Abstract
The authors examined the effects of smoking opportunity on neural responses to monetary outcomes in nicotine-deprived cigarette smokers. Participants who were told that they would be able to smoke during the study exhibited smaller responses to monetary gains and losses in the caudate nucleus than did those who anticipated having to wait several hours before having the opportunity to smoke. These findings highlight the importance of investigating the effects of perceived drug use opportunity on motivational processing in addicted populations.
Keywords: drug addiction, craving, motivation, reward, caudate nucleus
Aberrant processing of reward-related information is a core feature of drug addiction. Addicted individuals exhibit exaggerated affective, cognitive, and neurobiological responses to stimuli associated with drug-related rewards (Carter & Tiffany, 1999; Franken, 2003; Sayette, 2006). In contrast, they demonstrate blunted reactions to stimuli associated with non-drug rewards (e.g., money; Goldstein et al., 2007; Martin-Solch et al., 2001). Although these reward-processing biases are thought to be relatively enduring, transient factors (e.g., deprivation state) appear to moderate the degree to which addicted individuals exhibit hypersensitivity to drug-related rewards and hyposensitivity to non-drug rewards (Goldstein & Volkow, 2002).
Perceived drug use opportunity is one variable that influences the manner in which addicted individuals respond to stimuli associated with drug rewards (Hogarth & Duka, 2006; Wertz & Sayette, 2001b; Wilson, Sayette, & Fiez, 2004). For instance, substance users who anticipate an opportunity to use drugs in the near future report stronger cravings in the presence of drug cues (e.g., Carter & Tiffany, 2001; Sayette et al., 2003) and pay greater attention to drug-related stimuli (Wertz & Sayette, 2001a) than do those who expect a significant delay before drug use is possible. Similarly, individuals informed that they will be able to use drugs immediately after completion of experimental tasks exhibit more robust cue-elicited activation of the medial section of the orbito-frontal cortex, a brain region implicated in monitoring the reward value of stimuli (Kringelbach & Rolls, 2004), than do participants told they must wait several hours before consuming drugs (McBride, Barrett, Kelly, Aw, & Dagher, 2006; Wilson, Sayette, Delgado, & Fiez, 2005). Taken together, these data suggest that the salience and incentive value of cues associated with drug reward is enhanced when encountered in the context of an impending opportunity to engage in drug use.
While there is a growing body of research characterizing the robust effects that drug use expectancy has on the processing of drug cues, little is known about how responses to non-drug rewards are affected by whether or not drug use is anticipated. Indirect evidence suggests that non-drug reward stimuli may be processed differently when drug use is expected than when it is not. As noted above, drug-addicted individuals report stronger cravings when anticipating an opportunity to consume drugs, relative to when drug use is not expected. It is important that drug craving is associated with both an increased attentional focus on drug cues (e.g., Mogg, Field, & Bradley, 2005) and a reduced focus on non-drug cues (e.g., Cepeda-Benito & Tiffany, 1996; Sayette & Hufford, 1994). Thus, insofar as cues signaling the opportunity to consume drugs increase craving, such cues also may increase the use of cognitive resources for the processing of drug cues and reduce the availability of resources for processing non-drug information, including stimuli that predict non-drug rewards. Such expectancy effects, if they exist, would have significant implications for the design and interpretation of research investigating reward processing in addicted populations.
The present functional magnetic resonance imaging (fMRI) study aimed to examine the effects of smoking opportunity on the processing of monetary outcomes during the performance of a previously developed card-guessing task (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000). To elicit a strong motivation to smoke, we required non-treatment-seeking cigarette smokers to abstain from smoking for 8 hr prior to the experiment (Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). We focused on task-elicited responses in the caudate nucleus, the brain region shown to be most sensitive to reward-related information in several prior investigations using the card-guessing paradigm (Delgado, Locke, Stenger, & Fiez, 2003; Delgado et al., 2000; Delgado, Stenger, & Fiez, 2004; Tricomi, Delgado, McCandliss, McClelland, & Fiez, 2006). While the precise information encoded by neural activity in the caudate nucleus is still a matter of debate (cf. Davidson et al., 2004; O’Doherty, 2004; Schultz, 2000; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004), there is converging evidence that the caudate responds to stimuli with behavioral significance (Tricomi, Delgado, & Fiez, 2004). Further, previous studies that have used the card-guessing task have shown that reward-related responses in the caudate are remarkably sensitive to manipulations of reward magnitude (Delgado et al., 2003) and motivational significance (Delgado et al., 2004; Tricomi et al., 2006). Thus, the task is well suited for investigating the influence of drug use expectancy on the processing of non-drug rewards.
Method
Participants
Twenty-two right-handed, male, native English speaking cigarette smokers participated in the experiment. All participants had to report smoking between 20 and 40 cigarettes per day for at least 24 months continuously to be eligible for participation. Participants were recruited through advertisements in local newspapers. Written informed consent was obtained from all participants and all procedures were approved by the Institutional Review Board of the University of Pittsburgh. Data from 4 participants were excluded from all analyses because of excessive head motion during scanning (2 participants) and failing to comply with task instructions (2 participants). Subsequent analyses are reported on the remaining 18 participants.
Participants were invited to attend a 2-hr session. They were randomly assigned to one of two experimental conditions: (a) Half were told that they would be able to smoke during a break at the midpoint of the experimental session (Instructed-Yes; n = 9); (b) the other half were instructed that they could not smoke during the experimental session and would have to wait approximately 2 hr before smoking (Instructed-No; n = 9). Age, number of cigarettes smoked per day, number of quit attempts, and years of education were similar across instructed smoking opportunity conditions (ps > .05; see Table 1).
Table 1.
Participant Demographic Characteristics
| Instructed-Yes (n = 9) |
Instructed-No (n = 9) |
|||
|---|---|---|---|---|
| Characteristic | M | SD | M | SD |
| Age | 23.2 | 3.5 | 25.7 | 6.2 |
| Cigarettes per day | 21.1 | 2.2 | 21.7 | 3.5 |
| Years smoking | 7.8 | 2.0 | 8.1 | 5.1 |
| Quit attempts | 3.8 | 5.0 | 0.9 | 1.3 |
| Education | 13.2 | 1.7 | 13.1 | 1.4 |
Materials
Baseline assessment measures
Demographic and smoking history information was assessed with standard forms (Sayette et al., 2001).
Urge rating assessment
Participants verbally rated their urge to smoke on a scale ranging from 0 (absolutely no urge to smoke at all) to 100 (strongest urge to smoke I’ve ever experienced). Urge ratings were provided immediately before and after completing each of the two cue exposure runs (see below). Thus, a total of four urge ratings was obtained from each participant. Present analyses focus on the second and third urge ratings (Urge #2 and Urge #3), which were provided closest in time to the performance of the card-guessing task (described below). Complete urge data are reported elsewhere (Wilson et al., 2005).
Card-guessing task
Participants completed several trials of a card-guessing task adapted from previous research (see Delgado et al., 2000). For each trial, participants guessed whether the numerical value of a visually displayed “playing card” was higher or lower than 5. Participants were informed before beginning the task that each card would have a value ranging from 1 to 9, excluding the number 5. Thus, each guess was either correct or incorrect for each trial. After a choice-making period lasting 2,500 ms, a number from 1 to 9 (excluding 5) was presented for 500 ms, followed by feedback (also presented for 500 ms) informing participants whether or not their guess was correct. For trials in which a correct guess was made, feedback consisting of a green arrow pointing upward was presented. For trials in which an incorrect guess was made, feedback consisting of a red arrow pointing downward was presented. Trials concluded with the presentation of a fixation cross for 11.5 s.
Participants were informed that each correct guess led to the addition of $1.00 to the total payment they would receive, while each incorrect guess led to the loss of $0.50 from this total. Unbeknownst to participants, card values were selected only after the response was made for each trial to ensure an equal number of positive feedback and negative feedback trials. Participants completed a total of 90 interleaved trials (45 of each feedback condition) divided into five runs of 18 trials each.
Procedure
Participants visited the lab for three sessions: a screening session (Session 1), a session in which abstinence instructions were provided (Session 2), and the experimental session (Session 3). Participants completed Session 1 an average of 2.78 days (range = 1–9 days) before Sessions 2 and 3, which were held 8 hr apart on the same day. During Session 1, participants provided an expired air carbon monoxide (CO) sample, which was used to verify smoking status (≥10 ppm; BreathCo, Vitalograph, Lenexa, KS). Session 2 occurred 8 hr prior to the experimental session, during which participants returned to the laboratory to smoke one of their cigarettes. After smoking, a second CO sample was obtained to provide a baseline for comparison to levels obtained at the start of the experiment. Subsequently, all participants were instructed not to use tobacco products or other drugs for 8 hr prior to arriving at the laboratory for the experiment.
Experimental sessions were scheduled to begin between 4 p.m. and 6 p.m. Upon arriving for the experimental session, participants reported the last time they had smoked a cigarette, and a third CO sample was obtained to check compliance with deprivation instructions. For the third CO assessment, samples exceeding half of the second CO reading and/or 16 ppm resulted in exclusion from the study, on the basis of criteria derived from similar deprivation periods in comparable smoking samples (e.g., Sayette et al., 2001).
Immediately before scanning began, participants were told whether they would be permitted to smoke during the experimental session. Specifically, Instructed-Yes participants were informed that they would be removed from the scanner for a brief break during the study, at which point they would be able to smoke. Instructed-No participants were also told that they would be removed from the scanner for a break, but they were instructed that they would not be able to smoke during the study and therefore would have to wait about 2 hr before having the chance to smoke (see also Juliano & Brandon, 1998).
Next, participants completed the first of two cue presentation runs, during which they were asked to hold and look at a small yellow notepad and a white plastic golf ball. Participants held each of these objects, which were designed to elicit minimal changes in urge to smoke, for a period of 74 s (see Wilson et al., 2005). After completing the card-guessing task, participants completed a smoking cue presentation run. Results from this cue exposure paradigm have been published separately (Wilson et al., 2005).
Following smoking cue exposure, all participants were removed from the scanner for a brief break (about 5 min), and those participants told that they would be permitted to smoke were escorted outside where they could smoke a cigarette at their own pace. Afterwards, participants were returned to the scanner to complete approximately 45 additional minutes of the guessing task. There was significant variability in the subjective response to smoking during the break (e.g., some participants indicated that they wanted to smoke more than one cigarette, while others became light-headed and could not finish a whole cigarette) and in the fMRI data subsequently obtained. Accordingly, we focus on the effects of expectancy in this report and do not present post-break data; the data obtained following the break are consistent with the results and interpretations presented below (though at a lower level of significance) and do not alter conclusions drawn herein.
fMRI Data Acquisition and Analysis
Participants were scanned with a conventional 1.5 T GE Signa whole-body magnet and standard radio frequency coil. A structural series of 36 contiguous oblique-axial slices (3.75 × 3.75 × 3.8 mm voxels) parallel to the AC–PC (intercommissural) line was collected with a standard T1-weighted pulse sequence. We acquired functional images in the same plane as the structural series with coverage limited to the 20 center slices using a T2*-weighted one-shot spiral pulse sequence (echo time = 35 ms, repetition time = 1,500 ms, field of view = 24 cm, flip angle = 70°).
We conducted fMRI data analysis using the NeuroImaging Software package (Version 3.5; Fissell et al., 2003). Single-subject data were corrected for motion with Automated Image Registration (Version 3.08; Woods, Cherry, & Mazziotta, 1992) and adjusted for drift between and within runs. Structural images from each participant were coregistered to a common reference anatomy. Subsequently, functional images were transformed into the same space, globally mean-normalized, and smoothed with a three-dimensional Gaussian filter (4 mm full width at half maximum). Group-based statistical images were visualized and transformed into standard stereotaxic space (Talairach & Tournoux, 1988) with the Analysis of Functional NeuroImages software package (Version 2.6; Cox, 1996).
The primary aim of analysis was to examine the effects of smoking opportunity on the response to monetary gains and losses in the caudate nucleus. We first identified functionally defined regions of interest (ROIs) localized to the caudate using a repeated-measures analysis of variance (ANOVA) with time (10 sequential 1.5 s scans in a trial of 15 s) as a within-subjects factor. The voxel-wise significance threshold was set at p < .00001 for this contrast (uncorrected for multiple comparisons). Only those regions composed of at least five contiguous voxels exceeding this criterion were included in subsequent analyses. We have successfully used this approach in prior research to isolate caudate ROIs that are responsive to the card-guessing task for subsequent analysis (Delgado et al., 2004). To examine the effects of smoking opportunity on responses to feedback in the caudate nucleus, we performed additional analyses on the time series data extracted from caudate ROIs identified in this contrast. For these analyses, fMRI signal averaged over the 6–9 s following the presentation of feedback (the seventh and eighth scans) was the blood oxygen level-dependent response of interest, on the basis of our prior work indicating that differential responses to monetary gains and losses in the caudate are maximal within this temporal window (Delgado et al., 2000).
Results
Smoking Urge
A 2 × 2 mixed-model ANOVA, with instruction set (Instructed-Yes, Instructed-No) as a between-participants factor and time (Urge #2, Urge #3) as a within-participants factor, revealed a main effect of time, F(1, 16) = 17.5, p < .001, effect size d = 2.09. Urge ratings rose over time for both groups: Mean urge ratings collapsed across groups, Urge #2 = 60.7 (SD = 24.1), Urge #3 = 70.3 (SD = 26.7). The instruction set main effect and the Instruction Set × Time interaction were not significant.
Behavioral Data
Participants made a response for each trial of the card-guessing task. Responses and reaction times were collected for all trials. A two-tailed t test for independent samples revealed that reaction time did not differ significantly between smoking opportunity groups, t(16) = 1.05, p = .31, d = 0.53 (Instructed-No: M + SD = 643.14 ± 183.63 s; Instructed-Yes: M + SD = 573.31 ± 79.6 s). Similarly, the groups did not differ in choice allocation, t(16) = 0.83, p = .42, d = 0.41 (Instructed-No: M + SD = 59.15 ± 8.89% high choices; Instructed-Yes: M + SD = 54.70 ± 13.36% high choices).
fMRI Data
Brain regions exhibiting a main effect of time in our initial analysis are presented in Table 2. As expected, this analysis yielded ROIs bilaterally in the caudate nucleus (see Figure 1). Peak Talairach coordinates for these regions (see Table 2) are within 1 voxel of the peak coordinates for left and right caudate regions exhibiting a main effect of time in previously published studies from our laboratory using the card-guessing task. We extracted and further probed data from these ROIs using a mixed-model ANOVA with instruction set (Instructed-Yes, Instructed-No) as a between-participants factor and hemisphere (right, left) and feedback condition (monetary gain, monetary loss) as within-participants factors. There was a significant effect of hemisphere, F(1, 16) = 104.67, p < .001, d = 5.11, with signal intensity higher in the right than the left caudate. There was also a significant effect of feedback condition, F(1, 16) = 92.16, p < .001, d = 4.80. Signal intensity during the 6–9 s following feedback delivery was more robust following trials in which money was won than trials in which money was lost, irrespective of perceived smoking opportunity, which replicated prior work with the card-guessing task.
Table 2.
Regions Exhibiting a Main Effect of Time
| Region | BA | Size (No. of voxels) |
Peak Talairach coordinates (x, y, z) |
Maximum F value |
|---|---|---|---|---|
| Inferior frontal gyrus (R) | 9 | 13 | 39, 7, 29 | 26.90 |
| Thalamus (L) | 65 | −7, −16, 8 | 36.90 | |
| Thalamus (R) | 15 | 8, −26, 3 | 31.37 | |
| Caudate nucleus (L) | 10 | −12, 7, 8 | 23.53 | |
| Caudate nucleus (R) | 15 | 8, 6, 11 | 28.73 | |
| Lingual gyrus (L) | 17/18/19 | 25 | −21, −90, −6 | 27.54 |
| Fusiform gyrus (R) | 37 | 68 | 32, −42, −16 | 35.33 |
| Middle occipital gyrus (L) | 18/19 | 13 | −36, −76, −9 | 28.65 |
Note. BA = Brodmann area; L = left; R = right.
Figure 1.
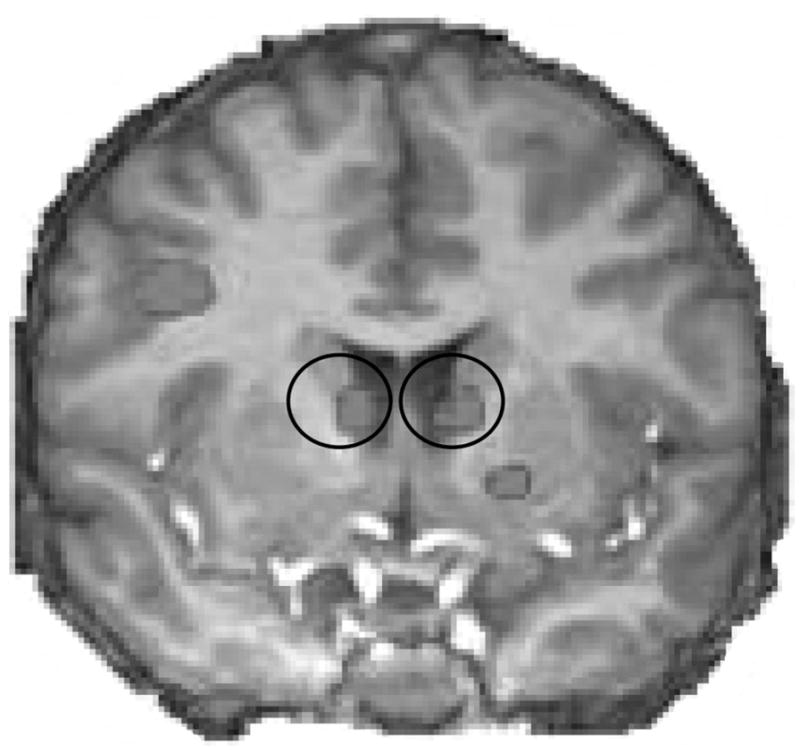
Caudate regions exhibiting a main effect of time (circled).
We also observed a significant main effect of instruction set, F(1, 16) = 25.86, p < .001, d = 2.54. Independent sample t tests (two-tailed) contrasting the response to feedback for each group (collapsed across hemisphere) revealed that individuals who were not expecting to smoke during the study (Instructed-No group) exhibited a larger response to both monetary gain, t(16) = 7.36, p < .001, d = 3.68, and loss, t(16) = 2.20, p = .043, d = 1.10, than did individuals who were expecting to smoke (Instructed-Yes group). Neither self-reported urge obtained before (Urge #2) nor those provided after (Urge #3) performance of the card-guessing was reliably correlated with the magnitude of the response to gains and losses in the left and right caudate nucleus (rs ranged from −.01 to −.30, ps > .20).
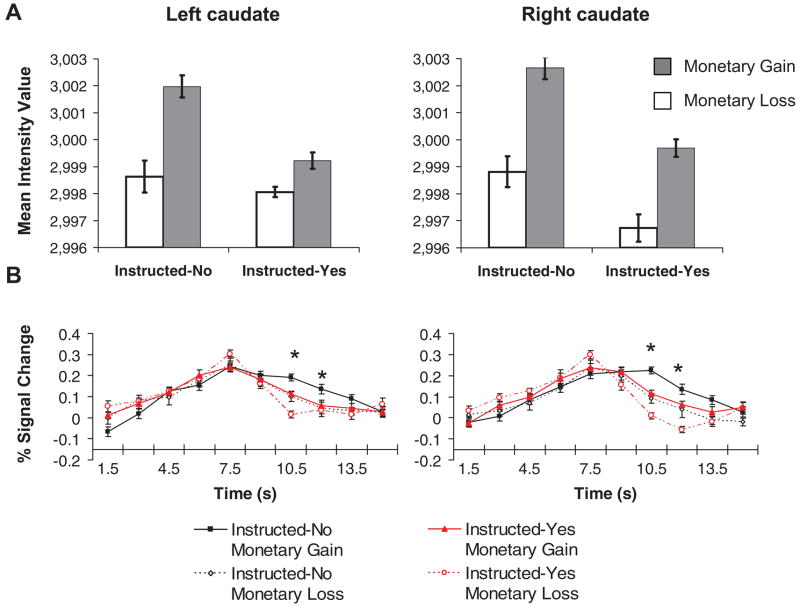
Finally, we found a significant interaction between instruction set and feedback condition, F(1, 16) = 6.79, p = .019, d = 1.30. As shown in Figure 2A, the response to gains in the caudate was more strongly affected by smoking expectancy than the response to losses. None of the remaining effects (Hemisphere × Instruction Set, Hemisphere × Feedback Condition, and Hemisphere × Feedback Condition × Instruction Set interactions) reached significance.
Figure 2.
(A) Response to monetary outcomes in the left and right caudate nucleus as a function of perceived smoking opportunity during the 6–9 s following feedback presentation. (B) Full time series of activation for the left and right caudate normalized to percentage signal change from the mean across conditions of signal intensity at trial onset. Asterisks denote the time points occurring during the 6–9 s postfeedback period. Error bars represent one standard error of the mean.
Several brain regions in addition to the a priori caudate ROIs were activated during performance of the task (see Table 2). To assess the specificity of the effects observed in the caudate, we performed exploratory analyses on signal extracted from these ROIs during the 6–9 s following the presentation of feedback. A mixed-model ANOVA with hemisphere (right, left) and feedback condition (positive feedback, negative feedback) as within-participants factors and smoking opportunity condition (Instructed-Yes, Instructed-No) as a between-participants factor was performed on data extracted from the bilateral thalamus ROIs. A similar approach was used for the remaining unilateral ROIs, but hemisphere was not included as a factor. Analysis on data from the thalamus ROIs yielded a significant effect of hemisphere, F(1, 16) = 30.78, p = .019, d = 2.77; as with the caudate, signal intensity was larger in the right than in the left hemisphere. A significant effect of feedback condition was observed in both the right inferior frontal gyrus, F(1, 16) = 11.56, p = .004, d = 1.70, and left lingual gyrus, F(1, 16) = 4.78, p = .044, d = 1.09. In both ROIs, responses to monetary gain were more robust than responses to monetary loss. No other effects were significant for these additional ROIs.
Discussion
Participants who were told they would be able to smoke during the study exhibited attenuated caudate responses to both monetary gains and losses, relative to those who anticipated having to wait several hours before having the opportunity to smoke. This effect was specific to the caudate and did not extend to other regions associated with performance of the task. Thus, as has been noted (Volkow et al., 2006; Wilson et al., 2004), it is becoming increasingly clear that contextual factors, such as perceived drug use opportunity, must be taken into account when investigating motivational processing in drug-addicted populations.
Present findings suggest that there was a shift in the caudate response to both monetary gains and losses as a consequence of perceived smoking opportunity. Prior research that has used the card-guessing task and similar paradigms suggests that, when actions may be associated with one of several potential outcomes, the magnitude of postfeedback responses in the caudate is maximal following receipt of the best possible outcome and smallest after delivery of the worst possible result (e.g., Delgado et al., 2003, 2004; Nieuwenhuis et al., 2005). Thus, the current data raise the intriguing possibility that monetary gains were processed as less rewarding and monetary losses as more punishing by individuals anticipating an opportunity to smoke soon, relative to those expecting a significant delay before having the chance to smoke (cf. Figure 2B; Delgado et al., 2003, Figure 2). As participants in the present study were not asked to describe the experience associated with winning and losing money, additional research is needed to examine this hypothesis.
Prior findings from our laboratory suggest that informing cigarette smokers that they can smoke soon may produce not just a desire to smoke but an impatient desire to smoke immediately (Sayette et al., 2003). Specifically, over the course of smoking cue exposure, smokers told they would be able to smoke soon became less likely to evince facial expressions associated with positive affect and more likely to display facial expressions associated with negative affect than did those informed that they could not smoke during the study. Thus, for the addicted individual, under certain conditions the delay between learning that drug use will be possible in the near future and actual drug consumption may be characterized by a high degree of negative affect. It has been proposed that alleviating negative affect, such as that associated with drug withdrawal, is an important source of motivation for the maintenance of drug-taking behavior (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Interestingly, nonhuman animal research indicates that conditioned cues repeatedly paired with withdrawal significantly decrease the activity of brain reward systems (e.g., Kenny & Markou, 2005). Although speculative, the present findings suggest that in addition to (or perhaps because of) the negative affect associated with delayed drug use, the value of non-drug rewards (e.g., money) is reduced when consumption is anticipated, an effect that would likely further increase the desire to engage in drug use.
Results also suggest that the response of the caudate response to monetary gains was particularly affected by smoking expectancy. This finding is in accord with recent data suggesting that the processing of positive and negative stimuli may be dissociable, at least under certain conditions (e.g., Liu et al., 2007). For instance, Dawkins, Acaster, and Powell (2007) found that abstinence from smoking influenced the subjective responses to film clips designed to elicit positive, but not negative, affect. As noted above, affective responses to feedback were not assessed in the present study. Thus, further research is needed to examine whether smoking expectancy differentially affects self-report, as well as neural, responses to monetary gains and losses.
Future research is also needed to clarify the mechanisms underlying the effects of perceived smoking opportunity on the caudate response to monetary gains and losses. Motivational responses may be influenced by myriad psychological processes (e.g., attention, arousal, outcome expectancies; Delgado et al., 2004). Explicating the effects observed herein by disentangling these processes is an important challenge for future research. For instance, recent research suggests that the neural correlates of reward value and degree of motivation are distinct (e.g., Roesch & Olson, 2004). Studies that use similar methods to further characterize the effects of perceived drug use opportunity would be valuable. (As in prior investigations that have used the card-guessing task, we failed to observe significant effects in the orbitofrontal cortex, one region that has been implicated in the representation of reward value by prior work; this is most likely due to susceptibility-related signal loss.)
Additional research examining how changes in craving and affect relate to the current findings would also be useful. While we failed to observe significant relationships between smoking expectancy, urge, and neural responses to monetary outcomes in the present study, the urge assessment strategy we employed was designed primarily to examine the effects of smoking expectancy on cue-elicited responses (Wilson et al., 2005). Moreover, because we sought to minimize the number of assessments because of concerns that craving state may be altered by repeatedly asking participants to rate their urge (Sayette et al., 2000), we only collected urge ratings from participants immediately before and just after completing the card-guessing task.
As described above, several studies have found that drug addicted individuals report stronger drug cravings when anticipating an opportunity to consume drugs than when drug use is not expected. Perhaps stronger linkages between craving and expectancy-related effects on feedback processing would be revealed through the use of a more time-sensitive urge assessment strategy. For example, craving that occurs when smokers expect to smoke may increase the salience of cues associated with drug-related reward, while simultaneously reducing the significance of stimuli associated with non-drug rewards, such as money (Kalivas & Volkow, 2005; Sayette, 2006). Alternatively, higher levels of craving may reduce the availability of cognitive resources necessary for task engagement and stimulus evaluation for those anticipating an opportunity to smoke (e.g., Cepeda-Benito & Tiffany, 1996; Sayette & Hufford, 1994). Still another possibility is that feedback processing may be altered by the manner in which perceived drug use opportunity influences the valence of an individual’s craving and/or affective state, as discussed above (Sayette et al., 2003). Additional research examining these and related alternatives is warranted, particularly given the limitations noted above.
It should be noted that the present sample was relatively small. Some null findings (e.g., those for reaction time) may have been due to power limitations. Accordingly, future research with larger samples would be useful. Another limitation of this study was that the subjective experience associated with the occurrence of monetary gains and losses was not assessed with self-report measures, as noted above. Consequently, our data do not address whether perceived smoking opportunity influences self-reported emotion while engaged in the card-guessing task. A third limitation of the current research concerns the inclusion of only male smokers. Research examining the effects of drug use opportunity on feedback processing in both male and female smokers is needed. Similarly, because the present study included only abstinent smokers, it is unclear whether perceived smoking opportunity would also affect feedback processing in nonabstinent smokers. Prior work in our laboratory suggests perceived smoking opportunity also can affect the urge ratings of nonabstinent smokers, albeit to a smaller degree (see Sayette et al., 2003, Table 1).
Studies focusing on treatment-seeking smokers also would be useful. As we have noted elsewhere (Wertz & Sayette, 2001b; Wilson et al., 2004), the effects of perceived drug use opportunity on information processing likely depend upon the degree to which drug use is desired or intended. For instance, the effects observed in individuals who intend to use drugs but who cannot do so because of situational restrictions may differ from those observed in individuals who do not anticipate using drugs as a result of self-imposed constraints (e.g., they are attempting to quit smoking; Wilson et al., 2005). Accordingly, research examining the effects of both drug use expectancy and drug use intentions on the processing of drug-related and non-drug rewards is indicated.
Notwithstanding these limitations, the present findings highlight the importance of perceived drug use opportunity as an area of investigation for researchers using functional neuroimaging to study motivation in addicted populations. Altogether, extant data suggest that anticipating drug use may lead to important changes in the way that addicted individuals process cues associated with both drug-related and non-drug rewards. Further investigation of the nature and implications of these changes would provide important data for understanding the maladaptive behavior patterns associated with drug addiction.
Footnotes
Portions of this data were presented at the annual meeting of the Society for Neuroscience (Orlando, Florida, November 2002). This article is based on research supported by National Institute of Drug Abuse Grants RO1 DA10605 (to Michael A. Sayette) and RO1 DA14103 (to Julie A. Fiez).
Contributor Information
Stephen J. Wilson, Department of Psychology, University of Pittsburgh, and Center for the Neural Basis of Cognition, Pittsburgh, Pennsylvania
Michael A. Sayette, Departments of Psychology and Psychiatry, University of Pittsburgh
Mauricio R. Delgado, Department of Psychology, Rutgers University–Newark
Julie A. Fiez, Departments of Psychology and Neuroscience and Learning Research and Development Center, University of Pittsburgh, and Center for the Neural Basis of Cognition, Pittsburgh, Pennsylvania
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Tiffany ST. The use of a dual-task procedure for the assessment of cognitive effort associated with cigarette craving. Psychopharmacology. 1996;127:155–163. doi: 10.1007/BF02805989. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional resonance neuroimages. Computational and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Horvitz JC, Tottenham N, Fossella JA, Watts R, Ulug AM, Casey BJ. Differential cingulate and caudate activation following unexpected nonrewarding stimuli. NeuroImage. 2004;23(3):1039–1045. doi: 10.1016/j.neuroimage.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addictive Behaviors. 2007;32(2):425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14(9):1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Fissell C, Tseytlin E, Cunningham D, Iyer K, Carter CS, Schneider W, Cohen JD. A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–125. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27(4):563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Duka T. Human nicotine conditioning requires explicit contingency knowledge: Is addictive behaviour cognitively mediated? Psychopharmacology. 2006;184(3–4):553–566. doi: 10.1007/s00213-005-0150-0. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. Journal of Neuroscience. 2005;25(26):6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. Journal of Neuroscience. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Experimental Brain Research. 2001;139(3):278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology. 2006;31(12):2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: An investigation of competing theoretical views of addiction. Psychopharmacology. 2005;180(2):333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. NeuroImage. 2005;25(4):1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004 April 9;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Craving, cognition, and the self-regulation of cigarette smoking. In: Sebanz N, Prinz W, editors. Disorders of volition. Cambridge, MA: MIT Press; 2006. pp. 419–438. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Tricomi E, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience. 2006;18(6):1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, et al. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. NeuroImage. 2006;32(4):1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine & Tobacco Research. 2005;7(4):637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Cherry S, Mazziotta J. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]