Abstract
Thrombocytosis is frequently encountered as an incidental laboratory finding. The most common etiology is reactive (secondary) thrombocytosis due to infections, trauma, surgery, or occult malignancy. Even though thrombocytosis is benign and self-limiting in most cases, it can result in hemorrhage or thrombosis. The hypercoagulable state is characterized by episodes of thrombosis and can be due to inherited or acquired conditions. Extreme thrombocytosis may result in thrombotic events such as acute myocardial infarction, mesenteric vein thrombosis, and pulmonary embolism. It is important for physicians to be familiar with the complications associated with thrombocytosis. Postsplenectomy reactive thrombocytosis has an incidence of about 75% to 82%. Thrombosis in association with elevated platelet count after splenectomy is well recognized, with an incidence of approximately 5%. This case report describes a 61-year-old patient who underwent emergent splenectomy and presented 1 week later with acute ST segment elevation myocardial infarction. Severe thrombocytosis, which was not present prior to splenectomy, was noted, and a diagnosis of reactive thrombocytosis was initially made. Involvement of the right coronary artery led to emergent percutaneous transluminal coronary angioplasty. Essential thrombocytosis was considered when treatment with hydroxyurea failed to lower the platelet count. A review of arterial and venous thrombosis in patients with severe thrombocytosis is presented, and the approach to the management of such patients is discussed.
The normal platelet count in adults ranges from 150 to 450 K/μL. The definition of thrombocytosis varies among authors (1, 2) but is most commonly defined as a platelet count >500 K/μL (3). Platelet counts >1000 K/μL are not unusual in a general hospital population (1); however, such extreme numbers are encountered more commonly in myeloproliferative disorders or after splenectomy (4). In one study, 75% of individuals without myeloproliferative disorders developed thrombocytosis after splenectomy (5). Platelet counts after splenectomy have been reported to increase 30% to 100%, with a peak reached at 7 to 20 days postoperatively (3). Common complications of thrombocytosis include thrombosis and hemorrhage. Postsplenectomy venous thrombosis is usually associated with platelet counts >600 to 800 K/μL (6, 7) and occurs in approximately 5% of patients (8). Less commonly, postsplenectomy thrombocytosis results in arterial thrombosis that leads to stroke or myocardial infarction (9, 10).
Regardless of the cause, thrombocytosis leads to platelet hyperaggregation; therefore, the first line of therapy is the administration of platelet-antiaggregating medication such as aspirin. For extreme thrombocytosis with evidence of arterial or venous thrombosis, patients may need cytoreductive agents such as hydroxyurea or anagrelide with close monitoring of platelet counts (11, 12).
CASE REPORT
A 61-year-old white man with previous systemic hypertension and hypothyroidism presented at the outpatient clinic with a 1-day history of left upper quadrant abdominal pain. The pain was described as constant, severe, and nonradiating. Examination showed tenderness to palpation in the left upper quadrant and a possible mass measuring 5 to 7 cm. An ultrasound of the abdomen showed a mass in the spleen. The patient was hospitalized.
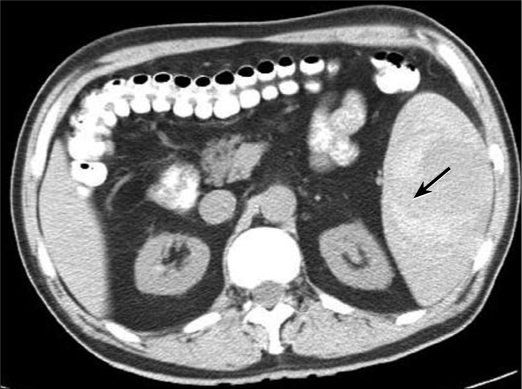
Laboratory values were significant for a hemoglobin level of 15.5 g/dL (reference range, 13.5–18.0 g/dL), a hematocrit of 43.4% (reference range, 4.0%–52.0%), and a platelet count of 125 K/μL (reference range, 140–440 K/μL). The patient's vitamin B12 level on admission was 50 pg/mL (reference range, 180–914 pg/mL). The low platelet count upon admission was suggestive of spleno-megaly, low levels of vitamin B12, and hyperfunction of the spleen. An echocardiogram ruled out endocarditis. A computed tomography scan of the abdomen without contrast revealed a hemorrhagic mass measuring 10 × 7.4 × 6.7 cm (Figure 1).
Figure 1.
Computed tomography scan without contrast at initial presentation. Note the hematoma (arrow).
The patient was started on aggressive vitamin B12 replacement. His hemoglobin dropped on the subsequent day from 15.5 to 10.1 g/dL, and he developed hypotension with no signs of sepsis. Due to the concern of continuous bleeding into his spleen and the possibility of an underlying neoplasm, a splenectomy was performed. Histological studies of the mass showed extensive hemorrhage and fibrin deposits, which supported the diagnosis of a splenic hemorrhage and hematoma.
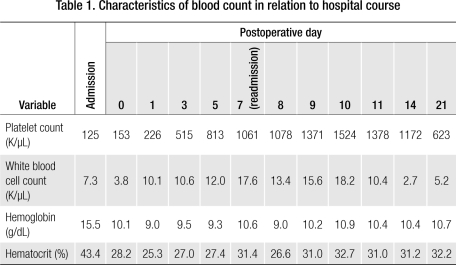
After the surgery, reactive thrombocytosis was observed in the patient. His platelet count was 226 K/μL immediately after splenectomy and was abnormally elevated to 813 K/μL on postoperative day 5, the day of his discharge (Table 1). The patient remained asymptomatic at discharge, and the recommendations for reactive thrombocytosis after splenectomy (e.g., mobilization, increased fluid intake) were discussed with him. He was started on 81 mg of aspirin per day.
Table 1.
Characteristics of blood count in relation to hospital course
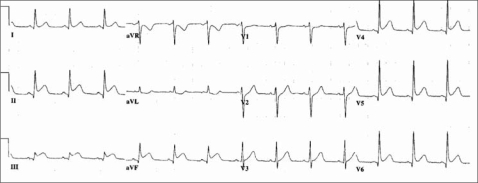
The patient presented to the emergency department 3 days after discharge with chest pain. Because the electrocardiogram suggested an inferior wall acute myocardial infarction (Figure 2), he underwent immediate cardiac catheterization, which revealed complete blockage of the distal right coronary artery. He was treated with balloon dilation without a stent.
Figure 2.
Electrocardiogram on readmission for acute chest pain. Acute ST segment elevation is shown in leads II, III, and aVF.
His platelet count was 1061 K/μL upon readmission and increased to 1524 K/μL during the 2 days after admission (Table 1). He was started on hydroxyurea, and 1 week after his readmission his platelet count began decreasing. He experienced severe nausea, vomiting, and leukopenia and was switched to 0.5 mg of anagrelide 3 times a day. On discharge, his platelet count was 623 K/μL. The patient also underwent bone marrow biopsy, which ruled out myeloproliferative disorders.
At 1-year follow-up, the patient's complete blood count remains normal. He continues his B12 supplement, and anagrelide has been titrated to 0.5 mg twice a day. He has been asymptomatic and working full-time with no recurrent cardiac events.
DISCUSSION
Reactive thrombocytosis is the presence of high platelet count in response to infection, trauma, or surgery (4, 13). The platelet count in reactive thrombocytosis is expected to normalize after resolution of the underlying condition (3). Secondary causes of an elevated platelet count (e.g., myeloproliferative disorders, splenectomy, and occult malignancy) must be ruled out in such patients.
Reactive thrombocytosis is a common cause of thrombocytosis (2, 4). In one study of patients with thrombocytosis, reactive thrombocytosis was diagnosed in 70% and primary thrombocytosis in only 22% (14). Similarly, in patients with extreme thrombocytosis (i.e., platelet count >1000 K/μL [15]), reactive thrombocytosis is a more common cause of thrombocytosis than is primary or essential thrombocytosis (1). In another study of 280 patients with a platelet count >1000 K/μL, reactive thrombocytosis was the cause in more than 80% and myeloproliferative disorder in only 14% (1). Splenectomy was found to be one of the main causes of extreme reactive thrombocytosis (1) (Table 2).
Table 2.
Etiology of extreme thrombocytosis∗
| Causes | Percentage reported |
| Infection | 31% |
| Postsplenectomy | 19% |
| Malignancy | 14% |
| Trauma | 14% |
| Inflammation | 9% |
| Blood loss | 6% |
| Rebound thrombocytosis | 3% |
| Uncertain etiology | 4% |
Essential thrombocytosis is the increased production of platelets in the absence of other myeloproliferative disorders. Essential thrombocytosis is characterized by persistent thrombocytosis >600 K/μL, confirmed by a bone marrow biopsy signifying megakaryocytic hyperplasia (16, 17). Essential thrombocytosis is diagnosed at a rate of 2 to 3 per 100,000 individuals every year (18). It usually affects middle-aged to elderly individuals, with an average age at diagnosis of 50 to 60 years (as in our patient), although it can affect any age population (19).
Essential thrombocytosis is not a well-recognized cause for arterial and venous thrombosis (20); however, case reports of patients with arterial and venous thrombosis requiring intervention have been reported in the literature (21, 22). Most cases of reported postsplenectomy thrombosis have been in portal, mesenteric, and splenic veins (8). Very few cases of myocardial infarction are reported with thrombocytosis, and, when it does occur, it is mainly in patients with essential thrombocytosis (10, 23, 24).
Essential thrombocytosis is a diagnosis of exclusion by ruling out known causes of reactive thrombocytosis. Even though our patient had a bone marrow biopsy that did not demonstrate megakaryocytic hyperplasia, we suspected essential thrombocytosis due to the absence of other myeloproliferative disorders and persistent thrombocytosis when the hematologist attempted to wean anagrelide. We also suspect a cumulative rebound effect from splenectomy, aggressive B12 treatment, and severe anemia.
Pathophysiology
Reactive thrombocytosis is thought to result from overproduction of one or more thrombopoietic factors that act on megakaryocytes or their precursors (3). Elevated levels of these growth factors are observed in various infectious, inflammatory, malignant, and traumatic processes (25). Of all the growth factors identified, interleukin (IL)-6 plays a primary role in reactive thrombocytosis (3).
Elevated IL-6 is found in some patients with iron-deficiency anemia, suggesting some other mechanism for reactive thrombocytosis in these patients. The association of iron-deficiency anemia with thrombocytosis points to an interrelationship between erythropoietic and thrombotic growth factors (3, 7). This may explain persistent thrombocytosis (as seen in our patient) in association with prolonged anemia.
The spleen plays a major role in platelet regulation, as it is the primary site of destruction of platelets, which is why thrombocytosis is seen with hyposplenism (26). Reactive thrombocytosis is a predictable finding after splenectomy, with the platelet count peaking at 1 to 3 weeks and returning to normal levels in weeks, months, and, rarely, years (3).
As pointed out above, the combination of hyposplenism, B12 replacement, and severe anemia could have precipitated extreme thrombocytosis in our patient.
Management
The first step in managing a patient who presents with elevated platelet count is to determine if the etiology is a primary process or a reactive response (27). The immediate risk to the patient from the increased platelet count should be assessed. Thereafter, management of the thrombocytosis and prevention of complications should be initiated. Patients with essential thrombocytosis who have had thrombotic events and cardiovascular risk factors should be treated (28). Some pharmacologic agents used for this purpose are listed in Table 3, along with their associated adverse effects. The anticipated reduction in risk of future thrombosis should outweigh the risk of complications from drug therapy. Hydroxyurea has been the treatment of choice (16, 17, 28) in many studies.
Table 3.
Pharmacologic agents for management of extreme thrombocytosis and associated adverse effects∗
| Drugs | Adverse effects |
| Acetyl salicylic acid | Bleeding |
| Hydroxyurea | Venous thrombosis, leukemic transformation |
| Anagrelide | Anemia, headache, tachycardia, edema |
| Interferon alpha | Immunosuppression |
| Ticlopidine | Bleeding, leukemic transformation |
| Enoxaparen | Bleeding, thrombocytopenia, headache |
| Plasmapheresis | Bleeding, infection, lung injury |
Anagrelide is a newer platelet-lowering agent that has been approved in patients with essential thrombocytosis. Harrison and colleagues compared hydroxyurea plus aspirin with anagrelide plus aspirin as initial therapy for essential thrombocytosis (11). The study showed that, compared with anagrelide, hydroxyurea was superior in lowering the risk of arterial thrombosis, hemorrhage, and transformation to myelofibrosis. However, it also had higher rates of venous thrombosis than anagrelide. The risk of bleeding associated with aspirin use should be kept in mind in patients with thrombocytosis. With hydroxyurea, patients should be monitored for leukemic transformation. Long-term data on the side effects and complications of anagrelide are lacking; however, preliminary data suggest it is well tolerated, with mild to moderate anemia as a frequent side effect (12). Plasmapheresis is another option for rapid reduction of platelet count in life-saving clinical situations.
Acknowledgments
We thank Dr. Shannon Moss, Dr. Kanti Agrawal, Dr. Benton R. Middleman, Debra Faber, and the Department of Radiology at Baylor Medical Center at Garland for their encouragement and support in the preparation of this report.
References
- 1.Buss DH, Cashell AW, O'Connor ML, Richards F, 2nd, Case LD. Occurrence, etiology, and clinical significance of extreme thrombocytosis: a study of 280 cases. Am J Med. 1994;96(3):247–253. doi: 10.1016/0002-9343(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 2.Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245(3):295–300. doi: 10.1046/j.1365-2796.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 3.Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 1981. pp. 1128–1134. [Google Scholar]
- 4.Santhosh-Kumar CR, Yohannan MD, Higgy KE, al-Mashhadani SA. Thrombocytosis in adults: analysis of 777 patients. J Intern Med. 1991;229(6):493–495. doi: 10.1111/j.1365-2796.1991.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 5.Boxer MA, Braun J, Ellman L. Thromboembolic risk of postsplenectomy thrombocytosis. Arch Surg. 1978;113(7):808–809. doi: 10.1001/archsurg.1978.01370190030004. [DOI] [PubMed] [Google Scholar]
- 6.Hayes DM, Spurr CL, Hutaff LW, Sheets JA. Post-splenectomy thrombocytosis. Ann Intern Med. 1963;58:259–267. doi: 10.7326/0003-4819-58-2-259. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh J, Dacie JV. Persistent post-splenectomy thrombocytosis and thrombo-embolism: a consequence of continuing anaemia. Br J Haematol. 1966;12(1):44–53. doi: 10.1111/j.1365-2141.1966.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 8.Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, Krespis E, Katsaragakis S, Bramis J. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006;141(7):663–669. doi: 10.1001/archsurg.141.7.663. [DOI] [PubMed] [Google Scholar]
- 9.Kim BJ, Park KW, Koh SB, Kim HK, Jung HL, Park MK, Lee DH. Stroke induced by splenectomy in hemoglobin Madrid: autopsy clues to the underlying mechanism. Blood Coagul Fibrinolysis. 2005;16(2):141–144. doi: 10.1097/01.mbc.0000161568.59140.a3. [DOI] [PubMed] [Google Scholar]
- 10.Daya SK, Gowda RM, Landis WA, Khan IA. Essential thrombocythemia-related acute ST-segment elevation myocardial infarction. A case report and literature review. Angiology. 2004;55(3):319–323. doi: 10.1177/000331970405500312. [DOI] [PubMed] [Google Scholar]
- 11.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, van der Walt JD, Reilly JT, Grigg AP, Revell P, Woodcock BE, Green AR, United Kingdom Medical Research Council Primary Thrombocythemia 1 Study Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353(1):33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 12.Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood. 2001;97(4):863–866. doi: 10.1182/blood.v97.4.863. [DOI] [PubMed] [Google Scholar]
- 13.Valade N, Decailliot F, Rébufat Y, Heurtematte Y, Duvaldestin P, Stéphan F. Thrombocytosis after trauma: incidence, aetiology, and clinical significance. Br J Anaesth. 2005;94(1):18–23. doi: 10.1093/bja/aeh286. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A, Ho TC, Ahmann GJ, Katzmann JA, Greipp PR. Plasma interleukin-6 and C-reactive protein levels in reactive versus clonal thrombocytosis. Am J Med. 1994;97(4):374–378. doi: 10.1016/0002-9343(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 15.Wiwanitkit V. Extreme thrombocytosis: what are the etiologies? Clin Appl Thromb Hemost. 2006;12(1):85–87. doi: 10.1177/107602960601200113. [DOI] [PubMed] [Google Scholar]
- 16.Murphy S, Iland H, Rosenthal D, Laszlo J. Essential thrombocythemia: an interim report from the Polycythemia Vera Study Group. Semin Hematol. 1986;23(3):177–182. [PubMed] [Google Scholar]
- 17.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34(1):29–39. [PubMed] [Google Scholar]
- 18.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61(1):10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Fruchtman SM, Hoffman R. Essential thrombocythemia. In: Hoffman R, Benz EJ Jr, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, editors. Hematology: Basic Principles and Practice. 4th ed. Philadelphia: Churchill Livingstone; 2005. chapter 71. [Google Scholar]
- 20.Johnson M, Gernsheimer T, Johansen K. Essential thrombocytosis: underemphasized cause of large-vessel thrombosis. J Vasc Surg. 1995;2(4):443–447. doi: 10.1016/s0741-5214(95)70013-7. discussion 448–449. [DOI] [PubMed] [Google Scholar]
- 21.Wu KK. Platelet hyperaggregability and thrombosis in patients with thrombocythemia. Ann Intern Med. 1978;88(1):7–11. doi: 10.7326/0003-4819-88-1-7. [DOI] [PubMed] [Google Scholar]
- 22.Kessler CM, Klein HG, Havlik RJ. Uncontrolled thrombocytosis in chronic myeloproliferative disorders. Br J Haematol. 1982;50(1):157–167. doi: 10.1111/j.1365-2141.1982.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre KJ, Hoagland HC, Silverstein MN, Petitt RM. Essential thrombocythemia in young adults. Mayo Clin Proc. 1991;66(2):149–154. doi: 10.1016/s0025-6196(12)60486-8. [DOI] [PubMed] [Google Scholar]
- 24.Koh KK, Cho SK, Kim SS, Oh BH, Lee YW. Coronary vasospasm, multiple coronary thrombosis, unstable angina and essential thrombocytosis. Int J Cardiol. 1993;41(2):168–170. doi: 10.1016/0167-5273(93)90158-d. [DOI] [PubMed] [Google Scholar]
- 25.Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19(7):757–760. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 26.Bullen AW, Losowsky MS. Consequences of impaired splenic function. Clin Sci (Lond) 1979;57(2):129–137. doi: 10.1042/cs0570129. [DOI] [PubMed] [Google Scholar]
- 27.Schafer AI. Thrombocytosis. N Engl J Med. 2004;350(12):1211–1219. doi: 10.1056/NEJMra035363. [DOI] [PubMed] [Google Scholar]
- 28.Tefferi A, Hoagland HC. Issues in the diagnosis and management of essential thrombocythemia. Mayo Clin Proc. 1994;69(7):651–655. doi: 10.1016/s0025-6196(12)61342-1. [DOI] [PubMed] [Google Scholar]