Abstract
Four isolates of spotted fever group rickettsiae isolated from ticks in China were compared with all known species and strains of spotted fever group rickettsiae by immunofluorescence assay, DNA polymerase chain reaction followed by restriction endonuclease fragment length polymorphism analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western immunoblot. The Chinese isolates belonged to three types, including a novel serotype which has not been described before. One isolate obtained from tick ova of Dermacentor nuttallii in Inner Mongolia was antigenically and genotypically identical to Rickettsia sibirica. Two isolates obtained from Dermacentor sinicus collected from Beijing were identical, different from other members of spotted fever group rickettsiae but apparently closely related to R. sibirica. HA-91, a strain isolated from Hyalomma asiaticum bv. kozlovi olenew, was antigenically and genotypically unique among spotted fever group rickettsiae, and we feel that data presented here should prompt consideration of it as a new species on the basis of current rickettsial taxonomy.
Full text
PDF

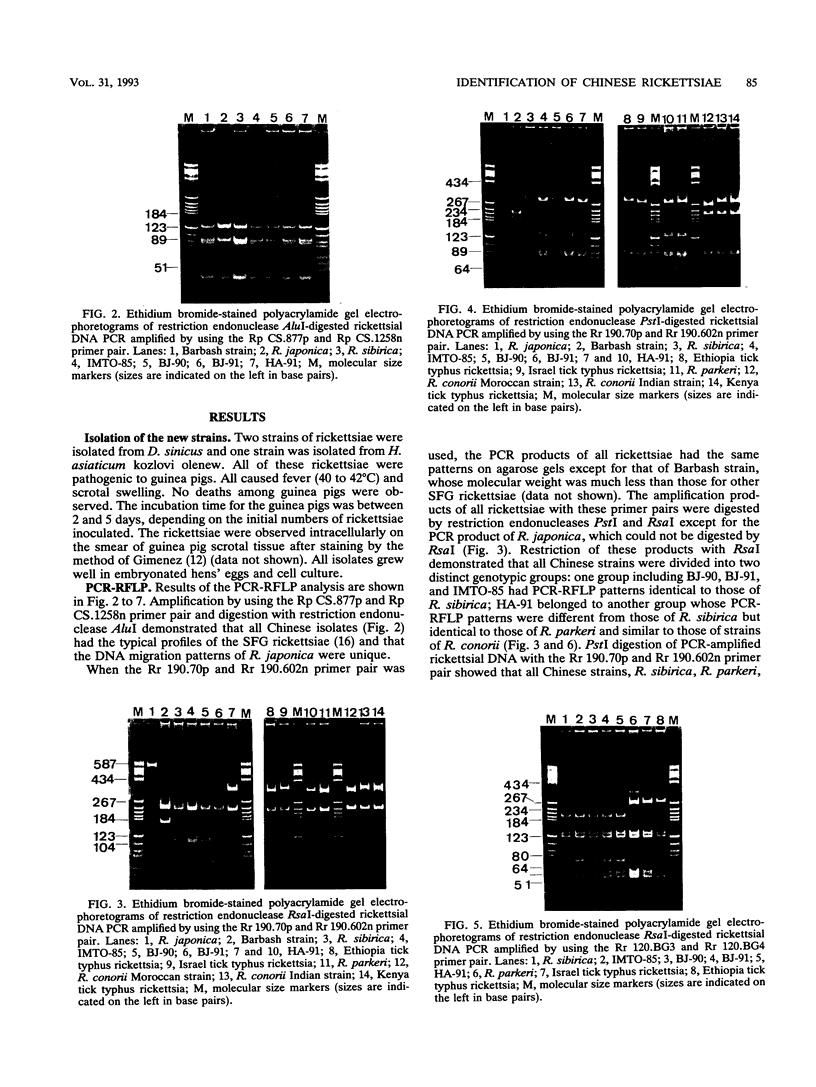

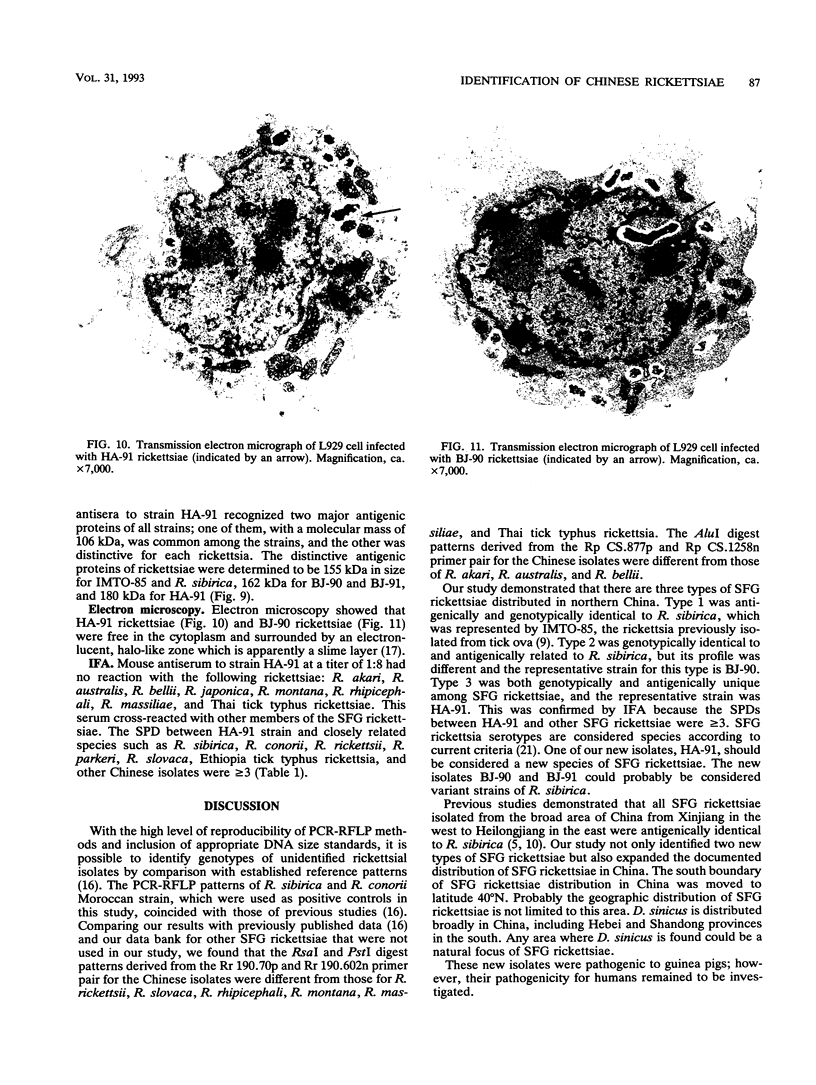

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beati L., Finidori J. P., Gilot B., Raoult D. Comparison of serologic typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: characterization of two new rickettsial strains. J Clin Microbiol. 1992 Aug;30(8):1922–1930. doi: 10.1128/jcm.30.8.1922-1930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W. Hemolymph test. A technique for detection of rickettsiae in ticks. Am J Trop Med Hyg. 1970 Nov;19(6):1010–1014. [PubMed] [Google Scholar]
- Dasch G. A., Ching W. M., Kim P. Y., Pham H., Stover C. K., Oaks E. V., Dobson M. E., Weiss E. A structural and immunological comparison of rickettsial HSP60 antigens with those of other species. Ann N Y Acad Sci. 1990;590:352–369. doi: 10.1111/j.1749-6632.1990.tb42242.x. [DOI] [PubMed] [Google Scholar]
- Drancourt M., Beati L., Tarasevich I., Raoult D. Astrakhan fever rickettsia is identical to Israel tick typhus rickettsia, a genotype of the Rickettsia conorii complex. J Infect Dis. 1992 Jun;165(6):1167–1168. doi: 10.1093/infdis/165.6.1167. [DOI] [PubMed] [Google Scholar]
- Fan M. Y., Walker D. H., Liu Q. H., Han L., Bai H. C., Zhang J. K., Lenz B., Hong C. Rickettsial and serologic evidence for prevalent spotted fever rickettsiosis in inner Mongolia. Am J Trop Med Hyg. 1987 May;36(3):615–620. doi: 10.4269/ajtmh.1987.36.615. [DOI] [PubMed] [Google Scholar]
- Fan M. Y., Yu X. J., Walker D. H. Antigenic analysis of Chinese strains of spotted fever group rickettsiae by protein immunoblotting. Am J Trop Med Hyg. 1988 Nov;39(5):497–501. doi: 10.4269/ajtmh.1988.39.497. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Joste N., McDonald G. A. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol Microbiol. 1989 Nov;3(11):1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Regnery R. L., Spruill C. L., Plikaytis B. D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991 Mar;173(5):1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]