Abstract
Microparticles (MPs) that circulate in blood may be a source of DNA for molecular analyses, including prenatal genetic diagnoses. Because MPs are heterogeneous in nature, however, further characterization is important before use in clinical settings. One key question is whether DNA is either bound to aggregates of blood proteins and lipid micelles or intrinsically associated with MPs from dying cells. To test the latter hypothesis, we asked whether MPs derived in vitro from dying cells were similar to those in maternal plasma. JEG-3 cells model extravillous trophoblasts, which predominate during the first trimester of pregnancy when prenatal diagnosis is most relevant. MPs were derived from apoptosis and increased over 48 hours. Compared with necrotic MPs, DNA in apoptotic MPs was more fragmented and resistant to plasma DNases. Membrane-specific dyes indicated that apoptotic MPs had more membranous material, which protects nucleic acids, including RNA. Flow cytometry showed that MPs derived from dying cells displayed light scatter and DNA staining similar to MPs found in maternal plasma. Quantification of maternal MPs using characteristics defined by MPs generated in vitro revealed a significant increase of DNA+ MPs in the plasma of women with preeclampsia compared with plasma from women with normal pregnancies. Apoptotic MPs are therefore a likely source of stable DNA that could be enriched for both early genetic diagnosis and monitoring of pathological pregnancies.
Microparticles (MPs) circulate in blood and have been associated with pathology in various diseases.1 However, the nature and physical characteristics of MPs are understudied. Most studies focus on quantity, origin, and biological activity of MPs, without specific analysis of the inherent organization. During normal placental development, fetal trophoblasts undergo apoptosis, making it plausible that apoptotic MPs could be generated. We propose that MPs generated specifically through apoptosis are a likely source of DNA suitable for PCR-based testing. Three types of membrane-bound vesicles are found in plasma: exosomes, activated MPs, and apoptotic MPs. All three are rich in phospholipids and can be derived from platelets, leukocytes, erythrocytes, or endothelial cells.2 Exosomes, which range in diameter from 40 to 80 nm, are released from activated cells when the membrane of intracellular multivesicular bodies fuses with the plasma membrane. MPs are larger (>100 nm in diameter) and originate from blebbing membranes of either activated cells or cells undergoing apoptosis.3,4,5 Apoptotic MPs have nuclear proteins as well as nucleic acids.5
Stable and PCR-amplifiable fetal cell-free DNA (cfDNA) has been detected in maternal plasma and used to predict6,7 and monitor8 the severity of complicated pregnancies. Yet, the low percentage of fetal cfDNA (3% to 6%) in maternal plasma limits its use for routine clinical applications. As a result, many groups, including ours, have focused on methods to enhance recovery and analysis of circulating fetal cfDNA. To achieve this goal, a better understanding of the molecular nature and kinetics of circulating (fetal) DNA is necessary. We hypothesize that circulating DNA could exist as free DNA subject to degradation as well as DNA contained in membrane bound MPs, providing protection from endonucleases. In 2005, we proposed that a significant portion of circulating DNA, including fetal DNA, was membrane bound and suitable for enrichment.9 We reported a 10-fold enrichment of fetal cfDNA from maternal plasma from normal pregnancies using the flow cytometric parameters of forward light scatter and acridine orange (AO) staining of nucleic acids. Although the nature and origin of the AO+ DNA was unknown, we suggested that the mechanism of release of fetal cfDNA might be due to placental apoptosis resulting in release of fetal DNA containing MPs into the circulation. The plausibility of this hypothesis was supported by data concerning turnover of syncytiotrophoblast and release of syncytiotrophoblast MPs (STBMs) into the maternal circulation.10 In addition, Gupta et al detected fetal nucleic acids in the supernatant of STBM samples prepared from placental explants.11 Other studies have also examined STBMs from preeclamptic patients using an enzyme-linked immunosorbent assay based method for quantification.12,13,14 Whether the STBMs contain nucleic acids similar to the AO+ MPs sorted from maternal plasma has not been studied.
One of the biochemical hallmarks of apoptosis is fragmented DNA. DNA is cleaved between nucleosomes, forming a “ladder” with spacing about 180 bp. Given that the majority of fetal cfDNA is relatively small (<300 bp, as detected by PCR) compared to maternal cfDNA,15 fetal DNA is likely released from apoptotic cells. However, the basis for fetal DNA stability has not been examined. More recently, Tjoa et al demonstrated an association of fetal cfDNA (as detected by β-globin via real-time PCR) and cell death in supernatants from third trimester placental cultures after oxidative stress,16 supporting the hypothesis that fetal cfDNA originates from the placenta via hypoxia-induced cell death, apoptosis and necrosis. However, the DNA detected was not examined as to source or characteristics.
Recovery of trophoblast MPs containing fetal DNA during the first trimester would be of clinical interest to prenatal genetic diagnosis. However, under hypoxic conditions, few syncytiotrophoblasts are detected in first trimester placental explants17 and circulating STBMs are not found until the second and third trimester.13 By contrast, invasive extravillous trophoblasts (EVTs) are most abundant during the first trimester.18 In addition, apoptosis limits invading EVTs during the first trimester,19 suggesting that MPs released by dying EVTs might enter the maternal circulation in the first trimester.
Primary trophoblasts isolated from villous explant cultures contain a heterogeneous population of cytotrophoblasts, syncytiotrophoblasts, fibroblasts, and macrophages.20,21 JEG-3 cells, which model invasive EVTs, most relevant for early genetic testing, have the advantage of being homogeneous with less spontaneous cell death than found with primary trophoblasts.22 Therefore, we used JEG-3 cells to represent EVTs.
Our studies show that cfDNA is contained within membranous MPs derived from dying JEG-3 cells. We also showed for the first time that the DNA within the apoptotic MPs contain fragmented DNA that is protected from plasma DNases. In addition, light scatter and DNA staining properties of in vitro cell derived MPs and maternal plasma MPs are similar, suggesting a common origin or mechanism of release. We also show that in vitro MPs and MPs from normal and preeclamptic pregnancies stain with the DNA dye, PicoGreen. Finally, we show for the first time that significantly higher numbers of DNA+ MPs are found in preeclamptic patients compared to control patients, suggesting that measuring such DNA+ MPs could help predict or monitor preeclampsia in a non-invasive manner. Other clinical and pathological conditions associated with increased levels of cell death (ie, trauma, stroke, organ transplantation, autoimmunity, and cancer) might also benefit from our discovery of circulating nucleic acids containing protected MPs.
Materials and Methods
Cell Culture
The human trophoblastic cell line, JEG-3 (male), was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in minimum essential medium, supplemented with heat inactivated 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 10 mmol/L HEPES, and 0.1 mmol/L non-essential amino acids (Invitrogen, Carlsbad, CA). The cultures were maintained at 37°C in an atmosphere of 5% CO2.
Induction of Cell Death
Two million cells were cultured in the presence or absence of either 50 μmol/L rotenone, which disrupts the mitochondrial electron transport chain to chemically mimic hypoxia (Sigma-Aldrich, St Louis, MO), or 30 μmol/L etoposide, a DNA topoisomerase II inhibitor (Sigma-Aldrich) that induces apoptosis. For necrosis, cells were heated for 1 hour at 60°C. Twenty-four and 48 hours after induction of cell death, floating cells were collected and adherent cells were detached with trypsin/EDTA (Invitrogen). The cells were pooled, centrifuged at 300 × g at room temperature, rinsed in PBS (pH 7.1; Invitrogen) and centrifuged at 300 × g.
Assessment of Apoptosis by Flow Cytometry
Quantification of Subdiploid Cells
Apoptotic cell death was confirmed by flow cytometric analysis of fixed (permeabilized) cells with a subdiploid DNA content.23 While gently vortexing, 1 × 106 cells (counted with a hemocytometer) were resuspended in 2 ml of 0.9% NaCl, followed by fixation with 5 ml ice cold 70% ethanol added dropwise. Cells were incubated for 1 hour at room temperature, centrifuged, and resuspended in 1 ml PBS containing 50 μg/ml propidium iodide (PI) (Sigma-Aldrich) and 0.1 mg/ml RNase A (Sigma-Aldrich), incubated for 1 hour at 37°C in the dark. Cells were analyzed using a Beckman Coulter EPICS XL2 (Beckman Coulter, Miami Lakes, FL). All data analysis was performed using EXPO32 software (Beckman Coulter).
Cell Viability
PI was used to test for membrane integrity using unfixed cells as previously described.23 One million (nonpermeabilized) cells were resuspended in PBS containing 50 μg/ml PI and analyzed by flow cytometry.
Isolation and Quantification of in Vitro MPs
Isolation and quantification of MPs are performed before all experiments. Double filtered (0.25 μm) PBS (dfPBS) was used in all MP experiments. MPs were centrifuged at room temperature, unless otherwise stated.
Isolation of MPs
MPs from 24 and 48 hours apoptotic and necrotic supernatants were separated from detached cells by two centrifugation steps (300 × g, 5 minutes; 800 × g, 5 minutes) and transferred to a 50 ml conical tube.
Quantification of MPs
MP concentration was determined as previously described.24 Briefly, 100 μl of fluorescent beads (counted at 500/μl with a hemocytometer) were added to 400 μl of each supernatant and 300 μl of dfPBS for a total volume of 800 μl and the number of beads counted was stopped at 10,000. After subtracting the number of background fluorescent beads, the concentration of MPs was determined and the appropriate number of MPs was centrifuged (25,000 × g, 1 hour at 4°C; in a SW40 Ti Swinging-Bucket Rotor in a Beckman L8-M, Class H, ultracentrifuge; Beckman Coulter).
Hoechst 33342 Labeling of MPs
Because lipid membrane MPs protect cell-free RNA (cfRNA) from RNase A activity (Table 1), we found it necessary to fix the MPs with 70% ethanol to permeabilize the membranes, before RNase treatment. Two million MPs were fixed with 70% ethanol (as described above) and centrifuged (13,000 rpm, 10 minutes) using an Eppendorf Centrifuge 5415c (Eppendorf, Westbury, NY). The MP pellets were resuspended in 500 μl PBS containing 10 μg/ml of Hoechst 33342 (Invitrogen), with or without 50 μl of 1 mg/ml RNase A. After incubating the samples for 20 minutes at 37°C in a water bath, the samples were placed on ice and analyzed using an LSR II flow cytometer (BD Instruments, San Jose, CA).
Table 1.
RNA from Apoptotic MPs Is Protected by Triton X-100 Extractable Lipids
| Sample | Treatments | Ct Value |
|---|---|---|
| A-MP | Untreated | 22 |
| A-MP | 200 μg/ml RNase | 21 |
| A-MP | Tx-100 + 200 μg/ml RNase | 40 |
| A-MP | 1000 μg/ml RNase | 19 |
| A-MP | Tx-100 + 1000 μg/ml RNase | 40 |
A-MP, apoptotic MPs.
Tx-100, Triton X-100.
n = 1.
Assessment of 4,6-Diamidino-2-Phenylindole Stained MPs by Fluorescence Microscopy Slide Preparation
MPs (2 × 106) in 50 μl were placed on a glass slide (Superfrost Plus Micro Slides; VWR Scientific, West Chester, PA) and air-dried at room temperature. The slides were fixed in a Coplin jar containing 25 ml of 100% methanol for 10 minutes and allowed to air-dry. The slides were immersed in a second Coplin jar containing 25 ml of 2× standard saline citrate with 0.2% NP40 for 1 minute and the edges were dried with a tissue. A final concentration of 4,6-diamidino-2-phenylindole (DAPI II, 1 μg/ml, Invitrogen) was placed on the surface of a glass coverslip, inverted onto a slide and analyzed by fluorescence microscopy.
Fluorescence Microscopy
Images of the DAPI-stained MPs were captured through an Olympus BX51 microscope with automated x-, y-, and z-stage movement and a Hamamatsu ORCA-2 Digital Camera using a ×100 objective (Hamamatsu Photonics, Hamamatsu, Japan). Data were analyzed using Advanced Digital Imaging Research automated interphase FISH Scanner software (Advanced Digital Imaging Research L.L.C., League City, TX). Each slide was autoscanned for single MPs ranging 1 μm to 6 μm in diameter to avoid clustering of MPs or possible cell contamination. An average of 3000 to 8000 images was taken per slide. Electronic gates (boxes 1 μm to 6 μm in length) were placed around each MP from randomly selected images and the integrated fluorescence intensity was determined. One hundred MPs per slide, per experiment were counted. The amount of DNA per particle was reported as the mean integrated fluorescence intensity (MIFI).
Real-Time PCR
DNA Extraction
DNA was extracted from 1 to 2 million MPs using the QIAamp DNA blood kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. All DNA samples were stored at 4°C before analysis.
PCR Analysis
Real-time PCR was performed as previously described.25 For both the β-globin (102 bp) and sex-determining region Y (SRY, 72 bp), quantitative real-time (RT)-PCR was performed using the Applied Biosystems 7700 sequence detection system (Applied Biosystems, Foster City, CA). Primer and probe sequences were as follows: SRY forward primer: 5′:-TGCACAGAGAGAAATACCCGAATTA-3′; SRY reverse primer: 5′:-TGCATTCTTCGGCAGCAT-3′; SRY TaqMan probe: 5′:-AAGTATCGACCTCGTCGGAAGGCGAA-3′; β-globin forward primer: 5′:-GTGCACCTGACTCCTGAGGAGA-3′; β-globin reverse primer: 5′:-CCTTGATACCAACCTGCCCAG-3′; β-globin TaqMan probe: 5′:-AAGGTGAACGTGGATGAAGTTGGTGG-3′.
Quantification of total and fetal DNA as genome equivalents was based on copies of β-globin and SRY sequences detected. Each reaction contained 5 μl of extracted DNA. Each reaction plate was run simultaneously with a duplicate calibration curve of titrated DNA (standard curve). Each sample was run in triplicate for both loci and the mean of the values was determined using the 7700 software and the standard curve of known DNA concentrations. To determine the relative amount of DNA per MP, the total (β-globin) and fetal DNA (SRY) genome equivalents were divided by the number of MPs.
Apoptotic DNA Ladder Assay
DNA was extracted from fifteen million MPs and analyzed on a 2% agarose gel with a 1-kb DNA ladder (Invitrogen). MPs were resuspended in 300 μl of lysis buffer (10 mmol/L Tris-HCl-EDTA) with 50 μg/ml RNase A for 1 hour at 37°C. MPs were incubated overnight (∼12 hours) at 37°C in 0.05% SDS with 2 mg/ml of Proteinase K (Sigma-Aldrich). An equal volume of phenol/chloroform/isoamyl alcohol (25:24:1; Invitrogen) was added and the tubes were gently inverted twenty times. The samples were centrifuged (13,000 rpm, 10 minutes) and the top layer (aqueous) was removed and transferred to second tube. An equal volume of chloroform (99+%; Sigma-Aldrich) was added and the samples were centrifuged (13,000 rpm, 10 minutes). The top layer (aqueous phase) was removed and transferred to a third tube. DNA was precipitated by adding one-third volume of 7.5 mol/L ammonium acetate (Sigma-Aldrich), followed by 2.5 volumes of absolute ethanol, and precipitated at −80°C for 30 minutes. The precipitates were collected by centrifugation (13,000 rpm, 10 minutes) and the pellets were washed with 70% ethanol and air-dried. The proteinase K, phenol/chloroform/isoamyl alcohol (25:24:1), and DNA precipitation steps were repeated a second time. The final DNA pellet was air-dried and resuspended in 60 μl of Tris-HCl-EDTA (10 mmol/L) and DNA concentration determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Three micrograms of DNA were electrophoresed on a 2% agarose gel (stained with 0.2 μg/ml ethidium bromide) and analyzed with Kodak Image Station 4000 mm Pro (Eastman Kodak Company, Molecular Imaging Systems, Rochester, NY).
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
DNA nicks were detected by the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay as previously described26 with slight modifications. Analysis was by flow cytometry and fluorescence microscopy with the following buffers: 5× TdT reaction buffer (1 mol/L potassium cacodylate [Sigma-Aldrich], 125 mmol/L Tris-Cl, pH 6.6, and 1.25 mg/ml bovine serum albumin [Sigma-Aldrich] stored at −20°C); and fluorescein isothiocyanate-conjugated anti-bromolated deoxyuridine triphosphates (Br-dUTP) monoclonal antibody solution (Anti-Br-dUTP fluorescein isothiocyanate-conjugated monoclonal antibody, 0.5 μg, Phoenix Flow Systems, San Diego, CA; 0.3% (v/v) Triton X-100; 1% (w/v) bovine serum albumin (Sigma-Aldrich); and PBS to 100 μl (stored at 4°C).
Flow Cytometry
Briefly, two million MPs from chemically-induced hypoxic cells and heat stress (HS)-treated cells (at 60°C) were pre-fixed in 1 ml of 2% methanol-free formaldehyde (Electron Microscopy Sciences, Hatfield, PA) diluted in PBS on ice for 1 hour. The MPs were centrifuged (25,000 × g, 1 hour at 4°C) and postfixed in 1 ml of ice-cold 70% ethanol for 5 hours at −20°C. MPs were centrifuged (13,000 RPM, 10 minutes), the ethanol removed, and the MPs washed in 1 ml of PBS. MPs were resuspended in 50 μl of labeling buffer (10 μl 5× TdT reaction buffer, 2 μl Br-dUTP stock solution [2 mmol/L Br-dUTP; Sigma-Aldrich], 0.5 μl [12.5U] TdT enzyme [Invitrogen], 5 μl CoCl2 [10 mmol/L; Sigma-Aldrich] and 33 μl distilled water) and incubated at room temperature overnight. Three hundred microliters of rinsing buffer (0.1% [v/v] Triton X-100, 5 mg/ml BSA, and PBS, pH 7.4) was added and the MPs centrifuged (13,000 RPM, 10 minutes). The supernatant was removed and 100 μl fluorescein isothiocyanate-conjugated anti-Br-dUTP monoclonal antibody was added to the MPs and incubated for 1 hour at room temperature. Four hundred microliters of PBS-based PI staining solution (50 μl of 1 mg/ml PI [Sigma-Aldrich] and 50 μl of 1 mg/ml RNase A [Sigma-Aldrich]) was added and incubated for 30 minutes at room temperature in the dark. The samples were analyzed by a Beckman Coulter EPICS XL2.
Fluorescence Microscopy
After labeling MPs with Br-dUTP incubated at room temperature overnight, a 50 μl aliquot of two million apoptotic or necrotic MPs was air-dried onto slides. MPs were counterstained with DAPI II (Invitrogen, as described above). The double labeled MPs were analyzed with an Olympus BX-51 Microscope and photographed with a Hamamatsu ORCA-2 Digital Camera using an ×100 objective (Hamamatsu, Photonics). Scale bars are 10 μm.
PKH26 and Hoechst 33342 Labeling of MPs
Two million MPs from each sample were labeled with PKH26 Red Fluorescent Cell Linker Kit (Sigma) as described by the manufacturer with slight modifications. MPs were washed once with PBS (Invitrogen) and stained with 100 μl of staining solution (5 × 10−5 mol/L PKH26 in diluent C) for 4 minutes at room temperature. The staining reaction was stopped by addition of 100 μl of fetal calf serum. One minute later, 100 μl of complete medium were added and MPs were washed twice in 10% minimum Essential medium, then once in PBS. MPs were resuspended in 500 μl PBS containing 10 μg/ml of Hoechst 33342 (Invitrogen), incubated for 20 minutes in a water bath at 37°C, placed on ice, and analyzed using an LSR II flow cytometer.
Cholera Toxin B and Hoechst 33342 Labeling of MPs
Two million MPs resuspended in 500 μl PBS were stained with 5 μl of cholera toxin B (CTB, 1 mg/ml, Invitrogen) and incubated on ice for 10 minutes. MPs were labeled with 4 μg/ml of Hoechst 33342 (Invitrogen), prepared as described on above, and analyzed using an LSR II flow cytometer.
Plasma DNase Assay
Plasma Samples
After obtaining Institutional Review Board approval from Baylor College of Medicine and written informed consent, 10 ml of peripheral blood was collected from a non-pregnant female in a syringe containing 10 μl/ml of anticoagulant heparin sulfate (5000 U/ml) and allowed to sit at room temperature for 1 hour. Blood was centrifuged for 10 minutes at 800 × g (room temperature), followed by a second centrifugation at 1600 × g. The cell-free plasma was used to incubate the in vitro MPs as described below.
Incubation of in Vitro MPs in Plasma
Five million MPs from each sample were incubated in 800 μl of the non-pregnant female plasma for 24 hours on ice and in a 37°C water bath. DNA was extracted using the MagNA Pure LC System (Roche Diagnostics) according to manufacturer’s instructions. Purified male gDNA (Promega, Madison, WI) and a water blank were used to create a standard curve (1.5 ng to 245 ng DNA) to determine the linearity and range of the PCR products.
PCR Analysis
PCR was performed using primers specific to a 193 bp SRY PCR product, using the following primers: SRY forward primer: 5′:-AAAGGCAACGTCCAGGATAGAG-3′; SRY reverse primer: 5′:-TGTAATTTCTGTGCCTCCTGGA-3′.
Controls used in all tests included a male positive control for SRY and no DNA (water) control. The PCR reaction (55 μl) included 5 μl 1× PCR buffer, 2 μl 50 mmol/L MgCl, 1.5 μl 100 mmol/L dNTPs, 2.5 μl 100 mmol/L SRY-Forward, 2.5 μl 100 mmol/L SRY-Reverse, 15 μl (30 ng) DNA, 0.3 μl (500 U) Taq polymerase, and 26.2 μl water (Invitrogen). PCR amplification was performed using a hot start method (10 minutes at 95°C.), followed by 35 cycles of denaturation at 94°C for 1 minute, re-annealing at 63.2°C for 1 minute, and extension at 72°C for 1 minute, and one final extension of 10 minutes at 72°C.
DNA Standard Curve
The PCR products were run on a 1% agarose gel, stained with 0.2 μg/ml ethidium bromide, and the band intensities were scanned using the Kodak Digital Science Image Station 440CF (Eastman Kodak Company, Molecular Imaging Systems). Band intensities were determined using the Image J version 1.37v software (developed at the National Institutes of Health). A standard curve was generated by plotting the log of the DNA concentration (x axis) versus ethidium bromide band intensity (y axis). Linear regression analysis was used to determine the slope and intercept for each experiment. The concentration of recovered male DNA was interpolated from a standard curve. The standard curve is representative of three individual experiments (Supplemental Figure 1, see http://ajp.amjpathol.org).
DNase I Sensitivity Assay
To reduce nonspecific binding of PKH26 to the microscope slide, two million MPs from each sample were labeled with 3.6 × 10−5 mol/L PKH26 (Sigma) and washed as previously described above. MPs were resuspended in 24 μl of Promega’s 1× Reaction Buffer and treated with or without 1 U of RNase-free DNase (Promega) for 20 minutes at 37°C. The reaction was stopped by adding 1 μl of Promega’s RQ1 DNase Stop Solution. MPs were fixed as described above, counterstained with DAPI II (Invitrogen), and analyzed with an Olympus BX-51 Microscope as described above.
RNase Protection Assay
Five million MPs were treated with 200 μg/ml or 1000 μg/ml RNase A with and without 0.2% Triton X-100 for 1 hour at 37°C. RNA was isolated using the RNeasy Micro kit (Qiagen Sciences). MPs were lysed using the Qiagen RLT buffer and the lysate homogenized by centrifuging through a QiaShredder (Qiagen Sciences, Valencia, CA). The flow-through was applied to the RNeasy column according to the manufacture’s instructions, treated with DNase I (Promega) and then washed. The RNA was eluted in 14 μl of RNase-free water and subjected to reverse transcription with random primers using Taqman Reverse Transcription Reagents (Applied Biosystems) according to the manufacture’s instructions.
RNA Isolation and Determination
RNA was isolated using the RNeasy Micro kit and treated with DNase I according to the manufacturer’s instructions (Qiagen Sciences, Maryland). RNA was reverse transcribed with random primers using Taqman Reagents (Applied Biosystems), according to the manufacture’s instructions. Reverse Transcription was performed in a PE9700 thermal cycler (Applied Biosystems). After Reverse Transcription, the cDNA products were amplified by real-time PCR using the GeneAmp 7700 sequence detection system and the Taqman Assay (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase mRNA transcript was amplified (Hs00266705-g1 Applied Biosystems) using cDNA. Cycling conditions consisted of a 2-minute incubation at 50°C to activate UNGErase, an initial denaturation step of 95°C for 10 minutes, and then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Threshold cycle (Ct) values were used as a measurement of the presence or absence of the transcript. Ct values reflect the cycle number at which the fluorescence generated from accumulating amplicons crosses the background fluorescence. Ct values ranging from 39 to 40 are considered negative for cDNA, suggesting that RNA is not present. At this point during the reaction the number of amplicons is statistically significant above the baseline, indicating the presence of copies of the transcript. Control specimens included reverse transcription blanks and PCR blanks.
Quantification of MPs in Preeclamptic Women
Frozen plasma samples known to come from normal or preeclamptic pregnancies were donated by Dr. Popek (Texas Children’s Hospital, Houston, TX). MP concentrations were determined as previously described in Materials and Methods. Briefly, 20,000 fluorescent beads (counted with a hemocytometer and resuspended in dfPBS) were added to a 1:53.3 dilution of (15 μl) plasma in dfPBS for a total volume of 800 μl and a final concentration of 25 beads/μl. The number of beads counted by flow cytometry (Beckman Coulter EPICS XL2) was stopped at 1000. Next, one million plasma MPs were resuspended in dfPBS for a final volume of 66 μl and labeled with 2 μl of DNA staining dye, PicoGreen (Invitrogen) at room temperature for 10 minutes in the dark. Unlabeled MPs were used as negative controls. Both labeled and unlabeled MPs were resuspended in 350 μl dfPBS and analyzed by flow cytometry (Beckman Coulter EPICS XL2). The number of events was stopped at 10,000 counts.
Statistical Analysis
Comparisons between two groups (Apoptotic MPs vs. Necrotic MPs) and (control pregnancy vs. preeclamptic pregnancy) were performed using the two-sample t-test. Two-sided P-values less than 0.05 were reported to be statistically significant. All statistical analyses were performed using Microsoft Office Excel®.
Results
Chemically Induced Hypoxia-Mediated Apoptosis
Preeclampsia is associated with hypoxia, which leads to apoptosis of trophoblasts and subsequent elevated levels of fetal cfDNA in maternal plasma.27,28 A trophoblastic cell line, JEG-3, was used to test the effect of chemically-induced hypoxic apoptosis on the release of cfDNA and MPs. Rotenone, which mimics hypoxia by disrupting the mitochondrial electron transport chain, was used.29 Etoposide was used as a positive control for mitochondria-mediated apoptosis,30 whereas 60°C HS for 1 hour was used to model necrosis.
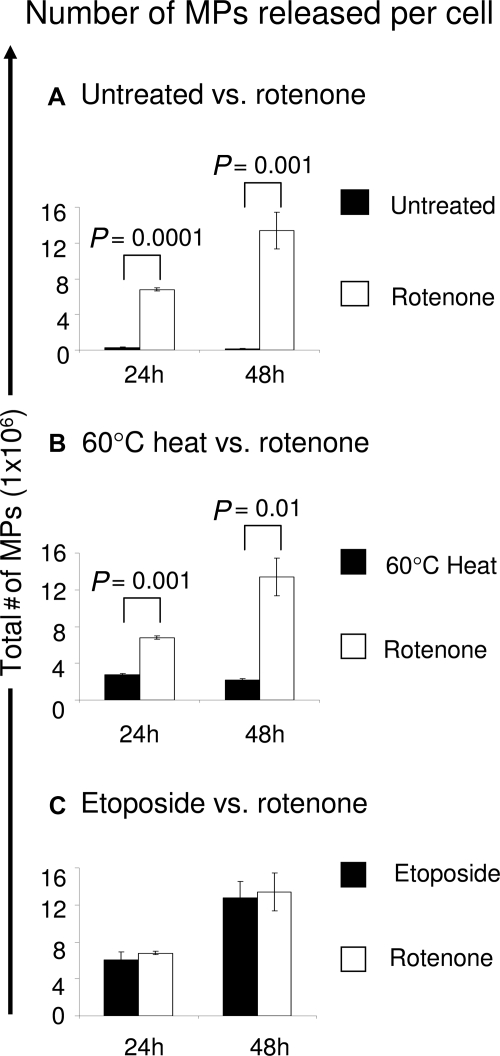
When apoptotic cells are fixed (permeabilized) with ethanol and stained with PI, they appear as a subdiploid peak by flow cytometry.23 Subdiploid DNA is shown in untreated cells (Figure 1A) and in cells treated with etoposide (Figure 1B), rotenone (Figure 1C), or HS (Figure 1D). Figure 1e is a composite figure indicating by 24 hours, 30 ± 4% SD of the rotenone-treated cells contained a subdiploid apoptotic region (% Sub-G1), whereas few of the HS-treated cells (9 ± 3%) had a subdiploid region, slightly more than untreated cells (4 ± 2%). These data show that rotenone and etoposide induce apoptosis, whereas HS does not.
Figure 1.
Cell death induction. Loss of DNA in apoptotic cells is reflected by the progressive increase of cells in the sub-G1 region. After induction of apoptosis by etoposide (30 μg/ml), rotenone (50 μg/ml), or necrosis with heat (60°C, 1 hour), cells were permeabilized with 70% ethanol and labeled with propidium iodide (PI) containing RNase A and analyzed the percentage of cells containing subdiploid DNA (% Sub G1) quantified by flow cytometry. The histograms show cell cycle data from one representative experiment of (A) untreated, (B) etoposide-, (C) rotenone-, and (D) 60°C heat- treated cells. The brackets within the histogram indicate the percentage of cells with subdiploid DNA. E: The bar graph shows the percent sub-G1+ cells of each treatment from three independent experiments and statistical significance (P value) was calculated by Student’s t-test. Standard deviations are indicated. PI uptake. Cells were labeled with PI without fixation and the change in light scatter and PI uptake measured by flow cytometry. The dot plots show PI uptake from one representative experiment of (F) untreated, (G) etoposide-, (H) rotenone-, and (I) 60°C heat- treated cells. PI+ cells are back-gated onto the light scatter and shown in red. J: The bar graph shows the mean PI uptake (± SD) of each treatment from three independent experiments and statistical significance (P value) was calculated by Student’s t-test.
PI uptake also was measured in non-fixed (non-permeabilized) cell samples after various treatments. Figure 1, F–I compare light scatter and PI uptake (back-gated on the light scatter and represented in red). Figure 1J shows that 26 ± 3% of rotenone-treated cells are PI+ after 24 hours, comparable to etoposide treated cells (26 ± 3% PI+). By contrast, nearly all of the HS-treated cells (Figure 1J) displayed a loss of plasma membrane integrity (99 ± 1% PI+) with light scatter changes and uptake of PI+, typical of necrosis.31 By 48 hours, both etoposide- and rotenone-treated cells were 70 ± 4% and 72 ± 1% PI+, respectively (Supplemental Figure 2, see http://ajp. amjpathol.org), suggesting late apoptosis/secondary necrosis. The vehicle for rotenone and etoposide, dimethyl sulfoxide, had no effect (data not shown).
Quantitation of MPs Generated under Chemically Induced Hypoxic Conditions
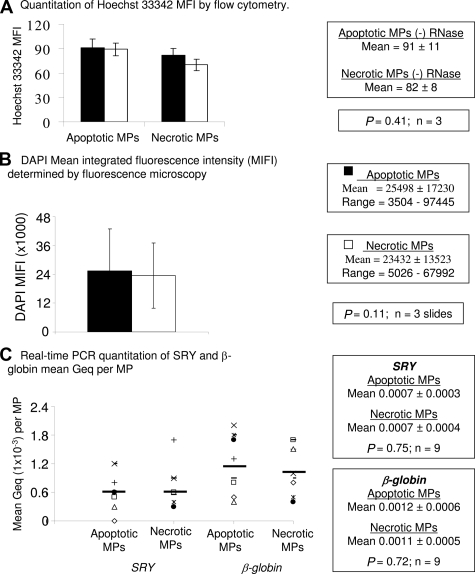
An increase in circulating DNA as well as endothelial MPs is associated with preeclampsia,32 suggesting that quantitation of MPs could be useful for diagnosing placental pathologies. To test whether the yield of released MPs is indicative of the type of cell death, two million JEG-3 cells were treated with rotenone, etoposide, or HS and cultured up to 48 hours. The numbers of MPs were detected by flow cytometry using a fluorescent bead counting assay.24 By 24 hours, rotenone-induced apoptosis generated 6.7 ± 0.1 × 106 MPs, significantly more MPs than both untreated cells (0.2 ± 0.1 × 106; P = 0.0001, n = 3, Figure 2A) and 60°C HS-induced necrosis (2.7 ± 0.1 × 106; P = 0.001, n = 3, Figure 2B). By 48 hours, rotenone-induced cells continued to produce significantly more MPs (13.3 ± 0.2 × 106) than untreated cells (0.2 ± 0.1 × 106; P = 0.001, n = 3, Figure 2A) or necrotic cells (2.1 ± 0.2 × 106; P = 0.01, n = 3, Figure 2B). No significant differences were observed between the number of rotenone-induced MPs and etoposide-induced MPs after 24 and 48 hours (Figure 2C). From the starting population of two million cells, we detected approximately 3 and 7 rotenone-induced MPs per cell at 24 and 48 hours, respectively (data not shown). By contrast, 60°C HS treatment resulted in only 1 necrotic MP per cell at 24 or 48 hours (data not shown) and untreated cells produced one MP per five cells by 24 and 48 hours (data not shown). The increase of rotenone-induced MPs is concordant with the changes in light scatter and the increase in the number of PI+ cells at 48 hours (Supplemental Figure 2, see http://ajp.amjpathol.org) suggesting that cells undergoing early to late apoptosis/secondary necrosis release continuous levels of MPs.
Figure 2.
Quantitation of cell death-induced MPs. JEG-3 cells were left untreated or treated with etoposide, rotenone, or 60°C heat and incubated for 24 and 48 hours at 37°C. Supernatants were collected and detached cells were pelleted (300 × g), followed by additional 800 × g centrifugation. MPs were quantitated as described in Materials and Methods. The total amount of cell-free MPs (y axis) released into the media were measured at 24 and 48 hours (x axis) by flow cytometry using a fluorescent bead assay. Number of MPs released over time. The bar graph compares the number of MPs found in the supernatant between rotenone-treated cells (A, B, C) and (A) untreated cells (B) 60°C heat- and (C) etoposide-treated cells. The results (mean ± SD) are from three independent experiments.
Analysis of Microparticle DNA
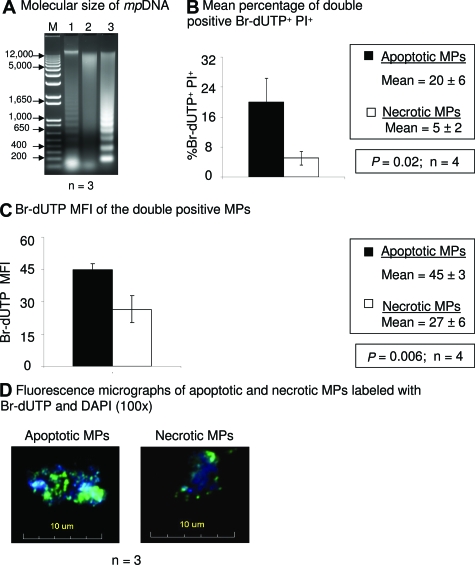
To test whether rotenone-induced or 60°C HS MPs contain DNA, MPs were labeled with the DNA dye, Hoechst 33342,33 and DNA content evaluated using the mean fluorescence intensity (MFI). Because we have determined that lipid membranes of rotenone-induced MPs protect cfRNA from RNase A activity (Table 1) the membranes of the MPs were permeabilzed with 70% ethanol, before RNase treatment. Rotenone-induced (MFI = 91 ± 11) and 60°C HS MPs (MFI = 82 ± 8) have similar amounts of DNA (Figure 3A). No significant difference was detected between rotenone-induced MPs treated with or without RNase (P = 0.75, n = 3) or between 60°C HS MPs with or without RNase (P = 0.07, n = 3), suggesting that Hoechst 33342 bound specifically to DNA (Figure 3A).
Figure 3.
Comparison of mpDNA content. Supernatants containing MPs were centrifuged at 300 × g to remove cells. The MPs were centrifuged again at 800 × g, followed by a final 25,000 × g centrifugation. A: Quantitation of Hoechst 33342 MFI by flow cytometry. MPs were treated with and without RNase A (1 mg/ml) and labeled with Hoechst 33342 (10 μg/μl). The results are Hoechst 33342 fluorescence as indicated by the MFI ± SD A total of 10,000 events were obtained for each experiment (n = 3). B: Amount of DAPI MIFI. MPs were labeled with DAPI and analyzed by an autoscanning fluorescence microscopy. One hundred MPs per slide per experiment were counted. The results are presented as the DAPI MIFI per MP ± SD for three independent experiments. C: SRY and β–globin DNA concentration per MP. DNA amplified for SRY and β–globin from apoptotic and necrotic MPs are indicated on the x axis. DNA concentrations are in Geq/MP and are plotted on the y axis (n = 9). The bars represent the mean value.
As an independent measure of DNA quantity, fluorescence microscopy was used. DAPI-labeled MPs from each sample show similar sizes (Supplemental Figure 3, see http://ajp.amjpathol.org). To determine the relative amount of DNA per MP, the MIFI was analyzed. No significant difference (P = 0.11; n = 3 slides, 100 MPs per slide) in DAPI MIFI between rotenone-induced MPs (25498 ± 17230; range, 3504 to 97445) and 60°C HS MPs (23432 ± 13523; range, 5026 to 67992) was observed (Figure 3B).
Concentrations of DNA based on amplification of two gene sequences (SRY and β-globin) were determined by real-time PCR (Figure 3C). The mean concentration of SRY DNA for rotenone-induced MPs (0.0007 Genome equivalents [Geq] MP ± 0.0003; range 0.0003 to 0.0013) and 60°C HS MPs (0.0007 Geq MP ± 0.0004; range 0.0003 to 0.0017) was similar (n = 9). The mean concentration of β-globin DNA for rotenone-induced MPs (0.0012 Geq MP ± 0.0006; range 0.0004 to 0.0020) and 60°C HS MPs (0.0011 Geq MP ± 0.0005; range 0.0004 to 0.0017) was similar (n = 9). Thus, both apoptosis and necrosis produce MPs with similar concentrations of DNA per particle. However, apoptotic cells generate many more MPs than do necrotic cells (Figure 2), so on a per cell basis, there would be 3 to 7 times more DNA from MPs produced after apoptosis induction.
Molecular Form of Micro-Particle DNA (mpDNA)
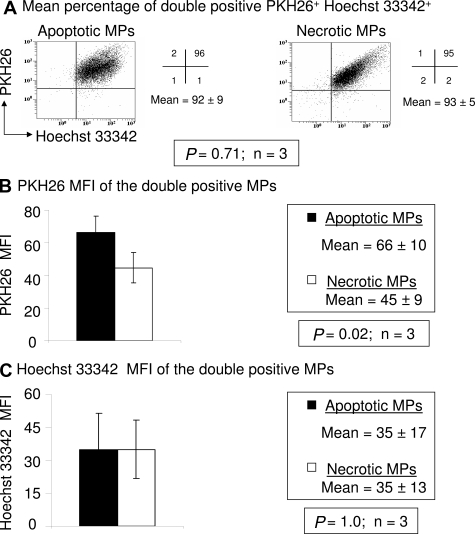
Apoptotic DNA contains a range of sizes with fragments cut into sizes of 180 bp, which produces a characteristic ladder on gel electrophoresis. Chan et al demonstrated by real-time PCR that the majority of fetal cfDNA detected in maternal plasma is less than 300 bp,15 suggesting that fetal cfDNA is fragmented and may come from apoptotic fetal cells. To determine the molecular form of mpDNA, DNA fragmentation was detected by gel electrophoresis. DNA from 60°C HS MPs has a random and general cleavage pattern (Figure 4A, Lane 2). By contrast, rotenone-induced mpDNA (Lane 3) is disrupted in a ladder pattern, similar to apoptotic cellular DNA (Lane 1).
Figure 4.
Molecular form of mpDNA. DNA fragmentation was demonstrated by the DNA ladder assay. Rotenone-induced and 60°C HS MPs were isolated from cell-free supernatants by two centrifugation steps (300 × g and 800 × g). After a final centrifugation (25,000 × g), mpDNA was purified by phenol/chloroform extraction and analyzed on a 2% agarose gel stained with ethidium bromide. A: Lane M, 1kb DNA size marker; lane 1, positive control apoptotic cells; lane 2, DNA from necrotic MPs; lane 3, DNA from apoptotic MPs. Results are representative of three independent experiments. TUNEL assay. Nicked mpDNA was detected by the TUNEL assay. MPs were isolated as mentioned above. The MPs were labeled with Br-dUTP and counterstained with PI for flow cytometric analysis or counterstained with DAPI II and analyzed by fluorescence microscopy. Flow cytometry. A total of 10,000 events were obtained in each of four independent experiments and the results reported as the mean ± SD B: The bar graph represents the percentage of Br-dUTP+ PI+ double positive apoptotic and necrotic MPs (n = 4). C: The bar graph represents the MFI of Br-dUTP from double positive (Br-dUTP versus PI) MPs (n = 4). Fluorescence microscopy. D: Br-dUTP labeled MPs (green) were fixed onto a slide with 100% methanol, counterstained with DAPI II (blue) and analyzed by fluorescence microscopy. Micrographs are representative of three independent experiments.
DNA fragmentation was also tested by the TUNEL assay. MPs were labeled with Br-dUTP, counterstained with PI and analyzed by flow cytometry. Figure 4B shows a significant difference in the percentage of Br-dUTP+ PI+ MPs between rotenone-induced (20 ± 6%) and 60°C HS MPs (5 ± 2%) (P = 0.02; n = 4). Furthermore, the Br-dUTP MFI (45 ± 3) of rotenone-induced MPs was significantly greater (P = 0.006; n = 4) than the Br-dUTP MFI (27 ± 6) of 60°C HS MPs (Figure 4C), suggesting more nicked mpDNA. Fluorescence micrographs also showed that Br-dUTP preferentially bound to apoptotic mpDNA more than necrotic mpDNA (Figure 4D). These data suggest that apoptotic mpDNA displays characteristic ladder patterns, indicative of the ordered process of DNA fragmentation during apoptosis.
Apoptotic MPs Have More Lipid Staining Membranes than Necrotic MPs
To test whether mpDNA contains lipid membranes, we performed flow cytometry after labeling MPs with membrane (PKH26) and DNA (Hoechst 33342) dyes. There was no significant difference in the percentage of PKH26+ Hoechst 33342+ MPs between apoptotic (92 ± 9%) and necrotic (93 ± 5%) MPs (P = 0.71; n = 3) (Figure 5A). However, the PKH26 MFI of apoptotic (66 ± 10) MPs was significantly greater (P = 0.02) than the PKH26 MFI (45 ± 9) of necrotic MPs (Figure 5B). Furthermore, apoptotic MPs (35 ± 17; n = 3) and necrotic MPs (35 ± 13; n = 3) bound similar levels of Hoechst 33342 dye (Figure 5C).
Figure 5.
Apoptotic MPs have more lipid membranes than necrotic MPs. A: Dot plots of apoptotic and necrotic MPs double stained for PKH26 (membrane dye) and Hoechst 33342 (DNA dye). The numbers shown in each quadrant are the percentage of the total population of MPs within each quadrant. A total of 10,000 events were obtained for each of three independent experiments and results reported as the mean ± SD of PKH26+ Hoechst 33342+ MPs. B: The bar graph represents the mean fluorescence intensity (MFI) of PKH26 from double positive (PKH26 vs. Hoechst 33342) MPs (n = 3). C: The bar graph represents the MFI of Hoechst 33342 from double positive (PKH26 vs. Hoechst 33342) MPs (n = 3).
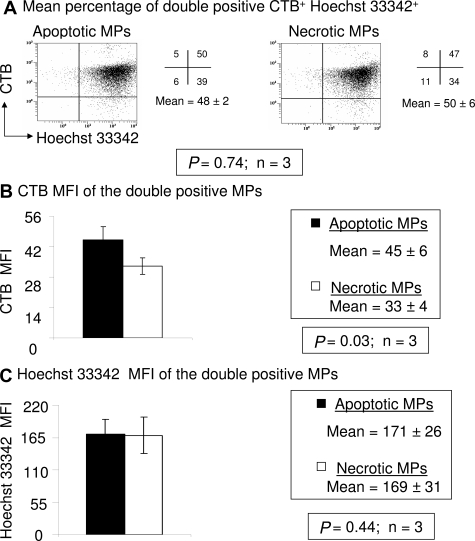
As an independent measure of membrane material, we also stained MPs with CTB to measure GM1 sphingolipids found in lipid rafts. MPs were counterstained with Hoechst 33342 as before. There was no significant difference in the percentage of CTB+ Hoechst 33342+ MPs between apoptotic (48 ± 2%) and necrotic (50 ± 5%) MPs (P = 0.74; n = 3) (Figure 6A). Similar to the PKH26 MFI results, Figure 6b shows that the CTB MFI was higher in apoptotic MPs (45 ± 6) than in necrotic (33 ± 4) MPs (P = 0.03; n = 3). There was no significant difference detected in Hoechst 33342 MFI between the double stained apoptotic (171 ± 26) and necrotic (169 ± 31) MPs (P = 0.44; n = 3) (Figure 6C). These data suggest the DNA+ apoptotic MPs have more material that stains with membrane specific dyes than the necrotic MPs, possibly providing a mechanism for greater DNA stability in maternal plasma.
Figure 6.
More cholera toxin B binding in apoptotic MPs. A: Dot plots of apoptotic MPs and necrotic MPs stained with CTB and Hoechst 33342. The numbers shown in each quadrant are the percentage of the total population of MPs within each quadrant. A total of 10,000 events were obtained in each of the three independent experiments and the results reported as the mean ± SD of CTB+ Hoechst 33342+ MPs. B: The bar graph represents the mean fluorescence intensity (MFI) of CTB from double positive (CTB versus Hoechst 33342) MPs (n = 3). C: The bar graph represents the MFI of Hoechst 33342 from double positive (CTB versus Hoechst 33342) MPs (n = 3).
Apoptotic MP DNA Is More Resistant to Endogenous Plasma DNase Activity than Necrotic MP or Naked DNA
To analyze the plasma stability of cfDNA associated with MPs, we incubated apoptotic MPs, necrotic MPs or free male genomic DNA in female plasma for 24 hours at 0°C (endogenous DNase activity is low34) or 37°C before amplifying the 193 bp SRY fragment. The concentration of recovered male DNA was interpolated from a standard curve as described (see Materials and Methods). More apoptotic mpDNA (28 ± 5%) was detectable by PCR compared to free DNA (6 ± 1%, P = 0.02; n = 3) and necrotic mpDNA (11 ± 1%, P = 0.03; n = 3) after incubation in female plasma for 24 hours at 37°C (Figure 7A). These data suggest that apoptotic mpDNA is more resistant to endogenous plasma DNase activity than necrotic mpDNA or free genomic DNA.
Figure 7.
Apoptotic MPs are more resistant to DNase activity in the plasma. A: MPs spiked into female plasma. Isolated MPs were resuspended in female plasma at two different temperatures, 0°C and 37°C, for 24 hours. In addition, naked male GDNA (JEG-3 cells) was spiked into female plasma under the same conditions. The SRY gene (Y chromosome) was amplified (193 bp fragment) and analyzed on a 1% agarose gel. The ethidium bromide band intensities were quantitated and the DNA concentrations were extrapolated using a PCR amplified standard curve (as described in Methods and Materials). The statistical significance of quantification was assessed using paired Student’s t-test (P < 0.05) and expressed in terms of the ratio of amount of DNA protected (37°C/0°C) for three independent experiments. B: DNase sensitivity assay. Isolated apoptotic and necrotic MPs labeled with membrane dye, PKH26 (red), incubated with and without DNase I for 20 minutes at 37°C and counterstained with and DNA dye, DAPI (blue). Micrographs are representative of three independent experiments.
To further evaluate which form of cfDNA is most resistant to DNase activity, we labeled JEG-3 cells with PKH26 and treated the cells with either rotenone or 60°C heat. The PKH26 pre-labeled apoptotic and necrotic MPs were treated with or without DNase in vitro, counterstained with DAPI and then analyzed using fluorescence microscopy. Apoptotic MPs treated with DNase I stained intensely with DAPI (Figure 7B). By contrast, necrotic MPs did not stain with DAPI after DNase I treatment (Figure 7B). Thus, DNA packaged in apoptotic MPs is more resistant to both endogenous plasma DNase activity as well as purified DNase I activity than DNA contained within necrotic MPs.
Membrane Lipid MPs Protect RNA from RNase Activity
Similar to cfDNA, cell-free RNA (cfRNA) has been suggested as a way of diagnosing fetal pathologies in vivo.35 One study indicated that cfRNA is associated with MPs.36 To determine whether RNA is also found in apoptotic MPs induced in vitro, mRNA was extracted from the MPs and amplified by real-time RT-PCR. To determine whether the RNA was protected by lipid protein complexes, we treated the apoptotic MPs with RNase ± Triton X-100, a nonionic detergent that solubilizes lipids, for 1 hour. Thereafter, the RNA was extracted and RT-PCR was performed using primers that bridge an exon-exon junction to prevent amplification of potential contaminating genomic DNA. Table 1 shows amplification of mRNA from apoptotic MPs treated with either 0.2 mg/ml RNase (Ct value of 21) or 1.0 mg/ml RNase (Ct value of 21). By contrast, the Ct value for apoptotic MPs treated with both Triton X-100 and RNase was 40, which indicates that no RNA could be detected above background. Together, the data suggest that protein/lipid membrane complexes likely protect against RNase activity.
In Vitro MPs Are Similar to Maternal Plasma MPs
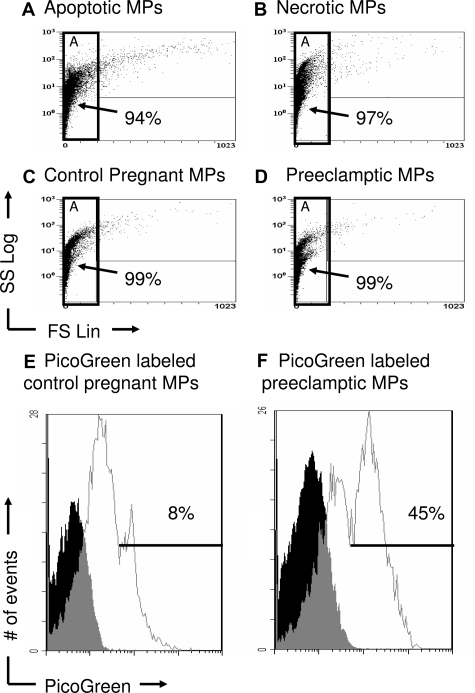
To compare similarities between MPs found in maternal plasma and in vitro MPs, samples from each were labeled with or without DNA dye, PicoGreen, and examined by flow cytometry. The dot plots, which display light scatter properties representing size (x axis) and granularity (y axis), are shown in Figures 8A–D. In vitro MPs (Figure 8, A and B) had forward light scatter patterns similar to MPs found in normal maternal plasma (Figure 8C) as well as preeclamptic plasma samples (Figure 8D). Although similar in light scatter patterns, dot plots of unlabeled PicoGreen MPs are not shown. These data suggest that the in vitro MPs were similar in size (forward scatter) and structure (side scatter) to the MPs found in maternal plasma. Next, an electronic gate (Gate A) was placed around PicoGreen labeled MPs (Figure 8, C and D) and unlabeled MPs (data not shown) from control pregnancies and preeclamptic pregnancies and the percentage of PicoGreen+ MPs was determined. Both PicoGreen histograms show three peaks; one negative, one intermediate, and one bright peak (Figure 4, E and F). The negative peak is the autofluorescence of unstained MPs as shown in the filled in histogram. The intermediate peak is a heterogeneous population of MPs containing intermediate amounts of DNA, the bright PicoGreen peak has high levels of DNA. To determine MPs with high levels of DNA, the cursor was placed on the PicoGreen bright peak. A threefold increase in the percentage of bright PicoGreen+ MPs from term preeclamptic pregnancies (46.2 ± 22.4%; n = 4) compared to term control pregnancies (14.3.± 7.2%; n = 4; P = 0.03) was detected, showing that the preeclamptic maternal MPs had more DNA per MP.
Figure 8.
MPs from maternal plasma display light scatter properties similar to in vitro MPs. In vitro MPs were centrifuged at 300 × g to remove cells, followed by a second 800 × g centrifugation. The MP supernatants were then centrifuged at 25,000 × g and resuspended in PBS. Frozen plasma samples were thawed, resuspended in PBS and labeled with or without PicoGreen. The light scatter properties of in vitro and plasma MPs were then compared by flow cytometry and displayed as dot plots (x axis, size; y axis, subcellular content). A: In vitro apoptotic MPs were resuspended in PBS and shown in Gate A. B: In vitro necrotic MPs were resuspended in PBS and shown in Gate A. C: Control maternal plasma samples were resuspended in PBS and shown in Gate A. D: Preeclamptic plasma samples were resuspended in PBS and shown in Gate A. (E, F) The PicoGreen negative peak is the autofluorescence of unstained MPs. The intermediate peak is heterogeneous containing MPs with intermediate amounts DNA, the bright PicoGreen peak has high levels of DNA. Histograms are representative of four independent experiments.
Total mpDNA Levels in Maternal Plasma
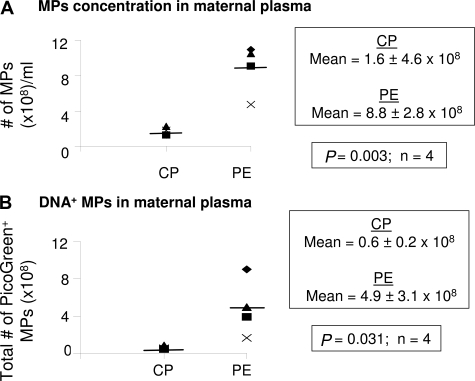
Using our fluorescent bead counting assay, we compared the levels of MPs in plasma from control pregnant women and women with preeclampsia. There were higher levels of MPs in maternal plasma from preeclamptic pregnancies (8.8 ± 2.8 × 108; n = 4, Figure 9A) compared to control pregnancies (1.6 ± 4.6 × 106; P = 0.003, n = 4, Figure 9A). Furthermore, the number of total PicoGreen+ MPs was significantly higher in preeclamptic women (4.9 ± 3.1 × 108; n = 4, Figure 9B) compared to control pregnant women (0.6 ± 0.2 × 108; P = 0.031, n = 4, Figure 9B). These data suggest higher levels of cell death occur in preeclampsia compared to normal control pregnancies.
Figure 9.
Quantitation of circulating mpDNA in normal pregnancy and preeclampsia. Frozen plasma samples were thawed and the concentration of MPs (# of MPs/ml) was quantitated by flow cytometry using a fluorescent bead assay as described in Materials and Methods. A: The scattergram compares the concentration of circulating MPs (MPs/ml) between control pregnant (CP) and preeclamptic (PE) women. The results are reported as the mean ± SD (n = 4). B: MPs labeled with PicoGreen (DNA binding dye) were analyzed by flow cytometry. A total of 10,000 events were counted for each of eight samples. The total number of PicoGreen+ MPs was obtained by multiplying the total number of MPs by the percentage of PicoGreen+ MPs in CP and PE women. The results are reported as the mean ± SD (n = 4). The bars represent the mean value.
Discussion
We sought to determine the cellular mechanisms and kinetics of circulating cfDNA associated with MPs. The current study confirms and expands our previous observations by demonstrating that MPs containing cfDNA are released from apoptotic (fetal) cells. Most importantly, the in vitro generated MPs are very similar to maternal MPs found in plasma. Apoptosis leads to continuous production of MPs so that as time progresses more appear in circulation (in vivo) or in the supernatant (in vitro). In addition, in vitro apoptotic mpDNA has fragmented low molecular weight DNA that is amplifiable and protected from DNases, similar to characteristics of apoptotic fetal cfDNA (Table 2). Interestingly, apoptotic MPs containing RNA are also protected from RNase activity (Table 2), similar to fetal cfRNA. Our observations further indicate that MPs released from apoptotic cells are similar to plasma MPs based on light scatter and DNA staining properties (Table 2), suggesting similar DNA content and cell death mechanism. This report also provides new and convincing evidence that preeclamptic patients have significantly higher amounts of mpDNA than found in normal control pregnancies, suggesting that quantitative analysis of DNA+ MPs could be used to clinically monitor or predict preeclampsia. Indeed, others have shown that levels of STBMs are similar in preeclamptic and control pregnancies at term,13,14 indicating that our methods may be more sensitive and relevant, than methods detecting STBMs.
Table 2.
Similarities between Maternal Plasma cfDNA, MPs, and in vitro mpDNA
| Similarities |
|---|
| Light scatter and DNA properties |
| Amplifiable DNA protected from DNases |
| Fragmented low molecular weight DNA |
| Amplifiable RNA protected from RNases |
In this study, we specifically show that hypoxia-induced MPs from JEG-3 cells, which model EVTs, are similar to plasma MPs found in preeclamptic and control pregnant women by light scatter properties, with similar size and DNA content based on PicoGreen staining. Similar results were seen with LnCaP prostate cells and Jurkat T cells, supporting our hypothesis that apoptotic MPs contain DNA (data not shown). In addition, we observed similar light scatter characteristics between flow sorted AO-labeled maternal plasma fetal DNA MPs and our in vitro MPs (Supplemental Figure 4, see http://ajp. amjpathol.org). AO-labeled maternal plasma showed a high forward light scatter pattern (FS Lin), resulting in a characteristic arc pattern (Supplemental Figure 4A, see http://ajp.amjpathol.org). AO+ nucleic acid material was also detected (Supplemental Figure 4B, see http://ajp. amjpathol.org). The AO+ DNA MPs were sorted and re-analyzed by flow cytometry. Postsorted AO+ MPs had less forward light scatter (Supplemental Figure 4A, 4C, see http://ajp.amjpathol.org). In vitro MPs (Supplemental Figure 4D, 4E, see http://ajp.amjpathol.org) also had forward light scatter patterns similar to the sorted maternal AO+ MPs (Supplemental Figure 4C, see http://ajp. amjpathol.org). Other experiments showed that the pH of the AO staining buffer (under 4.0) caused the characteristic arc pattern of forward scatter (data not shown). Together, these data suggest that the in vitro MPs are similar to MPs found in maternal plasma.
During normal pregnancy, fetal cfDNA is released into the maternal circulation from apoptotic fetal cells.37 Therefore, quantification of fetal cfDNA concentrations in maternal plasma has potential for monitoring pregnancy associated-disorders, including preeclampsia. Initial studies reported a five-fold increase in plasma fetal cfDNA concentrations in women with preeclampsia (mean gestational age [MGA] 32 weeks) compared to control pregnant women.38 It was also shown that higher combined maternal and fetal cfDNA concentrations correlated with the severity of preeclampsia (MGA 33 weeks)8 and that higher levels of fetal cfDNA alone were detected in maternal plasma before clinical symptoms (MGA 17 to 20 weeks).6,7 Together, these findings suggest that increased levels of fetal cfDNA in preeclampsia indicate placental damage/cell death and that the pathogenesis occurs before the onset of clinical symptoms.
The cellular mechanism most likely to give rise to circulating MPs is apoptosis. However, it has been suggested that trophoblasts undergo aponecrosis during preeclampsia.17 Aponecrosis is a form of cell death that has overlapping morphological features between apoptosis and necrosis in response to hypoxia39 and chemical-induced hypoxia.40,41 Huppertz et al reported that syncytiotrophoblasts exposed to severe hypoxia displayed both apoptotic and necrotic characteristics, suggesting aponecrosis.17 Consistent with this, our results show that cells treated with rotenone, which blocks the mitochondrial complex I, display fragmented DNA and PI uptake (26%) by 24 hours, whereas by 48 hours, the percentage of PI+ cells increased to 73% (Supplemental Figure 2, see http://ajp.amjpathol.org). Supportive of our results is a study reporting that fibroblastic cells treated with Antimycin A, an inhibitor of mitochondrial respiratory chain complex III, or rotenone, underwent aponecrosis.40 Tjoa et al demonstrated that placental explants challenged with oxidative stress displayed apoptotic as well as necrotic characteristics and released cfDNA into the supernatant, suggesting that aponecrosis may be an alternative mechanism for the release of fetal cfDNA.16 The authors did not address whether cfDNA was associated with MPs, which we conclusively show in this study.
We used JEG-3 cells because they are homogeneous and represent the EVT cells found in early gestation.18 Studies with primary trophoblasts show high background apoptosis,16,22 however background levels of death of JEG-3 cells are low, allowing more interpretable cell death experiments.
Few studies have examined the biochemical properties of cfDNA released from dying cells. Size fractionation of cfDNA extracted from maternal plasma demonstrated that the majority of fetal DNA fragments are less than 300 bp,42 supporting the idea that fetal cfDNA is released from apoptotic cells. Our data show that MPs form a DNA ladder when analyzed by gel electrophoresis. By contrast, necrotic cells released MPs with a DNA smear, consistent with necrotic DNA. Although rotenone-induced MPs are not true hypoxia-derived plasma MPs from preeclamptic women, their characteristics demonstrate that MPs released from apoptotic cells contain small fragments of DNA, similar to fetal DNA in the maternal circulation.42
Our studies show that rotenone-induced MPs contain significantly more membranes than necrotic-released MPs. Most importantly, these lipid membranes protect DNA from DNase activity, which accounts for MP stability in plasma. Others have shown that STBMs prepared by mechanical disruption, thought to represent an aponecrotic form of cell death, also contain high lipid levels,43 consistent with our results.
In addition to fetal DNA, fetal cfRNA is readily found in maternal plasma. Recently, we reported the feasibility of amplifying placental mRNA from dried blood spots,44 suggesting that fetal RNA is stable and easily amplified by reverse transcriptase real-time PCR. Although fetal transcripts have been found in the supernatant of STBMs prepared from placental explants,11 it is unclear whether the MPs contain cfRNA. By contrast, we demonstrate that apoptotic MPs contain cfRNA resistant to RNase activity, protected by lipids.
Syncytiotrophoblasts, which surround the outer layer of the placenta, constantly undergo apoptosis and release STBMs into the maternal blood circulation.12 Because the number of STBMs is increased in preeclampsia, compared to normal pregnancies, STBMs might be used as a predictive biomarker for preeclampsia. However, a limitation is that STBMs are not detected until the second and third trimester of pregnancy.13 Given that preeclampsia is associated with dysfunctional extravillous trophoblasts invasion of both the myometrium and the spiral arteries during the first trimester,18 MPs derived from EVTs might be a more informative biomarker in early gestation than STBMs.
An advantage of measuring total mpDNA from pathological pregnancies is that all sources of MPs would be included. Total cfDNA (maternal and fetal) has been quantitated using β–globin levels, which were higher (MGA 35.6 ± 2.8 weeks) in women with preeclampsia (MGA 32.0 ± 3.8 weeks),45 as well as higher in women (MGA 20.5 ± 3.3 weeks) who subsequently developed preeclampsia (MGA 33.5 ± 3.6 weeks).46 Goswami et al showed that STBMs were elevated in third trimester preeclamptic plasma samples compared to normal pregnancies.13,14 However, a significant difference in the number of STBMs was seen only in women with early-onset preeclampsia (<34 weeks of gestation), whereas no significant difference was seen in women with late-onset preeclampsia (>34 weeks of gestation).
Using our flow cytometry based mpDNA assay, we detected significantly elevated levels of total mpDNA in late-onset preeclampsia compared to control pregnancies, suggesting that our method is better than detecting elevated STBMs by enzyme-linked immunosorbent assay.13 Flow cytometric analysis of total mpDNA is advantageous given that the technique is simple, rapid (no processing of the plasma sample) and inexpensive as compared to real-time PCR. The method is also gender independent. Furthermore, this technique could be applied to other clinical and pathological conditions associated with increased levels of cell death (ie, trauma, stroke, organ transplantations, autoimmunity, and cancer). In its current form, quantifying mpDNA might not be applicable for prenatal diagnosis because it is not tissue specific nor does it discriminate between maternal and fetal DNA. However, such a method could be used to rapidly screen plasma samples before further analysis by more complex assays such as real-time PCR.
Future directions include addressing whether elevated levels of circulating mpDNA can be detected longitudinally in pregnancies that subsequently develop preeclampsia. Furthermore, it will be important to compare mpDNA levels with other known plasma/serum markers for preeclampsia such as Activin A, P-selectin, and vascular endothelial growth factor receptor.47 Finally, addressing whether mpDNA express surface markers from their original cells (ie, maternal endothelial cells or fetal trophoblasts) may help to understand the underlining pathology of preeclampsia or help to enrich fetal DNA from maternal plasma by isolating fetal-specific MPs.
Supplementary Material
Acknowledgments
We would like to thank the participants for donating blood, Dr. Edwina Popek (Texas Children’s Hospital, Houston, TX) for donating the preeclamptic and control plasma samples and Eleanor Chapman for preparation of the manuscript.
Footnotes
Address reprint requests to Dorothy E. Lewis, Ph.D., Department of Internal Medicine, Infectious Diseases Division, 301 University Boulevard, Mail Route 0435, Galveston, TX 77555.
Supported by grants NIH/NICHD HDO46623 and T32 AI007495. Accepted for publication August 14, 2008.
Supplementary material for this article can be found on http://ajp. amjpathol.org.
References
- Simak J, Gelderman MP. Cell Membrane Microparticles in Blood and Blood Products: potentially Pathogenic Agents and Diagnostic Markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Microparticles and Immunomodulation in Pregnancy and Pre-Eclampsia. J Reprod Immunol. 2007;76:61–67. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Toth B, Lok CA, Boing A, Diamant M, van der Post JA, Friese K, Nieuwland R. Microparticles and Exosomes: impact on Normal and Complicated Pregnancy. Am J Reprod Immunol. 2007;58:389–402. doi: 10.1111/j.1600-0897.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: a Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens Are Translocated into Small Apoptotic Bodies During Early Stages of Apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- Leung TN, Zhang J, Lau TK, Chan LY, Lo YM. Increased Maternal Plasma Fetal DNA Concentrations in Women Who Eventually Develop Preeclampsia. Clin Chem. 2001;47:137–139. [PubMed] [Google Scholar]
- Zhong XY, Holzgreve W, Hahn S. The Levels of Circulatory Cell Free Fetal DNA in Maternal Plasma Are Elevated Prior to the Onset of Preeclampsia. Hypertens Pregnancy. 2002;21:77–83. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- Swinkels DW, de Kok JB, Hendriks JC, Wiegerinck E, Zusterzeel PL, Steegers EA. Hemolysis. Elevated Liver Enzymes, and Low Platelet Count (Hellp) Syndrome as a Complication of Preeclampsia in Pregnant Women Increases the Amount of Cell-Free Fetal and Maternal DNA in Maternal Plasma and Serum. Clin Chem. 2002;48:650–653. [PubMed] [Google Scholar]
- Bischoff FZ, Lewis DE, Simpson JL. Cell-Free Fetal DNA in Maternal Blood: kinetics. Source and Structure Hum Reprod Update. 2005;11:59–67. doi: 10.1093/humupd/dmh053. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P. Villous Cytotrophoblast Regulation of the Syncytial Apoptotic Cascade in the Human Placenta. Histochem Cell Biol. 1998;110:495–508. doi: 10.1007/s004180050311. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Holzgreve W, Huppertz B, Malek A, Schneider H, Hahn S. Detection of Fetal DNA and Rna in Placenta-Derived Syncytiotrophoblast Microparticles Generated in Vitro. Clin Chem. 2004;50:2187–2190. doi: 10.1373/clinchem.2004.040196. [DOI] [PubMed] [Google Scholar]
- Knight M, Redman CW, Linton EA, Sargent IL. Shedding of Syncytiotrophoblast Microvilli into the Maternal Circulation in Pre-Eclamptic Pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess Syncytiotrophoblast Microparticle Shedding Is a Feature of Early-Onset Pre-Eclampsia, but Not Normotensive Intrauterine Growth Restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic Inflammatory Priming in Normal Pregnancy and Preeclampsia: the Role of Circulating Syncytiotrophoblast Microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- Chan KC, Zhang J, Hui AB, Wong N, Lau TK, Leung TN, Lo KW, Huang DW, Lo YM. Size Distributions of Maternal and Fetal DNA in Maternal Plasma. Clin Chem. 2004;50:88–92. doi: 10.1373/clinchem.2003.024893. [DOI] [PubMed] [Google Scholar]
- Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic Oxidative Stress and the Release of Cell-Free Feto-Placental DNA. Am J Pathol. 2006;169:400–404. doi: 10.2353/ajpath.2006.060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P. Hypoxia Favours Necrotic Versus Apoptotic Shedding of Placental Syncytiotrophoblast into the Maternal Circulation. Placenta. 2003;24:181–190. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A Class I Antigen. Hla-G, Expressed in Human Trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-Induced Apoptosis Limits Endovascular Trophoblast Invasion in the Uterine Wall of Preeclamptic Women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The Isolation and Characterization of a Population of Extravillous Trophoblast Progenitors from First Trimester Human Placenta. Hum Reprod. 2007;22:2111–2119. doi: 10.1093/humrep/dem144. [DOI] [PubMed] [Google Scholar]
- Chu W, Fant ME, Geraghty DE, Hunt JS. Soluble Hla-G in Human Placentas: synthesis in Trophoblasts and Interferon-Gamma-Activated Macrophages but Not Placental Fibroblasts. Hum Immunol. 1998;59:435–442. doi: 10.1016/s0198-8859(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Al-Nasiry S, Spitz B, Hanssens M, Luyten C, Pijnenborg R. Differential Effects of Inducers of Syncytialization and Apoptosis on Bewo and Jeg-3 Choriocarcinoma Cells. Hum Reprod. 2006;21:193–201. doi: 10.1093/humrep/dei272. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Asumendi A, Villar J, Gil MJ, Martinez-Merino V, Encio IJ, Migliaccio M. New Benzo(B)Thiophenesulphonamide 1,1-Dioxide Derivatives Induce a Reactive Oxygen Species-Mediated Process of Apoptosis in Tumour Cells. Oncogene. 2003;22:3759–3769. doi: 10.1038/sj.onc.1206435. [DOI] [PubMed] [Google Scholar]
- Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A General Method for Bead-Enhanced Quantitation by Flow Cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgez CJ, Dang DD, Simpson JL, Lewis DE, Bischoff FZ. Quantity Versus Quality: optimal Methods for Cell-Free DNA Isolation from Plasma of Pregnant Women. Genet Med. 2006;8:615–619. doi: 10.1097/01.gim.0000241904.32039.6f. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of Apoptosis by Cytometry Using Tunel Assay. Methods. 2008;44:250–254. doi: 10.1016/j.ymeth.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazell AE, Lacey HA, Jones CJ, Huppertz B, Baker PN, Crocker IP. Effects of Oxygen on Cell Turnover and Expression of Regulators of Apoptosis in Human Placental Trophoblast. Placenta. 2008;29:175–186. doi: 10.1016/j.placenta.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Crowley A, Martin C, Fitzpatrick P, Sheils O, O'Herlihy C, O'Leary JJ, Byrne BM. Free Fetal DNA Is Not Increased before 20 Weeks in Intrauterine Growth Restriction or Pre-Eclampsia. Prenat Diagn. 2007;27:174–179. doi: 10.1002/pd.1645. [DOI] [PubMed] [Google Scholar]
- Baby SM, Roy A, Lahiri S. Role of Mitochondria in the Regulation of Hypoxia-Inducible Factor-1α in the Rat Carotid Body Glomus Cells. Histochem Cell Biol. 2005;124:69–76. doi: 10.1007/s00418-005-0028-6. [DOI] [PubMed] [Google Scholar]
- Wang P, Song JH, Song DK, Zhang J, Hao C. Role of Death Receptor and Mitochondrial Pathways in Conventional Chemotherapy Drug Induction of Apoptosis. Cell Signal. 2006;18:1528–1535. doi: 10.1016/j.cellsig.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wolbers F, Buijtenhuijs P, Haanen C, Vermes I. Apoptotic Cell Death Kinetics in Vitro Depend on the Cell Types and the Inducers Used. Apoptosis. 2004;9:385–392. doi: 10.1023/b:appt.0000025816.16399.7a. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintero VH, Jimenez JJ, Jy W, Mauro LM, Hortman L, O'Sullivan MJ, Ahn Y. Elevated Plasma Endothelial Microparticles in Preeclampsia. Am J Obstet Gynecol. 2003;189:589–593. doi: 10.1067/s0002-9378(03)00469-1. [DOI] [PubMed] [Google Scholar]
- Tatischeff I, Lavialle F, Pigaglio-Deshayes S, Péchoux-Longin C, Chinsky L, Alfsen A. Dictyostelium Extracellular Vesicles Containing Hoechst 33342 Transfer the Dye into the Nuclei of Living Cells: A Fluorescence Study. J Fluoresc. 2007 doi: 10.1007/s10895-007-0271-4. [DOI] [PubMed] [Google Scholar]
- Pohl FM, Thomae R, Karst A. Temperature Dependence of the Activity of DNA-Modifying Enzymes: endonucleases and DNA Ligase. Eur J Biochem. 1982;123:141–152. doi: 10.1111/j.1432-1033.1982.tb06510.x. [DOI] [PubMed] [Google Scholar]
- Lo YM, Tsui NB, Chiu RW, Lau TK, Leung TN, Heung MM, Gerovassili A, Jin Y, Nicolaides KH, Cantor CR, Ding C. Plasma Placental Rna Allelic Ratio Permits Noninvasive Prenatal Chromosomal Aneuploidy Detection. Nat Med. 2007;13:218–223. doi: 10.1038/nm1530. [DOI] [PubMed] [Google Scholar]
- Ng EK, Tsui NB, Lam NY, Chiu RW, Yu SC, Wong SC, Lo ES, Rainer TH, Johnson PJ, Lo YM. Presence of Filterable and Nonfilterable Mrna in the Plasma of Cancer Patients and Healthy Individuals. Clin Chem. 2002;48:1212–1217. [PubMed] [Google Scholar]
- Maron JL, Bianchi DW. Prenatal Diagnosis Using Cell-Free Nucleic Acids in Maternal Body Fluids: a Decade of Progress. Am J Med Genet C Semin Med Genet. 2007;145:5–17. doi: 10.1002/ajmg.c.30115. [DOI] [PubMed] [Google Scholar]
- Lo YM, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative Abnormalities of Fetal DNA in Maternal Serum in Preeclampsia. Clin Chem. 1999;45:184–188. [PubMed] [Google Scholar]
- Formigli L, Zecchi Orlandini S, Capaccioli S, Poupon MF, Bani D. Energy-Dependent Types of Cell Death in Mcf-7 Breast Cancer Cell Tumors Implanted into Nude Mice. Cells Tissues Organs. 2002;170:99–110. doi: 10.1159/000046184. [DOI] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and Biochemical Exploration of a Syncretic Process of Cell Death Sharing Apoptosis and Necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Papucci L, Formigli L, Schiavone N, Tani A, Donnini M, Lapucci A, Perna F, Tempestini A, Witort E, Morganti M, Nosi D, Orlandini GE, Zecchi Orlandini S, Capaccioli S. Apoptosis Shifts to Necrosis Via Intermediate Types of Cell Death by a Mechanism Depending on C-Myc and Bcl-2 Expression. Cell Tissue Res. 2004;316:197–209. doi: 10.1007/s00441-004-0872-z. [DOI] [PubMed] [Google Scholar]
- Li Y, Zimmermann B, Rusterholz C, Kang A, Holzgreve W, Hahn S. Size Separation of Circulatory DNA in Maternal Plasma Permits Ready Detection of Fetal DNA Polymorphisms. Clin Chem. 2004;50:1002–1011. doi: 10.1373/clinchem.2003.029835. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Holzgreve W, Hahn S. Decrease in Lipid Levels of Syncytiotrophoblast Micro-Particles Reduced Their Potential to Inhibit Endothelial Cell Proliferation. Arch Gynecol Obstet. 2008;277:115–119. doi: 10.1007/s00404-007-0425-2. [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Simpson JL, Bischoff FZ. Recovery and Amplification of Placental Rna from Dried Maternal Blood Spots: utility for Non-Invasive Prenatal Diagnosis. Reprod Biomed Online. 2006;13:558–561. doi: 10.1016/s1472-6483(10)60645-1. [DOI] [PubMed] [Google Scholar]
- Sekizawa A, Farina A, Sugito Y, Matsuoka R, Iwasaki M, Saito H, Okai T. Proteinuria and Hypertension Are Independent Factors Affecting Fetal DNA Values: a Retrospective Analysis of Affected and Unaffected Patients. Clin Chem. 2004;50:221–224. doi: 10.1373/clinchem.2003.023259. [DOI] [PubMed] [Google Scholar]
- Farina A, Sekizawa A, Iwasaki M, Matsuoka R, Ichizuka K, Okai T. Total Cell-Free DNA (Beta-Globin Gene) Distribution in Maternal Plasma at the Second Trimester: a New Prospective for Preeclampsia Screening. Prenat Diagn. 2004;24:722–726. doi: 10.1002/pd.973. [DOI] [PubMed] [Google Scholar]
- Banzola I, Farina A, Concu M, Sekizawa A, Purwosunu Y, Strada I, Arcelli D, Simonazzi G, Caramelli E, Rizzo N. Performance of a Panel of Maternal Serum Markers in Predicting Preeclampsia at 11–15 Weeks’ Gestation. Prenat Diagn. 2007;27:1005–1010. doi: 10.1002/pd.1821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.