Abstract
Polycystic kidney (PCK) rats are a spontaneous model of autosomal recessive polycystic kidney disease that exhibit cholangiocyte-derived liver cysts. We have previously reported that in normal cholangiocytes a subset of vesicles contain three proteins (ie, the water channel AQP1, the chloride channel CFTR, and the anion exchanger AE2) that account for ion-driven water transport. Thus, we hypothesized that altered expression and location of these functionally related proteins contribute to hepatic cystogenesis. We show here that under basal conditions and in response to secretin and hypotonicity, cysts from PCK rats expanded to a greater degree than cysts formed by normal bile ducts. Quantitative reverse transcriptase-polymerase chain reaction, immunoblot analysis, and confocal and immunoelectron microscopy all indicated increased expression of these three proteins in PCK cholangiocytes versus normal cholangiocytes. AQP1, CFTR, and AE2 were localized preferentially to the apical membrane in normal rats while overexpressed at the basolateral membrane in PCK rats. Exposure of the cholangiocyte basolateral membrane to CFTR inhibitors [5-nitro-2-(3-phenylpropylamino)-benzoic acid and CFTRinh172], or Cl−/HCO3− exchange inhibitors (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate and 4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid disodium salt hydrate) blocked secretin-stimulated fluid accumulation in PCK but not in normal cysts. Our data suggest that hepatic cystogenesis in autosomal recessive polycystic kidney disease may involve increased fluid accumulation because of overexpression and abnormal location of AQP1, CFTR, and AE2 in cystic cholangiocytes. Therapeutic interventions that block the activation of these proteins might inhibit cyst expansion in polycystic liver disease.
The autosomal recessive polycystic kidney disease (ARPKD), the incidence of which is 1:20,000, often leads to fetal or neonatal death because of markedly enlarged kidneys, impaired lung function, and pulmonary hypoplasia. In surviving patients, hepatic lesions become progressively more severe, and the liver disease may be a major cause of morbidity and mortality. The liver pathology is characterized by congenital hepatic fibrosis, bile duct dilatation (Caroli’s disease), and/or cyst development.1,2,3,4,5 Hepatic cysts, which increase in number and size throughout life, are lined by cholangiocytes. Despite the identification of the genetic defect that causes this disease, the pathophysiology of cyst formation, growth, and expansion is still unclear; abnormalities in cell proliferation, fluid secretion, and extracellular matrix biology all likely contribute.1,4,6,7,8,9,10
The polycystic kidney (PCK) rat, a spontaneous mutant derived from a colony of Crj:SD rats, develops renal and hepatic pathological features that resemble human ARPKD.11 Linkage and gene cloning analysis showed that ARPKD and the liver and renal pathology in PCK rats are caused by mutations to orthologous genes, PKHD1/Pkhd1, respectively.12,13,14 The PKHD1 gene encodes fibrocystin,12,13,15 a large trans-membrane protein with unknown function located on cholangiocyte primary cilia. These nonmotile, antenna-like tubular organelles extend from the cholangiocyte apical membrane into the ductal lumen and function as mechano-, chemo-, and osmosensors,16,17,18 detecting and transmitting luminal bile stimuli into intracellular signals.
We recently reported that liver cysts in the PCK rat originate from cholangiocytes lining intrahepatic bile ducts and progressively grow and expand throughout time.19 We also found that bile ducts freshly isolated from the PCK rat and grown between two layers of collagen/Matrigel (three-dimensional culture) form cystic structures that expand at a threefold greater rate than bile ducts from normal rats.19 Furthermore, we demonstrated that increased intracellular cAMP (approximately twofold higher in cholangiocytes of the PCK rats compared to normal rats) contributes to cyst expansion. Indeed, reduction of intracellular cAMP levels by octreotide (an analog of somatostatin that binds and activates somatostatin receptors) led to a significant decrease in the hepatic cyst volume, hepatic fibrotic scores, and mitotic indices in PCK rats.19
It has been shown that secretin-stimulated activation of intracellular cAMP signaling in cholangiocytes results in apical membrane insertion of a subset of organelles that sequester three functionally related transport proteins (ie, the water channel AQP1, the chloride channel CFTR, and the anion exchanger AE2), a process that facilitates fluid secretion.20 Interestingly, a marked secretory response to the administration of secretin was observed in liver cysts from patients with autosomal dominant polycystic kidney disease (ADPKD).21 Although the mechanism of cyst expansion in polycystic liver diseases is not yet fully understood, increased fluid secretion into the cystic lumen might play a role. Therefore, we tested the hypothesis that cyst expansion in the PCK rat results from changes in expression and topographic location of AQP1, CFTR, and AE2, contributing to hepatic cystogenesis.
Materials and Methods
Animals and Diets
We maintained wild-type Sprague-Dawley and PCK rats on a standard laboratory diet after approval by the Mayo Institutional Animal Care and Use Committee. We anesthetized animals with pentobarbital (50 mg/kg body weight intraperitoneally) for in vivo procedures. We harvested livers, fixed them in 10% formaldehyde, and embedded them in paraffin for histology.
Experimental Models and Three-Dimensional Culture
We used the following experimental models: i) microdissected bile ducts from normal rats and microdissected single liver cysts (disconnected from the biliary tree) from the PCK rat; ii) bile ducts of both normal and PCK rats ranging in diameter between 40 and 100 μm; and iii) freshly isolated and short-term cultured normal and PCK cholangiocytes (100% purity).22,23 For three-dimensional culture, bile ducts or microdissected single liver cysts were grown between two layers of type I rat tail collagen (1.5 mg/ml; BD Biosciences, San Jose, CA) plus 10% Matrigel (BD Biosciences) to provide no spatial limits for expansion, and maintained the tissue in enriched Dulbecco’s modified Eagle’s medium-Ham’s F-12 medium.22 In three-dimensional culture, bile ducts isolated from normal and PCK rats formed cystic structures that expand throughout time.19
We assessed the development of cystic structures by light microscopy under several conditions: i) after luminal fluid aspiration. By the end of day 1 in three-dimensional culture, fluid was aspirated from the lumen of both normal and PCK cystic structures (ie, collapsed cysts) by inserting a needle attached to a syringe. Cysts were allowed to grow for an additional 4 days under similar conditions; and ii) in response to 1 μmol/L secretin (Bachem, Torrance, CA) for 30 minutes, with or without the presence of chloride channel blockers [either 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, 0.1 mmol/L; Sigma-Aldrich, St. Louis, MO) or 4-[4-oxo-2-thioxo-3-(3-trifluoromethyl-phenyl)-thiazolidin-5-ylidenemethyl]-benzoic acid (CFTRinh172, 2 μmol/L; Exclusive Chemistry Ltd., Obninsk, Russia] and Cl−/HCO3− exchange inhibitors [either 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS, 0.5 mmol/L; Sigma-Aldrich) or 4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid disodium salt hydrate (SITS, 0.5 mmol/L; Sigma-Aldrich)]. Each inhibitor was added 30 minutes before and during secretin stimulation.
PCK cholangiocytes exhibit well-developed junctional complexes, and distinct apical and basolateral membranes similar to normal cholangiocytes.24 Indeed, the transepithelial electrical resistance in cultured PCK cholangiocytes is higher than in cultured normal cholangiocytes.24 Furthermore, DIDS25 and SITS26,27 are impermeable to cell membranes; thus, the basolateral presence of these inhibitors does not affect apically located Cl−/HCO3− exchange. Likewise, CFTRinh172 is a recently discovered compound that induces a rapid, reversible, nontoxic, and highly specific inhibition of CFTR.24 At the concentration used here (2 μmol/L), basolateral administration of CFTRinh172 has been reported to not inhibit apical CFTR-dependent Cl− conductance.28
To study the effect of hypotonicity on cystic expansion and to mimic in vivo conditions, we used freshly isolated bile ducts from normal rats and freshly microdissected solitary PCK cysts. They were exposed for 60 and 370 seconds to hypoosmotic saline solutions (100 mOsm) prepared as previously described.29 Normal and PCK cystic structures of approximately similar dimensions and equal orientation in three-dimensional matrices were chosen for analysis. Sometimes cystic structures in three-dimensional culture might have irregular shape or even fused together and appear as a cyst cluster. In all cases, we calculated the circumferential areas of cystic structures by outlining only the external boundary of the structure. The circumferential area of each cyst was measured using the Image J software (National Institutes of Health, Bethesda, MD). To analyze the effect of stimuli on cystic expansion, we compared the areas of either normal or PCK cysts before and after the application of the stimulus. Data were expressed as percentage of change of cystic area.
RNA Isolation and Reverse-Transcriptase Real-Time Polymerase Chain Reaction (RT-PCR)
We obtained total RNA from pure short-term cultured normal and PCK cholangiocytes (100 purity) using Trizol reagent (Invitrogen, Carlsbad, CA). We estimated AQP1, CFTR, and AE2 mRNA levels by real-time RT-PCR (LightCycler Apparatus; Roche Molecular Biochemicals, Indianapolis, IN) using specific primers (Table 1). We measured 18S mRNA with the mRNA Universal 18S Internal Standard (Ambion, Austin, TX), and used 18S mRNA as a normalizing control.
Table 1.
Primer Sequences
| Name | Sequence |
|---|---|
| AQP1 Forward | 5′-ACTACACTGGCTGTGGGATC-3′ |
| AQP1 Reverse | 5′-CATGCGGTCTGTAAAGTCGC-3′ |
| CFTR Forward | 5′-GAATGGGACAGGGAACAAGC-3′ |
| CFTR Reverse | 5′-TTCCTAGCAAGACAGGCTGG-3′ |
| AE2 Forward | 5′-AGACTTTGAATACCACCGCC-3′ |
| AE2 Reverse | 5′-TCATCTTCCTCCCCTTCCTC-3′ |
Western Blot
Pure short-term cultured normal and PCK cholangiocytes22,23 were resuspended in Bio-Rad sample buffer (Bio-Rad, Hercules, CA). We ran aliquots of protein extracts (150 μg) in 12% [for proliferating cell nuclear antigen (PCNA)] or 4 to 15% gradient (for AQP1, CFTR, and AE2) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred the proteins to a nitrocellulose membrane. After blocking in 5% nonfat dried milk in phosphate-buffered saline (PBS) with 0.5% Tween, we incubated membranes with mouse antibodies against PCNA (1:200, Sigma-Aldrich) or rabbit antibodies against AQP1 (1:500; Alpha Diagnostics Intl. Inc., San Antonio, TX), CFTR (1:200; Chemicon International, Temecula, CA) and AE2 (1:500; Alpha Diagnostics), followed by incubation with peroxidase-conjugated secondary goat anti-mouse (1:1000) or anti-rabbit (1:2000) antibodies (Invitrogen). We visualized bands with the ECL Plus Western blotting detection kit (GE Health Care, Piscataway, NJ). Western blot for β-actin (Sigma-Aldrich) was used as a normalizing protein loading control.
Immunogold Electron Microscopy
We perfused normal and PCK livers with 4% paraformaldehyde in 0.1 mo/L PBS and processed them as previously described.30 We cut thin cryosections (60 nm) with a Leica cryomicrotome (Leica Microsystem Inc., Bannockburn, IL), and performed immunolabeling as previously described31 with the same rabbit affinity-purified polyclonal antibodies against AQP1 and AE2 as indicated above for Western blot analyses but at higher concentration (dilution of 1:30 and 1:20 in 10% fetal calf serum/PBS solution, respectively). Moreover, rabbit affinity-purified polyclonal antibodies against CFTR (1:30 in 10% fetal calf serum/PBS solution; Santa Cruz Biotechnology, Santa Cruz, CA) were used. Then we incubated sections with a 10-nm anti-rabbit IgG gold secondary antibody (Sigma-Aldrich) for 2 hours at room temperature, further fixed in 1% glutaraldehyde, and embedded in 2% methyl cellulose solution containing 0.3% uranyl acetate. We visualized the specimens with a Jeol 1200 electron microscope (Jeol USA Inc., Peabody, MA) operating between 60 to 80 kV. We printed immunogold electron microscopy images at a final magnification of × 60,000 and counted immunogold particles (IGPs). The average size of normal cholangiocytes (ie, distance from the basolateral membrane to apical membrane) is 6.62 μm and average size of cystic cholangiocytes is 8.87 μm (unpublished observation). The IGPs were counted in close proximity (not more than 500 nm) to apical or basolateral region and were defined as apical or basolateral immunogold labeling, respectively. Data are expressed as the number of gold particles in each group per × 60,000 magnification.
Immunofluorescence Confocal Microscopy
We performed immunofluorescence microscopy using a confocal microscope (Zeiss LSM 510; Carl Zeiss Inc., Thornwood, NY) with a × 100 Pan-Apochromat 1.4 nm oil objective and an epifluorescent Eclipse TE300 microscope (Nikon, Melville, NY) as previously described.16 We used antibodies against CFTR (for this technique from LifeSpan Biosciences, Inc., Seattle, WA) and against AQP1 and AE2 (all three at 1:50 dilution), followed by incubation for 1 hour with fluorescent secondary antibodies (1:100; Molecular Probes, Eugene, OR) at room temperature. We stained nuclei with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich). Corresponding negative controls (ie, with no primary antibodies) were performed in all experiments.
Statistics
All values are expressed as mean ± SE. Statistical analysis was performed by the Student’s t-test, and results were considered statistically different at P < 0.05.
Results
Microdissected Solitary PCK Cysts Expand More Rapidly in Three-Dimensional Culture than Normal Bile Ducts Both Spontaneously and in Response to Secretin
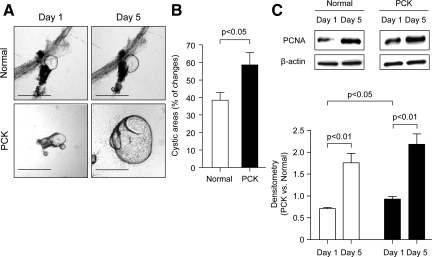
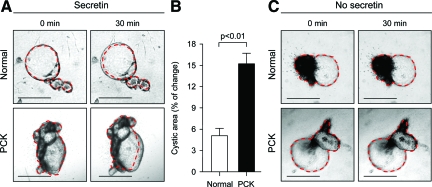
To study cyst expansion, freshly isolated bile ducts from normal rats and freshly dissected single solitary liver cysts from PCK rats were entered in three-dimensional culture. As shown in Figure 1A, at day 1 normal bile ducts form cysts when grown under these conditions. By the end of day 1, cystic fluid was aspirated from normal and PCK cysts and they remained in three-dimensional culture up to 5 days. Our data suggested that both normal and PCK cystic structures continue to expand throughout time. Importantly, PCK cysts accumulated fluid within the cystic lumen more rapidly than cystic structures derived from normal bile ducts (at day 5 their cystic areas increased by ∼59% and ∼38%, respectively; P < 0.05) (Figure 1B). As shown in Figure 1C, the rate of cholangiocyte proliferation assessed by the PCNA expression was increased in both normal and PCK cystic structures (P < 0.01 each). Interestingly, whereas at day 1 in three-dimensional culture PCK cholangiocytes proliferated at a greater rate than normal rat cholangiocytes (30.6%, P < 0.05), no significant differences in proliferation between normal and PCK cystic structures were found at day 5 (Figure 1C). Changes in cystic areas of normal bile ducts and PCK liver cysts grown in three-dimensional culture were also assessed after stimulation with secretin for 30 minutes. Secretin exposure induced an increase in the areas of PCK cysts that was threefold greater (15.3%) than in normal bile duct areas (5.1%, P < 0.01; Figure 2).
Figure 1.
In three-dimensional culture, isolated solitary PCK cysts expand more rapidly than cystic structures formed by normal bile ducts. A: Normal bile ducts and PCK solitary cysts were freshly microdissected and entered in three-dimensional culture. Normal bile ducts by day 1 formed cystic structures when grown in three-dimensional matrices. After measuring the areas of normal and PCK cysts at day 1, cystic fluid was aspirated and cystic areas were assessed again at day 5 to calculate luminal fluid accumulation. B: Quantitative analysis shows that areas of PCK cystic structures (n = 15) were increased by ∼59% compared to ∼38% increase in the areas of normal cystic structures (n = 19). C: Western blot analysis indicates that at day 1 in three-dimensional culture levels of the PCNA expression was 30.6% higher in PCK cysts than in normal cystic structures (n = 5 in both groups). However, by day 5, no differences in the PCNA expression were observed between normal and PCK cystic structures (n = 5 in both groups). β-Actin was used as a normalizing protein loading control. Scale bars = 500 μm.
Figure 2.
Secretin induces greater expansion of PCK cysts than normal cystic structures. A: Representative images of normal bile ducts and PCK cysts grown in three-dimensional culture. Secretin (1 μmol/L) was added to culture media for 30 minutes and cystic areas were measured before and after its addition. B: Quantitative analysis shows that in response to secretin PCK cystic areas (n = 10) were increased by 15.3% compared to 5.1% increase in normal bile ducts (n = 7). C: In the absence of secretin, no changes in the areas of both normal and PCK cystic structures were observed (during the same 30 minutes). Red lines surrounding cysts represent areas before secretin stimulation. Scale bars = 500 μm.
AQP1, CFTR, and AE2 Are Overexpressed in PCK Cholangiocytes
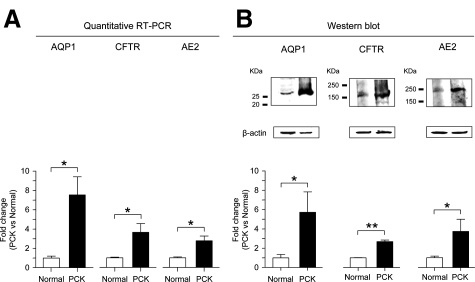
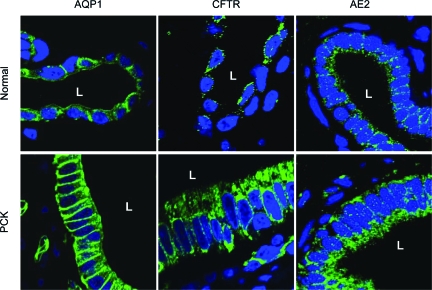
Quantitative RT-PCR revealed that AQP1, CFTR, and AE2 were increased (eight-, four-, and threefold, respectively) at mRNA levels in PCK cholangiocytes compared to normal cholangiocytes (P < 0.05, Figure 3A). Overexpression of AQP1, CFTR, and AE2 in PCK cholangiocytes was also confirmed at the protein level by Western blot analysis. As shown in Figure 3B, the expression of AQP1, CFTR, and AE2 were increased (six-, three-, and fourfold, respectively) in PCK rats compared to normal rats (P < 0.05). Moreover, immunofluorescent confocal microscopy further confirmed that AQP1, CFTR, and AE2 were all overexpressed in PCK cholangiocytes compared to normal cholangiocytes (Figure 4).
Figure 3.
AQP1, CFTR, and AE2 are overexpressed at mRNA and protein levels in PCK cholangiocytes. A: Quantitative RT-PCR indicates that AQP1, CFTR, and AE2 mRNA are overexpressed (eight-, four-, and threefold, respectively) in PCK cholangiocytes compared to normal cholangiocytes (n = 4 in all groups). 18S mRNA was used as a normalizing control. B: By Western blot, expression of AQP1, CFTR, and AE2 proteins are increased (six-, three-, and fourfold, respectively) in PCK cholangiocytes compared to normal cholangiocytes (n = 4, 3, and 6, respectively). β-Actin was used as a normalizing loading control.*P < 0.05, **P < 0.01.
Figure 4.
AQP1, CFTR, and AE2 are overexpressed in PCK cholangiocytes in vivo. Consistent with Western blot analysis, immunofluorescent confocal microscopy of whole rat liver shows that immunoreactivity of all three proteins (AQP1, CFTR, and AE2) was increased in cholangiocytes of the PCK rat compared to normal rats. AQP1, CFTR, and AE2 are preferentially localized to the apical membrane in normal cholangiocytes. In PCK cholangiocytes, greater accumulation of all these proteins in the basolateral membrane were observed; however, similarly to normal cholangiocytes they were also encountered in the apical membrane. AQP1, CFTR, and AE2 are shown in green and nuclei are stained in blue with 4′,6-diamidino-2-phenylindole. Please note that, consistent with our previous observation,30 cholangiocytes lining liver cysts are larger in size than cholangiocytes lining normal bile ducts. L, Lumen of bile duct or hepatic cyst. Original magnifications, × 100.
AQP1, CFTR, and AE2 Are Located Mainly on the Apical Domain in Normal Cholangiocytes, but Are Overexpressed at the Basolateral Domain in PCK Cholangiocytes
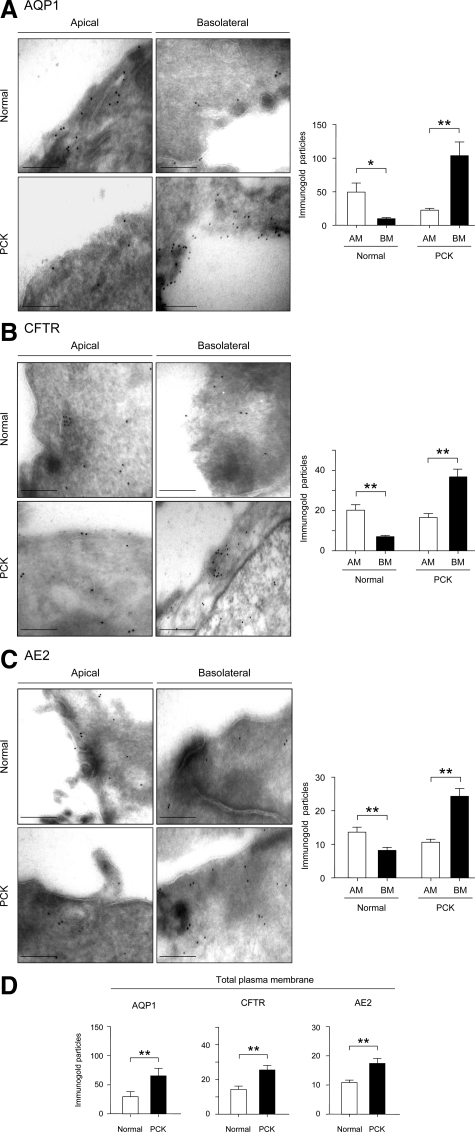
In normal rats, immunofluorescent confocal microscopy of liver tissue revealed that AQP1, CFTR, and AE2 proteins were mainly localized to the apical membrane of cholangiocytes as expected (Figure 4). In PCK cholangiocytes, however, these three proteins were found to be overexpressed at the basolateral membrane (Figure 4). We also used immunogold electron microscopy to characterize the apical and basolateral AQP1, CFTR, and AE2 distribution in detail. Using liver tissue from normal and PCK rats, we counted the number of gold particles for AQP1, CFTR, and AE2 at the apical and basolateral regions of normal and cystic cholangiocytes. In agreement with the immunofluorescent confocal microscopy, AQP1 (Figure 5A), CFTR (Figure 5B), and AE2 (Figure 5C) were localized preferentially to the apical membrane in normal cholangiocytes (P < 0.05). On the other hand, a greater accumulation of AQP1, CFTR, and AE2 gold particles was found in the basolateral membrane in PCK cholangiocytes (Figure 5, A–C; P < 0.05) lining the lumen. Moreover, the total number of gold particles for each protein (assessed as a sum of particles at both apical and basolateral membranes) was also found to be increased in PCK rats compared to normal rats (Figure 5D, P < 0.05).
Figure 5.
Immunogold electron microscopy confirming overexpression of AQP1, CFTR, and AE2 at the basolateral membrane of PCK cholangiocytes. Representative images of apical and basolateral membrane domains of normal and PCK cholangiocytes and quantitative assessment of IGPs showing the expression of AQP1, CFTR, and AE2. A: In normal cholangiocytes, AQP1 was mainly localized to the apical membrane (50 IGPs, n = 8) with significantly less expression on the basolateral membrane (10 IGPs, n = 8). In contrast, in PCK cholangiocytes, we observed more gold particles at the basolateral membrane (105 IGPs, n = 13) than at the apical membrane (23 IGPs, n = 14). B: In normal cholangiocytes, CFTR was mainly localized to the apical membrane (20 IGPs, n = 17), with significantly lower expression on the basolateral membrane (7 IGPs, n = 14). However, in PCK cholangiocytes more gold particles were observed basolaterally (37 IGPs, n = 20) than apically (17 IGPs, n = 14). C: In normal cholangiocytes, AE2 was mainly localized to the apical membrane (13 IGPs, n = 24), with less expression on the basolateral membrane (8 IGPs, n = 16). In PCK cholangiocytes, more gold particles were observed at the basolateral membrane (24 IGPs, n = 17) than at the apical membrane (10 IGPs, n = 15). D: The total number of IGPs for AQP1, CFTR, and AE2 was increased in PCK cholangiocytes (67, 26, and 18 IGPs for AQP1, CFTR, and AE2, respectively) compared to normal cholangiocytes (30, 14, and 11 IGPs for AQP1, CFTR, and AE2, respectively). AM, Apical membrane; BM, basolateral membrane. *P < 0.05, **P < 0.01. Scale bars = 250 nm.
Microdissected Solitary PCK Cysts Expand More in Response to Hypotonicity than Normal Bile Ducts
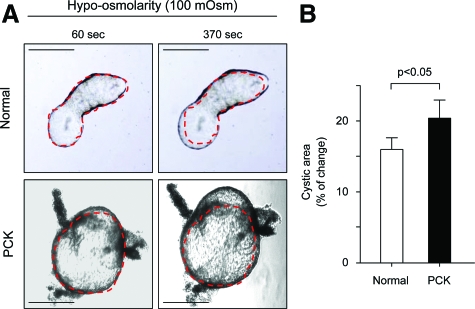
To analyze whether basolateral overexpression of AQP1 in PCK rats could lead to cyst expansion, we microdissected solitary PCK cysts and bile ducts of normal rats and exposed them to hypoosmotic (100 mOsm) solution. As shown in Figure 6, areas of the PCK cysts exposed to hypoosmotic solution for 370 seconds were increased by 21.2% compared to 14.8% increase in normal bile ducts (P < 0.05), indicating a facilitated entrance of water from the medium into the lumen of PCK cysts.
Figure 6.
Microdissected solitary PCK cysts expand more rapidly under hypotonic media compared to normal bile ducts. A: Representative images of normal bile ducts and microdissected PCK cysts exposed to hypoosmotic (100 mOsm) solution for 60 and 370 seconds. Red lines surrounding cystic structure represent areas before the addition of hypoosmotic solution. B: Quantitative assessment of areas of both normal and PCK structures shows that PCK cysts (n = 10) expand more rapidly in response to hypotonicity for 370 seconds (ie, 21.2% of increase in cystic areas) compared to lumen expansion (14.8%, n = 10) in normal bile ducts. Scale bars = 500 μm.
Secretin-Stimulated Expansion of PCK Cysts Is Inhibited by the Basolateral Application of NPPB, CFTRinh172, DIDS, or SITS
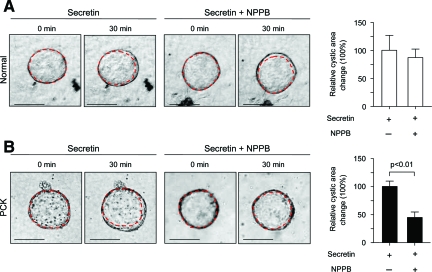
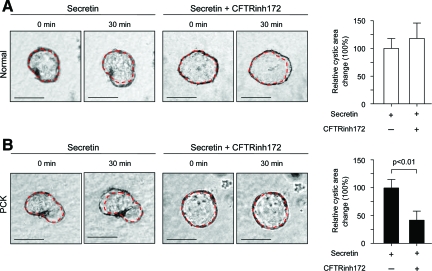
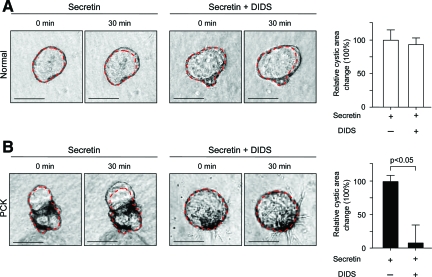
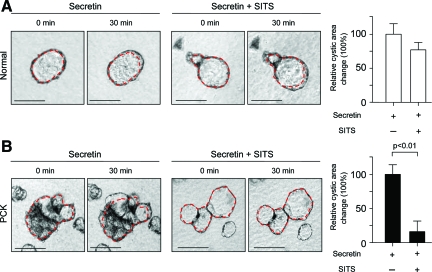
To analyze if the basolateral overexpression of the ion transporters CFTR and AE2 in PCK rats contribute to secretin-stimulated cyst expansion, isolated normal and PCK bile ducts were grown in three-dimensional culture to allow them to form cystic structures. These cystic structures were exposed during 30 minutes to the secretin alone or in combination with inhibitors of CFTR (NPPB and CFTRinh172, Figures 7 and 8) or AE2 (DIDS and SITS, Figures 9 and 10), each being added to the culture media, and changes in cystic areas were assessed. As shown in Figures 7A and 8A, both CFTR inhibitors had no effect on secretin-stimulated expansion of the cystic structures formed by normal bile ducts. Likewise, we found no effect of AE2 inhibitors (Figures 9A and 10A) on secretin-stimulated expansion of the normal cysts. By contrast, in PCK cysts we observed that both CFTR inhibitors, NPPB (Figure 7B) and CFTRinh172 (Figure 8B), suppressed secretin-stimulated expansion of the PCK cysts by 55.1% and 58%, respectively (P < 0.01). AE2 inhibitors had even a more dramatic effect—secretin-stimulated expansion of the PCK cysts was suppressed by 92.6% (P < 0.05) and 84% (P < 0.01) in response to DIDS (Figure 9B) or SITS (Figure 10B), respectively.
Figure 7.
Secretin-stimulated expansion of PCK cysts is inhibited in response to NPPB. Representative images of normal (A) and PCK (B) cystic structures grown in three-dimensional culture. Secretin alone or in combination with NPPB (an inhibitor of CFTR and other Cl− channels) was added for 30 minutes and changes in cystic areas assessed. A: In normal cystic structures (n = 16), basolateral presence of NPPB did not affect secretin-stimulated expansion of cystic structures. B: In contrast, in PCK cysts (n = 10) secretin-stimulated cystic areas were inhibited by 55.1% because of the basolateral presence of NPPB. Red lines surrounding cystic structure represent areas before NPPB and secretin exposure. Values are given as percentage of change of the cystic areas relative to the respective increase in the areas under the presence of secretin but in the absence of NPPB (considered to be equal to 100%). Scale bars = 250 μm.
Figure 8.
Secretin-stimulated expansion of PCK cysts is inhibited by the basolateral presence of CFTRinh172. Representative images of normal (A) and PCK (B) cystic structures grown in three-dimensional culture. Secretin alone or in combination with CFTRinh172 (a specific inhibitor of CFTR) was added to the culture media for 30 minutes and cystic areas were assessed. A: In normal cysts (n = 18) secretin-stimulated areas were not affected by the basolateral presence of CFTRinh172. B: In contrast, in PCK cysts (n = 24) secretin-stimulated cystic areas were inhibited by 58% in response to the basolateral application of CFTRinh172. Red lines surrounding cystic structure represent areas before CFTRinh172 and secretin exposure. Values are given as percentage of change of the cystic areas relative to the respective increase in the areas under the presence of secretin but in the absence of CFTRinh172 (considered to be equal to 100%). Scale bars = 250 μm.
Figure 9.
Secretin-stimulated expansion of PCK cysts is inhibited by the basolateral presence of DIDS. Representative images of normal (A) and PCK (B) cystic structures grown in three-dimensional culture. Secretin alone or in combination with DIDS (a Cl−/HCO3− exchange inhibitor) was added for 30 minutes and cystic areas were assessed. A: Basolateral presence of DIDS did not prevent secretin-stimulated expansion of normal cysts (n = 13). B: In PCK cysts (n = 14), DIDS inhibited the secretin-stimulated areas by 92.6%. Red lines surrounding cystic structure represent areas before DIDS and secretin exposure. Values are given as percentage of change of the cystic areas relative to the respective increase in the areas under the presence of secretin but in the absence of DIDS (considered to be equal to 100%). Scale bars = 250 μm.
Figure 10.
Secretin-stimulated expansion of PCK cysts is inhibited by the basolateral presence of SITS. Representative images of normal (A) and PCK (B) cystic structures grown in three-dimensional culture. Secretin alone or in combination with SITS (a Cl−/HCO3− exchange inhibitor) was added and changes in cystic areas assessed. A: In normal cystic structures (n = 30), secretin-stimulated areas were not affected by the basolateral application of SITS. B: In PCK cysts (n = 53), SITS inhibited cystic areas previously stimulated by secretin by 84%. Red lines surrounding cystic structure represent areas before SITS and secretin exposure. Values are given as percentage of change of the cystic areas relative to the respective increase in the areas under the presence of secretin but in the absence of SITS (considered to be equal to 100%). Scale bars = 250 μm.
Discussion
The results reported here show that: i) in three-dimensional culture, microdissected single PCK cysts expand more rapidly than normal bile ducts both spontaneously and in response to secretin; ii) although AQP1, CFTR, and AE2 are localized preferentially to the apical membrane in normal cholangiocytes, a striking overexpression of these three proteins is found at the basolateral membrane of PCK cholangiocytes; iii) PCK cysts secrete more fluid into the cystic lumen than normal bile ducts when exposed to hypoosmolarity; and iv) secretin-stimulated expansion of PCK cysts is inhibited by the basolateral application of CFTR and AE2 inhibitors. These data support the notion that hepatic cystogenesis in PCK rats may be the result of alterations in expression and topographic location of AQP1, CFTR, and AE2 proteins, accounting for more significant fluid accumulation within PCK cysts.
Two processes might be responsible for our findings that isolated PCK liver cysts expand more than normal bile ducts when grown in three-dimensional culture for 5 days: increased cell proliferation and/or increased luminal fluid accumulation. Western blot analysis (Figure 1C) shows that the rate of proliferation is initially (day 1) higher in PCK cholangiocytes. However, no significant differences in proliferation between normal and PCK cholangiocytes were found at day 5 of culture. These data might indicate that cell proliferation together with fluid accumulation is responsible for the greater expansion of the PCK cystic structures during the study period.
Our data suggest that, in the presence of secretin, PCK cysts expand to a greater extent than normal bile ducts. Previously published data have shown that stimulation of cholangiocytes by secretin results in increased levels of intracellular cAMP.32,33,34,35 We recently showed that elevated cholangiocyte cAMP levels are involved in hepatic and renal cystogenesis in the PCK rat, and that octreotide reduces cAMP levels and cyst expansion in vitro and inhibits hepatic and renal cystogenesis in vivo.19 Moreover, we previously reported that cholangiocytes contain intracellular vesicles with AQP1, CFTR, and AE2 proteins, which are inserted into the apical membrane in response to secretin and cAMP stimulation.20 Elevated cAMP subsequently activates the CFTR channel leading to the efflux of Cl− to the bile duct lumen, which in turn is exchanged with HCO3− through AE2 thus accounting for ion-driven water transport.35 These findings all provided the rationale for our present hypothesis that altered expression and location of these functionally-related proteins is associated with hepatic cystogenesis in PCK rats.
The message and protein levels of these three proteins are all overexpressed in PCK cholangiocytes by quantitative RT-PCR and quantitative Western blots; immunofluorescent confocal microscopy and immunoelectron microscopy provided additional evidence of overexpression. The intracellular location of the three proteins also appears to be disturbed in PCK cholangiocytes. AQP1, CFTR, and AE2 in normal rats are preferentially located to the apical membrane of cholangiocytes. In PCK cholangiocytes, these proteins are mainly overexpressed at the basolateral membrane. The presence of AQP1, CFTR, and AE2 in both membrane domains (apical and basolateral) of PCK cholangiocytes is consistent with previous observations in a related condition. Thus, Na+/K+-ATPase was reported to be mislocalized to the apical membrane of cystic renal tubular cells in a mouse model of ARPKD, suggesting its involvement in the net basal-to-apical vectorial transport of Na+ and fluid, resulting in tubular fluid secretion and cyst formation.36 Likewise, the epidermal growth factor receptor (EGFR) has been shown to be overexpressed and mislocalized to the apical membrane of cystic renal epithelial cells in the collecting tubules, suggesting that EGF-like factors in cystic fluid stimulate cystogenesis through an autocrine-paracrine cycle.37 In the early stages of ADPKD, AQP1 is found mainly on the apical plasma membrane of epithelial cells lining dilated proximal tubules in the kidney; with disease progression, AQP1 in cysts derived from proximal tubules is missorted to the basolateral membrane.38 Basolaterally expressed CFTR (through which Cl− can move in either direction depending on the Cl− gradient) may provide an additional pathway for Cl− entry in response to increased cAMP levels, thus increasing the net Cl− current to the apical lumen.39,40 Interestingly, cAMP-stimulated CFTR-dependent Cl− secretion has been reported to contribute to fluid accumulation in renal and hepatic cysts of ADPKD.21,41 Finally, AE2 is preferentially located to the apical membrane of both normal cholangiocytes35,42,43 and hepatocytes44 functioning as an acid loader facilitating the extrusion of HCO3− in exchange with Cl− influx. However, in many other tissues such as choroid plexus, distal nephrons and oxintic glands, AE2 is mainly expressed basolaterally working as a Cl− loader.45,46
We therefore investigated whether the overexpression of AQP1, CFTR, and AE2 at the basolateral membrane of cholangiocytes could facilitate more rapid cyst expansion in the PCK rat. Indeed, after exposure to a hypoosmotic solution, PCK cysts expanded more significantly than normal bile ducts. This finding together with our previous observation that in PCK cholangiocytes cell junctions are much tighter than in normal cholangiocytes24 suggest that basolateral overexpression of AQP1 might be responsible for greater changes in areas of PCK cystic structures compared to normal in response to hypotonicity.
Furthermore, to test the hypothesis that PCK cholangiocytes are characterized by an increased ion-driven water movement from the basolateral to the apical membrane, secretin-stimulated normal and PCK cysts were basolaterally exposed to NPPB (an inhibitor of CFTR and other Cl− channels), CFTRinh172 (a highly specific inhibitor of CFTR) and to DIDS and SITS (nonspecific inhibitors of Cl−/HCO3− exchange). Basolateral application of NPPB, CFTRinh172, DIDS, or SITS did not affect the secretin-stimulated cyst expansion in normal cysts. In PCK cysts, however, the presence of these inhibitors in the medium inhibited the increase in cystic areas in response to secretin. The results are consistent with overexpression of CFTR and AE2 at the basolateral membrane of PCK cholangiocytes and provide evidence that ion-driven transport from the basolateral to the apical cholangiocyte membrane after secretin-stimulation may account for accelerated fluid accumulation in hepatic cysts. The changes in cyst expansion in the presence of DIDS or SITS in the medium could also be linked to basolateral inhibition of the Na+-HCO3− co-transporter in addition to that of AE2 in the basolateral membrane of cholangiocytes.35,47 However, this possibility seems unlikely because the basolateral presence of DIDS or SITS had no effect on the secretin-stimulated cyst expansion in normal cholangiocytes. Although additional electrophysiological experiments (ie, patch-clamp techniques) using CFTR inhibitors could further support the notion of a basolateral to apical ion movement in PCK cholangiocytes, our in vitro experiments demonstrating basolateral CFTR overexpression in PCK cholangiocytes together with the physiological experiments in three-dimensional culture using two different CFTR inhibitors support this interpretation.
Thus, based on our findings, we suggest that elevated cAMP levels in PCK cholangiocytes19 might induce a permanent activation of apical CFTR, leading to continuous Cl− efflux into the bile duct lumen, which in turn is exchanged for HCO3− through AE2, thus accounting for ion-driven water transport via AQP1. Basolaterally overexpressed AQP1, CFTR, and AE2 facilitates basolateral to apical ion/water movement accounting for increased luminal fluid accumulation. The proposed model of basolateral to apical ion/water movement in the PCK biliary epithelium is consistent with previous findings reported in other epithelia.45,46
In conclusion, the present study provides evidence that elevated expression and altered topography of AQP1, CFTR, and AE2 are associated with hepatic cyst expansion in ARPKD. The data suggest the possibility of new therapeutic approaches to ameliorate the progression of the hepatic cystogenesis in ARPKD.
Footnotes
Address reprint requests to Nicholas F. LaRusso, M.D., Center for Basic Research in Digestive Diseases, Mayo Clinic College of Medicine, 200 First St., SW, Rochester, MN 55905. E-mail: larusso.nicholas@mayo.edu.
Supported by the National Institutes of Health (grant DK 24031 to N.F.L.), the PKD Foundation (to T.V.M. and S.A.G.), the Spanish Ramón Areces Foundation (to J.M.B.), the Chonbuk National University Medical Foundation (to S.L.), and the Mayo Foundation.
References
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol. 2005;289:F1159–F1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Sessa A, Righetti M, Battini G. Autosomal recessive and dominant polycystic kidney diseases. Minerva Urol Nefrol. 2004;56:329–338. [PubMed] [Google Scholar]
- Shneider BL, Magid MS. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant. 2005;9:634–639. doi: 10.1111/j.1399-3046.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Zerres K, Rudnik-Schoneborn S, Steinkamm C, Becker J, Mucher G. Autosomal recessive polycystic kidney disease. J Mol Med. 1998;76:303–309. doi: 10.1007/s001090050221. [DOI] [PubMed] [Google Scholar]
- Perrone RD, Grubman SA, Rogers LC, Lee DW, Moy E, Murray SL, Torres VE, Jefferson DM. Continuous epithelial cell lines from ADPKD liver cysts exhibit characteristics of intrahepatic biliary epithelium. Am J Physiol. 1995;269:G335–G345. doi: 10.1152/ajpgi.1995.269.3.G335. [DOI] [PubMed] [Google Scholar]
- Nichols MT, Gidey E, Matzakos T, Dahl R, Stiegmann G, Shah RJ, Grantham JJ, Fitz JG, Doctor RB. Secretion of cytokines and growth factors into autosomal dominant polycystic kidney disease liver cyst fluid. Hepatology. 2004;40:836–846. doi: 10.1002/hep.20401. [DOI] [PubMed] [Google Scholar]
- Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- Doctor RB, Johnson S, Brodsky KS, Amura CR, Gattone V, Fitz JG. Regulated ion transport in mouse liver cyst epithelial cells. Biochim Biophys Acta. 2007;1772:345–354. doi: 10.1016/j.bbadis.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Amura CR, Brodsky KS, Groff R, Gattone VH, Voelkel NF, Doctor RB. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/−) mice. Am J Physiol. 2007;293:C419–C428. doi: 10.1152/ajpcell.00038.2007. [DOI] [PubMed] [Google Scholar]
- Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y. Polycystic kidney rat is a novel animal model of Caroli’s disease associated with congenital hepatic fibrosis. Am J Pathol. 2001;158:1605–1612. doi: 10.1016/S0002-9440(10)64116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol. 2002;13:2246–2258. doi: 10.1097/01.asn.0000030392.19694.9d. [DOI] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk TV, Masyuk AI, Torres VE, Harris PC, LaRusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper S, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413–20419. doi: 10.1074/jbc.M302108200. [DOI] [PubMed] [Google Scholar]
- Everson GT, Emmett M, Brown WR, Redmond P, Thickman D. Functional similarities of hepatic cystic and biliary epithelium: studies of fluid constituents and in vivo secretion in response to secretin. Hepatology. 1990;11:557–565. doi: 10.1002/hep.1840110406. [DOI] [PubMed] [Google Scholar]
- Salter KD, Roman RM, LaRusso NR, Fitz JG, Doctor RB. Modified culture conditions enhance expression of differentiated phenotypic properties of normal rat cholangiocytes. Lab Invest. 2000;80:1775–1778. doi: 10.1038/labinvest.3780187. [DOI] [PubMed] [Google Scholar]
- Mennone A, Alvaro D, Cho W, Boyer JL. Isolation of small polarized bile duct units. Proc Natl Acad Sci USA. 1995;92:6527–6531. doi: 10.1073/pnas.92.14.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff MA, Masyuk TV, Stroope AJ, Huang BQ, Splinter PL, Lee SO, Larusso NF. Development and characterization of a cholangiocyte cell line from the PCK rat, an animal model of autosomal recessive polycystic kidney disease. Lab Invest. 2006;86:940–950. doi: 10.1038/labinvest.3700448. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972;10:311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Knauf PA, Rothstein A. Chemical modification of membranes. II. Permeation paths for sulfhydryl agents. J Gen Physiol. 1971;58:211–223. doi: 10.1085/jgp.58.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddy AH. A fluorescent label for the outer components of the plasma membrane. Biochim Biophys Acta. 1964;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong AY, Masyuk AI, Splinter PL, Huebert RC, Tietz PS, LaRusso NF. Channel-mediated water movement across enclosed or perfused mouse intrahepatic bile duct units. Am J Physiol. 2002;283:C338–C346. doi: 10.1152/ajpcell.00162.2001. [DOI] [PubMed] [Google Scholar]
- Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, Harris PC, Larusso NF. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol. 2004;165:1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras FI, Gradilone SA, Mazzone A, Garcia F, Huang BQ, Ochoa JE, Tietz PS, Larusso NF, Calamita G, Marinelli RA. Rat hepatocyte aquaporin-8 water channels are down-regulated in extrahepatic cholestasis. Hepatology. 2003;37:1026–1033. doi: 10.1053/jhep.2003.50170. [DOI] [PubMed] [Google Scholar]
- Roberts SK, Kuntz SM, Gores GJ, LaRusso NF. Regulation of bicarbonate-dependent ductular bile secretion assessed by lumenal micropuncture of isolated rodent intrahepatic bile ducts. Proc Natl Acad Sci USA. 1993;90:9080–9084. doi: 10.1073/pnas.90.19.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA, Hall RC. Cyclic AMP in secretin choleresis. Evidence for a regulatory role in man and baboons but not in dogs. Gastroenterology. 1976;70:537–544. [PubMed] [Google Scholar]
- Lenzen R, Alpini G, Tavoloni N. Secretin stimulates bile ductular secretory activity through the cAMP system. Am J Physiol. 1992;263:G527–G532. doi: 10.1152/ajpgi.1992.263.4.G527. [DOI] [PubMed] [Google Scholar]
- Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol. 2006;12:3496–3511. doi: 10.3748/wjg.v12.i22.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avner ED, Sweeney WE, Jr, Nelson WJ. Abnormal sodium pump distribution during renal tubulogenesis in congenital murine polycystic kidney disease. Proc Natl Acad Sci USA. 1992;89:7447–7451. doi: 10.1073/pnas.89.16.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490–499. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol. 1996;271:F169–F183. doi: 10.1152/ajprenal.1996.271.1.F169. [DOI] [PubMed] [Google Scholar]
- Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, Beaudet AL, Zabner J, Welsh MJ. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am J Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- Treharne KJ, Crawford RM, Mehta A. CFTR, chloride concentration and cell volume: could mammalian protein histidine phosphorylation play a latent role? Exp Physiol. 2006;91:131–139. doi: 10.1113/expphysiol.2005.031823. [DOI] [PubMed] [Google Scholar]
- Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 1996;50:208–218. doi: 10.1038/ki.1996.304. [DOI] [PubMed] [Google Scholar]
- Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, Prieto J, Medina JF. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- Aranda V, Martinez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040–1046. doi: 10.1016/j.bbrc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- Deng QS, Johanson CE. Stilbenes inhibit exchange of chloride between blood, choroid plexus and cerebrospinal fluid. Brain Res. 1989;501:183–187. doi: 10.1016/0006-8993(89)91041-x. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol. 2006;291:C59–C67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]