Abstract
Host genetic variations play a significant role in conferring predisposition to infection. In this study, we examined the immune mechanisms underlying the host genetic predisposition to severe Staphylococcus aureus infection in different mouse strains. Whereas C57BL/6 mice were the most resistant in terms of control of bacterial growth and survival, A/J, DBA/2, and BALB/c mice were highly susceptible and succumbed to infection shortly after bacterial inoculation. Other strains (C3H/HeN, CBA, and C57BL/10) exhibited intermediate susceptibility levels. Susceptibility of mice to S. aureus was associated with an inability to limit bacterial growth in the kidneys and development of pathology. Resistance to S. aureus in C57BL/6 mice was dependent on innate immune mechanisms because Rag2-IL2Rγ−/− C57BL/6 mice, which are deficient in B, T, and NK cells, were also resistant to infection. Indeed, neutrophil depletion or inhibition of neutrophil recruitment rendered C57BL/6 mice completely susceptible to S. aureus, indicating that neutrophils are essential for the observed resistance. Although neutrophil function is not inhibited in A/J mice, expression of neutrophil chemoattractants KC and MIP-2 peaked earlier in the kidneys of C57BL/6 mice than in A/J mice, indicating that a delay in neutrophil recruitment to the site of infection may underlie the increased susceptibility of A/J mice to S. aureus.
Staphylococcus aureus is an important human pathogen capable of causing a diverse spectrum of diseases ranging from mild cutaneous to severe invasive infections such as sepsis, pneumonia, or endocarditis. Estimates of the overall incidence of S. aureus infections vary, but some studies suggest that S. aureus accounts for 11 to 38% of cases of both community- and hospital-acquired bacteremia. The reported mortality from septicemia ranges from 20 to 90%.1,2,3,4,5 One of the most severe complications of S. aureus infections is the development of septic and toxic shock syndromes associated with vascular damage and multiple organ failure.6,7
Apart from its ability to cause a diverse range of life-threatening infections, an additional problem associated with S. aureus is its extraordinary potential to develop antimicrobial resistance.8 The emergence of multidrug-resistant S. aureus, especially methicillin-resistant and vancomycin-resistant strains is generating an enormous public health concern. S. aureus infections are usually hospital-acquired, although the incidence of community-acquired infections has been increasing in recent years.9,10,11,12 These pathogens are capable of causing severe and even fatal infections with limited options for therapy. Furthermore, such infections occur predominantly among persons without any known risk factors for acquisition of the infection.9,12,13,14 The reasons for the variability in human susceptibility to S. aureus are still unknown.
Bacterial infections are the result of complex interactions between invading bacteria and host defense mechanisms. A striking feature of most infections in human populations is their considerable interindividual phenotypic variability, ranging from asymptomatic to lethal infections. Virulence factors of the invading organism and genetic factors that influence the ability of the host to mount an appropriate immune response against the pathogen together determine the outcome of infection. Genetically determined variations in the immune system can make some individuals more susceptible to infection, or to develop a different form of disease, than others. Understanding the mechanisms underlying disease resistance or susceptibility is essential for the development of rational therapeutic interventions for many of these diseases.
Human resistance to infection is in general a multifactorial trait involving multiple gene interactions and strong environmental influences. Because of this, the laboratory mouse has been the experimental model of choice to study pathogenesis of infection, including innate and acquired host defense mechanisms. Inbred mouse strains differ significantly in their degree of susceptibility to infection with various human bacterial (eg, Mycobacterium tuberculosis,15,16 Salmonella enterica,17 Streptococcus pyogenes,18 Streptococcus pneumoniae19), fungal (eg, Histoplasma capsulatum,20 Aspergillus fumigatus21), protozoan (eg, Leishmania major,22 Plasmodium berghei,23 Plasmodium chabaudi24), helminthic (eg, Schistosoma mansoni25,26) as well as viral (eg, respiratory syncytial virus27,28) pathogens. This attribute has been exploited to identify novel loci influencing resistance/susceptibility to infection and to provide new insight on host mechanisms involved in response to those pathogens that ultimately affect the onset, progression, and outcome of the infection.
The aim of the study presented here is to uncover the immune mechanisms contributing to natural resistance to S. aureus using a murine model of infection. Identifying genetically regulated host immune responses is the first step in understanding the molecular targets and immunological mechanisms on which rational therapies to augment host resistance against pathogens can be developed.
Materials and Methods
Bacterial Strains
The rsbU+ derivative SH1000 of strain 8325-4, that has been shown to cause severe septic arthritis in NMRI mice and express low levels of exoproteins in a similar way to that observed for many clinical isolates was used in this study.29,30 Additionally, for in vivo bioluminescence analysis the previously described genetically engineered bioluminescent S. aureus strain SH1000 ALC2906 was used.31 Bacteria were grown to the stationary phase (15 hours) at 37°C with shaking (125 rpm) in brain-heart infusion medium, collected by centrifugation for 10 minutes at 3000 rpm, washed twice with sterile phosphate-buffered saline (PBS), and adjusted to a concentration of 5 × 108 cfu/ml. Aliquots of the S. aureus-suspension were frozen at −80°C for further use. The bacterial suspensions were further diluted with PBS to the required concentration and the number of viable bacteria (cfu) was determined after serial diluting and plating on blood agar containing 5% sheep blood (Invitrogen, Karlsruhe, Germany).
Mice
Naive inbred, specific pathogen-free (SPF status), 8- to 12-week-old mice were purchased from Harlan Winkelmann (Borchen, Germany): A/J OlaHsd (H-2a), BALB/c OlaHsd (H-2d), C57BL/6 JOlaHsd (H-2b), C57BL/10 ScSnOlaHsd (H-2b), C3H/HeNHsd (H-2k), CBA/J OlaHsd (H-2k), DBA/2 OlaHsd (H-2d). The B-, T-, and NK cell-deficient Rag2-IL-2Rγ−/− C57BL/6 mice were kindly provided by Werna Müller (Helmholtz Center for Infection Research, Braunschweig, Germany). These mice carry a deletion in Rag2 and the interleukin 2 receptor γ-chain (IL-2Rγ) rendering them deficient in B, T, and NK cell differentiation and function. Mice were maintained under standard conditions and according to institutional guidelines. All experiments were approved by the appropriate ethical board (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg, Germany).
Infection Model
Mice were inoculated with 4 × 107 cfu of S. aureus in 0.2 ml of PBS via a lateral tail vein. For determination of bacterial loads (cfu), infected mice were sacrificed by CO2 asphyxiation at different times of infection and the amount of bacteria determined by preparing kidney homogenates in 5 ml of PBS and plating 10-fold serial dilutions on blood agar. Bacteria colonies were counted after incubation for 24 hours at 37°C. For collection of plasma or serum, citrated or untreated blood, respectively, were collected at time of sacrifice, centrifuged at 4000 rpm for 10 minutes, and frozen at −80°C until use for further analysis.
Monitoring Bioluminescent S. aureus SH1000 in Infected Mice
Mice were intravenously infected with bioluminescent S. aureus strain SH1000 ALC2906,31 anesthetized via inhalation of Isoflurane (Isoba; Essex Tierarznei, München, Germany) and analyzed with the Xenogen Vivo Vision IVIS 200 system (Xenogen Corp., Hopkinton, MA). The amount of replicating S. aureus in infected mice was shown by the color scale overlaid on a gray-scale photograph of the infected animal.
Histopathological Examination
Kidneys or lungs of mice were fixed with 10% buffered neutral formalin solution, embedded in paraffin, and then cut into 3-μm-thick sections. Tissue sections were stained with hematoxylin and eosin (H&E) and then examined microscopically using an Olympus BX51 microscope (Olympus Europe GmbH, Hamburg, Germany) for pathological alterations.
Determination of Urea in Serum
Serum levels of urea were measured using the analyzer Olympus AU400 (Olympus Europe GmbH) according to the manufacturer’s instructions.
Measurement of Contact Activation
Activation of the intrinsic coagulation system (contact activation) was measured by incubating 50 μl of citrated plasma with 50 μl of aPTT reagent (aPTT Automate; Diagnostica Stago, Asnieres, France) for 60 seconds at 37°C. Clotting was initiated by the addition of 50 μl of a 25-mmol/L CaCl2 solution and activated partial thromboplastin time (aPTT) was measured using an Amelung coagulometer (Amelung, Lemgo, Germany). Levels of bradykinin in plasma were determined by enzyme-linked immunosorbent assay (MARKIT-M-Bradykinin; Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) according to the recommendations of the manufacturer.
Electron Microscopy
For scanning electron microscopy samples were fixed with 5% formaldehyde and 2% glutaraldehyde in cacodylate buffer (0.1 mol/L cacodylate, 0.01 mol/L CaCl2, 0.01 mol/L MgCl2, pH 6.9) for 1 hour on ice and washed in TE buffer (20 mmol/L Tris, 1 mmol/L ethylenediaminetetraacetic acid, pH 7.0). Samples were dehydrated with a graded series of acetone (10, 30, 50, 70, 90, 100%) and critical-point dried with CO2 (CPD030; Bal-Tec, Liechtenstein). Dried samples were then fractured and sputter-coated with gold (SCD500, Bal-Tec) before examination in a field emission scanning electron microscope DSM982 Gemini (Zeiss, Oberkochen, Germany) at 5 kV using the Everhart-Thornley secondary electron detector and the in-lens secondary electron detector in a 50:50 ratio. Images were recorded onto a MO-disk and contrast and brightness was adjusted with Adobe Photoshop 9.0.
Depletion or Inactivation of Neutrophils
Rat anti-mouse RB6 antibody specific for murine neutrophils and eosinophils32 was a kind gift from Siegfried Weiss (HZI, Braunschweig, Germany). Depletion of neutrophils by using anti-mouse RB6 antibodies was performed as previously described.33 Briefly, mice received an intravenous injection of 100 μg of anti-RB6 antibodies 1 day before bacterial inoculation. Control mice received equivalent amounts of isotype control antibodies in sterile PBS. The neutropenia was confirmed by collecting citrated blood at 24 and 48 hours after inoculation of the antibody. After lysis of erythrocytes, absence of neutrophils in blood was confirmed by staining of cells with fluorescein isothiocyanate-conjugated anti-RB6 antibodies (Serotec, Oxford, UK) and subsequent flow cytometry analysis.
Inhibition of Neutrophil Migration
For blockage of neutrophil migration from blood into infected tissue, mice were treated intravenously with 50 μg of both rat anti-mouse CD11b and anti-mouse CD18 antibodies (Leinco Technologies, Inc. St. Louis, MO) 4 hours before infection. Control mice received respective amounts of isotype control antibodies in sterile PBS. Blockage of neutrophil recruitment was verified by quantification of neutrophil recruitment into the peritoneal cavity after S. aureus challenge. For this purpose, mice were injected intraperitoneally with 4 × 107 cfu S. aureus SH1000 in 200 μl of PBS 4 hours before the analysis. Mice were then euthanized, the peritoneal cavity lavaged, collected exudates stained with fluorescein isothiocyanate-conjugated anti-RB6 antibodies, and subjected to flow cytometric analysis.
Gentamicin Protection Assay
To investigate the ability of peritoneal neutrophils to kill S. aureus, mice were injected intraperitoneally with 1 mg of carrageenan (type IV λ; Sigma, St. Louis, MO) 4 and 2 days before infection to induce macrophage depletion and infiltration of neutrophils in the peritoneal cavity. Mice were then intraperitoneally infected with 4 × 107 cfu of S. aureus SH1000 for 1 hour, sacrificed by CO2 inhalation and subjected to peritoneal lavage using Dulbecco’s modified Eagle’s medium. Peritoneal exudates were washed with sterile PBS, centrifuged at 1000 rpm for 10 minutes, resuspended in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 100 μg/ml of gentamicin, seeded in 24-well tissue culture plates at a density of 106 cells/well, and further incubated for 2 hours at 37°C and 5% CO2. Immediately (0 hours) or 1 hour thereafter, neutrophils were collected by centrifugation, washed with PBS, and disrupted with sterile water to release intracellular bacteria. The resulting suspension was serially diluted and bacterial numbers were determined after plating onto blood agar.
Cytokine and Chemokine Determination
Serum cytokine levels were determined by a solid phase sandwich immunoassay using the Mouse Cytokine Twenty Plex LMC0006 (Biosource Europe, Nivelles, Belgium) in conjunction with the 100 IS Total System (Luminex, Oosterhout, The Netherlands) according to the recommendations of the manufacturer. The levels of chemokines KC and MCP-1 were determined in kidney tissue after homogenization in lysis buffer (200 mmol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L Tris, 10% glycerol, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml leupeptide, and 28 μg/ml aprotinin, pH 7.4) at a concentration of 50 mg of tissue/ml. Tissue homogenates were centrifuged twice at 1500 × g for 15 minutes at 4°C and supernatants were stored at −80°C until use for further analysis. KC and MIP-2 were determined using the respective R&D Systems DuoSet Elisa Development Systems (R&D Systems, Minneapolis, MN) according to the recommendations of the manufacturer.
RNA Isolation, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), and Quantitative Real-Time PCR
Total RNAs were isolated from kidney tissue of infected and uninfected A/J and C57BL/6 mice after homogenization in RLT buffer by using a RNeasy Midi kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. cDNA synthesis was performed using a Gibco RT-PCR kit (Invitrogen) following the manufacturer’s instructions. The β-actin gene was used as an internal reference gene for RT-PCR. Rsp-9 was used as a housekeeping gene for quantitative real-time PCR. For the RT-PCR, the following oligonucleotides and annealing temperatures were used to generate the specific PCR products: for β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (58°C, 318 bp); for rsp-9, 5′-CTGGACGAGGGCAAGATGAAGC-3′ and 5′-TGACGTTGGCGGATGAGCACA-3′ (58°C, 143 bp); for KC 5′-GCTGGGATTCACCTCAAGAA-3′ and 5′-AGGTGCCATCAGAGCAGTCT-3′ (55°C, 206 bp); for MIP-2, 5′-ACCAACCACCAGGCTACAG-3′ and 5′-GCGTCACACTCAAGCTCT-3′ (54°C, 109 bp). Cycling conditions for PCR amplifications were 15 seconds at 94°C, 30 seconds annealing, and 45 seconds at 72°C for 32 cycles.
For quantitative real-time PCR, amplification was performed using a LightCycler 480 real-time PCR system (Roche Applied Science, Mannheim, Germany) and SYBR green PCR master mix (Roche). For each gene, a standard curve was constructed by using plasmids into which the full-length cDNA was cloned and subsequently subcloned into TOPO PCR2.1 cloning vector (Invitrogen). The resulting plasmids were prepared using a QIAprep Spin miniprep kit (Qiagen) following the manufacturer’s instructions and used for the generation of standard curves. Cycle threshold values for MIP-2 or KC were normalized to that of the housekeeping gene Rsp-9. Data are expressed as relative mRNA expression levels.
Statistical Analysis
Data were analyzed by using Excel 2000 (Microsoft Office; Microsoft, Redmond, WA) or GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Unless otherwise specified, all data are presented as mean ± SD. Each in vitro experiment was performed independently at least three times and within each experiment all samples were processed in duplicate. Comparison between groups was made by use of a variance analysis (F-test). Comparison of survival time curves was performed by use of log rank test. P values ≤0.05 were considered as significant.
Results
Different Inbred Mouse Strains Strongly Vary in Their Level of Susceptibility to Intravenous Infection with S. aureus
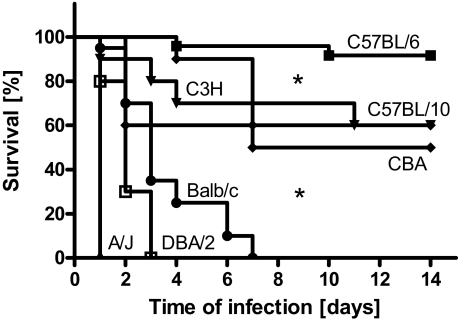
To determine the influence of the genetic background in susceptibility of mice to S. aureus infection, the survival times of mice from seven different inbred strains inoculated with 4 × 107 cfu of S. aureus SH1000 was determined throughout a period of 14 days. As shown in Figure 1, A/J, DBA/2, and BALB/c mice were very susceptible to S. aureus, exhibiting 100% mortality within 1 week of infection. Among these strains, the A/J mice were the most susceptible and all animals died at 24 hours after bacterial inoculation. In contrast, C57BL/6 was the most resistant mouse strain with only sporadic mortality (15%) observed in long-term experiments (up to 4 weeks). C3H/HeN, CBA, and C57BL/10 mice exhibited intermediate susceptibility and ∼50% of the mice survived the infection. Differences in survival times were statistically significant (P < 0.05) between the resistant, intermediate, and susceptible strains but not among strains belonging to the same group.
Figure 1.
Survival of C57BL/6, C57BL/10, C3H/HeN, CBA, BALB/c, DBA/2, and A/J mice after intravenous infection with 4 × 107 cfu of S. aureus strain SH1000. Each group contained a minimum of 10 mice. Comparison of survival curves was performed by use of log rank test. *P < 0.05.
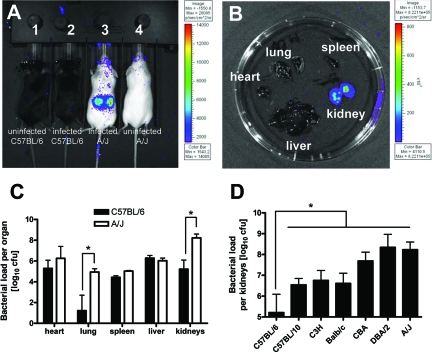
To determine whether the survival advantage of resistant C57BL/6 mice is related to a better capacity to control bacterial growth, C57BL/6 and highly susceptible A/J mice were infected with a genetically engineered bioluminescent strain of S. aureus SH1000 and bioluminescence of live, actively metabolizing bacteria was measured at 24 hours after bacteria inoculation using the Xenogen Vivo Vision IVIS 200 system. Whereas luminescence in C57BL/6 mice was below detection levels (Figure 2A), a very intense bioluminescent signal was recorded in kidneys of infected A/J mice (Figure 2B), the previously reported organ target of S. aureus in this model of infection.29,34,35,44,45,46 Quantification of bacterial loads in different organs isolated from susceptible A/J and resistant C57BL/6 mice at 24 hours of infection confirmed the significantly higher amount of bacteria in the kidneys of A/J mice (Figure 2C). In addition to the kidneys, significantly higher amount of S. aureus were detected in the lungs of A/J than in the lungs of C57/BL6 mice (Figure 2C), although the amount of bacteria was too low to be detected using the bioluminescence system (Figure 2B). Determination of the bacterial loads in the kidneys of several other inbred strains of mice at 24 hours after inoculation shows that susceptible or intermediate mouse strains had significantly higher bacterial burden in kidneys compared to resistant C57BL/6 mice (Figure 2D). These results suggest that resistance of C57BL/6 mice to S. aureus infection is associated with a superior capacity of these mice to control bacterial growth after intravenous inoculation. For further studies, A/J and C57BL/6 were chosen as representative susceptible and resistant mouse strains, respectively.
Figure 2.
A: Bacterial loads in resistant C57BL/6 (mouse 2) and susceptible A/J mice (mouse 3) infected for 24 hours with bioluminescent S. aureus strain SH1000 ALC2906. Uninfected C57BL/6 (mouse 1) and A/J (mouse 4) were used for comparison. B: Bacterial loads in systemic organs (heart, lung, spleen, kidney, or liver) isolated from a susceptible A/J mouse at 24 hours after bacterial inoculation. Visualization of luminescent bacteria was performed using the Xenogen Vivo Vision IVIS 200 system. C: Bacterial loads in systemic organs of C57BL/6 and A/J mice at 24 hours after intravenous infection with 4 × 107 cfu of S. aureus SH1000 determined after serial plating of the organ’s homogenates. D: Bacterial loads in kidneys of C57BL/6, C57BL/10, C3H/HeN, BALB/c, CBA, DBA/2, and A/J mice at 24 hours after intravenous infection with 4 × 107 cfu of S. aureus SH1000 determined after serial plating. Bars represent the mean ± SD of five mice per group. *P < 0.05, by F-test.
A/J Mice Develop More Severe Kidney and Lung Pathology after Intravenous Inoculation with S. aureus than C57BL/6 Mice
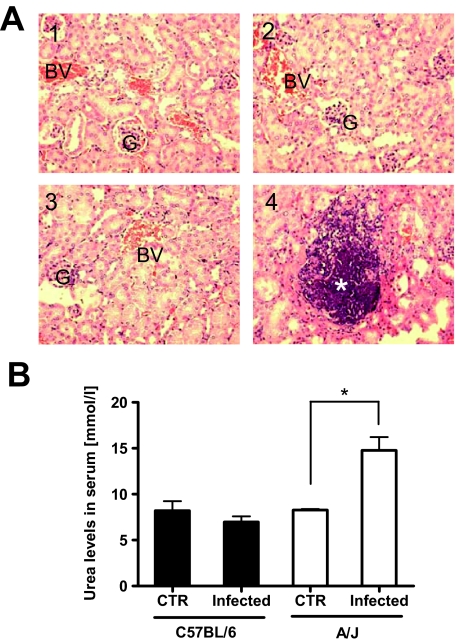
Because the kidney was the organ target for S. aureus in this model of infection, we performed histopathological evaluation of this organ from infected C57BL/6 and A/J mice to determine the extent of S. aureus-induced organ damage. Kidney tissues from uninfected C57BL/6 (Figure 3A1) and A/J (Figure 3A3) mice were included for comparison. Examination of kidney sections obtained from S. aureus-infected mice at 24 hours after bacteria inoculation revealed no signs of histological damage in C57BL/6 mice (Figure 3A2). In contrast, a diffuse and confluent inflammation with extensive areas of tissue destruction containing abundant bacteria was observed in the kidneys of infected A/J mice (Figure 3A4). In good correlation with the histopathological evaluation, A/J mice exhibited significantly higher levels of serum urea, a marker for renal dysfunction, than C57BL/6 mice at 24 hours after S. aureus inoculation (Figure 3B).
Figure 3.
A: Representative H&E-stained kidney sections collected from uninfected (A1, A3) or S. aureus-infected (A2, A4) C57BL/6 (A1, A2) or A/J (A3, A4) mice at 24 hours of infection with S. aureus. Glomeruli are indicated by G, blood vessels by BV, and bacteria by a white asterisk. B: Levels of urea in serum of infected or uninfected C57BL/6 (black bars) or A/J (white bars) mice at 24 hours of infection with S. aureus. *P < 0.05, by F-test. Original magnifications, × 200.
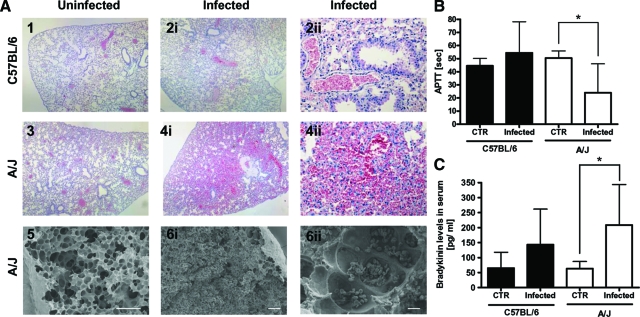
The general dysfunction of the endothelium characterized by microvascular permeability plays a central role in the pathogenesis of sepsis.36 The extent of vascular permeability in S. aureus-infected C57BL/6 and A/J mice was determined by histopathological examination of lung tissue. The alveolar architecture of lungs from uninfected C57BL/6 or A/J mice is shown in Figure 4, A1 and A3, respectively. Although widespread hemorrhages and massive erythrocyte infiltration of lung parenchyma and bronchioles were observed in lung sections of S. aureus-infected A/J mice (Figure 4A4), the lungs of infected C57BL/6 mice were unaffected (Figure 4A2). Scanning electron microscopy of lung tissue obtained from A/J mice confirmed the presence of massive erythrocyte infiltration into the alveoli and bronchioli in response to infection (Figure 4A6). Therefore, signs of endothelium dysfunction indicated by high levels of microvascular permeability were only evidenced in S. aureus-infected A/J but not in C57BL/6 mice.
Figure 4.
A: Representative light micrograph (H&E-stained) (A1 to A4) and scanning electron micrograph (A5 and A6) of lung tissue sections of uninfected (left) or S. aureus-infected (middle and right) C57BL/6 and A/J mice. The lungs of infected C57BL/6 mice were unaffected (A2) and show similar alveolar architecture as lung tissue from uninfected mice (A1, A3). Widespread hemorrhages and massive erythrocyte infiltration of lung parenchyma and bronchioles were observed in lung sections of S. aureus-infected A/J mice (A4, A6). B: Activated partial thromboplastin time (aPTT) in plasma. C: Bradykinin release in serum of C57BL/6 (black bars) and A/J (white bars) mice at 24 hours after intravenous infection with 4 × 107 cfu S. aureus. Bars represent the mean ± SD of five mice per group. *P < 0.05, by F-test. Scale bars: 100 μm (A5, A6i); 10 μm (A6ii). Original magnifications: × 40 (A1, A2i, A3, A4i); × 200 (A2ii, A4ii).
Activation of the Contact System in A/J but Not in C57BL/6 Mice in Response to S. aureus Infection
Because bradykinin is one of the most potent endogenous vasoactive peptides37 and previous studies have shown that S. aureus can induce the release of bradykinin by activating the contact system, also known as the intrinsic pathway of coagulation,38 we hypothesized that activation of the contact system in the A/J and the concomitant release of bradykinin may play an important role in the induction of the observed vascular leakage. To test this hypothesis, we determined the extent of contact activation in S. aureus-infected A/J and C57BL/6 mice by measuring the activated partial thromboplastin time (aPTT). As expected, aPTT was significantly shortened in A/J than in C57BL/6 mice at 24 hours after infection (Figure 4B) indicating a considerable activation of the intrinsic coagulation pathway. Accordingly, the levels of bradykinin were significantly higher in the serum of S. aureus-infected A/J mice than in serum of C57/BL/6 mice (Figure 4C). These higher levels of released vasoactive bradykinin in infected A/J mice may be responsible for the vascular leakage and hemorrhages observed in the lungs of these mice as already previously shown after intravenous Salmonella infections.39
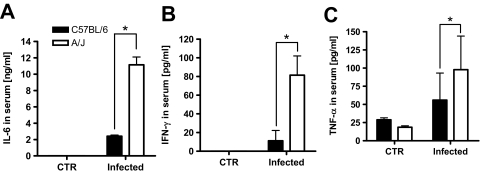
S. aureus-Infected A/J Mice Exhibit Higher Levels of Inflammatory Cytokines in Serum than Infected C57BL/6 Mice
Overproduction of inflammatory cytokines has been shown to contribute to severity of several bacterial infections.40,41 Analysis of the serum cytokine levels at 24 hours of infection by Luminex technology revealed significantly higher levels of inflammatory cytokines such as IL-6 (Figure 5A), interferon-γ (Figure 5B), and tumor necrosis factor-α (Figure 5C) in the serum of A/J mice than in the serum of C57BL/6 mice.
Figure 5.
Levels of IL-6 (A), interferon-γ (B), and tumor necrosis factor-α (C) in serum of uninfected or infected C57BL/6 (black bars) and A/J mice (white bars) at 24 hours of infection with 4 × 107 cfu of S. aureus. Bars represent the mean ± SD of three mice per group. *P < 0.05, by F-test.
The Resistance of C57BL/6 Mice to S. aureus Is Mediated by Innate Immune Mechanisms
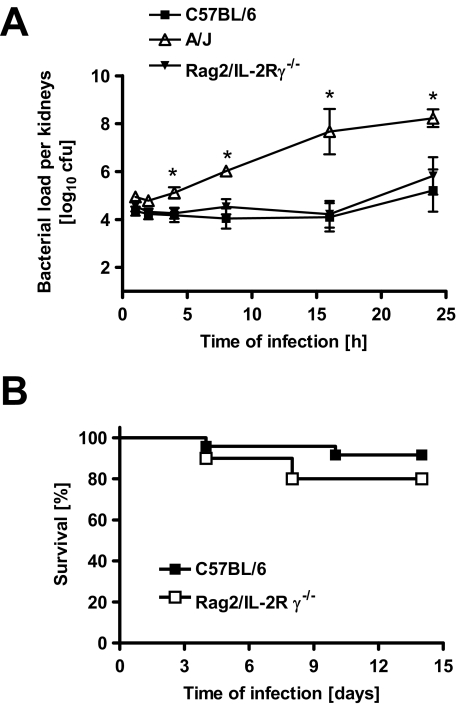
To determine whether the different levels of susceptibility exhibited by A/J and C57BL/6 mice to S. aureus was associated with an unequal hematogenous bacterial seeding in the kidneys after intravenous injection, bacterial loads in kidneys of A/J and C57BL/6 mice were compared starting at 1 hour after intravenous S. aureus inoculation. Results in Figure 6A show comparable bacterial loads in kidneys of A/J and C57BL/6 mice at 1 hour and 2 hours after bacterial inoculation indicating a similar bacterial seeding in both mouse strains. However, although C57BL/6 mice were fully able to keep bacterial growth under control in the later time points, S. aureus grew progressively in A/J mice (Figure 6A). At 4 hours of infection, significantly higher amounts of bacteria (10-fold) were detected in kidneys of A/J compared with C57BL/6 mice. These results indicate that the superior capacity of C57BL/6 mice over that of A/J mice to control bacterial growth is visible during the very early stage of infection. These early differences in the control of bacterial growth suggest that innate immune effector mechanisms might be particularly important for resistance to S. aureus.
Figure 6.
A: Kinetics of bacterial growth in the kidneys of C57BL/6, Rag2/IL-2Rγ−/− C57BL/6, and A/J mice after intravenous infection with S. aureus. Each point represents the mean ± SD of five mice per group. *P < 0.05, by F-test. B: Survival times of immunocompetent C57BL/6 and Rag2/IL-2Rγ−/− C57BL/6 mice after intravenous infection with 4 × 107 cfu S. aureus. Each group contained a minimum of 10 mice. Comparison of survival curves was performed by use of log rank test. *P < 0.05.
To confirm this hypothesis, Rag2/IL-2Rγ−/− C57BL/6 mice, which are deficient in B, T, and NK cells, were compared with the parental C57BL/6 mice for their resistance to infection with S. aureus. The obtained results show that Rag2/IL-2Rγ−/− mice were as resistant as C57BL/6 mice in terms of control of bacterial growth in the kidneys (Figure 6A) as well as survival (Figure 6B). These results confirm that the mechanisms of disease resistance in C57BL/6 mice are independent of adaptive immunity and mediated by the innate immune system.
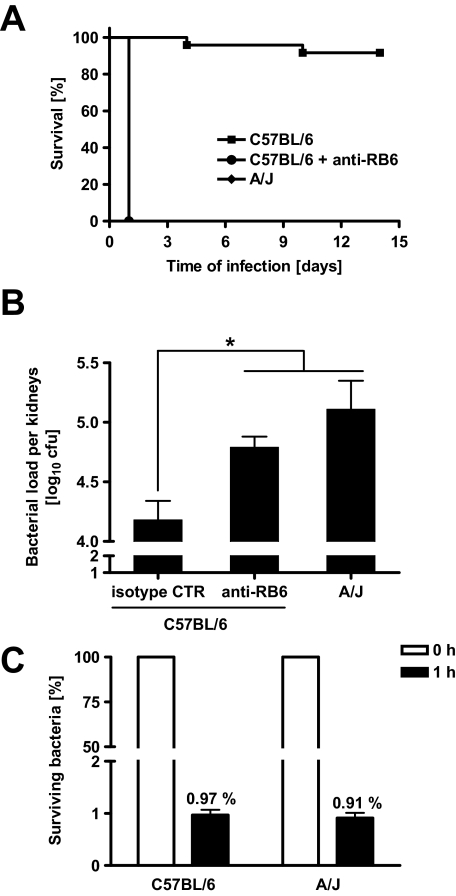
Neutrophil Depletion Rendered C57BL/6 Mice Fully Susceptible to S. aureus
During the acute phase of a local inflammatory response, peripheral blood neutrophils are the first responder cells to arrive in significant numbers, followed by mononuclear cells. To determine the role of neutrophils in resistance of C57BL/6 mice to S. aureus infection, we depleted neutrophils in these mice by injecting anti-RB6 antibodies. Depletion of neutrophils rendered C57BL/6 mice as susceptible as A/J mice to S. aureus infection as shown by the 100% mortality by 24 hours of infection (Figure 7A) and significantly higher bacterial loads in kidneys as early as 4 hours after bacterial inoculation (Figure 7B). Similar results were obtained when migration of neutrophils from the bloodstream into infected tissue was inhibited by treating C57BL/6 mice with anti-CD11b/CD18 antibodies (data not shown). These results suggest that neutrophils are required to control bacterial growth and to promote a protective response against S. aureus infection in C57BL/6 mice. To determine whether the susceptibility exhibited by A/J mice to S. aureus was attributable to an intrinsic defect of their neutrophils to kill this bacterium, we compared the capacity of neutrophils isolated from A/J mice with that of neutrophils from C57BL/6 mice to kill S. aureus in vitro. As shown in Figure 7C, the bactericidal activity of neutrophils isolated from both A/J and C57BL/6 mice was indistinguishable.
Figure 7.
A: Survival times of susceptible A/J mice, neutropenic C57BL/6 mice that were treated intravenously with anti-RB6 antibodies (100 μg, 1 day before infection) to deplete neutrophils, and immunocompetent C57BL/6 mice after intravenous infection with 4 × 107 cfu of S. aureus. B: Bacteria loads in kidneys of susceptible A/J mice, neutropenic C57BL/6 mice, and immunocompetent isotype control-treated C57BL/6 mice at 4h after intravenous infection with 4 × 107 cfu of S. aureus. Bars represent the mean ± SD of five mice per group. C: Capacity of neutrophils isolated from A/J or C57BL/6 mice to kill S. aureus. Carrageenan-treated mice were intraperitoneally infected with 4 × 107 cfu of S. aureus SH1000 for 1 hour. Peritoneal neutrophils were collected, resuspended in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 100 μg/ml of gentamicin to kill all extracellular bacteria, and further incubated for 2 hours at 37°C and 5% CO2. At 0 (100%) and 1 hour thereafter, neutrophils were disrupted with sterile water to release and quantify intracellular surviving bacteria. Bars represent the mean ± SD of three individual mice. *P < 0.05.
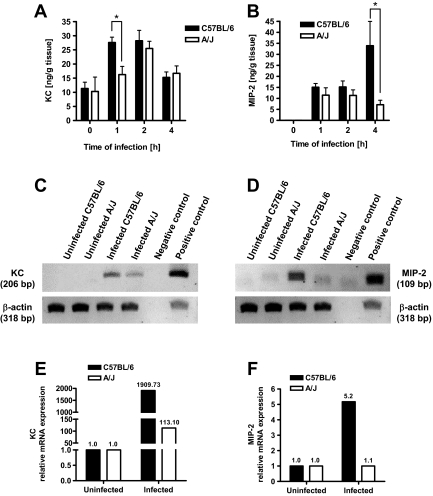
The observation that neutrophils from A/J mice did not display an intrinsic defect led us to hypothesize that recruitment of neutrophils in response to S. aureus infection might be impaired or delayed in A/J in respect to C57BL/6 mice. Because the regulation of neutrophil recruitment to sites of S. aureus infection is mediated, at least in part, by the production of chemokines such as KC and MIP-2,42 we determined the time course of KC and MIP-2 expression in kidney tissue of S. aureus-infected A/J and C57BL/6 mice during the very early phase of infection. As shown in Figure 8A, KC was very strongly induced at 1 hour after bacterial challenge in C57BL/6 mice, displayed a sustained production from 1 to 2 hours, and declined after 4 hours. The induction of KC in A/J mice, however, is slower and peaked at 2 hours of infection (Figure 8A). Therefore, C57BL/6 mice already exhibited significantly higher levels of KC in their kidneys than A/J mice at 1 hour after bacterial challenge. This difference may result in earlier neutrophil recruitment in C57BL/6 mice.
Figure 8.
Time course of KC (A) and MIP-2 (B) expression in kidney homogenates of uninfected or S. aureus-infected C57BL/6 (black bars) and A/J (white bars) mice after intravenous infection with 4 × 107 cfu of S. aureus. Bars represent the mean ± SD of five mice per group. *P < 0.05, by F-test. C: Representative agarose gel of KC-transcript expression (top lane) compared to β-actin loading control (bottom lane) in uninfected or infected C57BL/6 and A/J mice at 1 hour of infection. Plasmids containing the sequences encoding KC or β-actin were used as positive controls. D: Representative agarose gel of MIP-2-transcript expression (top lane) compared to β-actin loading control (bottom lane) in uninfected or infected C57BL/6 and A/J mice at 4 hours of infection. Plasmids containing the sequences encoding MIP-2 or β-actin were used as positive controls. E: RT-PCR amplification of KC-cDNA from uninfected and S. aureus-infected (1 hour of infection) kidney tissue from C57BL/6 (black bars) and A/J mice (white bars). Results are expressed as fold-changes in the ratio of KC-RNA to housekeeping gene mRNA in S. aureus-infected compared to uninfected tissue. F: RT-PCR amplification of MIP-2-cDNA from uninfected and S. aureus-infected (4 hours of infection) kidney tissue from C57BL/6 (black bars) and A/J mice (white bars). Results are expressed as fold-changes in the ratio of MIP-2-RNA to housekeeping gene mRNA in S. aureus-infected compared to uninfected tissue.
Interestingly, MIP-2 displayed a different expression pattern. As shown in Figure 8B, MIP-2 is weakly induced in both C57BL/6 and A/J mice during the first 2 hours after challenge and displayed a strong increase at 4 hours after inoculation only in C57BL/6 mice. To determine whether the differences in the expression of KC and MIP-2 observed in the kidneys of C57BL/6 and A/J mice at 1 hour and 4 hours after infection, respectively, are reflected by differences in the induction of the corresponding gene, the levels of mRNA in kidney tissue of infected animals were determined by RT-PCR and quantitative real-time PCR. The obtained results demonstrated that kidney tissue from infected C57BL/6 mice expressed significantly higher levels of mRNA coding for KC at 1 hour of infection (Figure 8, C and E) and MIP-2 at 4 hours of infection (Figure 8, D and F) than kidney tissue obtained from A/J mice at the corresponding time of infection.
Discussion
A better understanding of the pathogenesis of S. aureus infection has become increasingly relevant, particularly in the face of newly emerging epidemic clones as well as the increasing prevalence of multidrug-resistant strains. A prominent feature of S. aureus infections is their interindividual phenotypic variability, ranging from mild to lethal clinical manifestations. Common genetically determined variations in the immune system may contribute to the observed heterogeneity in the host response to this pathogen. Uncovering the immunological and molecular basis for these differences will provide useful insight into the natural immunity against S. aureus and aid the development of more efficient therapies and more useful vaccines.
Unfortunately, studies in human populations are extremely difficult and biased by population heterogeneity, environmental factors, and the lack of suitable controls. In this regard, progress in dissecting the mechanisms and identifying the genetic elements governing the host response to important pathogens have been made through the use of animal models of human disease.43 In the study presented here, we have used a mouse model of infection to explore the immunological basis underlying the differential levels of natural resistance exhibited by different inbred strains of mice to S. aureus. Our results confirmed a significant interstrain variation in resistance to this pathogen, with one mouse strain exceptionally resistant and able to survive infection (C57BL/6 mice), other strains such as C3H/HeN, CBA, and C57BL/10 mice exhibiting intermediate resistance (50 to 60% survival), and three strains (A/J, DBA/2, and BALB/c mice) highly susceptible with 100% mortality during the first week of infection. The levels of resistance to S. aureus significantly correlated with the capacity of the different mouse strains to limit bacterial growth in the kidney, previously reported to be the organ target for S. aureus in this model of systemic infection.29,34,35,44,45,46
Shortly after intravenous inoculation with S. aureus, the inability of susceptible A/J mice to destroy S. aureus caused an overwhelming systemic bacterial infection that led to severe sepsis and death. Mortality of A/J mice in this model of S. aureus infection resulted from synergism of more than one pathogenic pathway including activation of the contact system and the concomitant release of vasoactive bradykinin, production of high levels of inflammatory cytokines, and finally, organ damage involving kidneys and lungs. In contrast, C57BL/6 mice exhibited a remarkable capacity to control bacterial growth and showed very limited signs of disease. A comparison of the time course of bacterial proliferation in C57BL/6 and A/J mice showed that the enhanced inhibition of bacterial growth observed in C57BL/6 mice occurred very early during infection. This raised the question about the potential contribution of innate immune mechanisms to the enhanced host defense against S. aureus observed in C57BL/6 mice. The findings that Rag2/IL-2Rγ−/− C57BL/6 mice, which are deficient in the expression of specific immunity, were also resistant to S. aureus infection confirmed this assumption.
Among the different cellular components of the innate immune response, neutrophils have been shown to be important for protection against a variety of pathogenic bacteria including S. aureus.47,48,49,50,51,52,53,54 In the human situation, there is also evidence that neutrophil deficiency augments the susceptibility to S. aureus infection. This is best illustrated by the predisposition of chronic granulomatous disease (CGD) patients to suffer from recurrent and often life-threatening S. aureus infection.55,56 CGD is an inherited disorder in which the leukocyte respiratory burst is absent or markedly deficient resulting in inadequate generation of highly reactive oxidants (eg, superoxide, hydrogen peroxide, and hypochlorous acid) necessary for microbicidal activity within the phagosome.57
Depletion of neutrophils using anti-RB6 antibodies or inhibition of neutrophil migration into infected sites after treatment of mice with anti-CD18/CD11b antibodies rendered C57BL/6 animals as susceptible as A/J mice to S. aureus. Depletion of neutrophils in C57BL/6 mice was associated with significantly increased bacterial numbers in the kidneys already evident during very early infection. Together, these results indicate that resistance to S. aureus in C57BL76 mice is critically dependent on an effective and fast recruitment of neutrophils to the site of infection. In the absence of neutrophils, other antimicrobial defense mechanisms were unable to kill the bacterial inoculum or to limit bacterial growth in these mice.
After confirming that neutrophils from A/J and C57BL/6 mice did not differ in their direct bactericidal effector functions against S. aureus, we hypothesized that A/J mice may have a neutrophil recruitment deficiency, thus explaining their inability to control infection. Neutrophil migration from the bloodstream to the site of infection is initiated when bacteria stimulate the resident tissue, including stromal, epithelial, and endothelial cells to secrete chemokines.58,59 Chemokines play a central role in mediating the signaling cascade that targets neutrophils to sites of infection by binding to receptors on the surface of these cells.60,61,62 The murine chemokines KC (CXCL-1) and MIP-2 (CXCL-2), which are the functional murine homologues of the human IL-8, are the major chemoattractants responsible for recruiting neutrophils and both bind to chemokine receptor CXCR2.63,64 Therefore, we compared the time course of KC and MIP-2 expression in the kidneys of C57BL/6 and A/J mice during the early phase of S. aureus infection. Our results shown that expression of KC and MIP-2 displayed different kinetic profiles. Thus, the S. aureus challenge set in motion a rapid expression of KC in C57BL/6 mice, with peak levels already achieved 1 hour after bacterial inoculation. By this time, the level of KC in the kidneys of A/J mice still remain low. Four hours after the challenge, peak levels of MIP-2 were already evidenced in the kidneys of C57BL/6 whereas the levels of MIP-2 did not raise at all in the kidneys of A/J mice. The different kinetic profiles observed in the expression of KC and MIP-2 could be explained by differences in the mechanism of regulation of their synthesis, the cellular source responsible for their production, or both. Thus, whereas resident cells were probably the main source of KC detected at 1 hour of infection, infiltrating cells could be the source of MIP-2 detected at 4 hours of infection. Because neutrophils play a major role in the clearance of S. aureus, the superior resistance of C57BL/6 mice is likely to be the result of a fast response of resident cells to S. aureus that rapidly induce a sequence of leukocyte recruitment events and brings under control the infectious pathogen during the very early phase of infection.
Because the incidence of multidrug-resistant S. aureus is increasing, it is clear that new strategies and tactics are needed to combat this insidious pathogen. Identification of the host immune components that are essential for host defense against S. aureus will lay a foundation on which rational therapies that augment natural host resistance might be designed.
Acknowledgments
We thank Angelika Höhne, Anna Link, Claudia Höltje, and Ina Schleicher for excellent technical assistance; and Oliver Goldmann and Reinhard von Wasielewski for helpful scientific discussions.
Footnotes
Address reprint requests to Dr. Eva Medina, Infection Immunology Research Group, Helmholtz Centre for Infection Research, Inhoffenstrasse 7, 38124 Braunschweig, Germany. E-mail: eva.medina@helmholtz-hzi.de.
Supported by the Bundesministeriums für Bildung und Forschung (Suszeptibilität bei Infektionen: SkinStaph grant FKZ: 01kl07104).
References
- Chang FY. Staphylococcus aureus bacteremia and endocarditis. J Microbiol Immunol Infect. 2000;33:63–68. [PubMed] [Google Scholar]
- Pittet D, Wenzel RP. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis. 1993;16:567–573. doi: 10.1093/clind/16.4.567. [DOI] [PubMed] [Google Scholar]
- Bone R. How Gram-positive organisms cause sepsis. J Crit Care. 1993;8:51–59. doi: 10.1016/0883-9441(93)90033-h. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Wagner H. Arthritis and sepsis caused by Staphylococcus aureus: can the tissue injury be reduced by modulating the host’s immune system? Mol Med Today. 1998;4:15–18. doi: 10.1016/S1357-4310(97)80540-0. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- McCormick JK, Yarwood Jr, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold B, Immergluck L, Maranan M, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- Berman D, Eisner W, Kreiswirth B. Community-acquired methicillin resistant Staphylococcus aureus infection. N Engl J Med. 1993;329:1896. doi: 10.1056/NEJM199312163292517. [DOI] [PubMed] [Google Scholar]
- Pate K, Nolan R, Bannerman T, Feldman S. Methicillin-resistant Staphylococcus aureus in the community. Lancet. 1995;346:978. doi: 10.1016/s0140-6736(95)91605-9. [DOI] [PubMed] [Google Scholar]
- Gorak E, Yamada S, Brown J. Community-acquired methicillin resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. doi: 10.1086/520437. [DOI] [PubMed] [Google Scholar]
- Hussain F, Boyle-Vavra S, Daum R. Community-acquired methicillin resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr Infect Dis J. 2001;20:763–767. doi: 10.1097/00006454-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Kallen A, Driscoll T, Thornton S, Olson P, Wallace M. Increase in community-acquired methicillin-resistant Staphylococcus aureus at a naval medical center. Infect Control Hosp Epidemiol. 2000;21:223–226. doi: 10.1086/501750. [DOI] [PubMed] [Google Scholar]
- Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego A, Peters LC, Jensen JR, Ribeiro OG, Koury Cabrera WH, Starobinas N, Seman M, Ibañez OM, De Franco M. Genetic determinants of acute inflammation regulate Salmonella infection and modulate Slc11a1 gene (formerly Nramp1) effects in selected mouse lines. Microbes Infect. 2006;8:2766–2771. doi: 10.1016/j.micinf.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Medina E, Goldmann O, Rohde M, Lengeling A, Chhatwal GS. Genetic control of susceptibility to group A streptococcal infection in mice. J Infect Dis. 2001;184:846–852. doi: 10.1086/323292. [DOI] [PubMed] [Google Scholar]
- Gingles NA, Alexander JE, Kadioglu A, Andrew PW, Kerr A, Mitchell TJ, Hopes E, Denny P, Brown S, Jones HB, Little S, Booth GC, McPheat WL. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect Immun. 2001;69:426–434. doi: 10.1128/IAI.69.1.426-434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield JA, Rine J. The genetic basis of variation in susceptibility to infection with Histoplasma capsulatum in the mouse. Genes Immun. 2007;8:468–474. doi: 10.1038/sj.gene.6364411. [DOI] [PubMed] [Google Scholar]
- Zaas AK, Liao G, Chien JW, Weinberg C, Shore D, Giles SS, Marr KA, Usuka J, Burch LH, Perera L, Perfect JR, Peltz G, Schwartz DA. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLOS Genet. 2008;4:e1000101. doi: 10.1371/journal.pgen.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelková H, Badalová J, Svobodová M, Vojtiková J, Kurey I, Vladimirov V, Demant P, Lipoldová M. Genetics of susceptibility to leishmaniasis in mice: four novel loci and functional heterogeneity of gene effects. Genes Immun. 2006;7:220–233. doi: 10.1038/sj.gene.6364290. [DOI] [PubMed] [Google Scholar]
- Delahaye NF, Coltel N, Puthier D, Barbier M, Benech P, Joly F, Iraqi FA, Grau GE, Nguyen C, Rihet P. Gene expression analysis reveals early changes in several molecular pathways in cerebral malaria-susceptible mice versus cerebral malaria-resistant mice. BMC Genom. 2007;8:452. doi: 10.1186/1471-2164-8-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin A, Stevenson MM, Gros P. Complex genetic control of susceptibility to malaria in mice. Genes Immun. 2002;3:177–186. doi: 10.1038/sj.gene.6363841. [DOI] [PubMed] [Google Scholar]
- Nino Incani R, Morales G, Cesari IM. Parasite and vertebrate host genetic heterogeneity determine the outcome of infection by Schistosoma mansoni. Parasitol Res. 2001;87:131–137. doi: 10.1007/pl00008565. [DOI] [PubMed] [Google Scholar]
- Van de Vijver KK, Colpaert CG, Jacobs W, Kuypers K, Hokke CH, Deelder AM, Van Marck EA. The host’s genetic background determines the extent of angiogenesis induced by schistosome egg antigens. Acta Trop. 2006;99:243–251. doi: 10.1016/j.actatropica.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Anh DB, Faisca P, Desmecht DJ. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol. 2006;291:L426–L435. doi: 10.1152/ajplung.00483.2005. [DOI] [PubMed] [Google Scholar]
- Stark JM, McDowell SA, Koenigsknecht V, Prows DR, Leikauf JE, Le Vine AM, Leikauf GD. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol. 2002;67:92–100. doi: 10.1002/jmv.2196. [DOI] [PubMed] [Google Scholar]
- Jonsson IM, Arvidson S, Foster S, Tarkowski A. Sigma factor B and Rsbu are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect Immun. 2004;72:6106–6111. doi: 10.1128/IAI.72.10.6106-6111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. Sigma factor B modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–92. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Bokarewa M, Collins LV, Gjertsson I, Hultgren OH, Jin T, Jonsson IM, Josefsson E, Sakiniene E, Verdrengh M. Current status of pathogenic mechanisms in staphylococcal arthritis. FEMS Microbiol Lett. 2002;217:125–132. doi: 10.1111/j.1574-6968.2002.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Collins LV, Tarkowski A. Animal models of experimental Staphylococcus aureus infection. Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Washington DC: ASM Press,; Gram-Positive Pathogens. 2006:pp 535–543. [Google Scholar]
- Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 2001;29:S21–S27. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- Joseph K, Kaplan AP. Formation of bradykinin: a major contributor to the innate inflammatory response. Adv Immunol. 2005;86:159–208. doi: 10.1016/S0065-2776(04)86005-X. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Herwald H, Cramer H, Persson K, Sjöbring U, Björck L. Staphylococcus aureus induces release of bradykinin in human plasma. Infect Immun. 2001;69:3877–3882. doi: 10.1128/IAI.69.6.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K, Mörgelin M, Lindbom L, Alm P, Björck L, Herwald H. Severe lung lesions caused by Salmonella are prevented by inhibition of the contact system. J Exp Med. 2000;192:1415–1424. doi: 10.1084/jem.192.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfgott DC, Tatter SB, Santhanam U, Clarick RH, Bhardwaj N, May LT, Sehgal PB. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infections. J Immunol. 1989;142:948–953. [PubMed] [Google Scholar]
- Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. Am J Med. 1991;91:23–29. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Gros P. Genetic control of susceptibility to bacterial infections in mouse models. Cell Microbiol. 2003;5:299–313. doi: 10.1046/j.1462-5822.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- Gorrill RH. Experimental staphylococcal infections in mice. Br J Exp Pathol. 1958;32:151–155. [PMC free article] [PubMed] [Google Scholar]
- Smith IM, Wilson AP, Hazard EC, Hummer WK, Dewey ME. Death from staphylococci in mice. J Infect Dis. 1960;107:369–378. [Google Scholar]
- Smith JM, Dubos RJ. The behaviour of virulent and avirulent staphylococci in the tissue of normal mice. J Exp Med. 1956;103:87–108. doi: 10.1084/jem.103.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–6167. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Bayer AS, Yeaman M. Cellular and extracellular defense against staphylococcal infections. Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Washington DC: ASM Press,; Gram-Positive Pathogens. 2006:pp 544–549. [Google Scholar]
- Ferrante A, Martin AJ, Bates EJ, Goh DHB, Harvey DP, Parson D, Rathjen DA, Russ G, Dayer J-M. Killing of Staphylococcus aureus by tumor necrosis factor-α-activated neutrophils. J Immunol. 1993;151:4821–4828. [PubMed] [Google Scholar]
- Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil-mediated mycobactericidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2002;70:5322–5327. doi: 10.1128/IAI.70.9.5322-5327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan JW. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa J, Saunders B, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SK, Gavrilescu LC, Alcaraz A, Dekers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin JI, Buescher ES. Abnormal regulation of inflammatory skin responses in male patients with chronic granulomatous disease. Inflammation. 1983;7:227–232. doi: 10.1007/BF00917259. [DOI] [PubMed] [Google Scholar]
- Muorah M, Hinds R, Verma A, Yu D, Samyn M, Mieli-Vergani G, Hadzic N. Liver abscesses in children: a single center experience in the developed world. J Pediatr Gastroenterol Nutr. 2006;42:201–206. doi: 10.1097/01.mpg.0000189344.23387.26. [DOI] [PubMed] [Google Scholar]
- Rae J, Newburger PE, Dinauer MC, Noack D, Hopkins PJ, Kuruto R, Curnutte JT. X-linked chronic granulomatous disease: mutations in the CYBB gene encoding the gp91-phox component of respiratory-burst oxidase. Am J Hum Genet. 1998;62:1320–1331. doi: 10.1086/301874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- García-Ramallo E, Marques T, Prats N, Beleta J, Kunkel SL, Godessart N. Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J Immunol. 2002;169:6467–6473. doi: 10.4049/jimmunol.169.11.6467. [DOI] [PubMed] [Google Scholar]
- Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biological characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Koy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]