Abstract
Pemphigus foliaceus (PF) is a human autoimmune blistering disease in which a humoral immune response targeting the skin results in a loss of keratinocyte cell-cell adhesion in the superficial layers of the epidermal epithelium. In PF, desmoglein-1-specific autoantibodies induce blistering. Evidence is beginning to accumulate that activation of signaling may have an important role in the ability of pathogenic pemphigus IgGs to induce blistering and that both p38 mitogen-activated protein kinase (MAPK) and heat shock protein (HSP) 27 are part of this signaling pathway. This study was undertaken to investigate the ability of PF IgGs to activate signaling as well as the contribution of this signaling pathway to blister induction in an in vivo model of PF. Phosphorylation of both p38 MAPK and HSP25, the murine HSP27 homolog, was observed in the skin of PF IgG-treated mice. Furthermore, inhibition of p38 MAPK blocked the ability of PF IgGs to induce blistering in vivo. These results indicate that PF IgG-induced blistering is dependent on activation of p38 MAPK in the target keratinocyte. Rather than influencing the immune system, limiting the autoantibody-induced intracellular signaling response that leads to target end-organ damage may be a more viable therapeutic strategy for the treatment of autoimmune diseases. Inhibition of p38 MAPK may be an effective strategy for the treatment of PF.
The treatment of immunobullous disorders, in which an autoimmune response targets the skin to cause loss of epidermal integrity, has traditionally used agents that suppress the immune response. With a compromised epithelial barrier, patients are at risk for fluid and electrolyte imbalance and infection. Untreated, these disorders can be lethal. The introduction of corticosteroids revolutionized the treatment of autoimmune skin disease; however, chronic use of systemic corticosteroids carries significant morbidity and mortality. Steroid-sparing agents and therapies that target specific components of the immune response have been used to minimize these complications; however, immune suppression itself carries risk, including the potential for infectious complications. Rather than inhibit the immune system, an alternative strategy would be to block the ability of the autoimmune response to damage the target end- organ.
The pemphigus family of human autoimmune blistering diseases offers several advantages in the study of autoimmunity and the development of specific mechanism based therapies: i) end-organ damage is readily visible as a blister, ii) the pathogenic target autoantigens are defined,1,2,3 iii) in vivo models are available,4,5,6 and iv) the skin is accessible to topical as well as systemically delivered drugs. In pemphigus, a humoral immune response targets epithelial structures leading to loss of cell-cell adhesion and blister formation. The two major variants are pemphigus foliaceus (PF) and pemphigus vulgaris (PV).
Desmogleins, transmembrane nonclassical cadherin cell adhesion proteins, are the major pathogenic autoantibody targets of both pemphigus variants. Clinically, PV is characterized by flaccid vesicles and erosions of mucosa (mucosal PV) and subsequently mucosa and skin (mucocutaneous PV), whereas PF is characterized by superficial crusted vesicles and blisters. Histologically, loss of cell-cell adhesion (eg, acantholysis) occurs in the suprabasal epidermis in PV and in the subcorneal epidermis in PF. In PF, the autoantibody response is directed against desmoglein-1 (dsg1)2 and found in suprabasilar desmosomes of epidermal epithelia, whereas in PV, the autoantibody response is initially directed against desmoglein-3 (dsg3)1,3,7 and found in desmosomes of the basal layer of stratified epidermal epithelia and in mucosal epithelia. In PV, the autoantibody response subsequently evolves to include dsg1 as the disease transitions from mucosal to mucocutaneous PV.8,9,10 Dsg1 and dsg3 are similar in that they both are transmembrane proteins of the cadherin superfamily and are components of desmosome cell-cell adhesion junctions.11 The compensation hypothesis, the ability of dsg1 to compensate for loss of dsg3 function with the different distribution of dsg1 and dsg3 in epidermal epithelia and mucosa, has been proposed to explain the tissue distribution and location of the cleavage plane in PV and PF.12
Different mechanisms have been proposed to explain blister induction by Ig in autoimmune blistering diseases of the skin. For example, in the human subepidermal blistering disease bullous pemphigoid (BP), IgGs bind to the hemidesmosome-associated protein BP180 and trigger a complement-, mast cell-, and neutrophil-dependent inflammatory cascade culminating in human neutrophil elastase-dependent proteolytic cleavage of BP180 and separation at the dermal-epidermal junction.13,14,15,16,17,18 In contrast, pemphigus antibody binding to dsg does not require inflammatory components to mediate blister formation; pemphigus IgG induced epidermal cell detachment is neither Fc-,19 complement-,20 nor proteinase-dependent.21 Steric hindrance, the direct blocking of desmosome cadherin-adhesive interactions by pemphigus IgG, has also been suggested as a mechanism for the adhesive disrupting ability of these immunoglobulins22; however, steric hindrance alone may not be sufficient to explain the pathogenic effects because energy-requiring cellular events are required for pemphigus IgG to induce acantholysis.23,24
Binding of pemphigus IgG to the keratinocyte has been proposed to activate intracellular signaling within the target keratinocyte, and this may contribute to the loss of cell-cell adhesion.25 In our previous work on PV, we identified a series of intracellular phosphorylation events initiated by binding of PV IgG to keratinocytes. Incubation of cultured human keratinocytes with IgG purified from PV patient sera resulted in activation of p38 mitogen-activated protein kinase (MAPK), phosphorylation of the small heat shock protein (HSP) 27, reorganization of the actin cytoskeleton, and intermediate filament collapse. In tissue culture, inhibitors of p38MAPK prevented PV IgG-induced phosphorylation of HSP27 and reorganization of the cytoskeleton, events preceding the loss of cell-cell adhesion induced by PV IgG.26 These observations in tissue culture suggested that p38MAPK could be part of the acantholytic mechanism of PV IgG and that blocking p38MAPK activity might have a role in preventing PV IgG-induced blistering. Indeed, we then demonstrated that pharmacological inhibitors of p38MAPK prevented in vivo blister formation in the PV passive transfer mouse model.27 Furthermore, phosphorylation of both p38MAPK and HSP27 has been observed in perilesional epidermis of pemphigus patients.28
In PF, IgGs that target dsg1 are pathogenic. The similar immunobiology of PF and PV led us to hypothesize that like PV, PF IgGs might activate intracellular signaling via p38MAPK and HSP27. Using the passive transfer mouse model, this study was undertaken to determine if PF IgG induced activation of p38MAPK and HSP25, the murine HSP27 homolog, in the skin, and if pharmacological inhibition of p38MAPK could prevent blister formation in vivo. The results demonstrate that keratinocyte p38MAPK is activated in response to binding of pathogenic PF IgG to the skin and that inhibiting p38MAPK blocks the ability of the pathogenic IgG to cause end-organ damage, eg, blistering, in the skin. These observations suggest that targeting the mechanism by which the autoimmune response damages the end-organ may have utility in the treatment of autoimmune disorders.
Materials and Methods
Materials
Rabbit polyclonal anti-HSP25 antibodies were from StressGen (Ann Arbor, MI). Rabbit monoclonal anti-phospho HSP27 antibodies, rabbit monoclonal anti-phospho-p38MAPK (Thr180/Tyr182) antibodies, and horseradish peroxidase-linked anti-rabbit secondary antibodies were from Cell Signaling Technologies (Beverly, MA). Rabbit polyclonal anti-p38MAPK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA) and polyclonal anti-lactate dehydrogenase V antibodies were from Cortex Biochem (San Leandro, CA). Rabbit anti-sheep horseradish peroxidase-conjugated secondary antibodies were from Cortex Biochem. The p38MAPK inhibitors SB202190 and SB203580, and the inactive analog SB202474 were from Calbiochem (La Jolla, CA).
IgG Preparation
PF sera were previously described.29,30 Data presented in Figures 1 to 3 are from IgGs purified from a single PF patient, PF1, whose serum was available in sufficient quantities to perform the described studies; the activity of this serum was determined by indirect immunofluorescence on human skin with a titer of 1:160. A second independent series of experiments (Supplementary Figure S1, see http://ajp.amjpathol.org) used PF IgGs purified from sera of a second patient, PF2, (whose activity was determined by indirect immunofluorescence on human skin with a titer of 1:80) and showed similar results. Both sera were positive by dsg1 enzyme-linked immunosorbent assay (ELISA) and negative by dsg3 ELISA. PF IgGs were purified from PF patient sera by ammonium sulfate precipitation followed by affinity chromatography on Protein G (HiTrap; Pharmacia, Piscataway, NJ) as previously described.26 IgG fractions were dialyzed against phosphate-buffered saline (PBS) and sterile-filtered. Purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and activity assayed by indirect immunofluorescence and ELISA. Control IgGs (no activity by indirect immunofluorescence) were prepared in parallel from normal human sera.
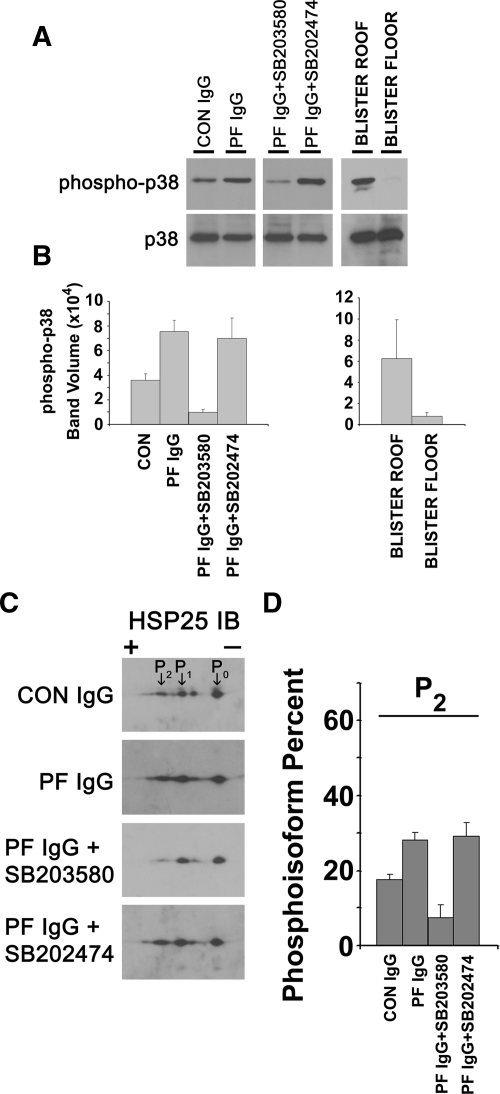
Figure 1.
Increased phosphorylation of p38MAPK in the skin of PF IgG-treated mice. Neonatal C57BL/6 wild-type mice were injected intradermally with control IgG (CON, 0.015 mg of IgG/g body weight), PF IgG (0.015 mg of IgG/g body weight), SB203580 and then PF IgG (PF IgG + SB203580), or the inactive analog SB202474 and then PF IgG (PF IgG + SB202474). Skin biopsies were obtained after 18 hours of treatment and extracted in IEF lysis buffer. A: Samples were equally loaded, separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride, and immunoblotted with antibodies to p38MAPK, phospho-p38MAPK (phospho-p38), or total p38MAPK (p38). Blots were developed by ECL reaction (Amersham Pharmacia). Band intensities of phospho-p38MAPK were normalized to the band intensities of total p38MAPK. Extracts prepared from the blister roof and floor of skin biopsies of PF IgG were similarly analyzed. A: Representative Western blot. B: Signal intensity from the ECL reaction for each band was quantified with a GeneGnome scanner (Syngene Bio Imaging) by using GeneSnap software (n = 3 mice per treatment condition; SD shown by error bars). P values are as follows: i) PF IgG compared to control, P = 0.002; ii) PF IgG + SB203580 compared to PF IgG, P = 0.004; iii) PF IgG + SB202474 compared to PF IgG, P = 0.181; iv) blister roof compared to blister floor, P = 0.03. P values were calculated by using the Student’s t-test. Increased phosphorylation of HSP25 in the skin of PF IgG-treated mice. Increased amounts of the most negatively charged HSP25 isoform (P2) were observed in PF IgG-treated mice and blocked in mice pretreated with SB203580, but not with the inactive analog SB202474. Neonatal C57BL/6 wild-type mice were injected intradermally with control IgG (CON, 0.015 mg of IgG/g body weight), PF IgG (0.015 mg of IgG/g body weight), SB203580 and then PF IgG (PF IgG + SB203580), or SB202474 and then PF IgG (PF IgG + SB202474). Skin biopsies were obtained after 18 hours of treatment and extracted in IEF lysis buffer. C: Skin extracts (30 μg) were prepared and separated in the first dimension by using 7 cm, pH 4 to 7, IPGphor strips (Amersham Pharmacia Biosciences) and in the second dimension by 10% SDS-PAGE, followed by immunoblotting with antibodies to murine HSP25 as described. Blots were developed by ECL reaction (Amersham Pharmacia). C: Representative Western blots of region of the two-dimensional gels showing HSP25 charge isoforms. D: Signal intensity from the ECL reaction for each spot corresponding to the two-dimensional gel HSP25 charge isoforms labeled P0, P1, and P2 were quantified as above with a GeneGnome scanner and GeneSnap software (n = 3 mice per treatment condition, SD shown by error bars). Values were expressed as a percentage of total HSP25 by using the formula Pn/(P0 + P1 + P2), where Pn corresponds to the signal intensity for n = spot 0, 1, or 2 and P0 + P1 + P2 is the summed signal intensity for all three HSP25 isoforms. An increase in the percentage of the most negatively charged HSP25 isoform, P2, is observed in skin extracts from PF IgG versus control-treated mice (P = 0.01). This increase is blocked in mice pretreated with SB203580 (P = 0.001 compared with PF IgG-treated mice), but not the inactive analog SB202474 (P = 0.4 compared with PF IgG-treated mice). P values were calculated by using the Student’s t-test.
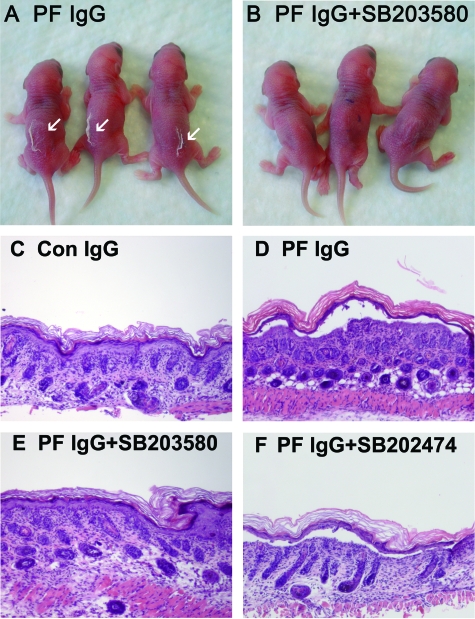
Figure 2.
Inhibiting p38MAPK prevents clinical blistering in PF passive transfer mice. Neonatal C57BL/6J mice were injected intradermally with either PF IgG (0.1 mg of IgG/g body weight) (A) or PF IgG (0.1 mg of IgG/g body weight) plus SB2023580 (B). After 18 hours, the skin of neonatal mice from the test and control groups was examined clinically. PF IgG-treated mice have a positive Nikolsky’s sign (white arrows), demonstrating loss of epithelial cell-cell adhesion. In contrast, mice treated with the SB203580 and PF IgG have a negative Nikolsky’s sign, indicating that epithelial adhesion remains intact. Inhibiting p38MAPK prevents histological blistering in PF passive transfer mice. Representative skin biopsies of mice treated with control IgG (0.015 mg of IgG/g body weight) (C), PF IgG (0.015 mg of IgG/g body weight) (D), SB203580 and then PF IgG (E), and SB202474 and then PF IgG (F), were fixed in formalin and stained with H&E. Acantholysis leading to blister formation is seen in PF IgG-treated mice, but is blocked in mice treated with the p38MAPK inhibitor SB203580, but not the inactive analog SB202474.
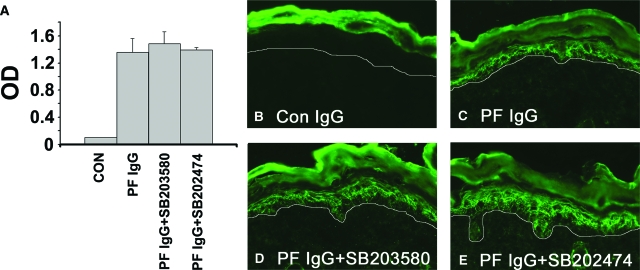
Figure 3.
The inhibitors do not prevent systemic absorption of the injected IgG: anti-dsg1 IgG serum levels. Serum samples obtained from control IgG, PF IgG-treated, PF IgG plus SB203580-treated, and PF IgG plus SB202470-treated mice were examined for the presence of anti-dsg1 autoantibodies by using a dsg1 ectodomain-based ELISA. (A) No significant differences in circulating anti-dsg1 IgG were found in mice pretreated with the active p38MAPK inhibitor or the inactive analog when compared to mice treated with PF IgG alone. P values are as follows: i) PF IgG compared to control, P = 0.004; ii) PF IgG + SB203580 compared to PF IgG, P = 0.27; iii) PF IgG + SB202474 compared to PF IgG, P = 0.4. SD is shown by error bars (n = 3 mice per treatment group); P values were calculated by using the Student’s t-test. The inhibitors do not prevent the diffusion of IgG to the epidermis. Perilesional skin biopsies from control IgG (B), PF IgG-treated (C), PF IgG plus SB203580-treated (D), and PF IgG plus SB202470-treated (E) mice were examined for the presence of human anti-dsg1 PF IgG by direct immunofluorescence by using a mouse anti-human Cy-2-conjugated monoclonal antibody. A honeycomb pattern of staining in the epidermis is seen in mice treated with PF IgG, PF IgG plus SB203580, and PF IgG plus SB202474, demonstrating that the inhibitors do notprevent binding of PF autoantibodies to the keratinocyte cell surface.
Passive Transfer Mouse Model
Breeding pairs of C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at the University of North Carolina DLAM Facility in accordance with Institutional Animal Care and Use Committee protocols. Neonatal mice (24 to 36 hours old with body weights between 1.4 g and 1.6 g) were used for passive transfer experiments. Neonates were injected intradermally with a sterile solution of either control IgGs or PF IgGs as described previously.4,31,32 For direct clinical examination, mice (n = 3) were injected with PF IgGs (PF1: 0.10 mg IgG/g animal weight) in a total volume of 50 μl of PBS. This dose of PF IgGs resulted in gross sloughing of the skin. Alternatively, mice (n = 3) were preinjected with 18.75 μg of SB203580 in 50 μl intradermally then at 2 hours re-injected intradermally with 18.75 μg of SB203580 + PF IgG/50 μl (total of 37.5 μg of SB203580). The skin of neonatal mice from PF IgG and PF IgG + inhibitor-treated groups was examined 18 hours after the injection of IgG for the presence of Nikolsky’s sign, in which gentle friction of perilesional skin causes sheet-like sloughing of the epidermis.
A second group of animals (n = 3 mice) received a lower dose of PF IgGs (PF1, 0.015 mg/g body weight in 50 μl of PBS) to preserve the cutaneous architecture lost by epithelial sloughing at the higher dose. After clinical examination, the animals were sacrificed, and skin and serum specimens obtained for routine histological examination using light microscopy (hematoxylin and eosin staining) and direct immunofluorescence assays to detect keratinocyte cell surface-bound pemphigus IgGs. Serum samples were assayed for the presence of circulating human anti-dsg1 IgGs by ELISA against baculovirus expressed ectodomains of human dsg1 as previously described.33 Additional skin samples were harvested to prepare protein extracts for SDS-PAGE and two-dimensional gel electrophoresis. After transfer to polyvinylidene difluoride, membranes were probed by immunoblot for proteins of interest. For in vivo inhibitor studies, mice (n = 3 mice per treatment group) were preinjected with 6.25 μg of SB202190 in 50 μl intradermally then at 2 hours re-injected intradermally with 6.25 μg of SB202190 + PF IgG/50 μl (total of 12.5 μg of SB202190). No increased mortality was observed in the inhibitor versus control mice. As a control, a separate group of mice (n = 3) were pretreated with the inactive analog SB202474 (total of 12.5 μg of SB202470)34 following the split dose protocol used for SB202190. Additionally, a third group of mice (n = 3) were pretreated with the p38MAPK inhibitor SB203580 (total of 25 μg of SB203580) following the split dose protocol used for SB202190. Typically, neonatal litters were groups of five to six mice. Because of the large numbers of mice used for this study, not all mice were injected at the same time, but rather, based on availability of neonatal litters.
Two-Dimensional Gel Electrophoresis
Extracts were prepared from skin biopsies by Dounce homogenization in IEF lysis buffer (8 mol/L urea, 4% CHAPS, 2.5 mmol/L dithiothreitol, 40 mmol/L Tris, 10 μmol/L pepstatin, 100 μmol/L leupeptin, 10 μmol/L E-64, 1 mmol/L phenylmethyl sulfonyl fluoride). Protein concentration was by modified Bradford as described.35 IPG buffer (pH 4 to 7, Pharmacia) was added to each sample to a final concentration of 0.5% before isoelectric focusing. Samples were separated in the first dimension using 7-cm, pH 4 to 7, linear IPGphor strips (Pharmacia) and in the second dimension by 10% SDS-PAGE. Gels were transferred to polyvinylidene difluoride membranes for immunoblot analysis. Western blots were developed by enhanced chemiluminescence (ECL) reaction and the signal intensity quantified by scanning chemiluminescence on a GeneGnome scanner (Syngene Bio Imaging, Frederick, MD) using GeneSnap software. The signal intensity for each HSP25 isoform was expressed as a percentage of total HSP25 using the formula Pn/(P0 + P1 + P2), where, n corresponds to the signal intensity for spot 0, 1, or 2, and P0 + P1 + P2 is the summed signal intensity for all three HSP25 isoforms. Statistical significance was determined using the Student’s t-test.
Results
Neonatal C57BL/6J mice were injected intradermally with PF IgGs purified from PF patient sera or control IgG from nonaffected individuals. After 18 hours, mice were examined clinically for the presence of blister formation. Additionally, skin biopsies were obtained for histological examination, immunohistochemical staining, and for biochemical analyses. Pretreatment by intradermal injection of the p38MAPK inhibitors SB203580, SB202190, or the inactive analog SB202474, followed by passive transfer of PF IgGs was used to determine whether inhibition of p38MAPK blocked PF IgG-mediated acantholysis in vivo.
Phosphorylation of p38MAPK and HSP25 Was Observed in Skin of PF IgG-Treated Mice
Extracts from biopsies were prepared by tissue homogenization in IEF lysis buffer and separated by SDS-PAGE and two-dimensional gel electrophoresis. After electrotransfer, polyvinylidene difluoride membranes were probed with antibodies to p38MAPK, phospho-p38MAPK, and HSP25. Compared to control IgG-treated mice, increased levels of phospho-p38MAPK were detected in skin biopsies from PF IgG-treated mice. The increase in phosphorylation was approximately twofold greater than baseline levels in controls. PF IgG-induced phosphorylation of p38MAPK was blocked when mice were pretreated with the p38MAPK inhibitors SB203580 (Figure 1, A and B) or SB202190 (data not shown), but not in mice pretreated with the inactive analog SB202474. In skin biopsies from PF IgG-treated mice, the blister roof and floor could be readily separated from one another and independently analyzed. As seen in Figure 1, phopsho-p38MAPK was found predominantly in the blister roof.
Phosphorylation of HSP25 occurs as a downstream consequence of p38MAPK-mediated kinase activity. Predicting that PF IgG-induced phosphorylation of p38MAPK would result in phosphorylation of HSP25, we next examined the phosphorylation state of HSP25 in PF IgG-treated mice. When skin extracts were separated by two-dimensional gel electrophoresis, three HSP25 charge isoforms were detected, P0, P1, and P2; P0 is nonphosphorylated HSP25; whereas, P1 and P2 represent phosphorylated isoforms of HSP25.26,27 Increased amounts of the most negatively charged P2 isoform were detected in skin of PF IgG-treated mice compared to controls. Additionally, PF IgG-induced phosphorylation of HSP25 was diminished in mice pretreated with either of two p38 inhibitors SB203580 (Figure 1, C and D) or SB202190 (data not shown), but not the inactive analog SB202474.
Inhibitors of p38MAPK Block PF IgG-Induced Blister Formation
Mice treated with PF IgGs were Nikolsky-positive, exhibiting gross blister formation. Importantly, inhibition of p38MAPK prevented the ability of PF IgGs to induce blister formation (Figure 2, Table 1). Similarly, routine histological examination of PF IgG-treated mice demonstrated acantholysis; however, PF IgG-induced acantholysis was prevented in mice pretreated with the p38MAPK inhibitor SB203590 (Figure 2, Table 2) or SB202190 (data not shown), but not the inactive analog SB202474.
Table 1.
Inhibition of Clinical Disease
| Nikolsky-positive | Nikolsky-negative | |
|---|---|---|
| PF1 IgG | 3 | 0 |
| PF1 IgG + SB203580 | 0 | 3 |
Neonatal mice were injected with PF IgG (0.1 mg/g) or PF IgG plus SB203580 and examined 18 hours later for Nikolsky’s sign.
Table 2.
Inhibition of Histological Disease
| Mice with blisters | Mice without blisters | |
|---|---|---|
| Control IgG | 0 | 3 |
| PF1 IgG | 3 | 0 |
| PF1 IgG + SB203580 | 0 | 3 |
| PF1 IgG + SB202474 | 3 | 0 |
Neonatal mice were injected with control IgG (0.015 mg/g), PF IgG (0.015 mg/g), or PF IgG and either the active p38 inhibitor SB203580 or the inactive analog SB202474. Mice were examined 18 hours later for the presence of blister formation by light microscopy of H&E-stained skin biopsy sections.
p38MAPK Inhibitors Do Not Block Systemic Absorption or Keratinocyte Binding of PF IgG
When assayed by ELISA against the recombinant dsg1 ectodomain, both PF IgG-treated and PF IgG + inhibitor-treated mice had similar levels of circulating human anti-dsg1 IgG (Figure 3A). Thus, the p38MAPK inhibitors did not prevent systemic absorption of the intradermally delivered autoantibodies. As expected, direct immunofluorescence of skin biopsies of PF IgG-treated mice demonstrated anti-human IgGs bound to the epidermal keratinocyte cell surface (Figure 3, B and C). Importantly, epidermal binding of PF IgGs was not blocked by pretreatment with SB203580 (Figure 3D), SB202190 (data not shown), or SB202474 (Figure 3E). These observations indicate that the p38MAPK inhibitors act downstream of antibody binding to the target keratinocytes consistent with the proposed role for p38MAPK and HSP25 in the mechanism of acantholysis.
A Second PF IgG Activates Signaling via p38 MAPK and HSP25
To exclude the possibility that the observed signaling events were unique to the particular PF IgG used in the above experiments, we examined the ability of a PF IgG purified from a second patient sera to activate signaling in vivo. Similar to the results observed with purified PF1 IgG, mice injected with purified PF2 IgG demonstrated acantholysis (Supplementary Figure S1A, see http://ajp. amjpathol.org), and increased phosphorylation of both p38MAPK and HSP25 compared to controls (Supplementary Figure S1, C–E, see http://ajp.amjpathol.org). Inhibitors of p38MAPK blocked the ability of PF IgG to induce both p38MAPK and HSP25 phosphorylation and blister formation (Supplementary Figure S1, B–E, see http://ajp.amjpathol.org) in the skin of test animals.
Discussion
In PF, autoantibodies directed against the human desmosomal cadherin dsg1 induce blistering. We used an in vivo model to investigate the hypothesis that PF IgGs activate signaling in target keratinocytes and that these signaling events are part of the mechanism by which autoantibodies induce acantholysis. Using the passive transfer mouse model of PF, increased phosphorylation of the MAP kinase p38 was observed in skin biopsies of PF IgG-treated mice.
In contrast to suprabasilar acantholysis observed in PV, pathogenic PF IgGs induce blistering in the more superficial spinous and granular layers of the epidermis. Separation of the blister roof and floor from skin of PF IgG-treated mice and subsequent analyses by immunoblot demonstrated that the increased phospho-p38MAPK signal was localized to the blister roof, indicating that the epidermal keratinocytes were the source of the increased phospho-p38MAPK signal.
One of the downstream targets for p38MAPK, via MAPKAP2 (MK2), is HSP27 (or the murine homolog, HSP25).35,36,37 Consistent with the activation of p38MAPK by PF IgG, increases in the amount of the most negatively charged HSP25 isoform were also observed in skin of PF IgG-treated mice. As previously shown,25,26 this most negatively charged isoform corresponds to phosphorylated HSP25. Furthermore, increased phospho-HSP25 immunoreactivity was detected on Western blots of skin extracts from PF IgG-treated mice compared to controls (Supplementary Figure S1, C and E, see http://ajp.amjpathol.org).
Use of either of the two p38MAPK inhibitors SB203580 or SB202190 inhibited the PF IgG-induced phosphorylation of both p38MAPK and HSP25; the structurally related but inactive analog SB202474 failed to block these PF IgG-induced phosphorylation events. Importantly, inhibition of p38MAPK activity blocked the ability of PF IgG to cause blister formation in the passive transfer mouse model. Inhibition of p38MAPK did not prevent systemic absorption of the PF IgGs nor the ability of the PF IgGs to diffuse and bind the epidermal keratinocytes indicating that the inhibitors blocked blister formation by inhibiting the ability of the bound antibody to activate signaling across the keratinocyte cell membrane. These observations suggest that similar to the related disease PV, PF IgGs activate intracellular signaling and that these signaling events are part of the mechanism by which pathogenic PF autoantibodies induce acantholysis. PF IgGs purified from a second PF patient serum demonstrated similar results.
Acantholysis represents a biological transition from a state of cell-cell adhesion to loss of adhesion, sharing some of the morphological and likely biological changes associated with the transition from a stationary to a motile cell. Consistent with this hypothesis is the observation that pemphigus IgG induces changes to both the intermediate filament and actin cytoskeleton.25 For example, the actin cytoskeleton transitions from a cortical staining pattern to one reminiscent of a migrating cell in primary human keratinocytes incubated with purified pemphigus IgG. Importantly, this pemphigus IgG-induced shift in the both the actin and intermediate filament cytoskeletons is p38MAPK-dependent because both cytoskeletal transitions were blocked when pemphigus IgG-incubated cells were first treated with SB202190 or SB203580, two small molecular weight inhibitors of p38MAPK.25
A signaling complex is formed by p38MAPK, MAP kinase-activated protein kinase 2 (MAPKAP2, MK2), and HSP27; activation of p38MAPK, with subsequent phosphorylation of MK2 and then HSP27, releases phosphorylated HSP27 from this complex.38 In retrospect, the role of the p38MAPK-MK2-HSP27 signaling cassette in pemphigus acantholysis should not be surprising. The p38MAPK-MK2-HSP27 signaling cassette has been shown to regulate the cytoskeleton. HSP27 itself regulates actin and intermediate filament organization.39 Additionally, HSP27 functions as an actin-capping protein.40 p38MAPK-MK2-dependent phosphorylation of HSP27 releases HSP27 mediated inhibition of actin polymerization41,42 and promotes cell migration.43,44,45 Similarly, p38MAPK and HSP27 also regulate the intermediate filament cytoskeleton. For example, a role for HSP27 in intermediate filament regulation is suggested by the observation that HSP27 missense mutations disrupt neurofilament assembly, leading to the neuromuscular disorder Charcot-Marie-Tooth disease and to distal hereditary motor neuropathy.47 Keratin intermediate filament phosphorylation is p38MAPK-dependent48,49 and keratin filament organization is regulated by p38MAPK phosphorylation.49,50 For example, phosphorylation of the basal cell layer keratin 5 by p38MAPK is associated with increased keratin solubility and keratin filament collapse.50
In addition to its ability to regulate the actin cytoskeleton through its effects on HSP27 phosphorylation,51,52,53 direct regulation of the keratin intermediate filament cytoskeleton by p38MAPK may be relevant to acantholysis. For example, phosphorylation of keratin 8 (K8) and K19 in glandular epithelia has been shown to be p38MAPK-dependent and to increase keratin solubility.48 Interestingly, Reichelt and colleagues54 reported that loss of keratin 10 leads to activation of p38MAPK in keratinocytes in the K10 knockout mouse model. Further biological links between keratins and p38MAPK were shown in a recent study in which orthovanadate-induced keratin filament network disassembly was monitored by live-cell fluorescence microscopy. Orthovanadate-induced keratin granules were observed to co-localize with p38MAPK. Furthermore, up-regulation of p38MAPK activity induced keratin granule formation, whereas down-regulation blocked disassembly of keratin filament networks.55
The role of p38MAPK and intracellular signaling in PV IgG-induced acantholysis has been recently confirmed by other investigators.23,56,57 Data from Washke and colleagues56 suggests a role for Rho GTPases in pemphigus acantholysis. Using an ex vivo human skin model and cultured HaCaT cells, they demonstrated that the Rho A activator cytotoxic necrotizing factor-y blocked pemphigus IgG-induced skin splitting. Incubation of cultured cells with PV or PF IgGs reduced the activity of Rho A to ∼50% of control levels. Moreover, activation of Rho A by cytotoxic necrotizing factor-1 was reduced in the presence of pemphigus IgG. Interestingly, the p38MAPK inhibitor SB202190 not only inhibited PV IgG-triggered keratinocyte dissociation, but also abolished PV IgG-triggered Rho A inactivation.56 Their results provide additional data to support a role for p38MAPK in pemphigus acantholysis and further suggest that Rho A may be one of the downstream targets for p38MAPK in this signaling cascade. It is worth noting that in other disorders, activation of p38MAPK in keratinocytes is observed in the absence of blistering. For example, activation of p38MAPK to approximately fourfold greater than baseline has been observed in lesional psoriatic keratinocytes58; however, acantholysis is not a feature of psoriasis. Thus, the role of p38MAPK activation and its relationship to changes in adhesion are likely to be context-dependent. One possibility is that different subcellular pools of p38MAPK are present in keratinocytes and that the subcellular location of p38MAPK, as well as its association with other cellular proteins, dictates its function.
Although the above data indicate a role for intracellular signaling in the mechanism of pemphigus acantholysis, the precise mechanism by which this signal is initiated has yet to be elucidated. PF IgGs, as well as PV IgGs, likely initiate signaling by acting as competitive inhibitors of the dsg adhesive interactions across the desmosome interface (Figure 4). Structural analysis of dsg cadherin interactions, including mapping of the polypeptide backbone of dsg extracellular domains/ectodomains to the crystal structure of E-cadherin as well as EM-tomograms of desmosomes indicate that the N-terminal EC1 domains are the site of desmosomal cadherin-adhesive interactions.59,60 Importantly, both pathogenic monoclonal IgG and IgG from patient sera bind to the EC1/2 epitopes within the N-terminus of dsg; whereas, nonpathogenic IgG and monoclonal antibodies bind to other regions of the dsg ectodomain. Thus, antibody binding to the dsg N-termini may compete for desmosomal cadherin adhesive interactions, either in cis- or trans. The binding of autoantibodies to dsg1 may initiate signaling by competing for binding to the region of the dsg ectodomain that forms the adhesive interface. This concept is supported by the observation that monovalent PV and PF IgG Fab′19,29 and anti-dsg single-chain variable region fragments (ScFvs),61 which are incapable of crosslinking desmosome cadherins, induce loss of adhesion.
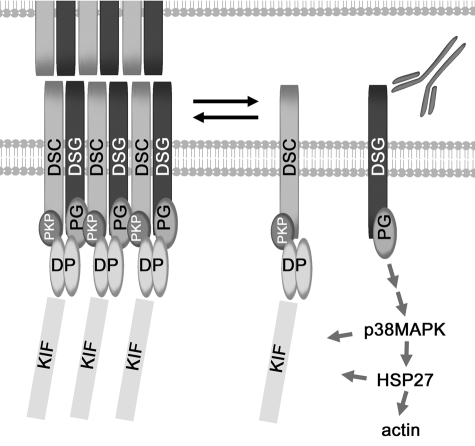
Figure 4.
Model of mechanism of pemphigus acantholysis. Pathogenic PF IgG may initiate signaling by acting as competitive inhibitors for trans and/or cis-interactions within the EC1/2 region of the desmosome cadherin dsg1 at the desmosome adhesive interface. The signal is likely propagated by modulation of a docking plexus on the cytoplasmic surface of the membrane and may include the cytoplasmic tail of dgs1 as well as associated proteins such as plakoglobin, desmoplakin, and plakophilin. Activation of p38MAPK and HSP25/27 contributes to reorganization of keratin intermediate filaments and the actin cytoskeleton with subsequent loss of keratinocyte cell-cell adhesion and clinical blister formation in the superficial layers of the epidermis. DSC, desmocollin; DSG, desmoglein; DP, desmoplakin; KIF, keratin intermediate filament; PG, plakoglobin; PKP, plakophilin.
The studies of the mechanism by which pemphigus autoantibodies lead to end-organ damage in the skin are beginning to define a series of biochemical events that lead to a coordinated cellular response that results in the decreased adhesion and subsequent blister formation in PV and PF. Our observations suggest that targeting specific components of this pathway can be used as a therapeutic strategy to treat autoimmune blistering diseases. Current treatments for pemphigus autoimmune diseases are directed at decreasing the autoimmune response, often in a nonspecific manner. In contrast, the observations presented in this study suggest an alternative approach to treating autoimmune disease that does not require immunosuppression. Rather, specific therapies targeted at key molecules that are part of the mechanism by which the autoimmune response mediates end-organ damage may represent an alternative approach to treating autoimmune disease without the need for broad immunosuppressive medications.
Supplementary Material
Footnotes
Address reprint requests to David S. Rubenstein, Department of Dermatology, The University of North Carolina School of Medicine, Suite 3100 Thurston-Bowles CB 7287, Chapel Hill, NC 27599-7287. E-mail: druben@med.unc.edu.
Supported by the National Institutes of Health (grants RO1 AI49427 to D.S.R., AI40768 to Z.L., and AR30281 and AR32599 to L.A.D.).
Disclosures (conflict of interest): P.B., L.A.D., and D.S.R. are co-inventors on a patent application identifying p38MAPK and HSP27 as druggable targets for the treatment of pemphigus.
Supplementary material for this article can be found on http://ajp. amjpathol.org.
References
- Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- Eyre RW, Stanley JR. Human autoantibodies against a desmosomal protein complex with a calcium-sensitive epitope are characteristic of pemphigus foliaceus patients. Journal of Experimental Medicine. 1987;165:1719–1724. doi: 10.1084/jem.165.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre RW, Stanley JR. Identification of pemphigus vulgaris antigen extracted from normal human epidermis and comparison with pemphigus foliaceus antigen. Journal of Clinical Investigation. 1988;81:807–812. doi: 10.1172/JCI113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. New England Journal of Medicine. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Patel HP, Labib RS, Diaz LA, Anhalt GJ. Experimentally induced pemphigus vulgaris in neonatal BALB/c mice: a time-course study of clinical, immunologic, ultrastructural, and cytochemical changes. Journal of Investigative Dermatology. 1985;84:41–46. doi: 10.1111/1523-1747.ep12274679. [DOI] [PubMed] [Google Scholar]
- Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. Journal of Clinical Investigation. 2000;105:625–631. doi: 10.1172/JCI8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M, Karpati S, Prussick R, Klaus-Kovtun V, Stanley JR. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. Journal of Clinical Investigation. 1992;90:919–926. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Aoki V, Mascaro JM, Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. Journal of Investigative Dermatology. 1997;109:592–596. doi: 10.1111/1523-1747.ep12337524. [DOI] [PubMed] [Google Scholar]
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. Journal of the American Academy of Dermatology. 1999;40:167–170. doi: 10.1016/s0190-9622(99)70183-0. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Amagai M, Iida T, Yamamoto Y, Nishikawa T, Shirai T. Late development of antidesmoglein 1 antibodies in pemphigus vulgaris: correlation with disease progression. British Journal of Dermatology. 1999;141:1084–1087. doi: 10.1046/j.1365-2133.1999.03209.x. [DOI] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. Journal of Clinical Investigation. 1999;103:461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KC, Zhao M, Schroeder PR, Li N, Wetsel RA, Diaz LA, Liu Z. Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest. 2006;116:2892–2900. doi: 10.1172/JCI17891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest. 2005;115:879–887. doi: 10.1172/JCI23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. Journal of Clinical Investigation. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, Diaz LA. The role of complement in experimental bullous pemphigoid. Journal of Clinical Investigation. 1995;95:1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. Journal of Clinical Investigation. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Mascaro JM, Jr, Espana A, Liu Z, Ding X, Swartz SJ, Fairley JA, Diaz LA. Mechanisms of acantholysis in pemphigus vulgaris: role of IgG valence. Clinical Immunology & Immunopathology. 1997;85:90–96. doi: 10.1006/clin.1997.4408. [DOI] [PubMed] [Google Scholar]
- Anhalt GJ, Till GO, Diaz LA, Labib RS, Patel HP, Eaglstein NF. Defining the role of complement in experimental pemphigus vulgaris in mice. Journal of Immunology. 1986;137:2835–2840. [PubMed] [Google Scholar]
- Mahoney MG, Wang ZH, Stanley JR. Pemphigus vulgaris and pemphigus foliaceus antibodies are pathogenic in plasminogen activator knockout mice. Journal of Investigative Dermatology. 1999;113:22–25. doi: 10.1046/j.1523-1747.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Ishiko A, Ota T, Tsunoda K, Amagai M, Nishikawa T. IgG binds to desmoglein 3 in desmosomes and causes a desmosomal split without keratin retraction in a pemphigus mouse model. J Invest Dermatol. 2004;122:1145–1153. doi: 10.1111/j.0022-202X.2004.22426.x. [DOI] [PubMed] [Google Scholar]
- Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- Seishima M, Esaki C, Osada K, Mori S, Hashimoto T, Kitajima Y. Pemphigus IgG, but not bullous pemphigoid IgG, causes a transient increase in intracellular calcium and inositol 1,4,5-triphosphate in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. 1995;104:33–37. doi: 10.1111/1523-1747.ep12613469. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome Signaling: INHIBITION OF p38MAPK PREVENTS PEMPHIGUS VULGARIS IgG-INDUCED CYTOSKELETON REORGANIZATION. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz P, Diaz LA, Hall RP, Rubenstein DS. Induction of p38MAPK and HSP27 phosphorylation in pemphigus patient skin. J Invest Dermatol. 2008;128:738–740. doi: 10.1038/sj.jid.5701080. [DOI] [PubMed] [Google Scholar]
- Rock B, Labib RS, Diaz LA. Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. Journal of Clinical Investigation. 1990;85:296–299. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, Rivitti EA, Santos V, Diaz LA. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. New England Journal of Medicine. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [comment] [DOI] [PubMed] [Google Scholar]
- Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, Patel H, Anhalt GJ. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. Journal of Investigative Dermatology. 1985;85:538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) New England Journal of Medicine. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- Arteaga LA, Prisayanh PS, Warren SJ, Liu Z, Diaz LA, Lin MS. A subset of pemphigus foliaceus patients exhibits pathogenic autoantibodies against both desmoglein-1 and desmoglein-3. J Invest Dermatol. 2002;118:806–811. doi: 10.1046/j.1523-1747.2002.01743.x. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Hu P, O'Keefe EJ, Rubenstein DS. Tyrosine phosphorylation of human keratinocyte beta-catenin and plakoglobin reversibly regulates their binding to E-cadherin and alpha-catenin. Journal of Investigative Dermatology. 2001;117:1059–1067. doi: 10.1046/j.0022-202x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Letters. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Engel K, Ahlers A, Brach MA, Herrmann F, Gaestel M. MAPKAP kinase 2 is activated by heat shock and TNF-alpha: in vivo phosphorylation of small heat shock protein results from stimulation of the MAP kinase cascade. J Cell Biochem. 1995;57:321–330. doi: 10.1002/jcb.240570216. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H, Shen X. MAPK-activated protein kinase-2 (MK2)-mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27. J Biol Chem. 2006;281:37215–37226. doi: 10.1074/jbc.M603622200. [DOI] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. Journal of Biological Chemistry. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. Journal of Biological Chemistry. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB Journal. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhou X, Liao J, Omary MB. Pervanadate-mediated tyrosine phosphorylation of keratins 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci. 1999;112(Pt 13):2081–2090. doi: 10.1242/jcs.112.13.2081. [DOI] [PubMed] [Google Scholar]
- Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J Biol Chem. 2002;277:10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Zhou Q, English LS, Omary MB. Type II keratins are phosphorylated on a unique motif during stress and mitosis in tissues and cultured cells. Mol Biol Cell. 2002;13:1857–1870. doi: 10.1091/mbc.01-12-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. Journal of Biological Chemistry. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Molecular & Cellular Biology. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. Journal of Cell Science. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Reichelt J, Furstenberger G, Magin TM. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J Invest Dermatol. 2004;123:973–981. doi: 10.1111/j.0022-202X.2004.23426.x. [DOI] [PubMed] [Google Scholar]
- Woll S, Windoffer R, Leube RE. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol. 2007;177:795–807. doi: 10.1083/jcb.200703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Aoyama Y, Tsunoda K, Amagai M, Kitajima Y. Pathogenic monoclonal antibody against desmoglein 3 augments desmoglein 3 and p38 MAPK phosphorylation in human squamous carcinoma cell line. Autoimmunity. 2006;39:587–590. doi: 10.1080/08916930600971943. [DOI] [PubMed] [Google Scholar]
- Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol. 2005;152:37–42. doi: 10.1111/j.1365-2133.2004.06304.x. [DOI] [PubMed] [Google Scholar]
- He W, Cowin P, Stokes DL. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.