Abstract
The interaction between platelets and the tumor microenvironment results in the modulation of angiogenesis, although the mechanisms governing this regulation remain unclear. This study explores the differences in the communication between wounded tissues and healthy, tumor-conditioned, and frozen platelets. Platelet-rich plasma obtained from healthy (PRP) or tumor-bearing (TPRP) mice was applied to dorsal, full-thickness wounds on diabetic mice. Wound healing was evaluated using macroscopic criteria and a staging system based on angiogenesis and stromal cell proliferation. Proteomic analysis was used to compare the levels of angiogenic proteins contained in the platelet preparations. TPRP-treated wounds reached 90% wound closure 5.6 to 9.5 days earlier than PRP-treated and nontreated wounds, respectively. TPRP induced a fourfold increase in stromal cell proliferation compared with nontreated wounds, and a 2.5-fold increase compared with PRP-treated wounds. TPRP induced the highest stimulation of angiogenesis with a fourfold increase compared with nontreated controls. On day 21, wounds treated with TPRP showed a typical architecture with thick collagen bundles. Although the levels of angiogenesis regulators detected via SELDI-ToF were similar between the PRP and TPRP treatment regimens, the enhanced healing capacity of TPRP suggests improved platelet delivery as indicated by frozen TPRP preparations that had lost most of their pro-angiogenic drive. In conclusion, these results show that intact tumor-conditioned platelets display an improved ability to deliver angiogenesis regulators to wounded tissues.
Circulating platelets are essential for the regulation of angiogenesis1 following injury. The mechanisms by which platelets support vascular growth and repair in wounds is still largely undefined. One leading hypotheses is that angiogenesis relies on platelet delivery of regulatory proteins. Several angiogenesis regulating proteins that are carried by platelets are present at different times during wound healing,2 and are stored in separate (pro- and anti-angiogenic) α-granule compartments in the platelet’s cytoplasm.3 Early evidence suggests that protease activated receptors may facilitate a selective release of pro- or anti-angiogenic factors from platelets.3,4 As a consequence, an orchestrated, temporal release of stimulators and/or inhibitors of angiogenesis may result during degranulation, rather than an acute delivery of all platelet proteins in a bolus. In addition, platelet levels of growth factors and cytokines are also variable.4,5 The concentration of some angiogenic growth factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, platelet-derived growth factor, and platelet factor-4, in platelets (but not in plasma) changes as a function of the stage of tumor growth4,5,6 and may have the potential to be used as an early diagnostic tool for cancer detection.4
Pro- and anti-angiogenic growth factors and cytokines are required to modulate vascular growth, but in the absence of an optimized delivery system; ie, reduced platelet numbers, they may be insufficient to sustain angiogenesis. In in vivo experimental models in which platelets were made dysfunctional, growing blood vessels exhibited moderate maturation and functionality.1 Similarly, our previous work using recombinant growth factors or sonicated platelets on experimental full-thickness diabetic wounds revealed less of a pro-angiogenic effect than using intact platelets.2,7 These data, therefore, suggest that the integrity of the platelet membranes or the open canallicular system may serve as the gateways controlling uptake and delivery of the various proteins that regulate angiogenesis.
It has been well established that platelets partake in vascular growth in primary tumors and metastasis8,9 by specific adhesion via fibronectin and Von Willebrand factor10 and possibly by secretion of platelet products.11 It is not clear whether platelet function changes on interaction with a tumor microenvironment in vivo. In particular, it is not known if the ability of platelets to store and release these factors is modulated by adherence to the activated endothelium or the extracellular matrix of a tumor in vivo. This study demonstrates that platelets conditioned in vivo by the presence of a tumor show an improved ability to deliver angiogenesis regulators, and enhance the healing of full-thickness wounds in diabetic mice, when compared to normal platelets.
Materials and Methods
In Vivo Preparation of Platelet-Rich Plasma
Wild-type, male, C57BL/6 male mice received an injection into the dorsal flanks of 1 × 106 Lewis lung carcinoma cells prepared in serum free media or an equal volume of serum free media alone. All tumor cell lines and tissues were tested for mycoplasma and viruses as required by guidelines set forth by the Animal Facility at Children’s Hospital Boston. Four to six weeks later, when tumors were ∼200 to 300 mm3, all mice were anesthetized and terminally bled through cardiac puncture. Blood was collected into 1:10 v/v sodium citrate and placed on a Nutator Mixer (BD Diagnostics) until processed. Platelet-rich plasma (PRP) from all groups was then obtained by centrifugation of the citrated blood at 180 × g for 20 minutes at room temperature, followed by collection of the top phase. The platelet concentration was then adjusted to 3 × 108/μl in the same plasma. PRP and PRP from tumor-conditioned mice (TPRP) preparations were stored at room temperature (22°C) on an Eberbach shaker until used. One hundred microliters of the adjusted PRP or TPRP was applied topically as described below in a blinded fashion. An aliquot of TPRP and PRP was also frozen and stored at −80°C before the topical application to test for the effect of platelet freezing on their delivery function.
Wound Model and Study Design
Homozygous, genetically diabetic, 10 week-old Lep/r-db/db male mice (strain C57BL/KsJ-Leprdb) were shaved and depilated (Nair, Church & Dwight Co., Princeton, NJ) one day before surgery. On the day of surgery, animals were weighed and anesthetized with 60 mg/kg Nembutal (Pentobarbital, Abbott Laboratories, Chicago, IL). Full-thickness, 1.0-cm2 areas of skin and panniculus carnosus were excised from the nape of the neck using a marked template. Wounds were then photographed and individually sealed with semiocclusive polyurethane dressings (Tegaderm, 3M, St. Paul, MN), followed by injection of PRP or TPRP (100 μl at ∼3 × 108 platelets/μl) using a 30 gauge needle through the dressing into randomly selected wounds. Platelet preparations were administered only on the day of the surgery. Groups were categorized and compared as follows: (1) wounds left untreated and left to heal spontaneously as negative controls (nontreated, NT, n = 15); (2) wounds treated with healthy PRP (PRP, n = 15); and (3) wounds treated with tumor-conditioned PRP (TPRP, n = 15). On days 10 and 21, randomly selected animals (n = 5 per group) were euthanized, while the remaining animals were followed until complete wound closure. In addition, a subset of animals was treated with frozen preparations of PRP (n = 5) or TPRP (n = 5) and euthanized on day 10. In all cases, the wounds were photographed for macroscopic morphology, and excised and fixed in 10% neutral-buffered formalin solution for histology and immunohistochemistry. All animal procedures were pre-approved by the institutional review board according to the Association for Assessment and Accreditation of Laboratory Animal Care.
Wound Closure Analysis
Three independent observers who were blinded to the treatment mode, compared, twice weekly, digital photographs of the wounds with initial photographs taken on day 0 by planimetric methods. Wound closure was assessed quantitatively by measuring contraction, re-epithelialization of the stromal compartment and extension of the open wound as a percentage of the original wound area. The sum of contracted, re-epithelialized, and open wound areas was made equal to 100% of the original wound size.12
Central wound cross-sections were embedded in paraffin, sectioned, and stained according to routine H&E protocols. Panoramic cross-sectional digital images of each wound were prepared using Adobe Photoshop CS Software (Adobe Systems Incorporated, San Jose, CA) to analyze granulation tissue area and thickness with digital planimetry (Image J, NIH, Bethesda, MD).
Immunohistochemistry
Paraffin-embedded sections were re-hydrated by microwaving the sections in 10 mmol/L sodium citrate (pH 6.0) for 10 minutes for the antigen retrieval of Ki-67 (antigen located on the surface of the chromosomes), and in proteinase K solution (14–22 mg/ml) at 37°C for platelet endothelial cell adhesion molecule one (PECAM-1). Sections were incubated with primary antibodies to PECAM-1 (CD31, Pharmingen, San Jose, CA), Ki-67 (LabVision, Freemont, Ca), and phospho-VEGFR-2 (Cell Signaling Technologies, Danvers, MA) at 4°C overnight or at 1 hour at room temperature. PECAM-1 signal was intensified using the tyramide amplification system (Perkin Elmer, Boston, MA). The total number of PECAM-1 positive blood vessels was counted in each high power field (hpf) using original magnification ×40. Similarly, a ratio of proliferating nuclei (positive for Ki-67) over total nuclei was quantified at original magnification ×40 to establish a proliferative index. For each treatment arm and immunolocalization condition, three fields per wound (n = 6 wounds per group), two from the edges and one from the middle of the wound, were evaluated at original magnification ×40.
Platelet and Plasma reparation for SELDI-ToF Mass Spectrometry
Platelets were separated from PRP and TPRP preparations by an additional centrifugation step at 900 g at room temperature, thereby giving rise to both platelet pellets and corresponding platelet-poor plasmas. Each preparation was then processed and analyzed by SELDI- ToF technology (Ciphergen, Fremont, California). Briefly, platelet pellets and 20 μl of platelet-poor plasma from each mouse were incubated with 25 μl and 40 μl, respectively, of U9 buffer (2% 3-[(3-cholamidopropyl) dimethylammonio]-1-propansulfonate), 50 mmol/L Tris-HCl, pH 9 (Ciphergen) for 1 hour at room temperature. Platelet lysates were then centrifuged at 10,000 × g for 1 minute at 4°C. Both platelet extracts and platelet-poor plasma were fractionated by anion-exchange chromatography modified after the expression difference mapping serum fractionation protocol (Ciphergen, Fremont, CA). The fractionation was performed in a 96-well format filter plate on a Beckman Biomek 2000 Laboratory Work Station equipped with a DPC Micromix 5 shaker. An aliquot of 20 μl of the platelet and 60 μl of denatured plasma diluted with 100 μl of 50 mmol/L Tris-HCl pH 9 was transferred to a filter bottom 96-well microplate pre-filled with BioSepra Q Ceramic HyperD F sorbent beads re-hydrated and pre-equilibrated with 50 mmol/L Tris-HCl, pH 9. All liquids were removed from the filtration plate using a multiscreen vacuum manifold (Millipore, Bedford, MA) into respective wells of 96-well microtiter plates with the capture of the initial flow-through as fraction 1. This step was repeated for subsequent incubations with 2 × 100 μl of the following buffers: pH 7 (50 mmol/L HEPES), pH 5 (50 mmol/L NaAcetate), pH 4 (50 mmol/L NaAcetate), pH 3 (50 mmol/L NaCitrate) (fractions 2, 3, 4 and 5, respectively), followed by a final organic wash (33% isopropanol/16.7% acetonitrile/0.1% trifluoroacetic acid).
Expression difference mapping on ProteinChip arrays was performed using weak cationic exchange chromatography protein arrays (CM10 ProteinChip arrays; Ciphergen, Fremont, CA) by loading sample fractions onto a 96-well bioprocessor, and equilibrating with 50 mmol/L sodium acetate (Sigma, St. Louis, MO), pH 4. A further dilution of 40 μl anion exchange chromatography fraction into 100 μl of the same buffer on each array spot was incubated for 1 hour. Array spots were washed for 3 minutes with 100 μl 50 mmol/L sodium acetate, pH 4. After rinsing with water, 2 × 1 μl of sinapinic acid matrix solution was added to each array spot. For protein profiling, all fractions were diluted 1:2.5 in their respective buffers used to pre-equilibrate ProteinChip arrays. This step was followed by readings using the Protein Ciphergen System 4000 (PCS4000) SELDI-ToF mass spectrometer (Ciphergen, Fremont, CA) and processed with the ProteinChip Software Biomarker Edition, Version 3.2.0 (Ciphergen, Fremont, CA). After baseline subtraction, spectra were normalized by means of a total ion current method. Peak detection was performed by using Biomarker Wizard software (Ciphergen, Fremont, CA) using a signal-to-noise ratio of 3:1.
Wound Watch Staging System: Relative Quantification of Vascular and Proliferative compartments
To compare the healing under different treatment conditions, high power images of stained sections were used to quantify the degree of proliferation and vascularization. Three digital images of PECAM-1 and Ki-67 stained slides were captured for each sample, one in the middle and two on the edges of the wound bed. Pictures were viewed with Adobe Photoshop CS Software, and blood vessels and proliferating nuclei in each high-powered field were marked and counted and Ki-67-positive cells were expressed as a ratio of proliferating nuclei to total nuclei. Ratios were calculated between results obtained from the center of the lesions and from the edges of each treatment group to the plasma treated group. Fifteen microscopic fields were evaluated at original magnification ×40 for each experimental treatment.
Statistical Analysis
In all text and figures, values are expressed as means ± SD One-way analysis of variance and ad hoc LSD tests were used to determine the significance of differences among treatments. Multivariate analysis was performed using Statistica v7.0 (StatSoft, Inc, Tulsa, OK).
Results
Faster Wound Closure Induced by TPRP
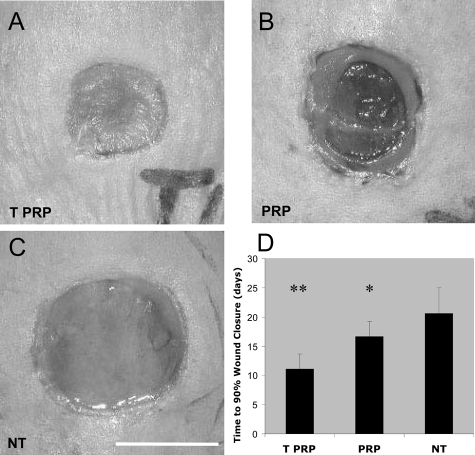
Wound healing kinetics were assessed over a 28-day period. Four days following full-thickness excisions, wounds treated with TPRP exhibited closure that was greater than 50% of the original size, compared with approximately 20% for wounds treated with PRP and less than 5% for NT control wounds, (P < 0.01, not shown). On day 10, TPRP treated wounds (Figure 1A) exhibited significant clinical improvements in wound closure over the NT controls (Figure 1C). Wounds treated with PRP showed an intermediate level of healing (TPRP>PRP>NT) (Figure 1B), exhibiting signs of macroscopic granulation and early re-epithelialization (Figure 1B). This trend continued for the first two weeks of follow-up (not shown). Overall, TPRP treated wounds reached 90% wound closure in approximately 11 days, reducing the time to 90% closure by 5.6 and 9.5 days when compared to PRP treatment (P < 0.05) and untreated (P < 0.01) wounds, respectively (Figure 1D).
Figure 1.
Clinical aspects of NT, PRP- and TPRP-treated wounds. Full-thickness wounds were excised on the dorsum of db/db mice and followed-up twice per week for 4 weeks. A: TPRP treated wounds reached more than 80% wound closure on day 10. PRP treated wounds were second to TPRP treatment in showing a highly granulating wound bed (B, 63% wound closure), while NT control wounds showed only minor signs of clinical healing (C, 49% wound closure). Scale bar = 0.5 cm. D: TPRP treatment achieved faster wound closure, starting from the first week following surgery. Treated wounds with TPRP achieved 90% wound closure within a shorter period of time (11.1 days) than wounds treated with PRP (16.7 days) and NT controls (20.6 days). ** P < 0.01 compared to PRP treatment and NT controls, *P < 0.01 compared to NT controls. NT = Non-treated, control wounds (n = 15); PRP = healthy platelet-rich plasma treated wounds (100 μl/wound, n = 15), TPRP = tumor conditioned platelet-rich plasma treated wounds (100 μl/wound, n = 15).
Maximal Stimulation of Angiogenesis and Stromal Cell Proliferation in Wounds Treated with TPRP
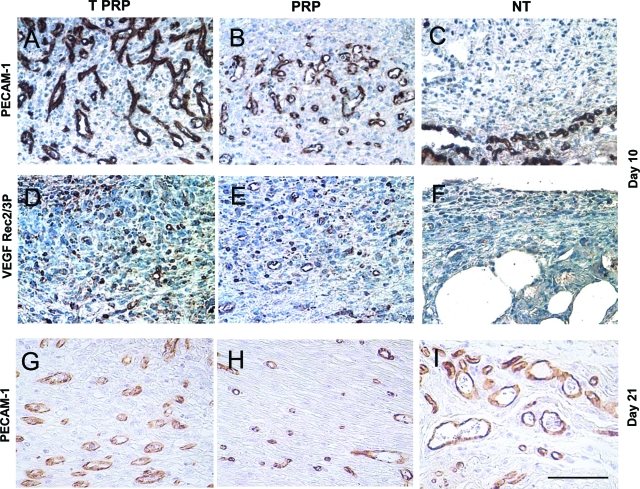
Developing organs, tumors and wounds rely on the formation of new vascular networks for growth and repair. Similar to the up-regulation of angiogenesis that leads to tumor growth and dissemination,13 early wound healing is dependent on active angiogenesis. These similarities led us to examine whether wound angiogenesis would be altered by addition of platelets from tumor-bearing mice. Immunohistochemistry with PECAM-1 (a pan-endothelial marker) showed induced vascularization averaging 61 blood vessels per hpf in TPRP treated wounds (Figure 2A, P < 0.01 compared to NT controls and P < 0.05 compared to PRP treatment), 44 vessels per hpf in PRP treated wounds (Figure 2B, P < 0.05 compared to NT controls), and only 15 blood vessels per hpf in the granulation tissue of NT control wounds (Figure 2C).
Figure 2.
Microphotographs of cross-sectional wound tissues stained for CD31 (PECAM-1). Wounds were harvested on day 10, and photographs were taken from the middle of the wound bed. Positive stained cells (brown) are endothelial cells. Quantification of blood vessels per high power field was performed for CD31 stained slides. TPRP treated wounds (A) showed a higher number of blood vessels when compared to both PRP treatment (B) and NT control wounds (C). B,D,F: Microphotographs of cross-sectional wound tissues stained for phosphorylated VEGFR-2/3 on day 10. Positive stained cells (brown) show activated VEGFR-2/3. Both TPRP (D) and PRP (E) treatments induced activation of the VEGF pathway as opposed to NT control wounds left to heal spontaneously (F), which showed a limited staining intensity. G, H, I: By day 21, while both TPRP (G) and PRP (H) treated wounds showed a decreased number of blood vessels, in NT wounds (I) blood vessels increased compared to day 10. Scale bar = 50 μm.
To determine the degree of platelet-mediated activation of angiogenesis we assessed the phosphorylation status of the cognate receptor for VEGF, VEGFR-2/3, one of the main regulators of angiogenesis, by immunohistochemistry. As seen in Figure 2, TPRP (Figure 2D) and PRP (Figure 2E) markedly increased VEGFR-2/3 phosphorylation in their wound beds over NT controls (Figure 2F), the former treatments displaying a similar gradient of intensity that peaked in TPRP treated wounds. By day 21, while wounds treated with either PRP or TPRP revealed a decreased number of vessels compared to that at day 10 (Figure 2, G and H), the NT controls showed an opposite trend (Figure 2I).
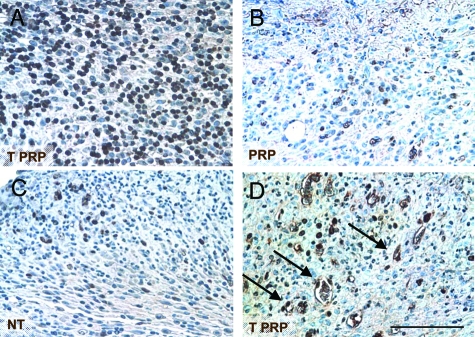
Stromal cells within the granulation tissue exposed to TPRP reached the highest proliferation rate, with up to 46% of cells actively proliferating on day 10 (P < 0.01 compared to PRP and NT, Figure 3A), as assessed by Ki-67 staining, a nuclear marker for actively replicating cells. Treatment with PRP stimulated stromal cell proliferation more so than NT wounds, but to a lesser extent than TPRP treatment (Figure 3, B and C). Among the proliferating cells in the granulation tissue, several endothelial-like cells were found to be positive in the TPRP wounds (Figure 3D).
Figure 3.
Microphotographs of cross-sectional wound tissues stained for Ki-67. Positive stained nuclei (brown) depict actively proliferating stromal cells. TPRP (A) treated wounds showed the highest levels of proliferation (46.2%), although PRP treatment (B) also stimulated cell proliferation over NT controls (C) on day 10. D: Many cells phenotypically resembling endothelial cells (arrows) were positively stained for Ki-67, but only in the TPRP treated wounds. Scale bar = 50 μm.
Unique Patterning of Collagen Deposition and of Cellularity in Wounds Treated with TPRP
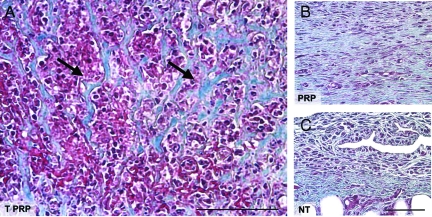
In the latter stages of wound healing, during wound tissue remodeling, collagen is deposited and organized to form a stable scaffold. This scaffold leads to the formation of a scar, the hallmark of skin repair. Both PRP and TPRP treatments resulted in increased collagen formation over NT control wounds (Figure 4A and B, respectively), however, the pattern of collagen deposition between the two treatments differed significantly (Figure 4, A and B). In addition to promoting collagen formation, TPRP also induced a unique pattern in the deposition of the collagenic matrix, characterized by thick bundles alternating with areas of high cellularity (Figure 4A). In contrast, PRP treated wounds exhibited collagenous matrix fibers in a parallel orientation and were relatively acellular, which is more typical of the formation of early scar tissue (Figure 4B). Untreated wounds showed limited ability to form a collagenous wound matrix, exhibiting only a friable and non-homogeneous collagen on day 21 (Figure 4C) by Trichrome Masson’s staining.
Figure 4.
Microphotographs of cross-sectional wound tissues harvested on day 21 and stained for collagen (Trichrome-Masson). A: TPRP-treated wounds showed a characteristic architecture of thick collagen bundles more focally distributed (arrows) with alternating areas of high cellularity. B: Wounds treated with PRP exhibited an increased amount and homogeneous organization of newly formed collagenic matrix, while NT controls left to heal spontaneously (C) showed irregularly deposited collagen, and friable wound tissue matrix. Scale bar = 50 μm.
Growth Factor Analysis of Platelets and Plasma from Healthy and Tumor-Bearing Animals
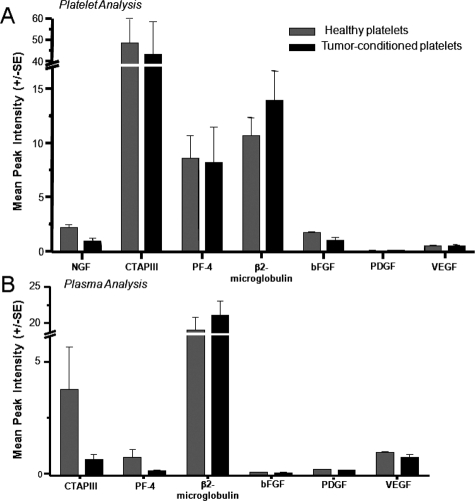
To determine whether the enhancement of angiogenesis by TPRP was due to increased levels of angiogenesis regulators or to an improved presentation of these regulators to the wounds, we separated PRP and TPRP into their respective platelet and plasma components and submitted these specimens to a complete proteomic analysis using SELDI-ToF technology. A number of previously identified angiogenesis regulators in platelets by the same technology in earlier studies4 were analyzed herein. Some of these proteins isolated and confirmed to be present in platelets in early tumor development are, β2-microglobulin, nerve growth factor, connective tissue activating protein-III, platelet factor-4, basic fibroblast growth factor, platelet-derived growth factor, and VEGF. The proteomic analysis of platelets (Figure 5A) and plasma (Figure 5B) preparations from non-tumor-bearing and tumor-bearing mice did not reveal any increase in these previously identified proteins, even though both platelets and plasma showed similar trends in the distribution of connective tissue activating protein-III and platelet factor-4 that were established for tumor growth. A number of other differentially expressed biomarkers were discovered, albeit unidentified. Sequencing these was beyond the scope of the analysis conducted for the purposes of this study, where angiogenesis related proteins were prioritized.
Figure 5.
Characterization of the proteome of platelets (A) and plasma (B) derived from tumor bearing and healthy mice. The angiogenic proteome was analyzed, showing a characteristic distribution between platelets and plasma. The contents of angiogenic factors analyzed were found to be comparable between healthy and tumor conditioned samples.
Improved Growth Factor Presentation by Platelets of TPRP
To explore the hypothesis that the enhanced healing observed with TPRP treatment was attributed to an improved bioavailability of angiogenesis regulators in response to enhanced platelet function, we tested the ability of intact platelets to deliver and present angiogenesis related proteins to the wound bed. While growth factors are preserved through freezing and thawing,7 platelets are subjected to an irreversible activation process (degranulation) and loss of function under such conditions.14 Frozen TPRP and PRP almost completely lost the ability to form new blood vessels, even though the levels of stromal cell proliferation were comparable between the two treatments (Figure 6). Frozen PRP showed an almost complete decay of stimulatory function (Figure 6).
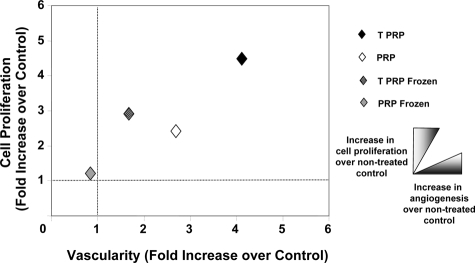
Figure 6.
The healing staging system. Wound tissues on day 10 were staged using a two dimensional plot representing vascularity on the x axis and cell proliferation on the y axis. NT control wound parameters were set to 1. TPRP treatment induced the highest histological stimulation of the wound tissues when compared to both PRP treatment and NT controls. Previously frozen TPRP and PRP failed to achieve the same levels of angiogenic stimulation, although frozen TPRP still exhibited some potential to induce stromal cell proliferation.
To better conceptualize and compare the effects of the studied treatments, we developed a 2D staging system in which vascularity was plotted against cell proliferation (Figure 6).15 The data presented are normalized to the histological stage of NT control wounds, which is given an arbitrary value of 1. TPRP treatment of wounds resulted in the highest histological stimulation of tissue repair, compared to NT control wounds (Figure 6). TPRP induced an over fourfold increase in cell proliferation (P < 0.01, compared to both PRP treatment and NT controls), while PRP induced a ∼2.5-fold increase over NT controls (P < 0.05) (Figure 6).
Discussion
When Dr. Dvorak postulated that “a tumor is a wound that never heals,”16 it was the first time that wounds and tumor lesions were acknowledged as similar biological processes. As a consequence, biological impairments in switching on and off the angiogenic drive may partially explain tumor growth as well as delayed healing in chronic wounds. Since platelets contribute to vascular growth in healthy and diseased tissues, in this study we described for the first time that an interaction between platelets and tumors (“tumor conditioning”) leads to an improved ability of intact platelets to stimulate angiogenesis.
Early in wound healing, angiogenesis is essential to initiate and support the repair process. Once healing has ceased, endothelial cells in the wound bed revert to a quiescent phenotype and start to undergo apoptosis in the early scar tissue, demonstrating that a timely switch between pro- and anti-angiogenesis is necessary to initiate and terminate the healing process, respectively.17 Platelets can likely be considered to partake in controlling this angiogenic switch as pro- and anti-angiogenic proteins are released in a sequential manner by intact platelets in response to protease-activated receptor 1 and protease-activated receptor 4, respectively.3 In our study, a similar consequence was evident; angiogenesis was stimulated on day 10, followed by a modulated down-regulation of vascular growth in the later stages of wound healing (day 21), which was not evident in NT control wounds. This is consistent with the concept that in later stages of healing in nonmalignant wounds, the increase of thrombin and collagen leads to activation of the receptor protease-activated receptor 4, subsequently releasing angiogenesis inhibitors from stromal cells and platelets.3,18,19 In contrast, the continuous proliferation and expression of pro-angiogenic factors20 by tumor cells prevents this ‘off’ switch in malignant tissues.16
The study of platelets spans over a century, but much of the acquired knowledge is focused on their behavior during clot formation. The contribution of platelets in the organization of extracellular matrix and the influence of this matrix on the formation of a neovasculature has been implied in many studies,6,8,21 but its mechanism is not completely elucidated. In this study, the distinctive architecture of the extracellular matrix of TPRP treated wounds suggests a direct stimulation of platelets on stromal formation. Stromal activation may have stimulated or followed angiogenesis; preliminary results indicate changes in transforming growth factor-β1 expression, the key fibrogenic growth factor, in TPRP treated wounds (not shown). Since there exists evidence that the invasion of some skin tumors depends on stromal cell activation,22 additional studies are warranted to understand the interplay between platelets and fibroblasts.
Although there were no significant quantitative differences in the contents of the reputed stimulators of angiogenesis between PRP and TPRP, such as VEGF, basic fibroblast growth factor, or platelet-derived growth factor, the proangiogenic response in wounds treated with TPRP was remarkably enhanced, suggesting a more effective presentation of these proteins. This is supported by our finding that angiogenesis was induced only by the use of intact platelets, and that this induction could not be similarly accomplished by simply exposing the wounds to the concentrated proteins alone, as was observed by using frozen platelets. It has to be noted that while the angiogenic properties of frozen TPRP and PRP faded almost completely, frozen TPRP still retained some proliferative stimulatory potential. It must also be noted that additional stimulatory factors that were not sequenced in the entire proteome may have contributed, at least in part, to the findings we have reported here.
Our wound healing staging system, developed to parallel wound healing and tumor biology, was based on the quantification of neovascularization and stromal cell proliferation in diabetic wounds.15 The diabetic mouse, with its impaired angiogenesis and wound healing,23 and decreased production of growth factors,24,25 served as a reproducible surrogate for the clinically relevant problem of recalcitrant wounds. Previous experiments have shown that diabetic mice have delayed wound contraction and hair re-growth, allowing for better wound dressing and closure analysis compared to wild-type mice.26 While diabetic wounds seem to lack an angiogenic drive, wounds induced by tumors seem to exhibit the opposite effect. As such, our findings suggest that discovering and exploiting the properties of tumors that can modulate platelet function may lead to the development of clinical methods that could circumvent impairments in the healing of recalcitrant wounds. We do recognize that such benefits may not transfer to cancer patients, given the very nature of the disease promoting a chronic pro-angiogenic state. However, a better understanding of the interplay we have described here may lead to improvements in the understanding of impaired wound healing.
Acknowledgments
We thank Drs. Arja Kaipainen, and Robert Valeri for their advice and for many useful discussions throughout the experimental work and in the preparation of this manuscript. We are also grateful to Dr. Judah Folkman for his enthusiastic support during these studies. The technical assistances of Jasmine Mathews, Laurie Bayer, and Dr. Dipak Panigrahy are also acknowledged.
Footnotes
Address reprint requests to Dennis P. Orgill, MD., PhD., Division of Plastic Surgery, 75 Francis Street, Brigham and Women’s Hospital, Boston, MA 02115. E-mail: dorgill@partners.org.
References
- Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, Saffaripour S, Ware J, Ruggeri ZM, Jain RK, Folkman J, Wagner DD. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci USA. 2006;103:855–860. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietramaggiori G, Kaipainen A, Ho D, Orser C, Pebley W, Rudolph A, Orgill DP. Trehalose lyophilized platelets for wound healing. Wound Repair Regen. 2007;15:213–220. doi: 10.1111/j.1524-475X.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: Pro- and anti-angiogenic proteins are organized into separate platelet {alpha}-granules and differentiall released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE, Jr, Folkman J, Klement GL. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111:1201–1207. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- Frechette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- Salgado R, Benoy I, van Marck E, Vermeulen P, Dirix L. The importance of the VEGF-load in platelets in cancer patients. Ann Oncol. 2002;13:1689–1690. doi: 10.1093/annonc/mdf320. [DOI] [PubMed] [Google Scholar]
- Pietramaggiori G, Kaipainen A, Czeczuga JM, Wagner CT, Orgill DP. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006;14:573–580. doi: 10.1111/j.1743-6109.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- Trikha M, Nakada MT. Platelets and cancer: implications for antiangiogenic therapy. Semin Thromb Hemost. 2002;28:39–44. doi: 10.1055/s-2002-20563. [DOI] [PubMed] [Google Scholar]
- Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3:99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- Yannas I. Tissue and Organ Regeneration in Adults. New York: Springer,; 2001:pp 63–91. [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion. 1973;13:61–68. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- Pietramaggiori G, Scherer SS, Mathews JC, Alperovich M, Yang HJ, Neuwalder J, Czeczuga JM, Chan RK, Wagner CT, Orgill DP. Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen. 2008;16:218–225. doi: 10.1111/j.1524-475X.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Ma L, Hollenberg MD, Wallace JL. Thrombin-induced platelet endostatin release is blocked by a proteinase activated receptor-4 (PAR4) antagonist. Br J Pharmacol. 2001;134:701–704. doi: 10.1038/sj.bjp.0704312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, Wallace JL. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci USA. 2005;102:216–220. doi: 10.1073/pnas.0406682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- Geng JG. Interaction of vascular endothelial cells with leukocytes, platelets and cancer cells in inflammation, thrombosis and cancer growth and metastasis. Acta Pharmacol Sin. 2003;24:1297–1300. [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26:293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109:132–138. doi: 10.1111/1523-1747.ep12319188. [DOI] [PubMed] [Google Scholar]
- Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, Krieg T, Eming SA. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- Scherer SPG, Mathews J, Chan R, Fiorina P, Orgill DP. Wound healing kinetics of genetically diabetic mouse. Wounds. 2007 [PubMed] [Google Scholar]