Abstract
Microbes and microbial products are closely associated with the pathogenesis of inflammatory bowel disease (IBD); however, the mechanisms behind this connection remain unclear. It has been previously reported that flagellin-specific antibodies are increased in IBD patient sera. As mastocytosis is one of the pathological features of IBD, we hypothesized that flagellin-specific immune responses might activate mast cells that then contribute to the initiation and maintenance of intestinal inflammation. Thirty-two colonic biopsy samples were collected from IBD patients. A flagellin/flagellin-specific IgG/Fc gamma receptor I complex was identified on biopsied mast cells using both immunohistochemistry and co-immunoprecipitation experiments; this complex was shown to co-localize on the surfaces of mast cells in the colonic mucosa of patients with IBD. In addition, an ex vivo study showed flagellin-IgG was able to bind to human mast cells. These cells were found to be sensitized to flagellin-specific IgG; re-exposure to flagellin induced the mast cells to release inflammatory mediators. An animal model of IBD was then used to examine flagellin-specific immune responses in the intestine. Mice could be sensitized to flagellin, and repeated challenges with flagellin induced an IBD-like T helper 1 pattern of intestinal inflammation that could be inhibited by pretreatment with anti-Fc gamma receptor I antibodies. Therefore, flagellin-specific immune responses activate mast cells in the intestine and play important roles in the pathogenesis of intestinal immune inflammation.
Growing evidence indicates that intestinal flora breaks host tolerance to induce an abnormal immune response that results in inflammatory bowel disease (IBD).1 The intestinal mucosa of the ileum and colon, the sites of inflammation in IBD,2 are exposed to the largest concentration of bacterial antigens of any tissue in the body, estimated to be up to 1012 organisms per gram of stool in the colon.3 Indeed, enteric bacteria or their products have been found within the inflamed mucosa of patients with Crohn’s disease.4,5 In addition, IBD patients can benefit from administration of antibiotics.6 Rats raised in germ-free conditions do not develop colitis.7,8 In contrast, colonization of these animals with commensal bacteria rapidly induces gut inflammation.9 It therefore seems likely that bacteria can be involved in the induction or/and maintenance of the chronic inflammatory status in IBD. Antigens from intestinal bacteria may be one of the many possible triggers of the pathological inflammatory reaction in the intestine.10 However, the detailed mechanisms by which bacteria break down the established tolerance and induce inflammation in the intestine remain unclear.
Conserved antigens of intestinal bacteria can drive chronic inflammation directly or via induction of immune disorders.11 Flagellins, which are targets of the host immune response during infection and immune disorder, are components of the flagella used by many microbial pathogens for their locomotion.12 It is proposed that flagellin interacts with Toll-like receptor (TLR)−5 and leads to the generation of a pro-inflammatory response and activation of host dendritic cells in vivo.12 Furthermore, flagellin is recognized by antibody and CD4+ T cells responses during bacterial infection.12 Specific antibodies against flagellin, which are at low levels or under detectable levels in healthy subjects, have been detected in the serum of IBD patients.13,14 Anti-flagellin IgG has the potential to activate immune cells such as dendritic cells, macrophages and mast cells via binding the Fcγ receptors (FcγR) on the surface of the cells. Mastocytosis is one of the pathological features of IBD.15 So far we have not fully understood the role of mast cells in the pathogenic processes of IBD. Based on the information above, we hypothesized that bacterial flagellin-specific immune responses can be an important factor in the initiation and maintenance of chronic immune inflammation in the intestine via activating FcγR pathway in mast cells.
Therefore, we attempted to provide insight into the mechanism by which flagellin induces immune inflammation in the intestine. The results demonstrate that flagellin-specific immune responses activate mast cells and induce inflammation in the intestine via the activation of the FcγRI pathway. A complex of flagellin/flagellin IgG/FcγRI was identified on colonic mast cells of IBD patients.
Materials and Methods
Mice and Reagents
BALB/c mice, 6 to 8 weeks old, were obtained from Charles River Canada (St. Constant, QC, Canada). W/Wv and +/+ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in a pathogen-free environment. The procedures of experiments in this study were approved by the Animal Care Committee at McMaster University. Reagents were provided as follows: Flagellin (Austral Biologicals; San Ramon, CA); anti-flagellin antibody (Biodesign, Saco, Maine); antibodies against CD16, CD32, and CD64 (Santa Cruz, Santa Cruz, CA); enzyme-linked immunosorbent assay (ELISA) kit for tumor necrosis factor (TNF)-α, interleukin (IL)-4, and interferon (IFN)-γ (R&D Systems, Burlington, ON, Canada); anti-TLR5 antibody (Abcam, Cambridge, MA); myeloperoxidase kit (Cell Sciences, Canton, MA); magnetic cell sorting reagents (Miltenyi Biotec, Auburn, CA); enhanced chemiluminescent reagents (GE Health care, Baie d’Urfe, QC, Canada); Oligo Fectamine (Invitrogen, Mississauga, ON, Canada). The other reagents used in this study, but not included in this list were purchased from Sigma Aldrich (Oakville, ON, Canada).
Collection of Colonic Tissue Biopsies from IBD Patients
Colonic tissue biopsy samples were obtained at the Department of Gastroenterology, Zhengzhou University from 32 patients with IBD undergoing colonoscopy including 9 patients with ulcerative colitis and 23 with Crohn’s disease during the remission period. Samples were fixed with 4% paraformaldehyde immediately for 4 hours. The diagnosis of IBD was based on the standard criteria of IBD.16,17 Six “normal” samples collected from surgically removed tissue from patients with colonic cancer were used as controls. The procedures in human study were approved by the Ethic Committee for Human Study at Zhengzhou University.
Confocal Microscopy
Paraffin sections were processed to rehydration. After blocking with 1% bull serum albumin for 30 minutes, sections were incubated with the primary antibodies at 4°C overnight. Fluorescently labeled secondary antibodies (1:100) were applied on the sections and incubated at room temperature for 1 hour. The nuclei were stained with propidium iodide (10 μg/ml) for 15 minutes. PBS washes were performed after each incubation. The sections were mounted with coverslips and observed under a confocal microscope (LSM510, Carl Zeiss MicroImaging GmbH, Germany). Mononuclear cell-derived mast cell (moMC) staining was performed in Eppendorf’s tubes following the same procedures above.
Generation of Human moMCs
CD34+ cells were separated from prepared peripheral blood mononuclear cells of healthy volunteers by magnetic cell sorting. The isolated cells were then transferred into 12-well plates at a density of 0.5 × 106 cells/ml and placed in a humidified incubator at 37°C. The CD34+ cells were grown in RPMI1640 media supplemented with 10% fetal cow serum, penicillin (100 U/ml) and streptomycin (100 μg/ml), IL-3 (10 ng/ml), IL-6 (50 ng/ml), and stem cell factor (100 ng/ml). The cytokine-supplemented medium was replaced weekly, and the non-adherent cells were collected and split to fresh medium. MoMC tryptase expression was used as an indicator of maturation. As shown by flow cytometry, over 95% cells were tryptase-positive in week 7; these cells were used for further experiments.
Co-Immunoprecipitation
Total proteins were extracted from colonic biopsy or cultured human peripheral moMCs with reported procedures.18 The immune complexes were formed between anti-flagellin (or anti-FcγRI; 1:100 for both Abs) and 100 μl of the extracts for 12 hours at 4°C, and then interacted for 1 hour with 50 μl of washed agarose beads containing covalently bound anti-IgG antibody. The agarose beads with bound flagellin/IgG/FcγRI complexes were washed three times with Tris buffer (pH 6.8). The washed beads with flagellin/IgG/FcγRI complexes were then interacted with Laemmili buffer containing 4% 2-mercaptoethanol and incubated at 57°C for 30 minutes to dissociate the IgG-agarose beads. The supernatants were separated on a precast NuPAGE gel system (Invitrogen, Mississauga, ON, Canada) and blotted onto nitrocellulose membrane (Bio-Rad, Mississauga, ON, Canada). The membranes were then blotted with antibodies against flagellin, or IgG (1:200), or FcγRI (1:100), or TLR5 (1:50) respectively. The proteins were detected using horseradish peroxidase-conjugated secondary antibodies. The horseradish peroxidase enzymatic reaction was detected with enhanced chemiluminescent reagents and recorded with X-ray films.
RNA Interference
For the silencing of FcγRI, moMCs were transfected with specific small interfering RNA (siRNA) duplexes. The chosen sequence of target mRNA used in this study was: 5′-AATATCTTGCATGTTACAG-3′ (1 through 19 from start codon); control siRNA 5′-AACGAAGCAACTAAGCTCG-3′ did not target any genes. The doses of all siRNAs were used at a final concentration of 100 or 300 nmol/L using Oligo Fectamine in serum-free RPMI1640 media for 4 hours. The maximal effect of RNA interference in moMCs occurred 40 to 60 hours after the transfection as determined by Western blot; the target protein expression was completely inhibited (data not shown). These moMCs were used for further experiments.
ELISA
Levels of histamine, IL-4, and IFN-γ in supernatant or serum were determined by ELISA with commercial reagent kits following the manufacturers’ instruction. Flagellin-specific IgG was assessed with the in-house ELISA with the procedures we reported previously.18
Sensitization
Mice received flagellin (100 μg/mouse, mixed in 100 μl complete Freund adjuvant) by intraperitoneal injection. Boost injections were repeated on day 3 and day 6. Mice were challenged with flagellin (100 μg/mouse) via gavage daily between days 28 through 32. The naïve control group was treated with the same protocol except using saline instead of flagellin. Another control group was sensitized to flagellin and challenged with ovalbumin. Mice were sacrificed on day 33. Samples of blood and intestinal segments were collected immediately after death.
Histology
Segments of the ileum and colon were fixed with 4% paraformaldehyde or Carnoy solution overnight and processed for paraffin embedding. Sections were stained with H&E or 0.5% toluidine blue. Tissue structure was observed under a light microscope. Mononuclear cells and mast cells were numerated at a magnification of × 200; 20 fields/mouse, 120 fields/group. Another segment of the ileum was processed for electron microscopy.18 Mast cell degranulation was observed under an electron microscope.
Mouse Colonic Mucosal Inflammatory Score
The degree of inflammation on microscopic intestinal tissue sections was scored (from 0 to 4; 0 indicated normal, 4 indicated severe condition) at a magnification of × 400 by a staff pathologist using the criteria reposted previously19: 0, no leukocyte infiltration; 1, low level of leukocyte infiltration; 2, moderate level of leukocyte infiltration; 3, high vascular density and thickening of the colon wall; and 4, transmural leukocyte infiltration, loss of goblet cells, high vascular density, and thickening of the colon wall. Grading was done in a blinded manner.
T Lymphocyte Proliferation Assays
Lamina propria mononuclear cells (LPMC) were isolated from intestinal segments of the experimental mice. CD4+ T cells were further isolated from the LPMC, and cultured in the presence of irradiated splenic dendritic cells (T cell:Dendtritic cell = 106:105/well) and flagellin (10 μg/ml; or ovalbumin using as a control) for 4 days. [3H] thymidine (0.5 μCi/well) was added at the last 16 hours. The incorporation of [3H] thymidine in CD4+ T cells was assessed with a scintillation counter. The supernatants were collected; levels of IL-4 and IFN-γ in the supernatants were determined with ELISA.
Flow Cytometry
Another batch of CD4+ T cells was cultured in the same condition above. Brefeldin (10 μg/ml) was added to the culture for the last 3 hours. Cells were stained with fluorescently labeled antibodies against IL-4 or IFN-γ. The stained cells were analyzed by flow cytometry.
Reconstitution of Mast Cell in W/Wv Mice
Bone marrow-derived mast cells were generated as we reported previously.20 Cultured mast cells (>95% purity) were injected to W/Wv mice via the tail vein at a dose of 50,000 cells/mouse in 0.25 saline; the transfer was executed once more, 1 month later. The mice were used for flagellin sensitization experiments 2 months after the first mast cell reconstitution. As examined with Carnoy staining and electron microscopy, mast cells were distributed in the intestine of W/Wv mice with mast cell reconstitution at a similar density of +/+ mice (data not shown).
Statistics
All values were expressed as the means ± SD of at least three independent experiments. The values were analyzed using the two-tailed unpaired Student’s t-test when data consisted of two groups or by analysis of variance when three or more groups were compared. P < 0.05 was accepted as statistically significant.
Results
Colocalization of Flagellin/Flagellin-Specific IgG/FcγRI on the Surface of Mast Cells
Previous reports13,14 suggested that bacterial flagellin might play a role in the pathogenesis of mucosal idiopathic inflammation such as IBD. To elucidate the immune pathogenic role of flagellin-specific immune response in the intestinal inflammation, colonic biopsy samples were collected from 23 Crohn’s disease patients; 6 “normal” samples were collected from surgical removed tissue from patients with colonic cancer to use as controls (the marginal normal tissue was used in this study). Since mast cells also play a role in IBD, we speculated that flagellin might activate mast cells via an immune mechanism. Our first attempt was to identify if flagellin bound to the surface of mast cells. Paraffin sections of the intestinal biopsy tissue were stained with antibodies against flagellin (specific for the flagellin from Helicobacter pylori) and tryptase. Confocal microscopy colocalized the positive immune products of both flagellin and tryptase on cells in the subepithelial region and the lamina propria. About 87.5 ± 14.6% of tryptase-positive cells were also flagellin-positive. The tryptase positive staining was localized in the cytoplasm; the positive products of flagellin were mainly localized on the surface of the cells (Figure 1Aa). Since tryptase is a specific molecule secreted by mast cells, we conclude that flagellin adheres to mast cells in the colon of IBD patients. Tryptase-positive cells were also localized in normal control samples, but none of these positive cells were flagellin-positive (Figure 1Ac). This fact indicates that flagellin does not adhere to mast cells in normal colonic tissue. Negative control slides were stained with an isotype control antibody or by omitting the primary antibody; no positive staining was identified in these negative control slides (data not shown).
Figure 1.
Immune staining of flagellin immune complex and FcγRI on the surface of mast cells. A: Colonic biopsy samples were obtained from patients with inflammatory bowel disease (IBD) (Aa, Ab) or normal controls (Ac, Ad). Consecutive sections were cut; a and b were from an IBD sample; c and d were from a control sample. Co-staining was performed with antibodies against tryptase and FcγRI (Aa and Ac) or tryptase and flagellin (Ab and Ad). Tryptase immune positive products were stained in red; FcγRI or flagellin was stained in blue. B: A confocal image of human mononuclear cell-derived mast cells (moMCs). The positive staining includes: Ba, flagellin; Bb, flagellin-specific IgG; Bc, FcγRI; Bd, merged by Ba and Bb; Be, merged by Ba, Bb and Bc. Bf is a negative control image.
We next sought to identify which components flagellin bound on the surface of mast cells. The putative targets might be the FcγRs, consisting of FcγRI, FcγRII, and FcγRIII subtypes. IgG antibodies may bind to FcγRs to form immune complexes that may be then bound by specific antigens. Thus, we speculated that flagellin might bind to such an immune complex on the surface of mast cell. In addition to staining for flagellin and tryptase, consecutive sections were also co-stained with antibodies against tryptase and FcγRI, or FcγRII, or FcγRIII. Confocal microscopy colocalized tryptase and FcγRI in colonic tissue of both IBD samples (Figure 1Ab) and normal control samples (Figure 1Ad). The binding sites of FcγRI were the same as those of flagellin as shown on consecutive sections (Figures 1Aa and 1Ac). FcγRII and FcγRIII were not detected in human colonic tissue (data not shown). The ratio of FcγRI positive mast cells was 84.5 ± 12.4% in IBD samples and 11.3 ± 3.6% in normal control samples (P < 0.001, as compared with IBD samples). No positive staining was found in the negative control slides that were stained with isotype control antibodies or by omitting the primary antibodies.
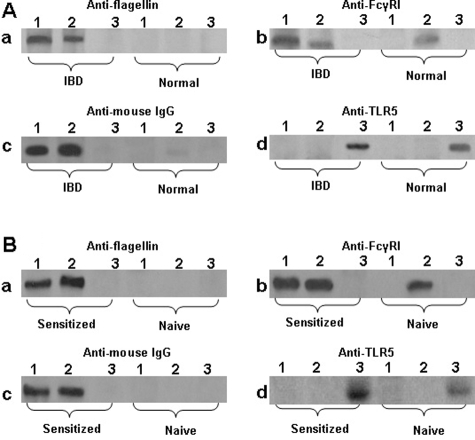
Since flagellin does not bind to FcγRI directly, we inferred that there might be a flagellin-specific IgG antibody mediating this action. The results presented in Figure 1, Aa–Ad implicate an immune complex of flagellin/flagellin-specific IgG forms on the surface of mast cells in IBD colonic mucosa. To support this speculation, protein extracts from another five colonic biopsies of CD patients were subjected to co-immunoprecipitation. The extracts were precipitated with antibodies against flagellin, FcγRI and TLR5 respectively. As expected, flagellin, IgG, and FcγRI were co-immunoprecipitated with anti-flagellin, or anti-FcγRI respectively, but not by anti-TLR5 (Figure 2A). This finding indicates that an immune complex of flagellin/flagellin-specific IgG is formed in colonic mucosa of patients with IBD; it is possible that the immune complex binds to FcγRI on mast cells.
Figure 2.
Co-immunoprecipitation of flagellin, IgG and FcγRI. Proteins were extracted from intestinal samples (A) or cultured cells (B) and analyzed by co-immunoprecipitation. Antibodies used for precipitation were: lane 1, anti-flagellin; lane 2, anti-FcγRI; lane 3, anti-TLR5. Antibodies used for blotting are presented above each gel. IBD, samples were taken from IBD patients. Normal, samples were taken from normal intestinal tissue. Sensitized, cells exposed to flagellin-specific IgG and then exposed to flagellin in culture. Naïve, naïve cells.
Having found that flagellin bound on the surface of mast cells (Figure 1A) and that flagellin, IgG and FcγR formed complexes in colonic mucosa (Figure 2A), we next performed an ex vivo experiment with human mast cells and flagellin to investigate this phenomenon in depth. Peripheral blood moMCs were generated. More than 95% of the mast cells express tryptase as shown by fluorescence-activated cell sorting (data not shown). These moMCs were treated with IFN-γ (20 ng/ml) for 24 hours to strengthen the expression of FcγRI that was proved by flow cytometry (over 96%). The moMCs were exposed to flagellin-specific IgG (1 μg/ml) for 30 minutes in culture. After washing, flagellin were then added to the culture (1 μg/ml) and incubated for another 30 minutes. Cells were washed and stained with antibodies against FcγRI and mouse IgG (the host of flagellin-specific IgG is mouse). As depicted in Figures 1Ba–Bd, flagellin, FcγRI and rabbit IgG were colocalized on the surface of moMCs. The finding indicates that a complex of flagellin/flagellin-specific IgG binds to FcγRI on the surface of moMCs. Some moMCs were treated with isotype control IgG instead of flagellin-specific IgG; no flagellin/IgG/FcγRI immune complexes were found on these cells (data not shown). To further verify the flagellin/IgG/FcγRI immune complexes in mast cells, another batch of moMCs were treated with flagellin/IgG as above. As shown by co-immunoprecipitation assay, flagellin, flagellin-specific IgG, and FcγRI were co-immunoprecipitated by either anti-flagellin antibody or anti-FcγRI antibody, but not by anti-TLR5 (Figure 2B). This result demonstrates that flagellin-specific IgG can bind to FcγRI on human mast cells, and that flagellin can bind to the flagellin-specific IgG that subsequently has the potential to activate the mast cells.
Flagellin-Specific Immune Response Induces moMCs to Release Inflammatory Mediators
We hypothesized that flagellin-specific IgG might bind to mast cells in the intestine to make mast cells sensitized to flagellin. In response to re-exposure to flagellin, sensitized mast cells might be activated to release inflammatory mediators that cause immune inflammation in the intestine. To verify this hypothesis, we generated moMCs. After exposure to flagellin-specific IgG, moMCs were challenged with the antigen flagellin, resulting in release of histamine and TNF-α. This fact verified that flagellin-specific IgG had bound onto these moMCs. Exposure to an irrelevant protein, ovalbumin, did not induce the mediator release from moMCs. The release of mediators occurred 30 minutes after the challenge, reached the peak value after 48 hours, and returned to the baseline 96 hours later. Some moMCs were exposed to flagellin-specific IgG alone, or to flagellin alone; the levels of histamine and TNF-α in culture did not increase as observed at time-points of 0, 0.5, 12, 24, and 48 hours after the exposure. Another batch of moMCs were sensitized with anti-flagellin IgG, pretreated with mast cell stabilizer ketotifen, and then challenged with flagellin. As expected, the release of mediators was abolished in these moMCs (Figure 3).
Figure 3.
Re-exposure to specific antigen flagellin activates sensitized moMCs. Flagellin-IgG sensitized moMCs were re-exposed to specific antigen flagellin; levels of histamine and TNF-α in supernatants were determined by ELISA. Bars indicate levels of histamine (A) and TNF-α (B). Naïve, moMCs were treated with media alone. F+IgG, moMCs were sensitized with flagellin-IgG and challenged with flagellin. F or IgG, moMCs were treated with flagellin alone or IgG alone. αTLR5, moMCs were treated with anti-TLR5 antibody. RNAi, moMCs were transfected with FcγRI siRNA at 300 nmol/L (RNAi1) or 100 nmol/L (RNAi2), or control siRNA (RNAi3). 48/80, moMCs were treated with 48/80 compound (5 μg/ml). Data are expressed as the means ± SD, *P < 0.05, compared with naïve cells.
As depicted in Figure 1, mast cells in the colon express FcγRI. We then took a further step to elucidate if FcγRI was indispensable to flagellin-specific immune response involved mast cell activation. FcγRI siRNA (or control siRNA) was transfected into moMCs at graded doses, and then exposed to flagellin-specific IgG. The moMCs were then exposed to flagellin in culture. As indicated by histamine and TNF-α release, FcγRI siRNA, but not the control siRNA, transfection inhibited flagellin-induced mediator release from moMCs in a dose-dependent manner (Figure 3). Another batch of moMC was pretreated with anti-FcγRI antibody before exposure to flagellin-specific IgG. These moMCs were not activated after the exposure to flagellin in culture (data not shown).
Mast cells express TLR5.20 One previous report indicated that TLR5 recognizes flagellin.21,22 We thus took another approach to elucidate if flagellin activated mast cell via acting on TLR5 to elicit the release of inflammatory mediators that might contribute to the initiation of mucosal inflammation. Anti-flagellin antibody- or anti-FcγRI antibody-precipitated moMC cellular extracts (as mentioned in Figure 2A) was processed by immune blotting. The staining with anti-TLR5 antibody yielded a negative result (Figure 2B). These data indicate that flagellin does not act on TLR5 to activate mast cells in colonic tissue.
To gain further insight into TLR5 involvement in flagellin-induced mast cell activation, we treated moMCs with flagellin at graded doses (0, 5, 10 and 20 μg/ml) at 37°C for 1, 6, and 24 hours respectively. The results showed that the effect of flagellin alone on the mediator release from moMCs was at the baseline (data not shown). We next pretreated moMCs with anti-TLR5 antibody; the moMCs were then treated with flagellin-IgG afterward and then exposed to flagellin in the same procedures as described above. The moMCs, however, were still activated as indicated by the increase in the release of histamine and TNF-α. The results confirmed that TLR5 was not involved in the moMC activation initiated by the flagellin-specific immune response (Figure 3).
Flagellin-Specific Immune Response Induces Inflammation in Mouse Intestine
Based on the facts that flagellin-specific IgG bound to FcγRI on moMCs (Figure 1 and Figure 2) and re-exposure to specific antigen flagellin activated mast cells (Figure 3), we hypothesized that flagellin-specific immune response induced inflammation in the intestinal mucosa via the activation of mast cells. To test this hypothesis, an animal experiment was performed. Mice were sensitized to flagellin. Four weeks later, mice were challenged with gavage-fed flagellin every other day for five treatments. The challenges with flagellin induced inflammatory response in the intestine that featured surface erosion on the mucosa of both the ileum and colon (Figure 4A) compared with naïve control (Figure 4C), high inflammatory scores (Figure 4D), weight loss (Figure 4E), increase in inflammatory cell infiltration in the intestine (Figure 5A and 5B, a–c), mast cell degranulation in the intestinal mucosa (Figures 5Bd and 5Be; ratio of mast cell degranulation: 68.5 ± 11.4% in flagellin group; 5.6 ± 2.3% in naïve group; P < 0.001. Data were collected in 20 randomly selected mast cells from each mouse, as shown by electron microscopy, five mice per group), and increase in myeloperoxidase contents in the intestinal mucosa (144.7 ± 25.6 U/g tissue in flagellin group that was higher than that in saline group, 36.8 ± 7.3 U/g; P < 0.05). Flagellin-specific Th1 cellular response was observed in LPMC in the presence of flagellin in the culture that manifested the increase in [3H] thymidine incorporation (Figure 6A) high levels of IFN-γ (but not IL-4) in culture supernatants (Figure 6B), and more IFN-γ+ T cells as shown by fluorescence-activated cell sorting (Figure 6C). Increase in flagellin-specific IgG in the serum was also observed (Figure 6D).
Figure 4.
Flagellin-specific immune response induces inflammation in mouse intestine. Mice were sensitized and challenged with flagellin. Intestinal segments were observed with light and electron microscopy. A–C: Representative images of colonic histology (×200) from mice sensitized to and challenged with flagellin (A), or mice sensitized to flagellin, pretreated with anti-FcγRI antibody, and then challenged with flagellin (B, ×100), or from naïve mice (C, ×100). D: Bars indicate the inflammatory scores in the intestine. E: Bars indicate the weight loss of the experimental mice that is presented as the percentage of the original body weight., *P < 0.05, compared with mice treated with saline. Balb, BALB/c mice. Wv1, W/Wv mice. Wv2, W/Wv mice were reconstituted with mast cells. Ab, pretreated with anti-FcγRI antibody. iAb, pretreated with isotype antibody. F+IgG, mice were sensitized and challenged with flagellin.
Figure 5.
Mast cell activation in the intestinal mucosa. Colonic sections were observed under a light microscope. A: Bars indicate numbers of mast cell and mononuclear cells in colonic tissue sections that are expressed as the means ± SD from 20 fields (×200) from each mouse., *P < 0.05, compared with naïve BALB/c mice. Ba–Bc: Photomicrographs show mast cell staining in colonic sections (stained by toludine blue) from naïve mouse (Ba), sensitized mice (Bb), and Wv/Wv mice (Bc). Bd–Be: Electron photomicrographs show mast cell degranulation (pointed by a solid arrow; Bd) from sensitized mice compared with normal mast cells with intact granules (Be, pointed by an open arrow) from naïve mice. Group legends are the same as that in Figure 4.
Figure 6.
Flagellin-specific cellular response in the intestine and serum IgG. A–C: LPMCs were cultured in the presence of flagellin for 72 hours. Cellular immune response was observed by antigen-specific T cell proliferation. Bars indicate the [3H] incorporation in isolated LPMC (A), cytokine levels in culture supernatants (B) and positively stained IFN-γ+ or IL-4+ T cells (C, analyzed by fluorescence-activated cell sorting). D: Bars indicate the levels of serum flagellin-specific IgG that were assessed by ELISA. Data are presented as the means ± SD, *P < 0.05, compared with mice treated with naïve BALB/c mice.
To further confirm the role of mast cell in flagellin-induced inflammation in the intestine, mast cell-deficient and +/+ littermates were sensitized and challenged with flagellin. W/Wv mice showed no inflammatory signs in the intestine (Figures 4–6). Some W/Wv mice were reconstituted with BMMCs; inflammation was induced in the intestine that was comparable to wild mice (Figures 4–6). Similar to BALB/c mice, +/+ mice also had induced inflammation in the intestine (data not shown) by sensitization to and challenge with flagellin.
As shown by Figures 1–3, flagellin/IgG complexes bound to FcγRI on mast cells to activate mast cells to release inflammatory mediators; sensitization to and challenge with flagellin induced inflammation in the intestine as presented in Figures 4–6. The data indicate that mast cells plays an important role in flagellin-specific immune response-induced inflammation in the intestine, and that FcγRI plays a critical role in the process. We thus speculated that blockade of FcγRI or stabilization of mast cells might abolish this type of immune inflammation in the intestine. To this end, another batch of sensitized mice was treated with anti-FcγRI antibody or mast cell stabilizer ketotifen before the challenge with flagellin. As expected, pretreatment with anti-FcγRI antibody (Figure 4B) or mast cell stabilizer ketotifen abolished the induced inflammation in the intestine (Figure 4–6).
Discussion
The present study has demonstrated that flagellin-specific immune response induces IBD-like inflammation in the intestine. The data show that flagellin/flagellin-specific IgG/FcγRI was colocalized on the surface of colonic mast cells of patients with IBD. Human moMCs can be sensitized by flagellin-specific IgG. Re-exposure to specific antigen-flagellin activates sensitized-moMCs to release inflammatory mediators. Furthermore, Th1 pattern inflammation was induced in mouse intestine with sensitization to and challenge with flagellin. FcγRI was detected on the surface of colonic mast cells that play an important role in the flagellin-specific immune inflammation in the intestine.
Previous studies indicate that mast cells express FcγRI,23 FcγRII,24 and FcγRIII.25 Our observation confirmed that mast cells in human colonic tissue express FcγRI, but we did not detect the other two types of FcγR in colonic mucosa. The differences between those studies and our observation can be local microenvironment-dependent. For instance, Zhao et al reported FcγRII expression in human skin, but the expression of FcγRI and FcγRIII was not observed.24 Exposure to IFN-γ or granulocyte colony-stimulating factor up-regulates FcγRI expression.26
A novel finding of this study is that flagellin/flagellin-specific IgG immune complexes have been observed on the surface of mast cells in the colon of patients with IBD. This complex further binds to FcγRI on mast cells. The phenomenon is confirmed by the ex vivo moMC experiments. It is noteworthy that this finding is that the flagellin/IgG complex was only detected on colonic mast cells of IBD patients, not in normal control samples. The phenomenon may be regarded as an immunopathological sign of IBD. The results are in line with previous reports, which indicate that serum bacterial antigen-specific IgG (including flagellin-specific IgG) can be a reference parameter in the diagnosis of IBD.27 It is possible that the colon is a major site of the flagellin-specific IgG production since its highest density of microbes in the body. The produced IgG may be partially absorbed into the blood stream and detected in the serum as reported,27,28 and partially bound to immune cells, such as mast cells, in local tissue as shown by the present study.
Based on the immune staining results (Figures 1 and 2) and the ex vivo experiments (Figure 3), this study has also proposed the notion that mast cells can be sensitized to the bacterial product flagellin by pretreatment of mast cells with flagellin-specific IgG. It is accepted that IgG can bind to FcγRs.29,30 Our results provided further evidence that flagellin, IgG and FcγRI can be co-immunoprecipitated by either anti-flagellin or anti-FcγRI antibody from human colonic tissue and moMCs. This phenomenon was confirmed by morphological evidence, as flagellin and FcγRI were colocalized at the same sites on the generated mast cells (Figure 2). These data support the notion that mast cells can be sensitized to flagellin by the binding of flagellin-specific IgG on the surface.
To demonstrate the significance of our finding that flagellin and FcγRI are structurally identified on IBD colonic mucosa, we generated moMCs, sensitized moMCs with flagellin-specific IgG, and challenged the moMCs with flagellin. The results supported our hypothesis that the flagellin-specific immune response could be generated in the intestine; re-exposure to flagellin could trigger a specific immune response to drive mast cells to release inflammatory mediators. The release of mediators occurred as early as 30 minutes after the challenge with flagellin; it was comparable to that evoked by 48/80 compound. Furthermore, the release of mediators lasted as long as 4 days after the challenge. This finding provides evidence that flagellin-specific immune responses in the intestine may contribute to the initiation and maintenance of immune inflammation such as IBD.
Data from the subsequent animal studies have provided further evidence to support the notion that flagellin-specific immune response contributes to the immune inflammation in the intestine. When flagellin-sensitized mice were re-exposed to flagellin, IBD-like pathogenic responses were evoked in the intestine. Since IFN-γ levels in the culture and IFN-γ secreting T cells in the intestine increased significantly, the immune response can be regarded as a Th1 pattern. The finding is in agreement with the immune pathological feature of human IBD that most IBD inflammation is categorized as Th1 pattern. Thus, the findings of this study have extended our knowledge in understanding the mechanism of IBD; bacterial antigen (including flagellin) may induce specific immune response in the intestine; re-exposure to specific bacterial antigens may initiate a destructive immune inflammation such as causing ulcers in the intestinal mucosa. Repeated exposures to specific bacterial antigens may sustain the inflammation in the intestine.
The results of this study indicate that FcγRI is an indispensable molecule in flagellin-specific immune response. Activation of FcγRI on moMCs triggers the release of inflammatory mediators. Others also reported that F(ab)2 fragments of IgG could aggregate this high affinity IgG receptor, FcγRI, to induce degranulation of mast cells, as well as increase mRNA expression for inflammatory cytokines such as TNF-α, granulocyte macrophage-colony stimulating factor, IL-3, IL-6, and IL-13.31,32 Therefore, it is conceivable that by blocking the function of FcγRI, this type of immune inflammation may be inhibited. Indeed, the pretreatment with anti-FcγRI antibody significantly blocked the flagellin-specific immune response, induced mediator release from moMCs, as well as inhibited the Th1 pattern inflammatory reaction in the intestine. Taken together, the treatment with anti-FcγRI antibody has therapeutic potential in the treatment of IBD.
Ketotifen is a mast cell stabilizer; it should have the capability to block the inflammation in the intestine if it is induced by mast cell activity. The present data agree with this speculation; pretreatment with ketotifen abolished the flagellin-specific immune response-induced intestinal inflammation. The results further confirm the role of mast cell in bacterial products, such as flagellin, in the pathogenesis of intestinal chronic immune inflammation. On the other hand, the results implicate that mast cells can be a therapeutic target in the treatment of the chronic immune inflammation in the intestine such as IBD.
It has been reported that mast cells express TLR5.20 TLR5 may recognize flagellin and initiate inflammatory response.33 It is thus possible that flagellin binds to TLR5 on mast cells and activates mast cells. Since blocking FcγRI abolished flagellin-induced inflammatory response as shown by our experiments, the flagellin-TLR5 pathway may contribute to intestinal inflammation via other mechanisms, rather than via activating on mast cells directly. For example, intestinal epithelial cells express TLR5.34 Flagellin may bind to TLR5 on the intestinal epithelium to activate the epithelial cells to cause the epithelial barrier dysfunction.29 As we observed in previous experiments, activated intestinal epithelium increased its permeability to macromolecular antigen,35 possibly speeding flagellin-transport to the subepithelial region where the absorbed flagellin has opportunity to contact immune cells and compromise the local immune homeostasis.
In summary, the present study indicates that bacterial flagellin binds to mast cells in colonic mucosa of patients with IBD. Flagellin-specific IgG can bind to FcγRI on the surface of mast cells to make the mast cells sensitized to flagellin. Re-exposure to specific antigen flagellin activates the sensitized mast cells to release inflammatory mediators and induce Th1 pattern immune inflammation in the intestine. Pretreatment with the antibody against FcγRI has an inhibitory effect in the flagellin-specific immune response induced inflammation that has therapeutic potential in the treatment of IBD.
Footnotes
Address reprint requests to Dr. Ping-Chang Yang, BBI-T3330, 50 Charlton Ave East, St. Joseph Hospital; Hamilton, ON, Canada L8N 4A6. E-mail: yangp@mcmaster.ca.
Supported by the Canadian Institutes of Health Research and the Nature and Science Foundation of China. Dr. P. Yang is a recipient of the New Investigator Award from the Canadian Institutes of Heath Research.
References
- Thompson-Chagoyán OC, Maldonado J, Gil A. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin Nutr. 2005;24:339–352. doi: 10.1016/j.clnu.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- Guarner F, Casellas F, Borruel N, Antolín M, Videla S, Vilaseca J, Malagelada JR. Role of microecology in chronic inflammatory bowel diseases. Eur J Clin Nutr. 2002;56 Suppl 4:S34–S38. doi: 10.1038/sj.ejcn.1601662. [DOI] [PubMed] [Google Scholar]
- Neut C, Bulois P, Desreumaux P, Membré JM, Lederman E, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel JF. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol. 2002;97:939–946. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Tejpar QZ, Soo I, Madsen K, Fedorak RN. The role of antibiotic and probiotic therapies in current and future management of inflammatory bowel disease. Curr Gastroenterol Rep. 2006;8:486–498. doi: 10.1007/s11894-006-0039-z. [DOI] [PubMed] [Google Scholar]
- Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, Pochart P, Doré J, Marteau P. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Latinne D, Fiasse R. New insights into the cellular immunology of the intestine in relation to the pathophysiology of inflammatory bowel diseases. Acta Gastroenterol Belg. 2006;69:393–405. [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101:117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RJ, Heazlewood SP, Gilshenan KS, O'Brien M, McGuckin MA, Florin TH. IgG antibodies against common gut bacteria are more diagnostic for Crohn’s disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–396. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318. doi: 10.3748/wjg.v10.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Yao T, Sakurai T, Yao K, Hirai F, Matake H, Tsuda S, Wada Y, Iwashita A, Kamachi S. Clinical features and pattern of indeterminate colitis: Crohn’s disease with ulcerative colitis-like clinical presentation. J Gastroenterol. 2003;38:647–655. doi: 10.1007/s00535-003-1117-8. [DOI] [PubMed] [Google Scholar]
- Cross SS, Harrison RF. Discriminant histological features in the diagnosis of chronic idiopathic inflammatory bowel disease: analysis of a large dataset by a novel data visualisation technique. J Clin Pathol. 2002;55:51–57. [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, Yeh C. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–1533. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng BS, He SH, Zheng PY, Wu L, Yang PC. Mast cells play a crucial role in Staphylococcus aureus peptidoglycan-induced diarrhea. Am J Pathol. 2007;171:537–547. doi: 10.2353/ajpath.2007.061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, van Aubel RA, van Putten JP. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol Immunol. 2008;45:1298–1307. doi: 10.1016/j.molimm.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, Kepley CL, Ryan JJ. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen N, Herbert DR, Lopata AL, Brombacher F. CD4+ T cell-specific deletion of IL-4 receptor alpha prevents ovalbumin-induced anaphylaxis by an IFN-gamma-dependent mechanism. J Immunol. 2007;179:2758–2765. doi: 10.4049/jimmunol.179.5.2758. [DOI] [PubMed] [Google Scholar]
- van der Meer W, Pickkers P, Scott CS, van der Hoeven JG, Gunnewiek JK. Hematological indices, inflammatory markers and neutrophil CD64 expression: comparative trends during experimental human endotoxemia. J Endotoxin Res. 2007;13:94–100. doi: 10.1177/0968051907079101. [DOI] [PubMed] [Google Scholar]
- Vernier G, Sendid B, Poulain D, Colombel JF. Relevance of serologic studies in inflammatory bowel disease. Curr Gastroenterol Rep. 2004;6:482–487. doi: 10.1007/s11894-004-0070-x. [DOI] [PubMed] [Google Scholar]
- Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, Wrobel I, Quiros A, Vasiliauskas EA, Grill B, Israel D, Bahar R, Christie D, Wahbeh G, Silber G, Dallazadeh S, Shah P, Thomas D, Kelts D, Hershberg RM, Elson CO, Targan SR, Taylor KD, Rotter JI, Yang H, Western Regional Pediatric IBD Research Alliance Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhorn M, Olin AI, Nimmerjahn F, Collin M. Human IgG/FcgammaR interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS ONE. 2008;3:e1413. doi: 10.1371/journal.pone.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z, Németh T, Verbeek JS, Mócsai A. Critical but overlapping role of FcgammaRIII and FcgammaRIV in activation of murine neutrophils by immobilized immune complexes. J Immunol. 2008;180:618–629. doi: 10.4049/jimmunol.180.1.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, Chiamolera M, Verbeek JS, Launay P, Monteiro RC. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Kumar A, Neish AS, Uematsu S, Akira S, Gewirtz AT. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Im E, Riegler M, Kokkotou E, O'brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]