Abstract
Foxp3+ CD4+ regulatory T (Treg) cells play a pivotal role in the maintenance of dominant self tolerance. Understanding how the failures of immune control by Treg cells are involved in autoimmune diseases is important for the development of effective immunotherapies. In the present study, we analyzed the characteristics of endogenous Treg cells in (NZB × NZW) F1 (BWF1) mice, a murine model of systemic lupus erythematosus. Unexpectedly, Treg number and frequency in aged BWF1 mice with developing lupus nephritis were increased, not decreased, and in vitro suppressive activity in lymphoid organs was intact. In addition, Treg cells trafficked to target organs because cells were present in the kidney and lung. Treg cells of aged BWF1 mice exhibited altered localization within lymph organs, however, and an altered phenotype, with higher expression levels of chemokine receptors and activation markers, suggesting a highly activated cellular state. Notably, the expression levels of co-stimulatory molecules were also markedly enhanced in the Treg cells of aged BWF1 mice. Furthermore, Treg cells of BWF1 mice did not show any suppressive effects on antibody production in vitro. Taken together, we conclude that Treg cells in BWF1 mice are not predisposed to functional incompetence but rather are present in a highly activated state.
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology characterized by a massive production of autoantibodies against various nuclear antigens. The deposit of immune complexes in the target organs, ie, skin, kidney, lung, joints, and central nervous system, is thought to cause fatal dysfunction of the body system. (NZB × NZW) F1 (BWF1) is a mouse strain that has been widely used as a model for SLE since the 1960s. These mice spontaneously develop severe autoimmune disease highly resembling human SLE in terms of serological and hematological abnormalities, and severe nephritis accompanying massive production of anti-nuclear antibodies.1
Reconstitution of SCID (severe combined immunodeficiency) mice with cultured pre-B cells of BWF1 mice recapitulates many symptoms of the disease of BWF1 mice. Cultured pre-B cells alone, however, are not sufficient to fully reproduce the disease.2 These data suggest that cellular subset(s) in addition to B cells are necessary for the development of the lupus-like syndrome of BWF1 mice, although abnormalities of the immune system predominantly lie within B cells. One of the possible candidates is CD4+ T cells, because depletion of CD4+ T cells with anti-CD4 antibody from 5 months old, slightly before the disease onset, prevents the development of the disease.3,4 CD4+ T cells are, therefore, also required for the development of the disease in BWF1 mice, possibly by providing help for the production of high-affinity autoantibodies.
Studies in this decade have clearly shown the key roles of naturally occurring regulatory T (Treg) cells in the maintenance of dominant self tolerance of the immune system.5 Treg cells in normal mice are mostly of thymic origin and are considered to be autoreactive T-cell clones that have bypassed negative selection by unknown mechanism(s).6 There also exists Treg cells of extra-thymic origin induced from conventional T cells during immune responses,7 although the underlying mechanisms of this process are still unclear. Foxp3, a member of forkhead-box family of transcription factors, is specifically expressed in the whole life of Treg cells and programs their functional properties.8,9,10 In contrast to the previously used marker CD25 or combination of CD25 and CD62L, expression of Foxp3 is specific for Treg cells, and thus can be used for the definitive identification of these cells.11 Immunoregulatory function of Treg cells is dependent on Foxp3 and genetic deficiency of Foxp3 causes fatal organ-specific autoimmune disease because of the lack of functional Treg cells.12,13,14 Furthermore, many groups have reported the reduced number and/or function of Treg cells in both organ-specific and systemic autoimmune diseases.15
A recent study has shown that the decreased frequency of Treg cells in the peripheral blood was associated with disease activity in SLE patients.16 Frequency of Treg cells identified as CD25+ CD62Lhi CD4+ T cells in the spleen was also decreased in aged BWF1 mice.17 Accordingly, adoptive transfer of in vitro-expanded Treg cells, or administration of histone-derived peptides or peptides derived from the complementarity-determining region 1 of anti-double-strand DNA immunoglobulin has been shown to ameliorate the disease in BWF1 mice by a mechanism involving Treg cells.17,18,19,20 These studies suggest that the function of endogenous Treg cells is, at least partially, abrogated by unidentified mechanisms in BWF1 mice.
Despite the effort to develop therapeutic methods involving Treg cells, their nature in BWF1 mice remains unclear. Here we performed a detailed characterization of Treg cells in BWF1 mice using Foxp3 as their marker. Our results demonstrated that aged BWF1 mice had increased frequency and number of Treg cells with apparently normal function, but with an activated phenotype including enhanced expression of co-stimulatory molecules and altered localization.
Materials and Methods
Mice
Female 6- to 8-week-old BWF1 and BALB/c mice were purchased from Japan SLC (Shizuoka, Japan) and were kept under specific pathogen-free conditions in the animal facility of our laboratory until analysis. Mice were used at 6 to 10 or 32 to 40 weeks of age as young or aged, respectively. All animal experiments were approved by the animal care committee of The University of Tokyo.
Antibodies
Monoclonal anti-mouse CD4 (clone RM4-5), CD5 (55-7.3), CD8α (53-6.7), CD11b (M1/70), CD16/32 (2.4G2), CD19 (1D3), CD23 (B3B4), CD25 (7D4), CD43 (S7), CD44 (IM7), CD45 (30-F11), CD45R (RA3-6B2), CD62L (MEL-14), CD69 (H1.2F3), CD90.2 (53-2.1), CD103 (M290), OX40 (OX-86), CXCR4 (2B11/CXCR4), CCR5 (C34-3448), NK1.1 (PK136), TER-119 (TER-119), and streptavidin were purchased from BD Biosciences (San Diego, CA); monoclonal anti-mouse 4-1BB (17B5), ICOS (7E.17G9), F4/80 (BM8), CCR7 (4B12), and Foxp3 (FJK-16s) were purchased from eBioscience (San Diego, CA); monoclonal anti-mouse CXCR3 (220803) was purchased from R&D Systems (Minneapolis, MN). Antiserum raised against mouse type IV collagen was purchased from LSL (Tokyo, Japan). Details of monoclonal anti-mouse CCR4 antibody (clone 2G11) will be described elsewhere by Nagakubo and colleagues.21,22
Cell Isolation
Single cell suspensions of the thymus, spleen, and lymph nodes were prepared by passing the tissue through a cell strainer (BD Bioscience). Single cell suspension of the kidney and lung were prepared by dissociating the tissue with collagenase D (Roche, Basel, Switzerland). Mononuclear cells in the kidney and lung were isolated from the single cell suspension by Percoll (Invitrogen, Carlsbad, CA) gradient centrifugation. CD25+ CD4+ T cells were isolated from the single cell suspension of various organs by magnetic enrichment of CD25+ cells followed by fluorescence-activated cell sorting with the Epics Altra cell sorter (Beckman Coulter, Fullerton, CA). CD25− CD4+ T cells were isolated from the single cell suspension of spleen by magnetic depletion of the cells positive for CD8α, CD11b, CD25, B220, CD138, NK1.1, or TER-119. B1 cells were isolated from peritoneal lavage cells by magnetic depletion of the cells positive for CD23, Thy-1.2, or F4/80. B2 cells were isolated from spleen by magnetic depletion of the cells positive for CD43, Thy-1.2, or TER-119. All procedures involving magnetic isolation were performed with an autoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany).
Flow Cytometry
Cells were incubated with fluorochrome- or biotin-labeled antibodies for 20 minutes at 4°C, following the blockade of FcγRII/III with unlabeled anti-CD16/32 for 10 minutes at 4°C; for the staining with biotin-labeled anti-CCR7, incubation after the blockade of Fc receptors was performed at 37°C. Biotin-labeled antibodies were visualized by further incubating with phycoerythrin-conjugated streptavidin for 15 minutes at 4°C. Staining of Foxp3 was performed according to the manufacturer’s instructions. Data were collected using BD LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Immunofluorescent Staining
Explanted tissues embedded in OCT compound were snap-frozen in liquid nitrogen and stored at −80°C until use. Six-μm-thick sections of frozen tissues were fixed with cold acetone for 10 minutes and rehydrated with phosphate-buffered saline (PBS) for 10 minutes at room temperature. Rehydrated sections were blocked for nonspecific binding of proteins with Blocking One (Nacalai Tesque, Kyoto, Japan) for 20 minutes at room temperature and incubated with unlabeled or biotinylated antibodies, or antisera for 60 minutes at room temperature. Sections were then incubated with Alexa Fluor-labeled anti-Ig secondary antibodies or streptavidin (Invitrogen) for 30 minutes at room temperature. After the staining, sections were fixed with phosphate-buffered 4% paraformaldehyde for 10 minutes at room temperature and were mounted with Prolong Gold Antifade Reagent (Invitrogen). Specimens were observed under IX70 confocal laser-scanning microscopy (Olympus, Tokyo, Japan).
Quantification of Histological Analysis
Images obtained from confocal microscopic observation were processed with Win ROOF software (Mitani Corporation, Fukui, Japan), and the number of the signals was counted manually or automatically using Win ROOF software.
In Vitro Proliferation and Suppression Assay
2 × 104 cells/well of purified CD25− CD4+ T cells were stimulated with 2 μg/ml of concanavalin A (Sigma-Aldrich, St. Louis, MO) and 8 × 104 cells/well of mitomycin-C (Sigma-Aldrich)-treated Thy1.2− splenocytes with or without titrated number of CD25+ CD4+ T cells were incubated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 10 mmol/L HEPES, 55 μmol/L 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin in a round-bottom 96-well plate for 72 hours at 37°C. CD25+ CD4+ T cells were cultured under the same conditions to measure their proliferative capacity in the absence of CD25− CD4+ T cells. Cells were pulsed with 1 μCi/well [3H-methyl]-thymidine (GE Health Care, Buckinghamshire, UK) for the last 6 to 8 hours of the culture, and proliferation was measured by cpm value of the harvested cells. Suppressive activity of CD25+ CD4+ T cells was expressed as percent suppression23 calculated as following: 100 × [cpm (responder) − cpm(CD25+ + CD25−)]/cpm(responder).
In Vitro Antibody Production Assay
In vitro antibody production by B cells was analyzed as previously described24 with several modifications. Briefly, 2 × 105 B1 or B2 cells isolated from young or aged BWF1 mice and equal numbers of CD25− CD4+ T cells isolated from the spleen of young or aged BWF1 mice were cultured with or without 1 × 105 CD25+ CD4+ T cells in supplemented RPMI 1640 medium for 5 days at 37°C. The concentration of IgG antibody in the culture supernatant was measured by enzyme-linked immunosorbent assay using a Mouse IgG quantitation kit (Bethyl, Montgomery, TX).
Preparation of cDNA and Real-Time Polymerase Chain Reaction (PCR)
Mice were perfused with 30 mL of PBS, and spleen, lymph nodes, kidney, and lung were excised. Tissues were homogenated with TRIzol reagent (Invitrogen), and purified total RNA was reverse-transcribed with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed with an ABI Prism 7500 (Applied Biosystems) using primers and Taq Man probes listed in Table 1.
Table 1.
Primers and Probes for Real-Time PCR
| Gene | Sense | Probe | Antisense |
|---|---|---|---|
| CCL19 | 5′-GAAAGCCTTCCGCTACCTTCT-3′ | 5′-CCCATCCCGGCAATCCTGTTCTTA-3′ | 5′-CCCTTAGTGTGGTGAACACAACA-3′ |
| CCL21 | 5′-GGCTATAGGAAGCAAGAACCAAGT-3′ | 5′-TTACTTCTACCGACGTCCCACGGA-3′ | 5′-TCAGGCTTAGAGTGCTTCCG-3′ |
| CXCL9 | 5′-TGATAAGGAATGCACGATGCTC-3′ | 5′-AGCCGAGGCACGATCCACTACAAATC-3′ | 5′-TTCCTTGAACGACGACGACTTT-3′ |
| CXCL10 | 5′-CGTCATTTTCTGCCTCATCCT-3′ | 5′-AAGCTTGAAATCATCCCTGCGAGCC-3′ | 5′-TGGTCTTAGATTCCGGATTCAG-3′ |
| CXCL12 | 5′-GCTCTGCATCAGTGACGGTAA-3′ | 5′-ATCGCCAGAGCCAACGTCAAGCAT CT-3′ | 5′-AGCCGTGCAACAATCTGAAG-3′ |
| GAPDH | 5′-AGTATGACTCCACTCACGGCAA-3′ | 5′-AACGGCACAGTCAAGGCCGAGAAT-3′ | 5′-TCTCGCTCCTGGAAGATGGT-3′ |
Statistical Analysis
Statistical significance of the difference between data sets was analyzed by Welch’s unpaired t-test for the comparison of two groups or by one-way analysis of variance with Bonferroni’s multiple comparison test for more than three groups. P < 0.05 was considered to be statistically significant.
Results
Increased Number and Frequency of Treg Cells in Aged BWF1 Mice
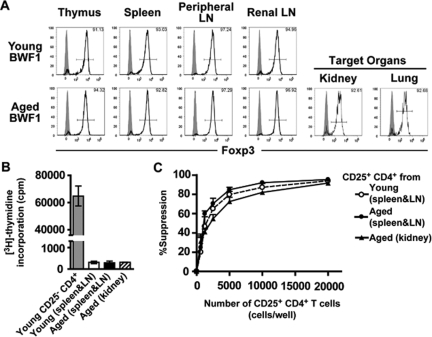
Suppressive activity of Treg cells is strongly correlated with the expression of Foxp3.11 To clarify whether an increase or decrease in the frequency and/or number of Treg cells exists, we analyzed the population of Foxp3+ CD4+ T cells by flow cytometry. We found that aged BWF1 mice had substantially increased frequency (Figure 1A) and number (Figure 1B) of Foxp3+ CD4+ T cells in the lymphoid organs compared with young BWF1 mice. A recent study has shown an age-dependent increase in CD25− Foxp3+ CD4+ T cells in 24-month-old normal mice,25 but increased Foxp3+ CD4+ T cells in aged BWF1 mice was not merely an age-dependent event because age-matched BALB/c mice did not show a marked increase in Foxp3+ CD4+ T cells (Figure 1, C and D).
Figure 1.
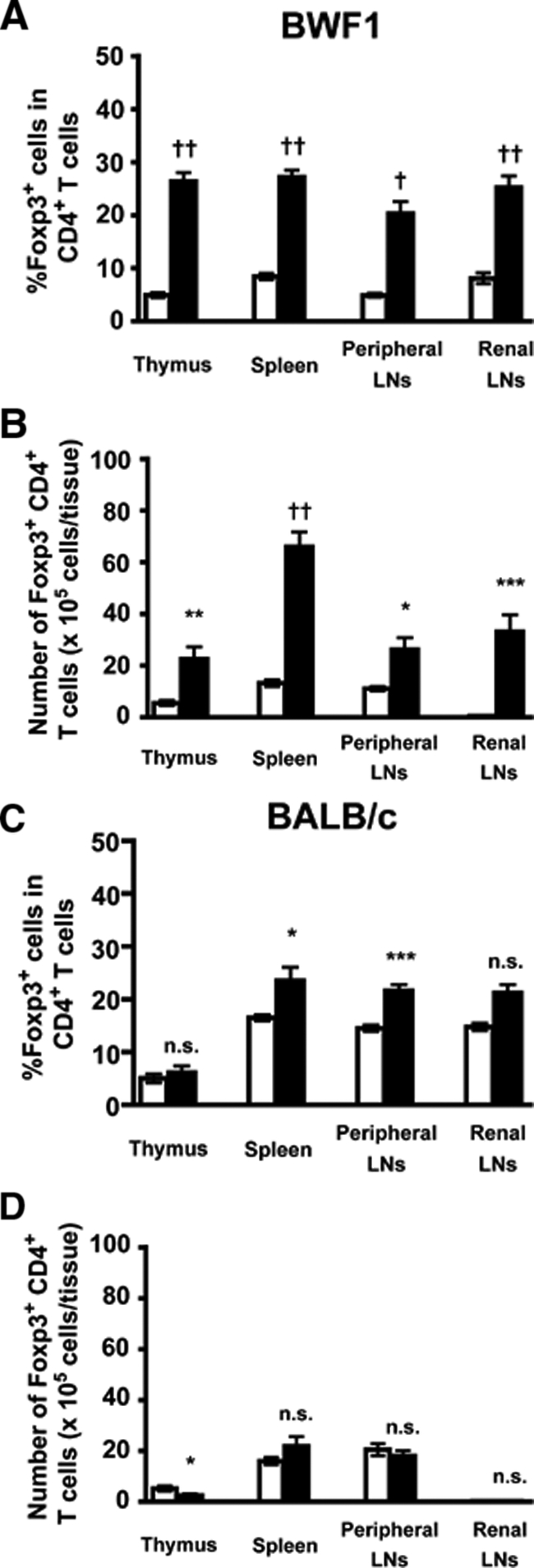
Increased Foxp3+ CD4+ Treg cells in aged BWF1 mice. Frequency (A, C) and number (B, D) of Foxp3+ CD4+ T cells within thymus, spleen, peripheral LNs (inguinal, axillary, brachial, submandibular, and cervical), and renal lymph node of BWF1 (A, B) or BALB/c (C, D) mice were analyzed by flow cytometry. Data were presented as mean ± SEM (n = 4 to 9 for each group). Open bar, young; filled bar, aged. Statistical significance of the difference between young and aged mice was analyzed by Welch’s unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.005, †P < 0.0005, ††P < 0.0001.
CD25+ CD4+ T Cells Showed Normal Suppressive Activity Both in Young and Aged BWF1 Mice
Valencia and colleagues16 reported a decreased suppressive activity of CD25+ CD4+ T cells in SLE patients, possibly because of the lower proportion of Foxp3+ cells among CD25+ CD4+ T cells. This result, however, does not exclude the possibility that a functional defect intrinsic to Treg cells exists as well. To test the functional competency of Treg cells of BWF1 mice, we performed an in vitro suppression assay. Because Foxp3 expression could be detected only in permeabilized cells, we used CD25+ CD4+ T cells as a surrogate for Foxp3+ CD4+ T cells. Concurrent with the high proportion of Foxp3+ cells among CD25+ CD4+ T cells even after disease onset (Figure 2A), CD25+ CD4+ T cells isolated from the spleen and lymph nodes of both young and aged BWF1 mice did not proliferate on stimulation (Figure 2B) and showed suppressive activity (Figure 2C). Furthermore, CD25+ CD4+ T cells isolated from the kidney (Figure 2C) and lung (data not shown), ie, the target organs, of aged BWF1 mice also showed suppressive activity comparable to those from the spleen and lymph nodes. CD25+ CD4+ T cells of thymus or lymph nodes showed similar suppressive activity (data not shown). We did not note a significant difference in the suppressive activity between young and aged, or lymphoid and nonlymphoid CD25+ CD4+ T cells in BWF1 mice at any dose of CD25+ CD4+ T cells. Taken together, our data suggest that aged BWF1 mice have an expanded pool size of Treg cells with intact suppressive activity.
Figure 2.
Suppressive activity of CD25+ CD4+ T cells. A: Representative profile of Foxp3 expression in CD25+ CD4+ T cells of young or aged BWF1 mice used for suppression assay (n = 3 for each group). Numbers in the histograms indicate the frequency of Foxp3+ cells. Shaded histogram indicates isotype control. Note that CD25+ CD4+ T cells are highly enriched for Foxp3+ Treg cells. B: Proliferation of CD25+ CD4+ T cells isolated from the spleen and lymph nodes of young or aged BWF1 mice or from the target organs of aged BWF1 mice. Data are presented as mean ± SEM. C: In vitro suppressive activity of CD25+ CD4+ T cells isolated from the spleen and lymph nodes of young or aged BWF1 mice or from the kidney of aged BWF1 mice. Data were presented as mean + SEM. Differences between the three groups presented in the graph were not significant when analyzed by one-way analysis of variance with Bonferroni’s multiple comparison test. A representative of three independent experiments that gave similar results is shown.
Treg Cells Infiltrated into the Target Organs
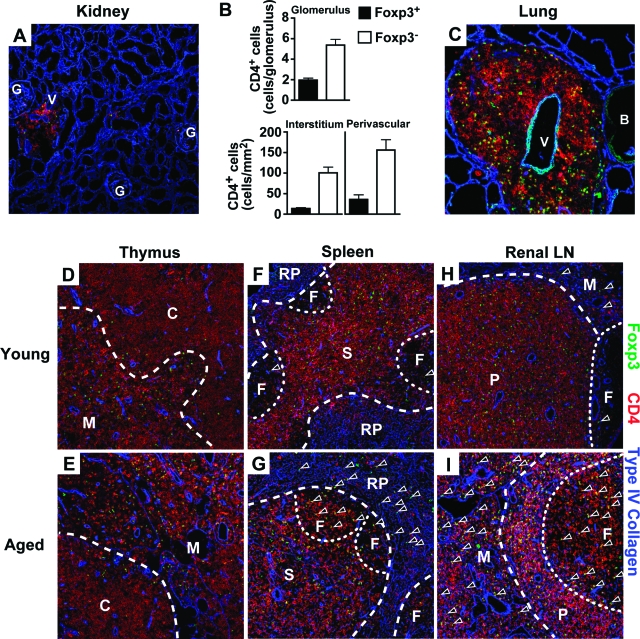
Defective migration into the site of inflammation is known to impair the in vivo suppressive activity of Treg cells even if they were functionally competent in vitro.26 Because our data indicated that Treg cells of BWF1 mice have intact suppressive activity in vitro, we asked whether Treg cells in aged BWF1 mice infiltrated into the target organs, ie, kidney and lung. Flow cytometric analysis of mononuclear cells within the target organs revealed that Foxp3+ as well as Foxp3− CD4+ T cells infiltrated into these organs, and that the frequency of Foxp3+ cells in CD4+ T cells was comparable to that in the lymph nodes of normal mice11 (18.76 ± 3.79% in the kidney and 14.08 ± 2.50% in the lung). Foxp3+ CD4+ T cells infiltrated into the glomeruli, interstitium, and perivascular region of the kidney along with Foxp3− CD4+ T cells (Figure 3B). Young BWF1 mice and nonautoimmune control mice did not show the infiltration of inflammatory cells (data not shown). Moreover, both Foxp3+ and Foxp3− CD4+ T cells were apparently distributed evenly within the infiltrating site of the target organs (Figure 3, A and C), indicating that clustering of these cells that were essential for Treg cell-mediated suppression26,27 would take place in the target organs as well as in the lymphoid organs.
Figure 3.
Altered localization of Treg cells in aged BWF1 mice. A–C: Histological analysis of the kidney and lung of aged BWF1 mice (n = 4). A and C: Triple immunofluorescent staining of a 6-μm-thick cryosection of the kidney (A) and lung (C) of aged BWF1 mice with anti-Foxp3 (green), anti-CD4 (red), and anti-type IV collagen (blue). Green signal on the vascular endothelium and bronchus of the lung was also detected in isotype control (data not shown). Such nonspecific signal was not observed in CD4+ cells. B: Summary of the number of Foxp3+ (filled bar) and Foxp3− (open bar) CD4+ T cells within renal compartments. Data were expressed as mean ± SEM. More than three fields were counted to calculate the mean value. D–I: Triple-immunofluorescent staining of 6-μm-thick cryosection of the thymus (D, E), spleen (F, G), and renal lymph node (H, I) of young (D, F, H) or aged (E, G, I) BWF1 mice with anti-Foxp3 (green), anti-CD4 (red), and anti-type IV collagen (blue). B, bronchus; C, cortex; F, follicle; G, glomerulus; M, medulla; RP, red pulp; P, paracortex; S, periaortic lymphoid sheath; V, blood vessel. Arrowheads in D–I indicate Foxp3+ CD4+ T cells located out of paracortex or periaortic lymphoid sheath. Representatives of the independent examination of four young and aged BWF1 mice are shown. Original magnifications, ×100.
Medullary Localization of Treg Cells within the Thymus
The thymus, another target organ of the disease in BWF1 mice, is the major site of the development of Treg cells.6 Disruption of the architecture of the thymic medulla where development of Treg cells occurs is known to impair that process.28 To determine whether Treg cells are properly located within the thymus, we analyzed thymic sections by immunofluorescent staining. In BWF1 mice, thymic architecture is strongly affected by the disease,4,29 but medullary localization of Treg cells remained virtually unchanged even after the manifestation of severe nephritis (Figure 3, D and E). Localization of Treg cells within the thymus is also confined to the medulla in BALB/c mice irrespective of their age (Supplemental Figure 1, A and B, see http://ajp.amjpathol.org).
Altered Distribution of Treg Cells within the Secondary Lymphoid Organs of Aged BWF1 Mice
Treg cells have to be located in the site of antigen presentation within the secondary lymphoid organs to make contacts with their target cells.26,27 Because our analyses on the number, function, and site of the development of Treg cells could not find any obvious defect, we examined the localization of Treg cells within the secondary lymphoid organs of BWF1 mice. Treg cells in aged BWF1 mice were located in the follicles and red pulp as well as periaortic lymphoid sheath in the spleen, whereas Treg cells in young BWF1 mice were mostly located in the periaortic lymphoid sheath (Figure 3, F and G; Supplemental Figure 2, see http://ajp.amjpathol.org). Similar localization of Treg cells were observed in the renal lymph node where Treg cells were located in the follicles and medulla as well as paracortex in aged BWF1 mice, whereas the localization of Treg cells in young BWF1 mice was relatively confined to paracortex (Figure 3, H and I; Supplemental Figure 2, see http://ajp.amjpathol.org). Such altered localization was not limited to Treg cells, but was also seen in Foxp3− conventional T cells. In contrast, localization of Treg cells in BALB/c mice was not altered with age and was similar to that of young BWF1 mice (Supplemental Figures 1, C–F, and 2, see http://ajp. amjpathol.org).
Changes in the Expression of Chemokine Receptors on Treg Cells in Aged BWF1 Mice
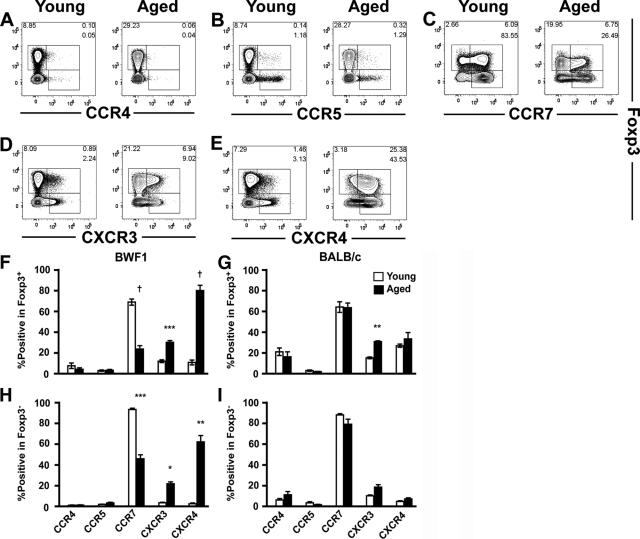
Localization of T cells is tightly regulated by various chemokines and their receptors to achieve efficient induction of immune response or tolerance.30 To elucidate the molecule(s) responsible for the altered localization of Treg cells, we next analyzed the expression of chemokine receptors involved in the function of Treg cells31,32,33,34 by flow cytometry. Both Foxp3+ and Foxp3− CD4+ T cells in the spleen showed decreased CCR7 expression (Figure 4C) and enhanced CXCR4 expression (Figure 4E) in aged BWF1 mice. On the other hand, the expression level of CCR4, CCR5, and CXCR3 did not show marked difference between young and aged BWF1 mice (Figure 4, A, B, and D), except that CXCR3 expression was slightly enhanced on both Foxp3+ and Foxp3− cells of aged BWF1 mice (Figure 4, F and H). These changes in the expression of chemokine receptors on CD4+ T cells were not observed in BALB/c mice (Figure 4, G and I). Expression pattern of chemokine receptors on CD4+ T cells in the target organs and lymph nodes was similar to that of splenic CD4+ T cells (data not shown). Aged BWF1 mice showed a 5 to 7 fold decrease in the expression of CCL19, CCL21, and CXCL12, ligands for CCR7 and CXCR4, in the lymphoid organs (Supplemental Figure 3, see http://ajp.amjpathol.org). On the other hand, expression of CXCL9 and CXCL10, ligands for CXCR3, were increased 2- to 3-fold and 8- to 28-fold, respectively, in the lymphoid organs and target organs, respectively (Supplemental Figure 3, see http://ajp.amjpathol.org).
Figure 4.
Expression of chemokine receptors on Foxp3+ and Foxp3− CD4+ T cells. Representative profile of the flow cytometric analysis of the expression of CCR4 (A), CCR5 (B), CCR7 (C), CXCR3 (D), and CXCR4 (E) on splenic CD4+ T cells of young (left) or aged (right) BWF1 mice. Gates were determined based on the profile of isotype control (data not shown). Numbers in the plots indicate the frequency of cells within the following gates: top right, Foxp3+ chemokine receptor+; bottom right, Foxp3− chemokine receptor+; left, Foxp3+ chemokine receptor−. Representative profiles of six mice are shown. F–I: Summary of the expression of the chemokine receptors on Foxp3+ (F and G) and Foxp3− (H and I) CD4+ T cells of young (open bars) and aged (filled bars) BWF1 (F and H) and BALB/c (G and I) mice (n = 6 for each group). Statistical significance of the difference between young and aged mice was analyzed by Welch’s unpaired t-test. *P < 0.005, **P < 0.001, ***P < 0.0005, †P < 0.0001.
Activated Phenotype of Both Foxp3+ and Foxp3− CD4+ T Cells in Aged BWF1 Mice
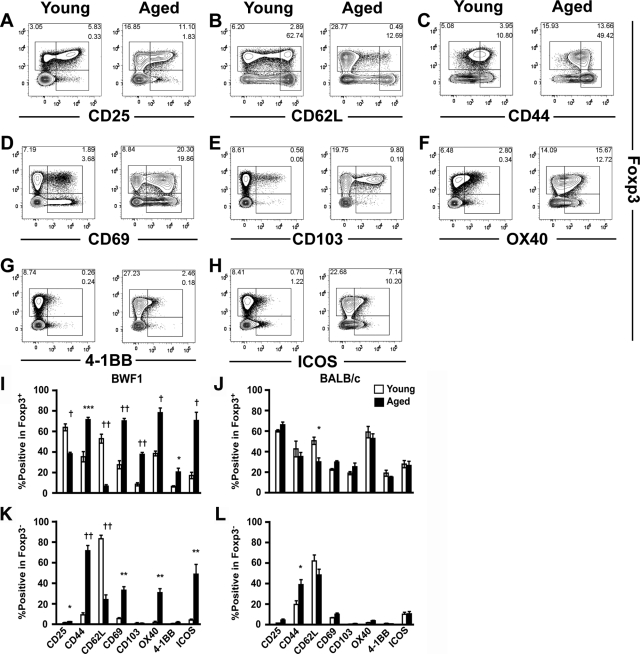
Altered localization of Treg cells in aged BWF1 mice per se does not explain the cause of their failure to control the autoimmunity. We found that Treg cells of aged BWF1 mice showed decreased expression of CD25 and CD62L (Figure 5, A, B, and I), in contrast to the enhanced or unaltered expression of activation markers CD44, CD69, and CD103 (Figure 5, C–E, and I). Various co-stimulatory molecules up-regulated on activation were reported to affect the function and/or number of Treg cells,35,36,37 therefore, we analyzed the expression of co-stimulatory molecules of Treg cells. Associated with their activated phenotype, co-stimulatory molecules OX40, 4-1BB, and ICOS were expressed on CD4+ T cells in aged BWF1 mice at higher level than young BWF1 (Figure 5, F–I). Among them, expression of OX40 and ICOS was enhanced on both Foxp3+ and Foxp3− T cells, whereas expression of 4-1BB was enhanced only on Foxp3+ Treg cells (Figure 5, I and K). Age-dependent alteration of surface phenotype in BALB/c mice was limited to slight changes in CD44 and CD62L (Figure 5, J and L).
Figure 5.
Expression of the surface markers on Foxp3+ and Foxp3− CD4+ T cells in BWF1 mice. Representative profile of the flow cytometric analysis of the expression of CD25 (A), CD62L (B), CD44 (C), CD69 (D), CD103 (E), OX40 (F), 4-1BB (G), and ICOS (H) on splenic CD4+ T cells of young (left) or aged (right) BWF1 mice (n = 6). Numbers in the plots indicate the frequency of cells within the following gates: top right, Foxp3+ marker+; bottom right, Foxp3− marker+; left, Foxp3+ marker−. I–L: Summary of the expression of the surface markers on Foxp3+ (I and J) and Foxp3− (K and L) CD4+ T cells of young (open bar) and aged (filled bar) BWF1 (I and K) and BALB/c (J and L) mice (n = 6 for each group). Data are presented as mean ± SEM. Statistical significance of the difference between young and aged mice was analyzed by Welch’s unpaired t-test. *P < 0.05, **P < 0.005, ***P < 0.001, †P < 0.0005, ††P < 0.0001.
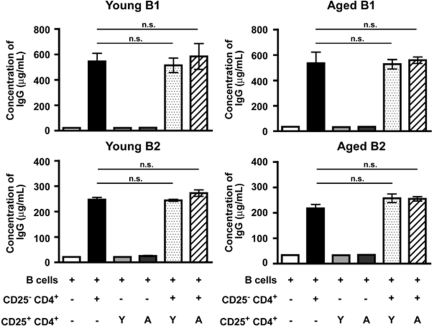
Inability of Treg Cells of BWF1 Mice to Suppress in Vitro IgG Antibody Production
Lastly, we assessed the impact of Treg cells on the antibody production by B cells. Sekigawa and colleagues24 demonstrated that CD4+ T cells of aged BWF1 mice induced IgG antibody production of splenic B cells on stimulation with concanavalin A and lipopolysaccharide in vitro. We used this method with several modifications and found that CD25− CD4+ T cells of aged, but not young, BWF1 mice induced IgG antibody production by B cells even in the absence of the stimuli (Figure 6 and data not shown). Because antibody production by B cells was totally dependent on the presence of CD4+ T cells in this assay, we assumed that Treg cells would suppress the antibody production by interfering with CD4 help. CD25+ CD4+ T cells, however, did not affect the amount of IgG antibody produced by B cells (Figure 6), demonstrating that Treg cells of both young and aged BWF1 mice are unable to suppress IgG antibody production induced by CD4+ T cells of aged BWF1 mice.
Figure 6.
Inability of Treg cells of BWF1 mice to suppress in vitro antibody production induced by CD25− CD4+ T cells of aged BWF1 mice. Concentration of IgG antibody in the culture supernatant was measured by enzyme-linked immunosorbent assay after co-culture of T cells and B cells in the following combinations for 5 days. B cells alone, (white column); B cells + CD25− CD4+ T cells (black column); B cells + CD25+ CD4+ T cells of young BWF1 (light gray column); B cells + CD25+ CD4+ T cells of aged BWF1 (dark gray column); B cells + CD25− CD4+ T cells + CD25+ CD4+ T cells of young BWF1 (dotted column); B cells + CD25− CD4+ T cells + CD25+ CD4+ T cells of aged BWF1 (striped column). CD25− CD4+ T cells of aged BWF1 mice were used for all combinations. B-cell subsets used for each combination were indicated above each panel. Data are presented as mean ± SEM. n.s., not significant by one-way analysis of variance with Bonferroni’s multiple comparison test. Representative of three independent experiments is shown.
Discussion
Foxp3+ CD4+ Treg cells play a pivotal role in the maintenance of dominant self tolerance, and lack of functional Treg cells is associated with various autoimmune diseases. In contrast, our present study in a murine model of SLE revealed a substantially expanded pool size of Treg cells with a phenotype suggesting their highly activated state, and their inability to suppress antibody production in vitro.
We could not detect any obvious defect in the suppressive activity of Treg cells in BWF1 mice. In addition, localization of both Treg cells and Foxp3− conventional CD4+ T cells within the lymphoid organs was altered, but they showed concomitant migratory behavior. These data collectively suggest that Treg cells in BWF1 mice had little defect in their function, and the failure of Treg cells to control the disease might be predominantly caused by the extrinsic factors, such as cytokine milieu and co-stimulatory signals provided by antigen-presenting cells (APCs). On the other hand, it is reported that treatment of BWF1 mice with the Treg cell-inducing molecules such as all-trans-retinoic acid or tolerogenic peptides delays or prevents the onset of murine lupus.18,19,20,38 One possible explanation for the failure of Treg cells to control the disease is that presence of Treg cells capable of controlling the disease at an earlier stage is critical, as suggested by the previous reports in which induction of Treg cells in BWF1 mice was conducted well before the onset of the disease. Another possibility is the antigen specificity of Treg cells. La Cava and colleagues19 showed that induction of Treg cells specific for the peptide derived from anti-DNA antibody were associated with the therapeutic effect of this peptide in BWF1 mice. This report raises the possibility that endogenous Treg cells in prediseased BWF1 mice lack population(s) with such antigen specificity, and expansion of the pool size of Treg cells in aged BWF1 mice with severe nephritis does not compensate for that repertoire. It is therefore feasible that accumulation of Treg cells is too late to control the pathogenic autoimmune response in aged BWF1 mice, or that antigen specificity of Treg cells in aged BWF1 mice differ from those in young BWF1 mice. However, there are other possible mechanisms for the inability of Treg cells to control the pathogenic autoimmune response in aged BWF1 mice as described below.
There are several reports suggesting a possible effect of Treg cells on T-dependent B-cell responses.19,39,40,41 It was, therefore, surprising that Treg cells of BWF1 mice could not suppress the in vitro antibody production induced by CD25− CD4+ T cells despite their intact suppressive activity against the proliferation of T cells in vitro. Possible explanations for our result are as follows: first, loss of the sensitivity of CD25− CD4+ T cells of aged BWF1 mice to Treg cell-mediated suppression; second, reversal of Treg cell-mediated suppression by signaling through co-stimulatory molecules. OX40, 4-1BB, and ICOS have been implicated in the pathogenesis of lupus.42,43,44 OX40 and 4-1BB magnify the T-cell response through induction of the proliferation of conventional T cells and inhibition of Treg cell-mediated immune suppression.37,45 The ICOS-mediated signal is essential for the induction of follicular helper T cells, thus it functions as an enhancer of B-cell response.46 On the contrary, these molecules as well as ICOS also facilitate the expansion of Treg cells.36,45,47 B cells of aged BWF1 mice, however, did not show significant expression of ligands for these co-stimulatory molecules (data not shown). This observation implies that reversal of the suppression, if any, might take place through the other pathway(s). Also, CD25− CD4+ T cells of aged, but not young, BWF1 mice contain CXCR5+ ICOS+ follicular helper T cells whose function may be resistant to Treg cell-mediated suppression. Further studies with regard to the impact of Treg cells on humoral immune response as well as the interaction between Treg cells and their target cells will be required to clarify their role in antibody-mediated autoimmune diseases such as SLE.
Concomitant migratory behavior of Treg cells and conventional T cells was shown to be crucial for the immunoregulatory function of Treg cells.26,31,32 Chemokines and their receptors, as well as the activation markers CD44, CD62L, CD69, and CD103, are the possible regulators of the migration of T cells. Our present data demonstrating similar localization of Treg cells and conventional T cells with the comparable expression of chemokine receptors and activation markers between these cells suggest that regulation of the migratory behavior of these cells were not impaired; however, BWF1 mice still develop the fatal autoimmune response. This idea, together with our notion of intact suppressive activity, further suggests that failure of Treg cells to control the disease is because of the other factor(s) residing in the microenvironment.
Collectively, we demonstrated that aged BWF1 mice developing lupus nephritis had increased Foxp3+ CD4+ Treg cells with highly activated phenotype and altered localization, but with intact suppressive activity. Our present results may provide a clue to understanding the nature of Treg cells in the lupus and also help to unveil the mechanisms of the failure of Treg cells to control autoimmune responses. Further studies directed at these points would facilitate the development of novel strategies for the treatment of SLE.
Supplementary Material
Acknowledgments
We thank the Center for NanoBio Integration of The University of Tokyo for the use of flow cytometer BD LSR II; Dr. Yoshie for kindly providing monoclonal ant-mouse CCR4 antibody; and Drs. Makoto Kurachi and Tetsu Nishiwaki for helpful discussion.
Footnotes
Address reprint requests to Sho Ishikawa, 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan. E-mail: yamasho@m.u-tokyo.ac.jp.
Supported by the Long-Ranged Research Initiative of the Japan Chemical Industry Association and the Ministry of Health, Labor, and Welfare of Japan.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, Mcconahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes—clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L, Radaszkiewicz T, Kosco M, Melchers F, Rolink AG. Development of autoimmune-disease in SCID mice populated with long-term in vitro proliferating (NZB × NZW)F1 pre-B cells. J Exp Med. 1992;176:1343–1353. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D, Chiang NY, Greenspan JS, Ermak TH. Treatment of murine lupus with monoclonal antibody to L3T4. I. Effects on the distribution and function of lymphocyte subsets and on the histopathology of autoimmune disease. J Autoimmun. 1988;1:415–431. doi: 10.1016/0896-8411(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Ermak TH, Steger HJ, Wofsy D. Treatment of murine lupus with monoclonal-antibody to L3T4. II. Effects on immunohistopathology of thymus, spleen, and lymph node. Lab Invest. 1989;61:447–456. [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FoxP3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- Scalapino KJ, Tang QZ, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-β. Proc Natl Acad Sci USA. 2006;103:8810–8815. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+ CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F-1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- Tsunemi Y, Saeki H, Nakamura K, Nagakubo D, Nakayama T, Yoshie O, Kagami S, Shimazu K, Kadono T, Sugaya M, Komine M, Matsushima K, Tamaki K. CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli. Eur J Immunol. 2006;36:2116–2127. doi: 10.1002/eji.200535564. [DOI] [PubMed] [Google Scholar]
- Ueha S, Yoneyama H, Hontsu S, Kurachi M, Kitabatake M, Abe J, Yoshie O, Shibayama S, Sugiyama T, Matsushima K. CCR7 mediates the migration of Foxp3+ regulatory T cells to the paracortical areas of peripheral lymph nodes through high endothelial venule. J Leukoc Biol. 2007;82:1230–1238. doi: 10.1189/jlb.0906574. [DOI] [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Sekigawa I, Ishida Y, Hirose S, Sato H, Shirai T. Cellular basis of in vitro anti-DNA antibody-production—evidence for T-cell dependence of IgG-class anti-DNA antibody-synthesis in the (NZB × NZW)F1 hybrid. J Immunol. 1986;136:1247–1252. [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Tang QZ, Adams JY, Tooley AJ, Bi MY, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro CE, Shakhar G, Shen SQ, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ishikawa S, Akadegawa K, Ito T, Yurino H, Kitabatake M, Yoneyama H, Matsushima K. Aberrant B1 cell migration into the thymus results in activation of CD4 T cells through its potent antigen-presenting activity in the development of murine lupus. Eur J Immunol. 2004;34:3346–3358. doi: 10.1002/eji.200425373. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204:1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su LS, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJC, Campbell IL. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yang XJ, Chiorazzi N, Hoffmann MK, Mittler RS. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F-1 mice. J Clin Invest. 2003;111:1505–1518. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- Vu MD, Xiao X, Gao WD, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Yoo BS, Nozaki Y, Sugiyama M, Ikoma S, Ohno M, Funauchi M, Kanamaru A. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F-1 mice. J Immunol. 2003;170:5793–5798. doi: 10.4049/jimmunol.170.11.5793. [DOI] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- Hahn BH, Ebling F, Singh RR, Singh RP, Karpouzas G, La Cava A. Cellular and molecular mechanisms of regulation of autoantibody production in lupus. Ann NY Acad Sci. 2005;1051:433–441. doi: 10.1196/annals.1361.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DS, Graham RR, Manku H, Wong AK, Whittaker JC, Gaffney PM, Moser KL, Rioux JD, Altshuler D, Behrens TW, Vyse TJ. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui LX, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Yu D, Tan AHM, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, Goodnow CC, Vinuesa CG. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Elpek KG, Yolcu ES, Franke DDH, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.