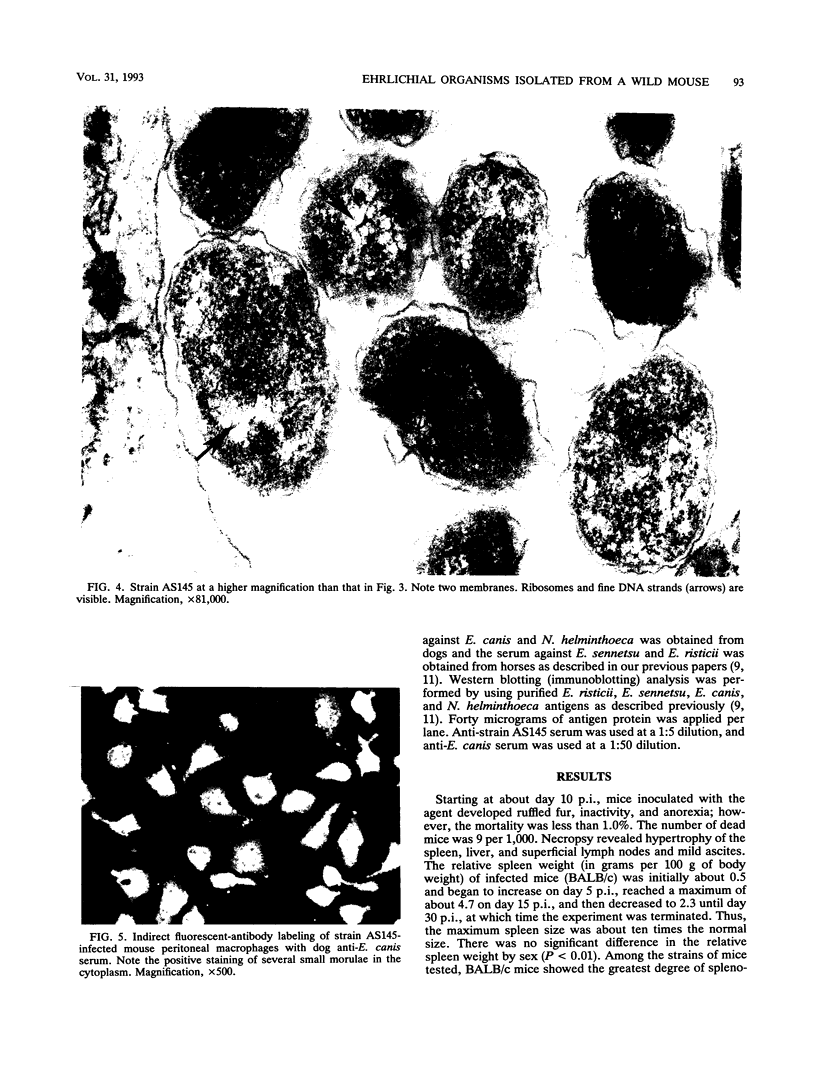
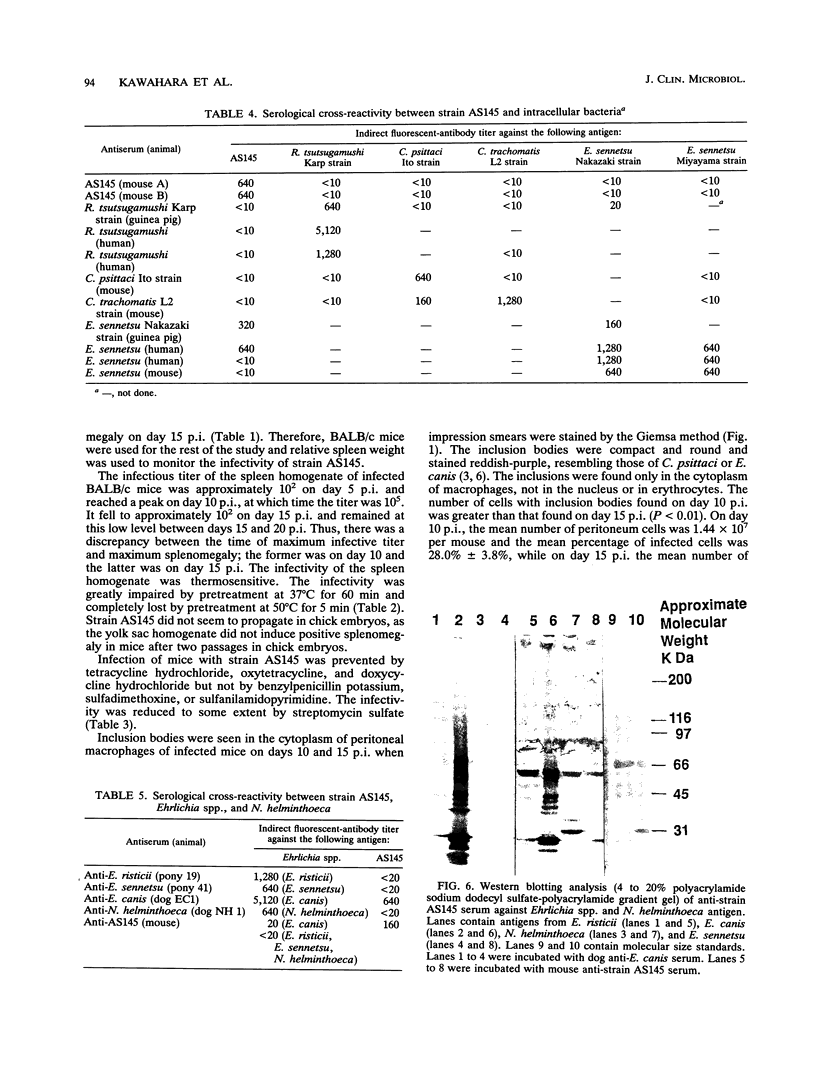
Abstract
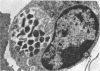
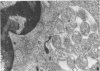
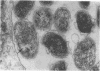
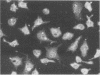
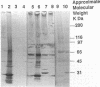
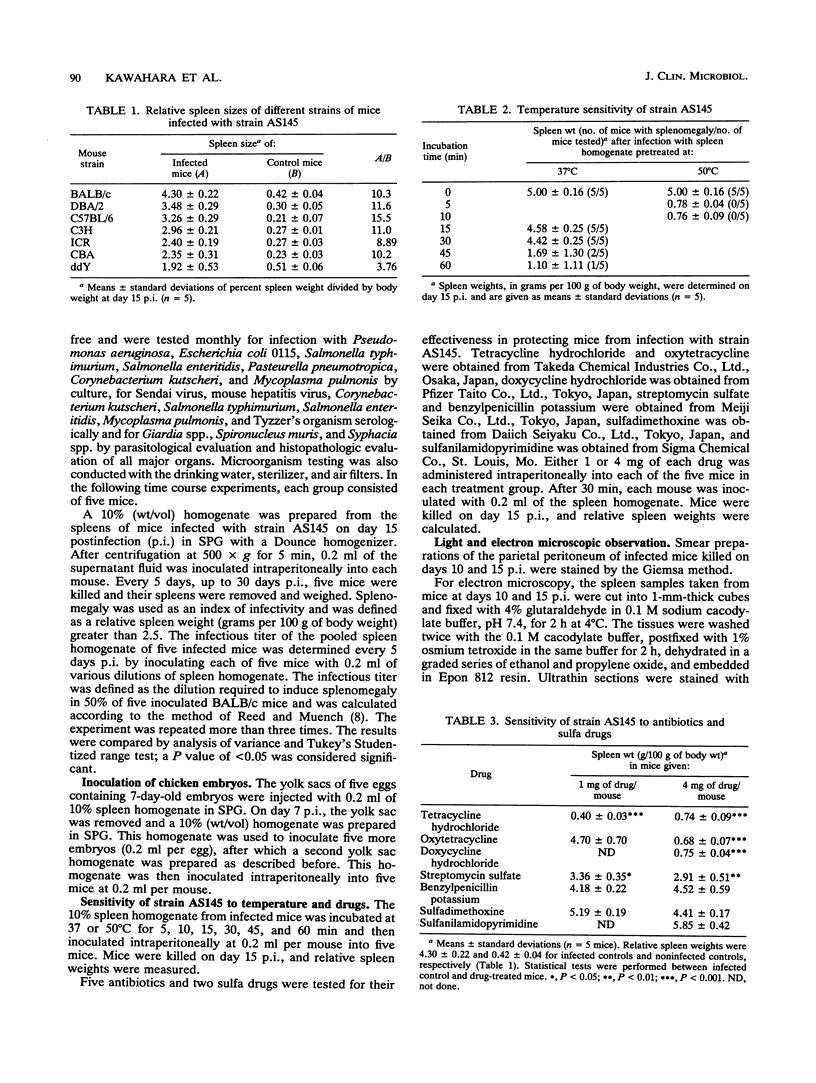
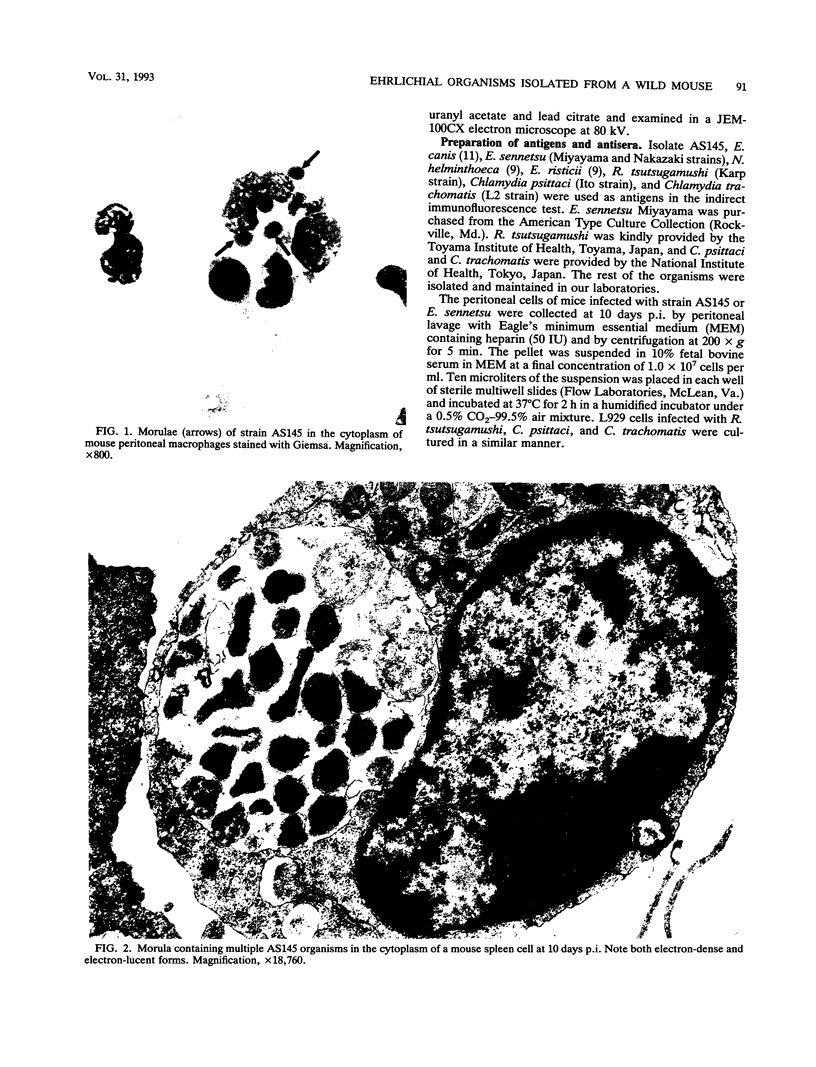
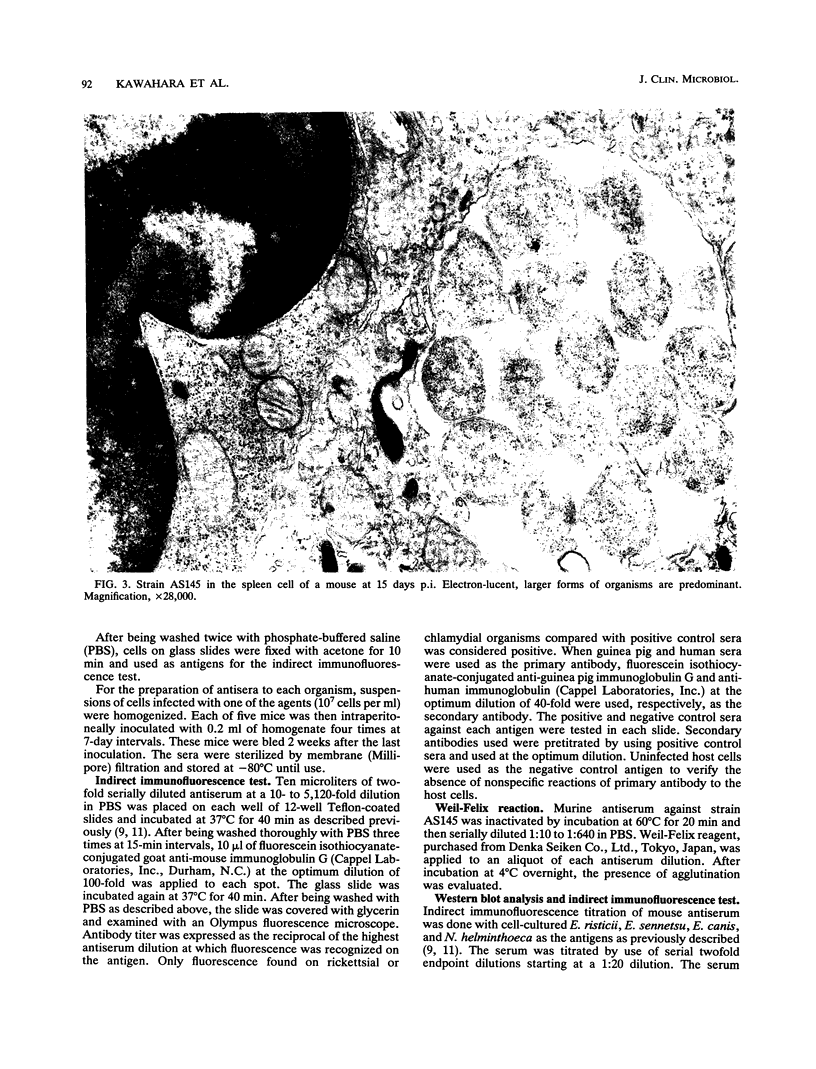
An infectious agent was isolated from the enlarged spleen of a wild mouse, Eothenomys kageus, by intraperitoneal inoculation of the spleen homogenate into laboratory mice. The laboratory mice developed splenomegaly, and the agent was maintained by serial passage of spleen homogenates in laboratory mice. The agent in the spleen homogenate was inactivated after incubation at 37 or 50 degrees C. Tetracyclines were effective in preventing infection of mice with this agent, but penicillin and sulfonamides were ineffective. Cytoplasmic inclusion bodies were observed in the peritoneal macrophages of infected mice. Electron microscopy revealed numerous small pleomorphic cocci within membrane-lined vacuoles in the cytoplasm of splenic macrophages. Morphologically similar to the ehrlichial organisms, each organism was surrounded by a distinct plasma membrane and rippled outer cell membrane without a distinct peptidoglycan layer. The agent did not grow in chicken embryos, and the Weil-Felix test result was negative. In the indirect fluorescent-antibody test, the agent reciprocally cross-reacted with Ehrlichia canis and cross-reacted somewhat with Ehrlichia sennetsu but did not cross-react with Ehrlichia risticii, Neorickettsia helminthoeca, Rickettsia tsutsugamushi, or Chlamydia spp. The mouse antiserum against this agent reacted with 64-, 47-, 46-, 44-, and 40-kDa proteins of E. canis by Western blotting (immunoblotting). Since E. canis and closely related Ehrlichia chaffeensis and Ehrlichia ewingii are not known to proliferate or cause splenomegaly in mice, these results suggest that the agent is a new species within the tribe Ehrlichieae of the family Rickettsiaceae. The finding suggests that wild rodents may serve as reservoirs for pathogenic ehrlichiae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. E., Dawson J. E., Jones D. C., Wilson K. H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991 Dec;29(12):2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., Greene C. E., Jones D. C., Dawson J. E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992 Apr;42(2):299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- Hildebrandt P. K., Conroy J. D., McKee A. E., Nyindo M. B., Huxsoll D. L. Ultrastructure of Ehrlichia canis. Infect Immun. 1973 Feb;7(2):265–271. doi: 10.1128/iai.7.2.265-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Huxsoll D. L., Cole A. I., Rapmund G. Adaptation of Ehrlichia sennetsu to canine blood monocytes: preliminary structural and serological studies with cell culture-derived Ehrlichia sennetsu. Infect Immun. 1985 May;48(2):366–371. doi: 10.1128/iai.48.2.366-371.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON J. W., DUFF J. T., GEARINGER N. F., ROBBINS M. L. GROWTH CHARACTERISTICS OF THREE AGENTS OF THE PSITTACOSIS GROUP IN HUMAN DIPLOID CELL CULTURES. J Infect Dis. 1965 Feb;115:49–58. doi: 10.1093/infdis/115.1.49. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J Clin Microbiol. 1991 Sep;29(9):2024–2029. doi: 10.1128/jcm.29.9.2024-2029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Ewing S. A., Fox J. C., Siregar A. G., Pasaribu F. H., Malole M. B. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J Clin Microbiol. 1992 Jan;30(1):143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Johnson G. C., Burger C. J. Reduced immune responsiveness and lymphoid depletion in mice infected with Ehrlichia risticii. Infect Immun. 1987 Sep;55(9):2215–2222. doi: 10.1128/iai.55.9.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Pretzman C. I., Johnson G. C., Reed S. M., Yamamoto S., Andrews F. Clinical, histopathological, and immunological responses of ponies to Ehrlichia sennetsu and subsequent Ehrlichia risticii challenge. Infect Immun. 1988 Nov;56(11):2960–2966. doi: 10.1128/iai.56.11.2960-2966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991 Jul;4(3):286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]