Abstract
After pacemaker/implantable cardioverter-defibrillator (pacemaker/ICD) implantation, patients are often required to immobilize the affected arm with a sling to minimize the risk of lead displacement. We examined whether performing a resistive range-of-motion exercise protocol after pacemaker/ICD surgery would result in lead displacement and, therefore, whether sling immobilization and activity restrictions are justified. Ten subjects who had undergone pacemaker/ICD surgery performed four individual resistive range-of-motion exercises (three sets of 10 repetitions for each: one warm-up set without weight and two sets with a 1- or 2-pound hand weight) with the affected arm prior to hospital discharge. For each subject, an electrophysiology nurse specialist used a noninvasive device programmer to evaluate surgical lead placement before and after the exercises. As an adjunct to the study, we queried clinicians at 48 US hospitals about sling immobilization and activity restrictions after pacemaker/ICD implantation at their institutions. No lead displacement occurred after the weightlifting exercises were performed. Based on these results in a small group of patients, it appears that requiring the use of a joint immobilization sling is overly restrictive, promotes fear, and hinders recovery. We encourage the development of consistent discharge instructions that will promote early mobility and a safe and rapid return to normal activities.
When a joint is immobilized, the muscles, bones, and surrounding connective tissue begin to degenerate quickly (1). Connective tissue, which tends to shorten very slowly and progressively if it is not opposed by a considerable force (2), contains chondrocytes that are responsible for the formation, maintenance, and repair of articular cartilage (3). Within the first day of immobilization, chondrocyte activity changes, signaling the beginning of degeneration (1). The second day brings a noticeable decrease in proteoglycans (4), which contribute to the stiffness of cartilage. By the third day (5), degenerative changes are seen in chondrocytes in the areas of contact between articular surfaces, and by the fourth day, there is a marked decrease in proteoglycan content (4). As the tissue contracts and reorganizes, it becomes denser and, usually within a week, results in restricted range of motion (6).
Degenerative changes to bones and muscles also occur rapidly during immobilization. It has been shown that changes in the mechanical properties of the spine occur after only 4 days of bed rest (7). Research has shown that a dramatic change in muscle mass occurs within 4 to 6 weeks of bed rest. This decrease in muscle mass is accompanied by decreases of 6% to 40% in muscle strength (8). Skeletal muscle strength declines by 1% to 1.5% per day after strict bed rest (9), and the decline in strength is even more profound (1.3% to 5.5% per day) with cast immobilization (10). It is important to understand the negative consequences of joint immobilization and the need for early motion. Immobilization has clearly detrimental effects on the surrounding tissues, including shortening, decreased tensile strength, edema formation, venous stasis, and atrophy (11). These changes may lead to situations such as persistent stiffness and pain, muscle atrophy, and decreased range of motion—conditions that inevitably produce dysfunction and/or disability (12, 13). In one study, subjects spent 9 days wearing a cast suspended from the neck by a sling that immobilized muscles acting on the wrist. The cross-sectional area of the forearm muscle decreased by 4.1%. Isometric strength for flexion and extension decreased by 29.3% and 32.5%, respectively (14).
With shorter lengths of stay following surgery, patients returning home need to have the byproducts of surgery addressed promptly and efficiently (11). When joint mobility is lost, patients become fearful of and have difficulty performing activities of daily living.
Slings have been used to treat dislocated shoulders for thousands of years, although there is little information on their efficacy (15). Using a sling to immobilize the arm of the accessed shoulder after pacemaker/implantable cardioverter-defibrillator (pacemaker/ICD) surgery is intended to reduce the risk of lead displacement (16), which is the most common complication of transvenous pacing (17). Displacement rates should be <2% for ventricular leads and <3% for atrial leads (17). Pacemaker lead displacement can result in myocardial irritability (18), increased pacing thresholds, failure to capture, or failure to sense (19). Despite the importance of the topic, studies of lead displacement are rare (20). Information about causes is scarce, and it is often difficult to relate lead displacements to a specific etiology. Some that have been identified are “Twiddler's syndrome,” in which the patient manipulates the pulse generator; “Reel syndrome,” in which the patient rotates the generator (20); and direct trauma over the system, caused by intense respiratory therapy such as clapping (21). Interestingly, weightlifting and excessive activity with the arm on the accessed side were not mentioned as causes of lead displacement.
During the first 24 to 48 hours following pacemaker/ICD implantation, the arm of the accessed shoulder is often immobilized with a sling (16). Upon discharge, patients may be given this common postsurgical advice: “Don't lift more than 10 pounds for the first 2 weeks after surgery” (22) or “Avoid lifting the affected arm higher than the shoulder level for the first few weeks after implantation” (17). Physicians differ in their opinions about the use of slings. Some require their patients to use them; others do not and instead encourage their patients to perform range-of-motion exercises. Neither of these practices has been thoroughly investigated.
Our study tested whether lead displacement would occur in patients who not only did without an immobilization sling during the first 24 hours after pacemaker/ICD implantation but also performed a series of resistive range-of-motion exercises.
METHODS
Ten white subjects (four women and six men) gave consent and participated in the study, which was approved by the institutional review board. A study investigator and an electrophysiology nurse specialist led the experimental protocol in each subject's hospital room between 2 and 24 hours after the procedure but before hospital discharge. Appropriate postsurgical lead placement was confirmed by use of a noninvasive device programmer both before and after the subjects performed the exercise protocol. An electrophysiology nurse specialist provided the programmer (Medtronic model 2090, Medtronic, Inc., Minneapolis, MN) at the subject's bedside. Subjects were informed that the lead placement for their device was to be tested before and confirmed after performance of the weightlifting activity.
The subject was connected to the programmer using a standard monitoring lead configuration. The programming head of the programmer was placed over the device. The device was interrogated, and the initial programmed parameters, including mode, sensitivity, amplitude, and impedance, were printed and reviewed.
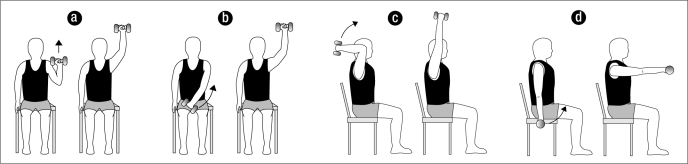
The subjects were asked to sit on their hospital bed, put their feet flat on the floor, and perform four resistive range-of-motion exercises using the arm of the affected shoulder. The subjects were coached by the study investigator on proper technique and were led through a warm-up/educational range-of-motion set of 10 repetitions of the following exercises: military press, diagonal raise, overhead triceps extension, and frontal raise (Figure). Subjects then performed two sets of 10 repetitions of the same resistive exercises while holding either a 1- or 2-pound hand weight. The subjects' ability to tolerate the exercise load determined which weight was used. Exercises were performed to the cadence of 2 seconds up and 2 seconds down and were completed within 15 minutes.
Figure.
The four exercises performed by the study subjects: (a) military press, (b) diagonal raise, (c) overhead triceps extension, and (d) frontal raise.
The electrophysiology nurse examined the pre- and post activity parameters—including sensitivity threshold, pacing threshold, and impedance for the leads and device—to confirm that no change in lead status had occurred following the weightlifting protocol. At implantation, the values for these parameters were considered normal and appropriate according to the following criteria: 1) the sensitivity threshold was >2 mV in the atrium (P waves) and >5 mV in the ventricle (R waves); 2) for pacing threshold, the loss of capture was <2 V/.5 ms in the atrium and <1 V/.5 ms in the ventricle; and 3) lead impedance values were approximately 300 to 1000 Ω The criteria for dislodgment were as follows:
Sensitivity threshold was assessed by printing intracardiac electrograms and manually measuring P and R waves on a 25 mm/s scale with an amplitude of 1 mV/mm. Dislodgment would be indicated by a value that was 15% lower than the value at implantation.
Pacing threshold was evaluated starting at an output of 5 V/.5 ms. Utilizing automatic testing, the amplitude was decremented until loss of capture was noted or a minimum of .5 V/.5 ms was reached. Dislodgment would be indicated by a value that was 15% higher than the value at implantation.
Lead impedance was assessed through automatic testing to confirm stability of the lead and to assure set screw connection at the header. Dislodgment would be indicated by a value that was 15% higher or lower than the value at implantation.
All parameters were reviewed with the evaluation findings to ensure appropriate safety margins and programming.
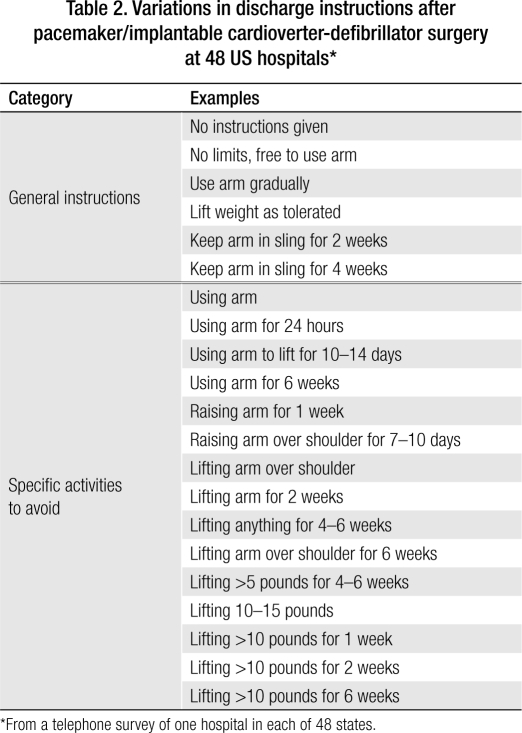
As an adjunct to this study, we investigated the instructions given to patients by physicians and surgeons after pacemaker/ICD implantation nationwide and whether immobilization was used. In a telephone survey of clinicians at 48 US hospitals where these procedures are commonly done, we asked about sling use and about the discharge instructions given after pacemaker/ICD implantation. The hospitals were randomly chosen, and each was in a different state.
RESULTS
Ten patients participated as subjects in this study. Demographic characteristics are summarized in Table 1. Each subject successfully performed a warm-up set of each exercise without weights, then two sets of 10 repetitions with a 1- or 2-pound weight during each of the four exercises. No lead displacement occurred after the resistive range-of-motion exercises were performed.
Table 1.
Demographic characteristics of the study subjects∗
| Characteristic | Patients (n = 10) |
| Age (y) | 60 ± 13 |
| Weight (lb) | 215 ± 51 |
| Height (in) | 69 ± 3 |
| Sex: female (n) | 4 |
| Race: white (n) | 10 |
∗Mean ± SD unless otherwise indicated.
We posed the following question to clinicians in 48 states via an informal telephone survey: “Do you use a sling on patients after pacemaker/ICD implantation, and if so, what are your recommendations?” The most common response from clinicians at hospitals where slings are used was that inpatients wear the sling “24 hours until hospital discharge,” although some advised use of the sling for 2 to 3 days and one hospital advised use of it for nearly a month. Each hospital clinician was also asked, “What are your discharge instructions to patients following pacemaker/ICD surgery?” The most common discharge instructions were “Don't lift your arm over your shoulder” and/or “Don't lift more than 10 pounds for 2 weeks.” The responses about discharge instructions are listed in Table 2.
Table 2.
Variations in discharge instructions after pacemaker/implantable cardioverter-defibrillator surgery at 48 US hospitals∗
∗From a telephone survey of one hospital in each of 48 states.
DISCUSSION
We found that the activity advice given to patients after pacemaker/ICD surgery was often overly restrictive and did not take into account patients' need to lift the affected arm over their head or how quickly their joint mobility might be compromised. As the results of our informal survey show, sling use and discharge activity instructions vary widely among different US hospitals. In fact, so many different responses were given that it was impossible to categorize them. Some hospitals never use the sling, while others require it for 4 weeks. Four hospitals, each in a different state, use an elastic bandage wrap to restrain the arm and shoulder (more restrictively than a sling) for times ranging from 24 to 72 hours. Patients at one hospital are told not to lift their affected arm over the shoulder for 6 weeks, while those at another hospital are told they have unrestricted use of the arm immediately after surgery (see Table 2).
The American College of Sports Medicine guidelines recommend that patients not lift their affected arm above shoulder height for 2 to 3 weeks (23). Following these guidelines would preclude patients from necessary daily activities like washing their hair or reaching into a kitchen cabinet, making them feel vulnerable and dependent on others for even basic tasks. For those who live alone, this dependence can be emotionally devastating. Although activity restrictions are intended to protect patients, they may have unintended consequences: fear and anxiety about potential self-injury can lead to complete inactivity.
The study investigator who led the subjects through the exercise protocol observed an interesting phenomenon. The subjects (and often their spouse or significant other) seemed extremely anxious and fearful at the start of the resistive exercises. By the session's end, however, their fear had been replaced by complete confidence as the protocol was successfully completed.
Pacemaker/ICD procedures are performed under local anesthesia and take 1 to 5 hours to complete, depending on the number and location of pacing leads being inserted. The pulse generator is placed in a subcutaneous pocket in the pectoral area, and the pacing lead is inserted either through the cephalic vein or through the subclavian vein into the right ventricular apex, the septum, or occasionally the right ventricular outflow tract (24). Some complications of pacemaker/ICD implantation include pneumothorax, embolism, perforation of the heart, arrhythmia, heart valve damage, infection, and pain (25). We are neither minimizing the significance of the surgery nor advocating that physicians ignore potential complications by encouraging patients to perform exercises that would exacerbate them; however, sling immobilization represents the opposite extreme.
Sling immobilization following pacemaker/ICD surgery may result in muscle atrophy and weakness. Patients typically avoid movement in the area of their surgical sites, exacerbating the problem and making it difficult to regain previous strength and range of motion. If joints, muscles, and other supporting tissues are not taken through full range of motion, adhesions develop and musculature becomes weaker and may foreshorten. Exercise increases blood flow to the injured area and accelerates healing. The longer exercising is delayed, the more difficult it is for the patient to fully recover (26).
The main limitation of our study was the small sample size, which was related to two factors. First, we were restricted to uncomplicated cases performed by the two participating cardiologists. Second, patients who needed cardiac resynchronization therapy and revision (generator change) were excluded from the study, and most who needed these procedures were patients of the participating cardiologists.
Challenging conventional wisdom in clinical practice is not easy, and doing so often results in a classic catch-22 scenario: large-scale studies are needed to validate a new practice; however, the perceived risks involved in testing that new practice can make enrolling large numbers of subjects difficult if not impossible. Although a larger investigation is needed to substantiate our results, our study is an important first step for two reasons. First, we have demonstrated that patients can safely perform resistive exercises with the affected arm within 24 hours of receiving a pacemaker/ICD. Second, we have documented wide variations in the use of sling immobilization after pacemaker/ICD implantation in the USA and thus shown the need for change.
CONCLUSION
Our study results show that patients can safely perform resistive range-of-motion exercises soon after pacemaker/ICD surgery, but our findings require validation by further research. Nevertheless, we believe strongly that the immobilization practiced at many institutions is overly restrictive, promotes fear, and hinders recovery. Instead of focusing on joint immobilization and activity restrictions, health care providers in cardiac electrophysiology should develop consistent guidelines that encourage patients to perform safe activities that promote healing and help them return quickly to normal daily life.
References
- 1.Videman T, Michelsson J, Rauhamäki R, Langenskiöld A. Changes in 35S-sulphate uptake in different tissues in the knee and hip regions of rabbits during immobilization, remobilization and the development of osteoarthritis. Acta Orthop Scand. 1976;47(3):290–298. doi: 10.3109/17453677608991993. [DOI] [PubMed] [Google Scholar]
- 2.Hepburn GR. Case studies: contracture and stiff joint management with Dynasplint™. J Orthop Sports Phys Ther. 1987;8(10):498–504. [Google Scholar]
- 3.Percival M. Nutritional support for connective tissue repair and wound healing. Clinical Nutrition Insights. 1997:1–5. Available at http://acudoc.com/Injury%20Healing.PDF; accessed April 29, 2008. [Google Scholar]
- 4.Troyer H. The effect of short-term immobilization on the rabbit knee joint cartilage. A histochemical study. Clin Orthop Relat Res. 1975;(107):249–257. doi: 10.1097/00003086-197503000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz RW. Experimental models of degenerative joint disease. Semin Arthritis Rheum. 1972;2(1):95–116. doi: 10.1016/0049-0172(72)90017-0. [DOI] [PubMed] [Google Scholar]
- 6.Kottke FJ, Pauley DL, Ptak RA. The rationale for prolonged stretching for correction of shortening of connective tissue. Arch Phys Med Rehabil. 1966;47(6):345–352. [PubMed] [Google Scholar]
- 7.LeBlanc AD, Evans HJ, Schneider VS, Wendt RE, 3rd, Hedrick TD. Changes in intervertebral disc cross-sectional area with bed rest and space flight. Spine. 1994;19(7):812–817. doi: 10.1097/00007632-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Müller EA. Influence of training and of inactivity on muscle strength. Arch Phys Med Rehabil. 1970;51(8):449–462. [PubMed] [Google Scholar]
- 10.Müller EA, Hettinger T. The difference of training speed of atrophied and normal muscles. Arbeitsphysiologie. 1953;15(3):223–230. [PubMed] [Google Scholar]
- 11.Hammesfahr R, Serafino MT. CPM: the key to successful rehabilitation. Reprinted from Orthopedic Technology Review. 2002;3(2) Available at http://www.arthroscopy.com/sp06005.htm; accessed January 25, 2008. [Google Scholar]
- 12.Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989;(242):12–25. [PubMed] [Google Scholar]
- 13.De Deyne PG. Application of passive stretch and its implications for muscle fibers. Phys Ther. 2001;81(2):819–827. doi: 10.1093/ptj/81.2.819. [DOI] [PubMed] [Google Scholar]
- 14.Miles MP, Clarkson PM, Bean M, Ambach K, Mulroy J, Vincent K. Muscle function at the wrist following 9 d of immobilization and suspension. Med Sci Sports Exerc. 1994;26(5):615–623. [PubMed] [Google Scholar]
- 15.Murrell GA. Treatment of shoulder dislocation: is a sling appropriate? Med J Aust. 2003;179(7):370–371. doi: 10.5694/j.1326-5377.2003.tb05596.x. [DOI] [PubMed] [Google Scholar]
- 16.Sunderlin MK. Keeping pace with cardiac devices. RN. 2006;69(7):40–46. Available at http://rn.modernmedicine.com/rnweb/article/articleDetail.jsp?id=339912; accessed July 29, 2008. [PubMed] [Google Scholar]
- 17.Hayes DL, Lloyd MA, Friedman PA. Cardiac Pacing and Defibrillation: A Clinical Approach. Vol. 199. Armonk, NY: Futura Publishing Co; 2000. p. 453. [Google Scholar]
- 18.Kaiser Permanente. The implantation procedure Available at http://www.kpep.org/icd/icd_implantation.htm; accessed April 18, 2008.
- 19.Vijayaramen P, Ellenbogen KA. Bradyarrhythmias and pacemakers. In: Fuster V, Alexander RW, O'Rourke RA, Roberts R, King SB 3rd, Nash IS, Prystowsky EN, editors. Hurst's the Heart. 11th ed. Vol. 2. New York: McGraw-Hill; 2004. p. 919. [Google Scholar]
- 20.Fuertes B, Toquero J, Arroyo-Espliguero R, Lozano IF. Pacemaker lead displacement: mechanisms and management. Indian Pacing Electrophysiol J. 2003;3(4):231–238. [PMC free article] [PubMed] [Google Scholar]
- 21.Arroyo Espliguero R, Oteo Domínguez JF, Castedo Mejuto E, Antorrena Miranda I, Ortigosa Aso J, Cañas Cañas A, Artaza Andrade M. Late displacement of a ventricular pacing lead after respiratory therapy. Pacing Clin Electrophysiol. 2001;24(11):1693–1695. doi: 10.1046/j.1460-9592.2001.01693.x. [DOI] [PubMed] [Google Scholar]
- 22.Allina Hospitals and Clinics. Self care after implantable device surgery Available at http://www.medformation.com/ac/hearthealth.nsf/page/scaids; accessed April 18, 2008.
- 23.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 195. [Google Scholar]
- 24.Woods SL, Froelicher ESS, Motzer SU, Bridges EJ. Cardiac Nursing. 5th ed. Vol. 713. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 721. [Google Scholar]
- 25.Ellenbogen KA. Practical Cardiac Diagnosis Series: Cardiac Pacing. 2nd ed. Cambridge, MA: Blackwell Science; 1996. pp. 247–256. [Google Scholar]
- 26.Pollock ML, Wilmore JH. Exercise in Health and Disease: Evaluation and Prescription for Prevention and Rehabilitation. 2nd ed. Philadelphia: WB Saunders; 1990. p. 523. [Google Scholar]