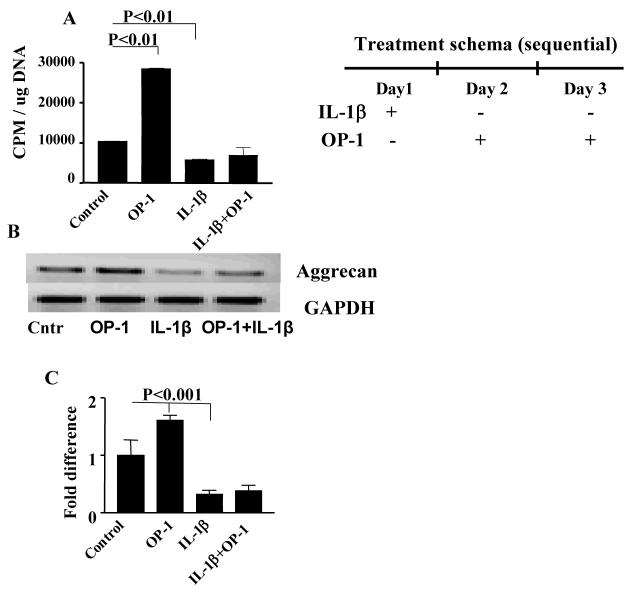
PG synthesis and aggrecan gene expression were selected as markers of OP-1 anabolic activity (3,5). OP-1 alone stimulated PG synthesis in chondrocyte cultures by 3-fold (P<0.001), while IL-1β alone caused a 2-fold inhibition of this parameter (P<0.001) in comparison with culture control (Fig.1A). However, when the two cytokines were given in combination, OP-1 was not able to statistically recover IL-1β inhibited PG synthesis. A similar effect was observed for aggrecan expression (Fig.1B and C). These results prompted the focus of the study on the mechanisms of OP-1 signaling in the presence of high dose of IL-1β.
Figure 1. Effect of IL-1β, OP-1 or combined IL-1β+OP-1 on PG synthesis (Fig. A) and aggrecan gene expression (Fig. B-C).
A, The data are presented as CPM normalized to the cellular DNA content. The histograms represent the mean ± SD of four independent experiments; OP-1 vs mini-ITS, P<0.01; IL-1β vs mini-ITS, P<0.01. B, representative PCR gel for aggrecan expression. C, corresponding histograms represent the mean ± SD of the relative density of the bands after densitometric scanning of 1% agarose gel from four independent experiments. The relative densities of the bands were normalized to that of GAPDH to control sample variations. OP-1 vs mini-ITS, P<0.001; IL-1β vs mini-ITS, P<0.001.
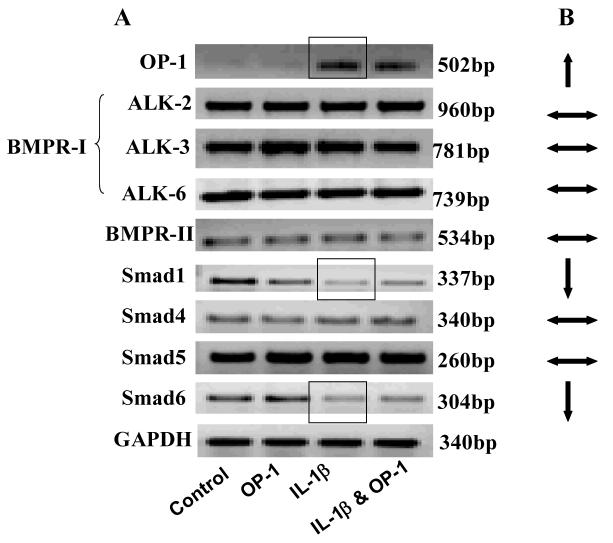
Effect of IL-1β on gene expression of OP-1 and its signaling mediators
The most conclusive results were observed in the cells treated with IL-1β only (Fig.2) where IL-1β stimulated OP-1 expression, while expression of BMP receptors was not influenced. Among Smads, Smad1 and 6 genes were down-regulated by 3- (P<0.02) and 2.5-fold (P<0.03) respectively. Expression of Smad4 and 5 remained unchanged.
Figure 2. Gene expression for endogenous OP-1 and its downstream signaling mediators.
A, PCR gels with corresponding size of the band. B. Summary of the effect of IL-1β on the gene expression of OP-1 and its downstream signaling mediators.  Expression was up-regulated;
Expression was up-regulated; expression was down-regulated;
expression was down-regulated;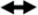 expression did not change.
expression did not change.
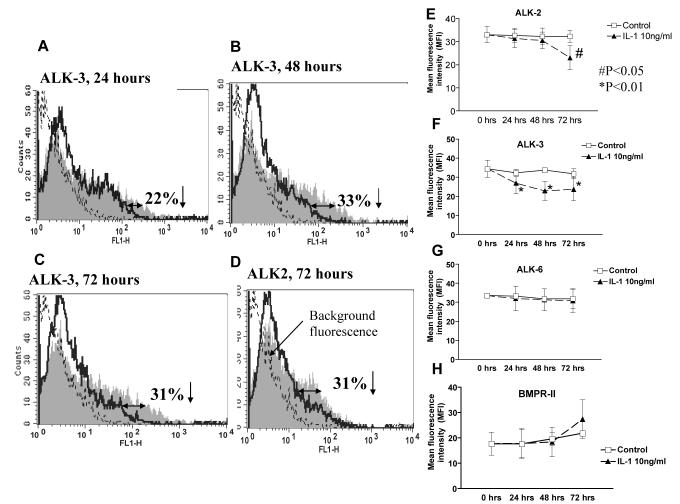
Effect of IL-1β on BMP receptors. Flow cytometry
Next, we investigated how IL-1β affects the number of BMP receptors on chondrocyte surface. Flow cytometry showed that after 24hrs IL-1β significantly decreased the percentage of cells to which anti-ALK-3 antibody was bound to in comparison with corresponding untreated control (22% decrease; P<0.01; Fig.3A&F). Longer cultures with IL-1β further reduced the percentage of ALK-3 positive cells: after 48hrs there was a 33% decrease (P<0.01; Fig.3B&F) which remained at this level for another 24hrs (P<0.01; Fig.3C&F). Changes in ALK-2 receptor were observed only after 72hrs IL-1β culture, where the number of ALK-2 positive chondrocytes was reduced by 31% (P<0.05; Fig.3D&E). No significant changes were found for ALK-6 receptor (Fig.3G). The percentage of chondrocytes that bound the BMPR-II antibody also remained relatively constant after 24 and 48hrs of IL-1β treatment (17.8±16.04, P=0.99 for 24hrs; 18.35±16.97, P=0.68) in comparison with the corresponding control (Fig.3H). After 72hrs, there was a trend towards an increase in the BMPR-II positive cells in comparison to day0 control, however statistical significance was not achieved (P=0.53; Fig.3H). These data indicate that, although IL-1β did not change gene expression of OP-1 receptors, it decreased the number of ALK-2 and 3 receptors which might contribute to the reduced responsiveness to OP-1 in the presence of IL-1β.
Figure 3. Changes in the number of BMP receptors after IL-1β stimulation.
Flow cytometric analysis of ALK-2, 3, 6 and BMPR-II receptors with IL-1β treatment. A, B & C, histograms represent changes in ALK-3 (BMPR-IA) receptor in chondrocytes treated with IL-1β for 24, 48 and 72 hours respectively. D, Histogram represents changes in ALK-2 receptor in chondrocytes treated with IL-1β for 72 hours. Changes in the number of receptors are shown by the shift in the fluorescence (clear histograms, solid lines) to the left as compared to the corresponding non-stimulated culture control (shaded histograms); dotted lines reflect the background fluorescence produced by the secondary antibody only (negative control). E-H, Linear graphs for each receptor separately represent the mean florescence intensity of seven independent experiments (mean ± SD); in each case ▴ represent cells treated with IL-1β; □ indicates corresponding non-stimulated control. The data were corrected for the negative background fluorescence caused by secondary antibody at each time point.
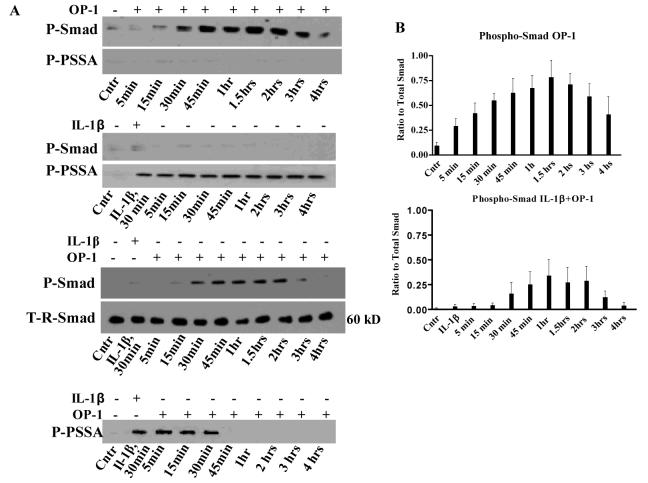
Effect of IL-1β on OP-1-induced Smad phosphorylation
BMPs activate identical amino acid sequence at C-terminal domain of R-Smads (11-13); therefore the same antibody recognizes the phosphorylated forms of all three R-Smads. The phosphorylation of R-Smads became noticeable at 15min of OP-1 treatment and continued for up to 4hrs (Fig.4A). The size of the specific band was about 60 kD. IL-1β itself did not induce any visible R-Smad phosphorylation at the BMP site. However, addition of IL-1β prior to OP-1 altered the kinetics of R-Smads activation in comparison with the individual treatment controls (Fig.4A). First, the initial R-Smad phosphorylation was delayed and did not occur until 30min of culture with OP-1. Second, an early termination of phosphorylation was noticed (after 2hrs rather than 4hrs with OP-1). Third, the overall levels of phosphorylation were also reduced (Fig.4B). Yet, no changes in total R-Smads were found in OP-1 and IL-1β alone controls (data not shown) or in their combination (Fig.4A). Similar patterns were seen with antibodies against total Smad1, 5, or 8 (data shown only for Smad1).
Figure 4. Phosphorylation of R-Smads at the BMP-specific C-terminal motif (P-Smad) or within the linker region (P-PSSA) by OP-1, IL-1β, and the sequential treatment with IL-1β and OP-1.
A, representative gels of chondrocytes treated with either OP-1 only for 4 hours, IL-1β only for 30 minutes replaced with fresh media contained no IL-1β, or chondrocytes pre-treated for 30 minutes with IL-1β followed by the treatment with OP-1 for another 4 hours. T-R-Smad, representative control gel with the antibody against total non-phosphorylated Smad1, where the same samples were used as in gels with combined treatment. Western blot with the antibody against total Smad1 (as a control) was performed separately for each treatment group and is similar in appearance to the blots with total Smad 5 or 8. B, Densitometry of gels stained with P-Smad antibody of chondrocytes cultured in the presence of OP-1 only or combined IL-1β and OP-1. The histograms represent the relative amounts of phosphosmad protein levels from densitometric scanning of immunoblots (mean ± SD) from three independent experiments normalized to total Smad1.
IL-1β induced linker region phosphorylation
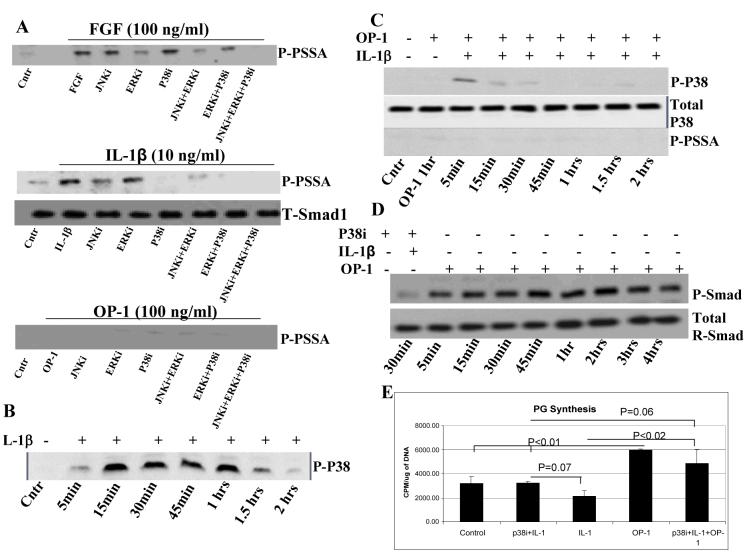
We investigated whether IL-1β could activate linker region and thereby interfere with the BMP-mediated R-Smad phosphorylation. To characterize the PSSA antibody that recognizes phosphorylated serine residues in the PXSP motif of the linker region we performed the following steps. The antibody specificity was tested by inhibition control studies on a slot blot where the antibody was pre-absorbed to the peptide. The complete inhibition of the PSSA antibody by the recombinant peptide occurred at an antibody dilution of 1:12,800 (data not shown). Since growth factors (EGF, FGF and others) were shown to induce linker region phosphorylation via the MAPK pathway (18,20), here we used FGF as a positive control. To verify the signal specificity and to identify which MAP kinases were involved in phosphorylation of the linker region by bFGF, chondrocytes were pre-incubated for 1hr with specific inhibitors of JNK, ERK, and p38 prior to treatment with bFGF (100ng/ml for 1hr; Fig.5A). We found that bFGF induced linker region phosphorylation which was inhibited in part by the inhibitor of MAP/ERK kinase, by the combination of JNK and ERK inhibitors (Fig.5A), or completely abolished when all three inhibitors were combined. Thus, we documented that linker region activation in articular chondrocytes by bFGF is MAPK-dependent. Then we tested whether IL-1β activates linker region through the same MAPK pathway. Treatment of chondrocytes with IL-1β caused MAPK-dependent phosphorylation of the linker region, which was inhibited mostly by p38 inhibitor and to a lesser extent by JNK inhibitor (Fig.5A). Combinations of either two or all three inhibitors totally abrogated the signal induced by IL-1β. As expected, OP-1 alone did not induce noticeable linker region phosphorylation (Fig.5A). The concentration of the MAPK inhibitors used in this study did not result in significant cell death (data not shown) and was the same as previously reported (32).
Figure. 5. The mechanism of linker region phosphorylation.
A, Human articular chondrocytes were pre-incubated for 1 hour with specific inhibitors of MAP kinases, JNK, ERK, and p38, prior to stimulation with bFGF, IL-1β, or OP-1; all for 1 hour. Cell lysates were blotted with PSSA antibody or antibody against total Smad1 (as a control). Western blotting with anti-total Smad1 antibody was performed for each experimental condition separately. B, Western blot with the antibody against phosphorylated p38 kinase (IL-1β control). Chondrocytes treated with IL-1β for 2 hours and collected at 5, 15, 30, 45, 60, 90, and 120 minutes. C, Combined OP-1 and IL-1β treatment. Chondrocytes pre-treated with OP-1 for 1 hour followed by the addition of IL-1β for 2 hours (both factors were present together in culture). Sample collection was at 1 hour of OP-1 treatment and then at 5, 15, 30, 45, 60, 90, and 120 minutes of combined treatment. Cell lysates were immunoblotted with antibodies against phosphorylated p38 (p-p38; upper panel) and Smad linker region (p-PSSA; bottom panel). As a control, cell lysates were blotted with an antibody for non-phosphorylated p38 (middle panel). D, Phosphorylation of R-Smad at C-terminal motif (P-Smad) by combined IL-1β and OP-1. Chondrocytes were pre-treated with p38 inhibitor (20 μM SB203580) for 1 hour and IL-1β for 30 min (total 1.5 hours) before the media was replaced with fresh media contained OP-1. Cells were cultured in the presence of OP-1 for 4 hours and collected according to schema used in Figure 4. Cell lysates were immunoblotted with the antibodies against phosphorylated (BMP-mediated) and total Smad1. E. Restoration of PG synthesis by p38 inhibitor. Experimental design is the same as in Fig. 1. Chondrocytes were pretreated with either IL-1β alone or in combination with p38 inhibitor for 24 hours, then the media were replaced with the fresh media contained OP-1 for another 48 hours. The data are presented as CPM normalized to the cellular DNA content. The histograms represent the mean ± SD of 2 independent experiments.
To confirm antibody specificity, the lysates from cells treated with either bFGF or IL-1β were also blotted with P-Smad antibody; no phosphorylation was observed (data not shown). Next, we investigated whether OP-1 interferes with the IL-1β mediated linker region phosphorylation (Fig.4A). Linker region phosphorylation was apparent within 5min of treatment with IL-1β alone and remained such during the entire culture period (for up to 4hrs) regardless whether IL-1β was removed after 30min (Fig.4A) or remained in the culture (data not shown). OP-1 alone did not cause any noticeable linker region activation; only weak, barely visible, baseline bands were detected (Fig.4A). Addition of OP-1 to the chondrocytes pre-treated with IL-1β changed the pattern of IL-1β induced linker region phosphorylation; it remained activated for only another 30min in the presence of OP-1 (Fig.4A); longer cultures with OP-1 completely abolished linker region phosphorylation (Fig.4A, low panel). Comparing R-Smad phosphorylation pattern at both sites (the BMP and MAPK) after the sequential treatment with IL-1β and OP-1 (Fig.4A) we found that IL-1β initially activates R-Smads via linker region and prevents BMP-mediated phosphorylation; then OP-1-induced signaling apparently prevails by promoting the BMP-specific activation of R-Smads and inhibiting (directly or indirectly) MAPK-dependent linker region phosphorylation.
The mechanism of OP-1 inhibition of IL-1β dependent linker region phosphorylation
To address this matter, we tested the ability of OP-1 to directly impact MAPK activity. Chondrocytes were pre-treated with OP-1 for 1hr and then cultured with IL-1β for another 2hrs. The cell lysates were used for Western blots with the antibodies against phosphorylated and non-phosphorylated p38 and JNK (Cell Signaling Technology, Inc and Biosource respectively), the kinases that mediate linker region activation by IL-1β (Fig.5A, middle panel). Pre-treatment with OP-1 inhibited p38 phosphorylation after 5min of culture with IL-1β (Fig.5C), while in the presence of IL-1β alone it lasted from 5min to 1.5hrs (Fig.5B). Similar results were observed for JNK phosphorylation, although its phosphorylation was inhibited by OP-1 to a lesser extent (data not shown). The same cell lysates were tested with the PSSA antibody. Though pre-treatment with OP-1 completely abrogated IL-1β induced linker region activation (compare Fig.5C with Fig.4A, forth gel from the top), PG synthesis was only partially restored (data not shown).
To prove that activation of p38 is one of the key mechanisms through which IL-1β impairs BMP signaling and responses to OP-1, phosphorylation and metabolic experiments were repeated in the presence of p38 inhibitor. In signaling studies, cells were pre-treated with p38 inhibitor for 1hr prior to treatment with IL-1β and OP-1. P38 inhibitor completely abolished the inhibitory effect of IL-1β on OP-1 mediated R-Smad phosphorylation (Fig.5D); the pattern of R-Smad activation was restored and was similar to that induced by OP-1 only (upper gel in Fig.4A). In metabolic studies, p38 inhibitor was added simultaneously with IL-1β for 1 day, which then was replaced with OP-1 for 2 days as in Fig.1. As anticipated, p38 inhibitor prevented IL-1β induced inhibition of PG synthesis (Fig.5E); the levels of PG synthesis induced by OP-1 under the combined p38 inhibitor and IL-1β treatments were comparable to those stimulated by OP-1 alone (Fig.5E) confirming that IL-1β inhibits OP-1 mediated responses via activation of p38 kinase.
Smad4 translocation under combined IL-1β and OP-1 treatment
When uncoupled with R-Smads, Smad4 is localized in the cytoplasm; however in a complex with phosphorylated R-Smads, it translocates into the nucleus (33). Smad4 appeared inside the cells within 15min of stimulation with OP-1 (Fig.6A). After 30min of OP-1 treatment, the majority of cells contained fluorescently labeled Smad4 in the nucleus, which remained there for up to 4hrs (Fig.6A and 6B, a-e). In the untreated control (Fig.6A and 6B, a), there were some cells that contained baseline levels of Smad4 thus representing perhaps signaling controlled by endogenous growth factors. The kinetics of OP-1 induced nuclear translocation of Smad4 and R-Smad phosphorylation were similar, suggesting that these two events are tightly connected. In the presence of IL-1β alone (Fig.6A and 6B, f), the number of cells contained Smad4 in the nucleus remained at baseline levels confirming that IL-1β does not activate BMP pathway. However, it greatly affected OP-1-induced signaling. When chondrocytes were pre-treated with IL-1β prior to stimulation with OP-1, the number of cells with fluorescently labeled Smad4 in their nuclei was decreased by 50% (P<0.01) in comparison with OP-1 alone (Fig.6A and 6B, g-j). The pattern of changes in Smad4 translocation in chondrocytes treated with both IL-1β and OP-1 correlated with changes in the BMP-specific R-Smad phosphorylation.
Figure 6. Effect of IL-1β on OP-1 induced Smad4 nuclear translocation.
Chondrocytes were stimulated with OP-1 ( ), IL-1β (o) alone or pre-treated with IL-1β for 30 min followed by the treatment with OP-1 (Δ) according to general design of the study. A, Graphical representation of the percentage of chondrocytes contained fluorescently labeled Smad4 in the nuclei (red dots over the blue DAPI stain) at each time point under each treatment. The data presented as the mean ± SD from four independent experiments. B, Representative photomicrographs of Smad 4 translocation induced by either OP-1 alone from 0 min to 4 h (upper panel), IL-1β alone for 30 min (control; lower panel, f) or by combined IL-1β+OP-1 (lower panel, g-j). a, media control, 0 min; b, chondrocytes stimulated with OP-1 for 30 minutes; c, chondrocytes stimulated with OP-1 for 1 hour; d, chondrocytes stimulated with OP-1 for 2 hours; e, chondrocytes stimulated with OP-1 for 4 hours; f, IL-1β control; g, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 30 minutes; h, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 1 hour; i, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 2 hours; j, chondrocytes pre-treated with IL-1β for 30 minutes and cultured in the presence of OP-1 for 4 hours.
Discussion
The current studies were aimed to understand the mechanism of OP-1 signaling under the conditions of altered cartilage metabolism. Under high doses of IL-1β used here (10ng/ml vs 0.1ng/ml; 100-fold higher than in [5,28]) OP-1 failed to overcome the deleterious effects of IL-1β on PG synthesis and aggrecan expression.
To avoid the effect of growth factors present in serum and to identify responses induced solely by IL-1β and OP-1 we selected serum-free cultures. Although IL-1β up-regulated expression of endogenous BMP-2 and OP-1 in human (presented data; 28,29) and rabbit (34) cartilage, it had no stimulatory effect on the gene expression of their receptors. In contrast, it caused a decrease in the number of ALK-2 and ALK-3 receptors on the chondrocyte surface, while ALK-6 appeared to be a constitutively-expressed kinase that is not affected by IL-1β or degenerative processes (35). Surprisingly, BMPRI antibodies were bound to only about one third of the chondrocytes, while even lower number of cells bound anti-BMPR-II antibody (<20%). Observed differences may be due to the detection method, sensitivity and affinity of the antibodies, isolation procedures, type and duration of culture, and the possibility that the receptor expression is cell cycle dependent. In particular, the latter would explain why after prolonged incubation the majority of cells display altered responsiveness to stimuli. Furthermore, the IL-1β and OP-1 receptors could also be expressed on a different cell subpopulation, which would suggest that only cells bearing receptors for both ligands will be the responsive population. The mechanism by which IL-1β down-regulated BMP receptors is yet unclear, although IL-1β-induced post-translational shedding of the receptors was suggested to modulate their expression and thereby, at least in part, determine the altered responsiveness to BMPs (36). Internalization via endocytosis could be another possible mechanism that regulates BMP receptors (37-40). However, this needs further exploration in chondrocytes.
Besides BMP receptors, IL-1β had a profound effect on gene expression and activity of Smads. It inhibited Smad1 and Smad6 mRNA supporting previous findings (41). Most importantly, IL-1β interfered with OP-1-dependent phosphorylation of R-Smads and nuclear translocation of Smad4: it delayed the onset and reduced the overall levels of activation as well as decreased the number of chondrocytes that contained R-Smad-Smad4 complexes in the nuclei which directly depends upon the presence of activated R-Smads (42). Such effects of IL-1β on R-Smad phosphorylation may be also due to the reduced number of type I BMP receptors available for subsequent binding to R-Smads (43).
The most challenging aim of our study was to understand the mechanisms by which IL-1β inhibits OP-1 mediated R-Smad phosphorylation. Recent reports on the alternative MAPK signaling (18-20) in Smad1 led us to hypothesize that the inhibition of OP-1 signaling by IL-1β in human chondrocytes could occur due to MAPK signaling within linker region. Utilizing custom-made antibody we found that in chondrocytes, p38 phosphorylation is one of the mechanisms thereby IL-1β transduces signal in the linker region. Importance of this kinase in the canonical IL-1β signaling has been documented earlier (44). Here, the inhibition of p38 completely abrogated IL-1β-induced activation of the linker region, re-established normal pattern of OP-1 mediated R-Smad phosphorylation, and thus restored anabolic responses to OP-1. Previous studies (19) showed that linker region phosphorylation is catalyzed by the Erk family of MAP kinases; however, our experiments with articular chondrocytes suggest the involvement of JNK and especially p38 pathways as well. Comparing phosphorylation pattern of R-Smad at both sites, we noticed that pretreatment with IL-1β initially activates MAPK signaling and even the presence of OP-1 does not change it within the first 30min of culture. Longer cultures with OP-1 inhibited MAPK signaling and promoted BMP mediated R-Smad phosphorylation. Our unpublished immunoprecipitation data suggest that phosphorylation at both sites may occur in the same cell population. According to our most recent findings, the mechanism responsible for the ability of OP-1 to reverse MAPK signaling may lie at least in part in its direct inhibition of IL-1β-induced p38 phosphorylation. This may be yet another example of crosstalk between different signaling pathways, which appears to be common in signal transduction, especially considering that (i) TGF-β and BMP-4 have already been implicated in TAK-1, ERK, and JNK of MAPK signaling (45), (ii) OP-1 was shown to inhibit IL-1β-induced phosphorylation of JNK in mesangial cells (46), and (iii) to inhibit p38 and AT-2 transcription factor in murine inner medullary collecting duct (47). An alternative activation of R-Smad by IL-1β reported in this study may provide additional clues to the understanding of the reduced anabolism and enhanced catabolism observed in human cartilage under high doses of pro-inflammatory cytokines (48). Linker region phosphorylation of Smad2/3 has also been shown to negatively control TGF-β (49). How this paradigm functions and how it is coordinated in vivo is an important question in chondrocyte signaling that remains to be investigated.
In conclusion, we propose that OP-1 and IL-1β interact in a highly regulated manner, in which each factor signals, not only through its recognized pathway, but also modulates the signaling of the other factor. The studies reported here propose mechanisms for how IL-1β controls OP-1/BMP signaling and provide an explanation for a limited repair in a model of acute inflammation (high concentration of IL-1β). They also may suggest novel therapeutic modalities/new targets for treatment of cartilage defects and OA.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants AR47654 and SCOR AR 39239 and Stryker Biotech grant SC-001. The authors would like to thank the Gift of Hope Organ and Tissue Donor Network and the donor families. We also thank Dr. Arkady Margulis for the procurement of human donor tissues and Stryker Biotech for providing OP-1. We acknowledge Drs. Katalin Mikecz and Bara Saraj for their help with flow cytometry and Dr. Im-Sampen for providing basic FGF.
References
- 1.Chambers MG, Bayliss MT, Mason RM. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis Cartilage. 1997;5:301–8. doi: 10.1016/s1063-4584(97)80034-9. [DOI] [PubMed] [Google Scholar]
- 2.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Relat. Res. 1998:26–37. [PubMed] [Google Scholar]
- 3.Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 4.Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 2000;43:206–14. doi: 10.1002/1529-0131(200001)43:1<206::AID-ANR25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, et al. Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1beta. Arthritis Rheum. 1997;40:2157–61. doi: 10.1002/art.1780401209. [DOI] [PubMed] [Google Scholar]
- 6.Koepp HE, Sampath KT, Kuettner KE, Homandberg GA. Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm. Res. 1999;48:199–204. doi: 10.1007/s000110050446. [DOI] [PubMed] [Google Scholar]
- 7.Nishida Y, Knudson CB, Knudson W. Osteogenic Protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthritis Cartilage. 2004;12:374–82. doi: 10.1016/j.joca.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Review Int Orthop. 2007;31(6):773–81. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys. Acta. 2002;1588:126–34. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 10.Merrihew C, Kumar B, Heretis K, Rueger DC, Kuettner KE, Chubinskaya S. Alterations in endogenous osteogenic protein-1 with degeneration of human articular cartilage. J. Orthop. Res. 2003;21:899–907. doi: 10.1016/S0736-0266(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 11.ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994;269:16985–8. [PubMed] [Google Scholar]
- 12.Miyazono K, ten Dijke P, Yamashita H, Heldin CH. Signal transduction via serine/threonine kinase receptors. Semin. Cell Biol. 1994;5:389–98. doi: 10.1006/scel.1994.1046. [DOI] [PubMed] [Google Scholar]
- 13.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata M, Miyazono K. Signal transduction of the TGF-beta superfamily by Smad proteins. J. Biochem. (Tokyo) 1999;125:9–16. doi: 10.1093/oxfordjournals.jbchem.a022273. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, et al. Smad6 inhibits signaling by the TGF-beta superfamily. Nature. 1997;389:622–6. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 17.Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–97. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signaling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–22. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17(24):3023–8. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 22.Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–49. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 23.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J. Clin. Invest. 1996;98:2425–30. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003;48(1):119–33. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- 25.Arner EC, Pratta MA. Modulation of interleukin-1-induced alterations in cartilage proteoglycan metabolism by activation of protein kinase C. Arthritis Rheum. 1991;34:1006–13. doi: 10.1002/art.1780340810. [DOI] [PubMed] [Google Scholar]
- 26.Blanco FJ, Lotz M. IL-1-induced nitric oxide inhibits chondrocyte proliferation via PGE2. Exp. Cell Res. 1995;218:319–25. doi: 10.1006/excr.1995.1161. [DOI] [PubMed] [Google Scholar]
- 27.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, et al. Cytokine regulation of chondrocyte functions. J. Rheumatol. 1995;43(Suppl.):104–8. [PubMed] [Google Scholar]
- 28.Merrihew C, Soeder S, Rueger DC, Kuettner KE, Chubinskaya S. Modulation of endogenous osteogenic protein-1 (OP-1) by interleukin-1 in adult human articular cartilage. J. Bone Joint Surg. Am. 2003;85-A(Suppl 3):67–74. doi: 10.2106/00004623-200300003-00012. [DOI] [PubMed] [Google Scholar]
- 29.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J. Bone Joint Surg. Am. 2003;85-A(Suppl 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 30.Masuda K, Shirota H, Thonar EJ. Quantification of 35S-labeled proteoglycans complexed to alcian blue by rapid filtration in multiwell plates. Anal Biochem. 1994;217(2):167–75. doi: 10.1006/abio.1994.1105. [DOI] [PubMed] [Google Scholar]
- 31.Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, et al. CD44 modulates Smad1 activation in the BMP-7 signaling pathway J Cell Biol 2004. Sep 27;16671081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005;174(9):5781–8. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Pouponnot C, Massague J. Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes. Genes Dev. 1997;11:3157–67. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida S, Kubota Y, Toba T, Horiuchi S, Shimamura T. Induction of osteogenic protein-1 expression by interleukin-1beta in cultured rabbit articular chondrocytes. Tohoku J. Exp. Med. 2002;197:101–9. doi: 10.1620/tjem.197.101. [DOI] [PubMed] [Google Scholar]
- 35.Chubinskaya S, Merrihew C, Mikhail R, ten Dijke P, Rueger D, Kuettner KE.BMP receptors specific for OP-1 are identified in human normal, degenerative and OA cartilage Trans ORS 200147, 262678 [Google Scholar]
- 36.Singhatanadgit W, Salih V, Olsen I. Shedding of a soluble form of BMP receptor-IB controls bone cell responses to BMP. Bone. 2006;39(5):1008–17. doi: 10.1016/j.bone.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–49. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signaling and turnover Nat. Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 39.Dore JJ, Jr., Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB. Mechanisms of transforming growth factor-beta receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol. Biol. Cell. 2001;12:675–84. doi: 10.1091/mbc.12.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jortikka L, Laitinen M, Lindholm TS, Marttinen A. Internalization and intracellular processing of bone morphogenetic protein (BMP) in rat skeletal muscle myoblasts (L6) Cell Signal. 1997;9:47–51. doi: 10.1016/s0898-6568(96)00094-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser M, Haag J, Söder S, Bau B, Aigner T. Bone morphogenetic protein and transforming growth factor beta inhibitory Smads 6 and 7 are expressed in human adult normal and osteoarthritic cartilage in vivo and are differentially regulated in vitro by interleukin-1beta. Arthritis Rheum. 2004;50(11):3535–40. doi: 10.1002/art.20750. [DOI] [PubMed] [Google Scholar]
- 42.Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J. Biol. Chem. 1997;272:27678–85. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- 43.Kang YM, Yeh YL, Graves DT. Interleukin-1 modulates phosphorylation of proteins in human osteoblastic cells. J Bone Miner. Res. 1995;10:96–105. doi: 10.1002/jbmr.5650100114. [DOI] [PubMed] [Google Scholar]
- 44.Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 2005;389(Pt 3):723–9. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirota Y, Tsukazaki T, Yonekura A, Miyazaki Y, Osaki M, Shindo H, et al. Activation of specific MEK-ERK cascade is necessary for TGF-beta signaling and crosstalk with PKA and PKC pathways in cultured rat articular chondrocytes. Osteoarthritis Cart. 2000;8:241–7. doi: 10.1053/joca.1999.0297. [DOI] [PubMed] [Google Scholar]
- 46.Lee MJ, Yang CW, Jin DC, Chang YS, Bang BK, Kim YS. Bone morphogenetic protein-7 inhibits constitutive and interleukin-1 beta-induced monocyte chemoattractant protein-1 expression in human mesangial cells: role for JNK/AP-1 pathway. J. Immunol. 2003;170:2557–63. doi: 10.4049/jimmunol.170.5.2557. [DOI] [PubMed] [Google Scholar]
- 47.Hu MC, Wasserman D, Hartwig S, Rosenblum ND. p38MAPK acts in the BMP7-dependent stimulatory pathway during epithelial cell morphogenesis and is regulated by Smad1. J. Biol. Chem. 2004;279:12051–9. doi: 10.1074/jbc.M310526200. [DOI] [PubMed] [Google Scholar]
- 48.Aigner T, Soeder S, Haag J. IL-1β and BMPs - interactive players of cartilage matrix degradation and regeneration. European Cells and Materials. 2006;12:49–56. doi: 10.22203/ecm.v012a06. [DOI] [PubMed] [Google Scholar]
- 49.Grimm OH, Gurdon JB. Nuclear exclusion of Smad2 is a mechanism leading to loss of competence. Nat. Cell Biol. 2002;4(7):519–22. doi: 10.1038/ncb812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.