Abstract
Mast cells participate in pathophysiological processes that range from antimicrobial defense to anaphylaxis and inflammatory arthritis. Much of the groundwork for the understanding of mast cells was established in mice that lacked mast cells through defects in either stem cell factor or its receptor, Kit. Among available strains, C57BL/6-KitW-sh (Wsh) mice are experimentally advantageous because of their background strain and fertility. However, the genetic inversion responsible for the Wsh phenotype remains poorly defined, and its effects beyond the mast cell have been incompletely characterized. We report that Wsh animals exhibit splenomegaly with expanded myeloid and megakaryocyte populations. Hematopoietic abnormalities extend to the bone marrow and are reflected by neutrophilia and thrombocytosis. In contrast, mast cell-deficient WBB6F1-KitW/KitW-v (W/Wv) mice display mild neutropenia, but no changes in circulating platelet numbers. To help define the basis for the Wsh phenotype, a “DNA walking” strategy was used to identify the precise location of the 3′ breakpoint, which was found to reside 67.5 kb upstream of Kit. The 5′ breakpoint disrupts corin, a cardiac protease responsible for the activation of atrial natriuretic peptide. Consistent with this result, transcription of full-length corin is ablated and Wsh mice develop symptoms of cardiomegaly. Studies performed using mast cell-deficient strains must consider the capacity of associated abnormalities to either expose or compensate for the missing mast cell lineage.
Mast cells arise as precursors in the bone marrow and mature within the tissues, where they participate in both allergic and nonallergic immune processes. Phylogenetic studies confirm that this lineage predates the development of humoral immunity, implying that participation in IgE-mediated responses is a rather late specialization.1 Indeed, mast cells have now been implicated in a broad range of pathophysiologic processes, where they most typically initiate or amplify immune responses via rapid release of preformed and newly synthesized mediators. One important role in this regard is the mobilization of defenses against bacteria and parasites.2,3,4,5,6 Rapid mediator release also has pathogenic consequences, such as anaphylaxis or the initiation phase of inflammatory arthritis.7,8 Beyond this “sentinel” role, mast cells participate in the neutralization of venoms and inflammatory mediators as well as other biological processes (reviewed in9).
These conclusions have been reached largely through experiments in mice with spontaneous genetic mutations that perturb mast cell differentiation. Mast cells at all stages of maturation express the receptor tyrosine kinase Kit (CD117) and require the Kit ligand, stem cell factor (SCF), for their survival. Most laboratory strains deficient in mast cells arise from mutations affecting the Kit/SCF axis. For example, the WCB6F1/J-KitlSl/KitlSl-d mouse lacks SCF on the surface of fibroblasts and other cells.10 The W/Wv mouse bears a compound mutation (one allele null, the other impaired) at the Kit locus W (white spotting), while the Wsh mouse carries an incompletely characterized inversion upstream of Kit that affects a key regulatory element.11,12,13,14,15,16 Mice with mutations affecting Kit (rather than SCF) are particularly useful because they can be engrafted with cultured mast cells.17,18 An abnormal phenotype that can be corrected with such engraftment may presumptively be attributed to mast cell deficiency.
Such complementation studies are important because mutations affecting Kit have effects beyond the mast cell lineage. For example, the W/Wv mouse is white, anemic, partially deaf, prone to dermatitis and gastritis, and lacks intestinal interstitial cells of Cajal, as well as intraepithelial γδ T lymphocytes (reviewed in18). Bone marrow and circulating neutropenia have also been described.19,20 Further, W/Wv mice are sterile, so colony maintenance and breeding are cumbersome, and control mice are WBB6 heterozygotes. Wsh mice are also white (the heterozygote exhibiting a white abdominal sash, providing the strain name, the homozygote having residual ear pigment), but they are not anemic and are fully fertile. Other hematological lineages have been considered normal based on the comparison of limited numbers of Wsh and C57BL/6 animals.18,20 The breeding advantage and C57BL/6 background strain (from backcrossing, after the mutation arose spontaneously during a cross between C3H/HeH and 101/H)21 have made Wsh mice recent favorites for work in the mast cell field.
In initial analyses of Wsh mice, it was noted that some animals had strikingly enlarged and histologically abnormal spleens. Given the increasing importance of the Wsh strain in mast cell research, we wished to better understand “off-target” hematological effects of the large gene inversion in these animals, since associated abnormalities could alter experimental interpretation. The results presented herein provide an expanded phenotypic and genotypic characterization of this important experimental strain, and suggest that experimental results obtained in both Wsh and W/Wv must be interpreted in light of the potential role of accompanying abnormalities on the phenotype of interest.
Materials and Methods
Mice
WBB6 F1-Kitw/KitW-v (W/Wv), WBB6 littermate controls and C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-KitW-sh/W-sh mice22 were maintained at The Jackson Laboratory. Animals were housed for at least 2 weeks in the specific-pathogen-free animal facility of the Dana-Farber Cancer Institute before sacrifice for phenotyping experiments. All procedures were approved by the animal care and use committees of the Dana-Farber Cancer Institute or Harvard Medical School.
Hematological and Cardiac Phenotyping
Age-matched male mice (W/Wv and WBB6: 12 to 20 weeks, Wsh and B6: 10 to 15 weeks, except as noted) were anesthetized with isofluorane for bleeding by cardiac puncture. Whole blood was collected in the presence of EDTA to prevent clotting. A complete blood count and automated leukocyte differential was obtained using an Advia 120 Hematology System (Siemens, Tarrytown, NY) and appropriate species-specific standards and software. The accuracy of the automated neutrophil assessment was confirmed by parallel flow cytometric examination of selected blood samples stained for CD45 and Gr-1 (data not shown). Spleens were removed, cleaned of attached tissues, and weighed. Remaining splenic tissue was disaggregated and filtered through 100 μm mesh for cytofluorometric analysis. Hearts were removed, cleaned of excess soft tissue, and compressed to expel luminal blood before weighing. Femoral bone marrow was obtained by flush and passed through 100-μm mesh to separate adherent cells and exclude bone fragments. Tissue taken for histology was fixed in 4% paraformaldehyde in PBS.
Cytofluorimetry
Samples were washed in PBS with 10% fetal bovine serum and stained with appropriate antibodies and isotype controls. Cytofluorometric analysis was performed using a FACSDiva cytometer (Becton-Dickinson, Franklin Lakes, NJ). All samples were co-stained with 7-amino-actinomycin D (7-AAD) and anti-CD45.2 (BD Biosciences, Franklin Lakes, NJ), and gated to include only viable (7-AAD negative) CD45+ cells. Other antibodies used were as follows: CD3-flurorescein isothiocyanate, CD41-phycoerythrin (BD Biosciences), CD11b-flurorescein isothiocyanate, CD117-Alexa647, F4/80-phycoerythrin, Gr-1-flurorescein isothiocyanate, Gr-1-Alexa647 (Invitrogen, Carlsbad, CA), CD11c-allophycocyanin, CD19-phycoerythrin (eBioscience, San Diego, CA).
Mast Cell Progenitor Assay
Mast cell progenitors were enumerated by limiting dilution analysis, as described.23 Briefly, splenic mononuclear cells were isolated from disaggregated splenocytes via Percoll gradient, enumerated, and cultured by limiting dilution in the presence of irradiated syngenic feeder splenocytes and baculovirus-generated SCF and interleukin-3 (both at 20 ng/ml final). Culture media was RPMI 1640 supplemented with 10% fetal calf serum, glutamine (2 mmol/L), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), pyruvate (1 mmol/L), nonessential amino acids, Hepes (10 mmol/L), and 2-mercaptoethanol (50 μmol/L). The mast cell colonies with their distinct morphology were counted at 10 to 14 days. While committed mast cell progenitors have typically been enumerated in peripheral tissues with this technique,24 myeloid progenitors upstream of committed mast cell progenitors that are SCF- and/or interleukin-3-responsive would also be expected to form colonies in this assay.25 Thus this assay enumerates the cells capable of giving rise to committed mast cells within this tissue.
Microscopy
Histological sections were stained with H&E, and examined at ×100 and ×400 using a Leica DM LB2 microscope and Leica digital camera.
Inversion Breakpoint Identification
The 5′-breakpoint of the Wsh inversion mutation was located by PCR with primers that yield a 600-bp product from wild-type (C57BL/6J), but not Wsh template (WT-for, 5′-TTTGCACGTGCTA GTTACAC-3′; WT-rev, 5′-TTAAGATGGCACCCTGCTG-3′). A series of three nested PCR primers was designed for sequencing 5′ to the distal breakpoint (TSP1, 5′-C CTCAGCCTGTCACACTTATG-3′; TSP2, 5′-GACAACGAAATGATACAGAG GATTC-3′; TSP3, 5′-GAGGATTCATAGTTGTTCAATGTCC-3′), and were used with the DNA Walking Speedup Kit (Seegene, Rockville, MD) to amplify a single 500-bp product from Wsh, but not WT template. Confirmatory PCR primers flanking the predicted breakpoint were designed (Wsh-for, 5′-AGGCTTGCAGCGCATTAT-3′; Wsh-rev, 5′-GAGGATTCATAGTTGTTCAATGTCC-3′). The “WT” and “Wsh” primers can be used for genotyping purposes with the PCR program: 94°C for 5 minutes, followed by 35 cycles of 94°C for 20 seconds; 57°C for 30 seconds; 72°C for 1 minute, followed by 72°C for 10 minutes. Regions of corin intron 5 and exon 6 were amplified using the following primers: Int 5A-for, 5′-GGGGGATTGTTTCCAATTCT-3′; Int 5A-rev, 5′-TGGA CCTAGAGGGCATCATC-3′, Int 5B-for, 5′-TCAGCCATTCGGTATTCCTC-3′; Int 5B-rev, 5′-AAAAGGCCACCAACAGATTG-3′; Exo 6-for, 5′-GGGGTTCT GGAAGGAAATCT-3′; Exo 6-rev, 5′-TCGCTCCAGTCAT CACAGTC-3′.
Quantitative PCR of Corin Transcription
Total RNA was prepared from atria dissected from wild-type or Wsh mice and cDNA synthesized using random primers. PCR was performed with SYBR Green Mastermix (Applied Biosystems, UK) using primers designed to corin exons 3 to 4 (forward-5′-TCCT TCTCCAGAGGACCAGA-3′; reverse-5′-AGGGCAGAATTTGACACTGG-3′), 5 to 6 (forward-5′-GCAGGAACATGGAAAGCAAT-3′; reverse-5′-TGGTACACAGGAA GCTCTCG-3′), or 10 to 11 (forward-5′-GACAGCAGCCTGAGTAACTGC-3′; reverse-5′-TGTAGGG CAAATTCATGCAG-3′) on a Mx3000p PCR machine (Stratagene, La Jolla, CA). Data were analyzed using the 2−ΔΔCt method normalized to a single wild-type data set.26
Statistical Analysis
Experimental strains were compared with background-control animals using the Student’s t-test without correction for multiple comparisons. Data are expressed as mean ± SEM. P values ≤0.05 were considered significant.
Results
Expanded Myeloid and Megakaryocyte Populations in Wsh Spleen
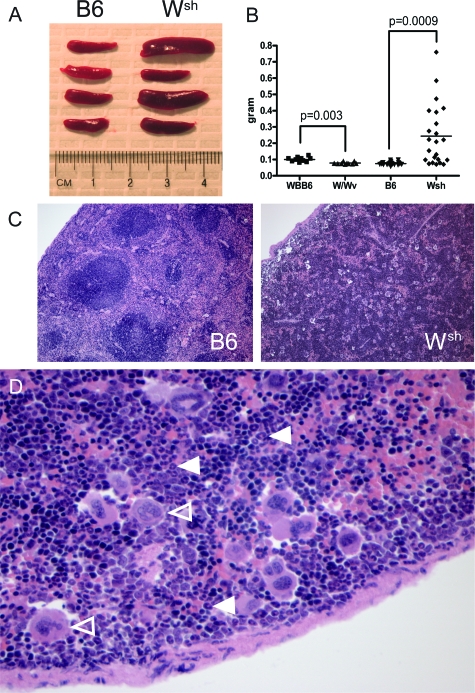
Initial work with the Wsh mice was notable for several splenic abnormalities. Even among littermates, a striking divergence of spleen size was readily observed (Figure 1A–B). These changes were not explained by body mass, which was equivalent between Wsh and B6 animals (body weight mean of male 12-week old mice n = 15 to 18/group: Wsh 25.5 ± 0.5g, B6 26.1 ± 0.4g, P = 0.32). Histologically, splenic architecture ranged from normal to strikingly abnormal, with disrupted white pulp and abundant large multinucleated cells (Figure 1C). At higher power, these large cells were seen to be normal-appearing megakaryocytes, while abundant neutrophils were also evident (Figure 1D). Erythroid precursors were increased as well, indicating exaggerated trilineage hematopoiesis. In contrast, W/Wv spleens were slightly smaller than WBB6 controls and appeared histologically normal (Figure 1B and data not shown).
Figure 1.
Wsh animals exhibit gross and microscopic splenic abnormalities. A: Representative spleens from 12 week male B6 and matching Wsh mice showing variable enlargement of Wsh spleens even among littermates. B: Quantitation of spleen mass from age-matched mast cell-deficient mice and controls, n = 10 (WBB6 and W/Wv), n = 22 (B6), n = 23 (Wsh). C: H&E staining of B6 and Wsh spleens from 12-week-old mice (magnification = original ×100). Note the clear separation of white pulp and red pulp in B6 that is lost in Wsh, along with the appearance of large multinucleated cells. This phenotype is observed typically in larger Wsh spleens, while others may show relatively normal histology. D: High power image of Wsh spleen (magnification = original ×400) demonstrating large multinucleated megakaryocytes (open arrowheads) and an abundance of neutrophil-lineage forms (solid arrowheads).
To evaluate these abnormalities, splenocyte populations were quantified by cytofluorimetry and histomorphometry. Consistent with an expansion of the myeloid compartment in many of the Wsh mice, the proportion of cells expressing CD11b was elevated 2- to 3-fold, accompanied by a fivefold increase in cells expressing the myeloid marker Gr-1 and an almost twofold increase in cells expressing F4/80 (Figure 2, A–C). Reciprocal decreases were found in the proportion of splenic B and T cells (data not shown). Cells expressing the dendritic cell marker CD11c were present in normal proportions (data not shown). Histomorphometric enumeration of megakaryocytes demonstrated a greater than 20-fold increase in the number of cells visible in a splenic cross section, corresponding to a more than hundred-fold expansion extrapolated across three dimensions (Figure 2D). Since expression of CD11b together with Gr-1 is thought to mark a regulatory myeloid phenotype, co-expression of these antigens was examined.27 Both Gr-1hiCD11bhi and Gr-1intCD11bint populations were expanded in Wsh animals (Figure 2, E–F). Unlike Wsh animals, W/Wv mice showed a reduction in splenic CD11b+ cells, Gr-1+ cells, and Gr-1hi CD11bhi splenocytes compared with their control strain (Figure 2, A, B, F).
Figure 2.
W/Wv and Wsh mice display contrasting myeloid aberrancy in spleen. Spleens from mast-cell deficient animals and age-matched controls were disaggregated and analyzed by cytofluorimetry and histomorphometry. Cytofluorimetric data reported are the percentage of viable, CD45+ cells in individual mice (n = 10 to 26 W/Wv and WBB6 mice and n = 13 to 29 Wsh and B6 mice pooled from 2 to 7 independent experiments). A: CD11b (component of Mac-1). B: Gr-1. C: F4/80. D: Megakaryocyte quantitation by histomorphometry (B6 3.0 ± 0.7 vs. Wsh 66.3 ± 11.4 cells/10x hpf, n = 13 male mice aged 10 to 14 weeks/group pooled from three experiments). E: Representative dot plots of viable CD45+ splenocytes showing two Gr-1+CD11b+ populations defined by expression level of these markers. F: Quantitation of Gr-1/CD11b-expressing populations.
Expanded Splenic Mast Cell Progenitor Population in Wsh
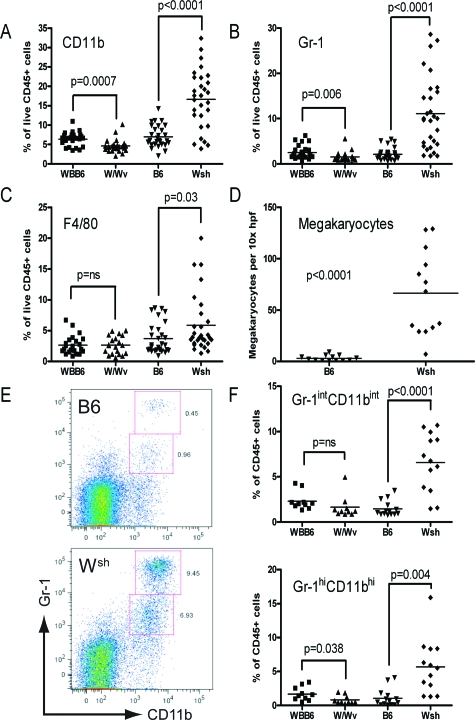
Although the Wsh mutation impairs Kit expression in mast cells, its effect on Kit expression is variable among tissues, and enhanced expression may be observed in a regionally and developmentally regulated manner.12 Further, signaling via Kit has been implicated in the recruitment of myeloid precursors to the spleen.28 Accordingly, splenocyte expression of Kit was studied. Unlike splenocytes from W/Wv mice, where surface Kit was rare, Kit+ cells were present in elevated proportion in Wsh mice compared to control (Figure 3A). This finding was supported by analysis of the mast cell potential of splenocytes by stimulation with SCF and interleukin-3. These conditions are optimized to grow mast cell progenitors and thus the numbers represent the number of cells potentially capable of becoming committed mast cell progenitors, which includes the early myeloid progenitors as well as those committed to the mast cell lineage.23,25 Indeed, the density of mast cell colonies emerging from Wsh spleen was fourfold greater than that from B6 and 20-fold greater than that from W/Wv mice (Figure 3B).
Figure 3.
Wsh animals exhibit an elevated proportion of cells expressing functional Kit. A: Surface expression of Kit in n = 15 to 17 W/Wv and WBB6 mice and n = 22 Wsh and B6 mice pooled from 3 to 6 independent experiments. B: Quantitation of splenic mast cell progenitor populations in W/Wv, WBB6, Wsh, and B6 mice. Note that Wsh mice demonstrate a fourfold higher frequency per 106 mononuclear cells as compared with B6 and a 20-fold excess compared to W/Wv (n = 5 to 7 mice per group pooled from 2 separate experiments with similar results). MNC, mononuclear cells.
Divergent Aberrancy in Marrow and Blood in Wsh and W/Wv
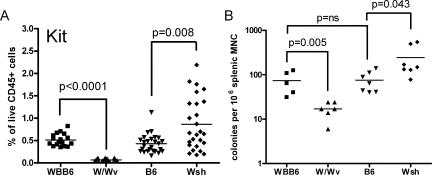
Whether these hematological abnormalities extended to the bone marrow was then investigated. Consistent with earlier results, cells expressing CD11b and Gr-1 were expanded in Wsh marrow and diminished in W/Wv marrow, though F4/80 expression was inversely affected (Figure 4A–C). Both Gr-1hiCD11bhi and Gr-1intCD11bint populations were expanded in Wsh mice (Figure 4, D and E). Kit expression in Wsh was similar or slightly decreased, while it was essentially undetectable in W/Wv individuals (Figure 4F). Again, B and T cells proportions were reciprocally reduced while CD11c expression was equivalent (data not shown).
Figure 4.
Bone marrow is neutropenic in W/Wv and neutrophilic in Wsh mice. Cytofluorimetric analysis of CD45+ bone marrow populations in W/Wv, WBB6, Wsh, and B6 mice (n = 10 to 26 W/Wv and WBB6 mice and n = 9–22 Wsh and B6 mice pooled from 2 to 6 independent experiments). A: CD11b. B: Gr-1. C: F4/80. D: Cells co-expressing Gr-1 and CD11b at an intermediate level. E: Cells co-expressing Gr-1 and CD11b at a high level. F: C-kit.
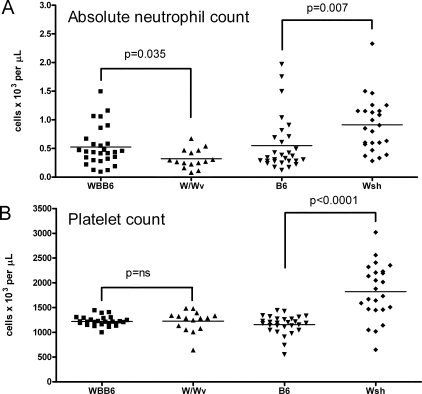
Finally, circulating blood parameters in Wsh and W/Wv mice were quantified. As has been described, W/Wv mice were anemic, while Wsh mice displayed mean hematocrit values similar to those of B6 animals, though with a wider scatter among individuals (data not shown). Consistent with observations in the spleen and marrow, Wsh mice exhibited an almost 70% increase in absolute circulating neutrophils compared with B6 controls (0.92 ± 0.10 vs. 0.55 ± 0.09 × 103 cells/μL, P = 0.008), while W/Wv mice manifested a 40% decrease relative to WBB6 (0.32 ± 0.05 vs. 0.53 ± 0.07 × 103 cells/μL, P = 0.03) (Figure 5A). Finally, in keeping with the megakaryocytosis observed in spleen, and quite distinct from the other strains tested, Wsh mice exhibited a previously undescribed thrombocytosis (1824 ± 114 vs. 1155 ± 37 × 103 cells/μL, P < 0.0001) (Figure 5B).
Figure 5.
Wsh mice exhibit circulating neutrophilia and thrombocytosis, while W/Wv mice are mildly neutropenic. Blood from cardiac puncture was analyzed by automated cytometer to obtain complete blood count and leukocyte differential. A: Absolute neutrophil count. B: Platelet count by automated cytometer demonstrating marked elevation in Wsh mice. Data shown represent individual mice pooled from at least 5 independent experiments (n = 15 to 28 mice/group).
Since these results differed in certain respects from those of other authors who have compared Wsh and B6 mice aged 7 to 9 weeks,20 a potential age-dependence of the hematopoietic phenotype was examined using 7 to 8 week-old mice (9 male animals pooled from 2 independent experiments). Indeed, splenomegaly was less pronounced in the younger animals, though still evident (Wsh 0.086 ± 0.004g versus B6 0.069 ± 0.002g, P = 0.0016). However, the relative neutrophilia in the blood and bone marrow occurred at a magnitude similar to older animals (bone marrow %Gr-1+ Wsh 51.9 ± 2.5 vs. B6 39.4 ± 1.0, P = 0.0003; blood absolute circulating neutrophil count Wsh 0.34 ± 0.04 × 103 cells/μL versus B6 0.14 ± 0.01 × 103 cells/μL, P < 0.0001). Similarly, splenic neutrophilia and circulating thrombocytosis were also observed at this younger age (data not shown). Accordingly, these findings suggest that younger Wsh animals are not free of hematopoietic aberrancy.
Identification of the 3′ Inversion Breakpoint and Disruption of Corin in Wsh
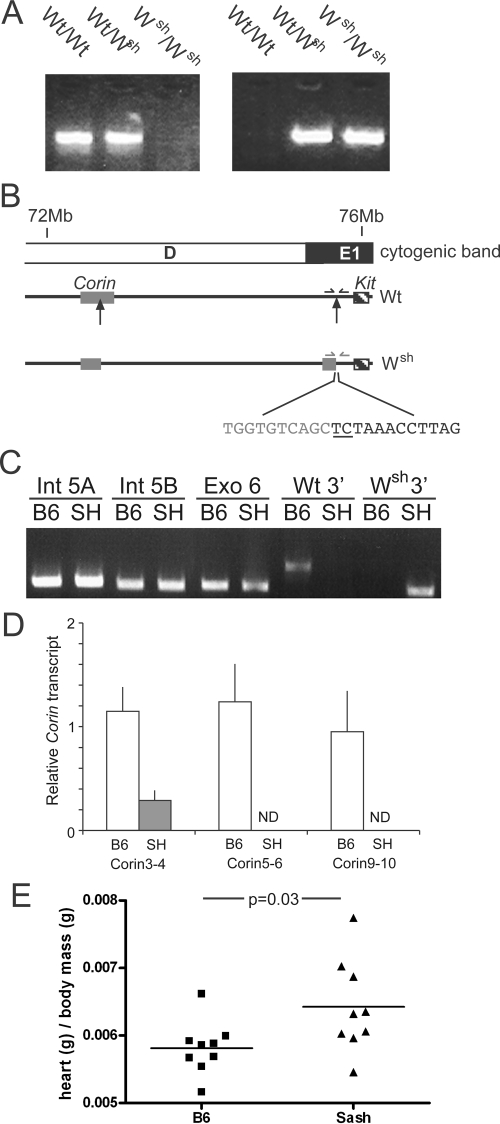
To understand further the genetic basis for the hematological defects observed in Wsh mice, the causative inversion on chromosome 5 was defined. The 5′-breakpoint of the inversion had been shown to reside 2.8 to 3.3 Mb upstream of the 3′-breakpoint, somewhere between gabrb1 and tec, and thereby with the potential to directly interrupt the coding sequence of 10 genes.14 The 3′ breakpoint had been localized approximately 72 kb upstream of Kit, extinguishing the influence of a locus control region important for mast cell Kit expression.15,16 PCR analysis with primers flanking this region amplified product from wild-type genomic DNA, but not Wsh template (Figure 6, A and B), thus confirming these findings. Three nested reverse primers for sequencing immediately downstream of the predicted 3′ breakpoint were used to perform a “DNA walking” reaction using wild-type or Wsh template. Sequencing of a single 500-bp product amplified from Wsh, but not wild-type, genomic DNA revealed that the entire fragment was homologous to an inverted intron of corin, a gene residing within the region proposed to contain the 5′ breakpoint. PCR using several pairs of primers designed to flank the predicted 3′ breakpoint amplified the expected products from Wsh but not wild-type template (eg, Figure 6A). Sequence analysis confirmed the exact site of the 3′ breakpoint at Ensembl (release 49) position 75,903,511(+) between bases C and T, 67.5 kB upstream of the Kit start sequence, with an insertion of the bases TC (Figure 6B).
Figure 6.
Precise definition of the Wsh inversion mutation. A: Products from PCR of Wt/Wt, Wt/Wsh and Wsh/Wsh genomic DNA using primers flanking the sites of the 3′ breakpoint in the wild-type allele (Wt = C57BL/6J) or Wsh allele. B: Schematic representation (not to scale) of Wsh inversion on chromosome 5 showing only corin and Kit (at least 21 genes intervene). Arrows point to sites of 5′ and 3′ breakpoints on W and consequent inversion on Wsh. Gray nucleotides belong to inverted corin, underlined and italicized bases are insertions and black nucleotides are bases upstream of Kit unaffected by inversion. C: Products from genomic PCR using primers designed to corin regions immediately upstream of the 5′ breakpoint. Two regions of intron 5 (Int 5A and Int 5B) and most of exon 6 (Exo 6) were assayed alongside controls for the Wt and Wsh alleles. D: Quantitative PCR analyses of transcripts for various corin exon pairs were performed using RNA prepared from wild-type (n = 5) or Wsh (n = 6) atria. Data were normalized to a single wild-type data set and the mean and SD are shown. ND, not detected. E: Heart weight relative to total body weight from wild-type (n = 9) and Wsh (n = 9) male mice aged 7 to 8 weeks pooled from 2 experiments.
Based on the sequence brought to the 3′ breakpoint from corin, the predicted site of the 5′ breakpoint resides at Ensembl position 72,790,584(−) between bases C and G (reverse strand). This position lies between exons 5 and 6 of corin. Multiple attempts to amplify a PCR product using primers expected to flank the 5′ endpoint were unsuccessful, raising concerns about potential loss or addition of genetic material at that breakpoint. To delimit the extent of such anomaly, we performed genomic PCR for sequences in intron 5 and exon 6 of corin, immediately upstream of the inversion, and found both to be intact (Figure 6C). To evaluate for substantial loss within the inversion itself, we evaluated expression of the first potentially vulnerable gene at the newly 5′ end (pdgfra) and found expression of PDGF receptor on thymic stromal cells to be normal (data not shown).
Disruption of corin itself was confirmed by quantitative PCR analysis of transcripts encompassing exons 3 to 4, 5 to 6, and 10 to 11. These analyses revealed low levels of a truncated transcript corresponding to the inverted exons in atria of Wsh mice; no mRNA from remaining corin exons was detected (Figure 6D). As a consequence, Wsh mice exhibit cardiac hypertrophy, a phenotype characteristic of corin-deficient mice (Figure 6E).24 These data confirm that the mutation underlying the Wsh phenotype interrupts corin between exons 5 and 6, and encompasses 27 other genes in an inversion spanning approximately 3.1 Mb (Table 1). We cannot exclude the loss of a limited amount of genetic material at the newly 5′ end of the inversion, though any such loss cannot extend as far as pdgfra.
Table 1.
Genes Inverted in Wsh (c-kit → corin)
| Gene abbreviation | Full name |
|---|---|
| Pdgfra | Platelet derived growth factor, alpha polypeptide |
| Gsx2 | GS homeobox 2 |
| Chic2 | Cysteine-rich hydrophobic domain 2 |
| EG619945 | Unknown gene |
| Lnx1 | Ligand of numb-protein X 1 |
| Fip1l1 | FIP1 like 1 |
| Scfd2 | Sec1 family domain containing 2 |
| Rasl11b | RAS-like, family 1, member B |
| 2700023E23Rik | Unknown gene |
| Usp46 | Ubiquitin specific peptidase 46 |
| Spata18 | Spermatogenesis associated 18 |
| Sgcb | Sarcoglycan, beta (dystrophin-associated glycoprotein) |
| BC0319011 | Unknown gene |
| Dcun1d4 | Defective in cullin neddylation 1, domain containing 4 |
| C130090K23Rik | Unknown gene |
| Ociad2 | OCIA domain containing 2 |
| Ociad1 | OCIA domain containing 1 |
| Fryl | Furry homolog-like |
| Slc10a4 | Solute carrier family 10 |
| Slain2 | SLAIN motif family, member 2 |
| Tec | Cytoplasmic tyrosine kinase |
| Txk | TXK tyrosine kinase |
| Npal1 | NIPA-like domain containing 1 |
| Cnga1 | Cyclic nucleotide gated channel alpha 1 |
| EG545758 | Unknown gene |
| Zar1 | Zygote arrest 1 |
| Nfxl1 | Nuclear transcription factor, X-box binding-like 1 |
Discussion
The absence of mast cells in several strains of mice has proven invaluable to elucidate the function of this lineage. Most of this work, including studies from our own group,8,29 has been performed in W/Wv animals, though increasingly the breeding and background advantages of Wsh have rendered this mouse the experimental strain of choice for many investigators. Given the importance of Kit in hematopoiesis, it is perhaps not surprising that both W/Wv and Wsh mice bear abnormalities beyond the mast cell lineage. Unexpected, however, was the finding that these two strains diverge so sharply in the direction of their hematopoietic phenotypes. Specifically, W/Wv animals are neutropenic in spleen, marrow and blood, while Wsh animals exhibit a marked expansion of Gr-1+CD11b+ cells in spleen and marrow, as well as an elevated number of circulating neutrophils and platelets.
The impact of these contrasting myeloid and megakaryocyte abnormalities on the results of experiments performed with these mice will likely vary depending on the experimental system. In a mast cell-dependent asthma model, W/Wv and Wsh animals were found to be largely equivalent.30 By contrast, while W/Wv mice are resistant to autoantibody-induced arthritis unless engrafted with mast cells, Wsh mice remain susceptible.8,20,29 It is possible that in such a system the relative neutropenia of the W/Wv could expose a requirement for the mast cell, as has been suggested.20 Alternately, myeloid and megakaryocyte expansion in the Wsh might compensate aberrantly for the missing mast cells. In yet other contexts, the Wsh strain could exhibit an unexpected phenotype because of the expansion of Gr-1+CD11b+ cells in the spleen. These cells have been termed “regulatory myeloid cells” because of their observed impact in limiting anti-tumor immunity and generating skewing of immune responses in the Th2 direction in septic animals.27,31,32 The relative importance of the Gr-1/CD11b-intermediate and -high populations has not been defined. Given these competing abnormalities in W/Wv and Wsh mice, drawing conclusions from either strain regarding the role of mast cells in the “normal animal” cannot be straightforward. However, correction of an abnormal phenotype in either strain by mast cell engraftment will remain strong prima facie evidence that mast cells have the capacity to fulfill a particular physiological role, at least under certain circumstances.
What underlies the phenotypic differences between W/Wv and Wsh mice? The W/Wv genetic lesion is well defined: W is a null allele of Kit, while Wv bears a point mutation in the cytoplasmic tail of the receptor, which greatly reduces its functional capacity as a kinase.11 The result is reduced expression of a hypofunctional receptor. In contrast, the Wsh mutation had not been fully defined. This strain was shown to bear an inversion of approximately 3Mb upstream of, but not involving, the Kit coding region.12,13,14,15 Using restriction fragment analysis, the 3′ breakpoint was found to reside approximately 72kb upstream of c-kit, while the 5′ breakpoint was undefined, lying between gabrb1 and tec. The inversion included a regulatory locus located approximately 150kb upstream of Kit that controls the expression of this receptor in mast cells, and elegant experimental work demonstrated that interference with this regulatory element is the probable cause for mast cell deficiency in the Wsh mouse.16
The current results provide an improved understanding of the Wsh inversion. The 3′ end of the inversion resides 67.5 kb 5′ to the coding region of Kit in a region devoid of known genes. The 5′ breakpoint resides between exons 5 and 6 of corin, disrupting that gene. While it is possible that some genetic material has been lost at the newly 5′ end of the inversion, this loss does not encompass the most proximal of the 27 inverted genes.
In addition to defining the genetic basis for the Wsh phenotype, the precise identification of the 3′ breakpoint provides a rapid and reliable PCR genotyping assay not previously available. This assay enables breeding of the Wsh mutation into white mice, such as BALB/c and NOD, where the characteristic coat phenotype of Wsh heterozygotes and homozygotes would not be observed. PCR genotyping opens the door to further studies of the impact of mast cell deficiency on a variety of spontaneous diseases.
Although dysregulation of Kit is the likely cause of the observed hematopoietic abnormalities, the inactivation of corin by the 5′ breakpoint revealed here must also be considered. Corin encodes a cardiac transmembrane serine protease that activates pro-atrial natriuretic peptide by cleavage.33 Roles in the regulation of adipocytes and hair color have also been reported, and expression of the gene in kidney, testis, gravid uterus, and developing bone has been identified by Northern blot and in situ hybridization.34,35,36 Corin-deficient mice exhibit mild hypertension and cardiac hypertrophy, but otherwise appear normal, although no hematological characterization has been reported.33
Beyond corin itself, it is possible that regulatory elements associated with genes in the immediate neighborhood of corin may have been disturbed, or that genes within the inversion may manifest altered activity. Unfortunately, little is known about potential hematopoietic functions for most of these genes. The exception is tec, encoding a cytoplasmic src-related kinase and strongly expressed in hematopoietic cells, including murine bone-marrow-derived mast cells and fetal liver megakaryocytes.37,38 Interestingly, tec protein physically associates with Kit and is phosphorylated in response to SCF stimulation.39 Tec is also phosphorylated in response to ligation of c-Mpl by thrombopoietin, and has therefore been implicated in the homeostasis of the megakaryocyte population, an association of potential relevance given the megakaryocytosis and thrombocytosis phenotype demonstrated here.40 Beyond tec, the presence in the inversion of a potential X-box binding transcription factor (Nfxl1) is notable. It is therefore possible that defects in corin or other genes modify the outcome of Kit dysregulation in Wsh mice to give rise to the unique phenotype observed in this strain.
A further contributor to the Wsh phenotype is its mixed genetic background. The Wsh mutation arose in an F1 C3H/HeH × 101/H. The commercial source for these animals reports that the animal has been backcrossed at least 10 generations to C57BL/6, leaving the thoroughness of this backcross in some uncertainty. Further, the immediate genetic environment of the Wsh locus will remain that of the founder strain. For example, the first gene immediately upstream of the inversion, Atp10 d encoding a P-type ATPase, is mutated to include a premature stop codon in B6, while no such mutation is present in other strains including C3H/He.41
The present results contrast with prior reports of an equivalence of hematological parameters between Wsh and B6 mice.18,20 Although colony conditions might explain a certain degree of divergence among mice, results from these smaller studies fall within the ranges observed in the current analysis, which was numerically powered to detect interstrain differences. Thus, there exists no meaningful conflict between these data and those published previously.
In conclusion, W/Wv and Wsh mice exhibit abnormalities affecting hematopoietic lineages beyond the mast cell. The W/Wv strain exhibits anemia and mild neutropenia, while the Wsh manifests neutrophilia, thrombocytosis, splenomegaly and cardiomegaly. The genetic basis for these differences remains uncertain, but in Wsh mice may involve defects beyond the regulation of Kit, given the size and complexity of the genetic inversion, including disruption of corin. The importance of these phenotypic differences is likely to vary with experimental context. Consideration of this aberrancy is warranted when using these important murine strains for functional study of the participation of mast cells in experimental models of disease.
Footnotes
Address reprint requests to David M. Lee, M.D., Ph.D., Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, One Jimmy Fund Way, Smith 552B, Boston MA 02115. E-mail: dlee@rics.bwh.harvard.edu.
Supported by K08-AR051321 (P.A.N.); Australian National Health and Medical Research Council CJ Martin Overseas Biomedical Fellowship (D.H.D.G.); Juvenile Diabetes Research Foundation, P01 AI065858-02, Young Chair funds (D.M., C.B.); and R01-AI059745, P01 AI065858-02, and the Cogan Family Foundation (D.M.L.).
References
- McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TR, Galli SJ, Katona IM, Drazen JM. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice, J Clin Invest. 1989;83:1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, Benoist C, Mathis D, Lee DM. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1, Proc Natl Acad Sci USA. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC, Frederick W, Jacobsen, Langley Keith E, Smith Kent A, Takeish Takashi, Cattanach Bruce M, Galli Stephen J, Suggs Sidney V. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor, Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: w37. Wv, W41 and W. EMBO J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–717. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- Duttlinger R, Manova K, Berrozpe G, Chu TY, DeLeon V, Timokhina I, Chaganti RS, Zelenetz AD, Bachvarova RF, Besmer P. The Wsh and Ph mutations affect the c-kit expression profile: c-kit misexpression in embryogenesis impairs melanogenesis in Wsh and Ph mutant mice. Proc Natl Acad Sci USA. 1995;92:3754–3758. doi: 10.1073/pnas.92.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DL, Kozak CA, Mano H, Chapman VM, Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum Mol Genet. 1995;4:2073–2079. doi: 10.1093/hmg/4.11.2073. [DOI] [PubMed] [Google Scholar]
- Berrozpe G, Timokhina I, Yukl S, Tajima Y, Ono M, Zelenetz AD, Besmer P. The W(sh), W(57), and Ph Kit expression mutations define tissue-specific control elements located between -23 and -154 kb upstream of Kit. Blood. 1999;94:2658–2666. [PubMed] [Google Scholar]
- Berrozpe G, Agosti V, Tucker C, Blanpain C, Manova K, Besmer P. A distant upstream locus control region is critical for expression of the Kit receptor gene in mast cells. Mol Cell Biol. 2006;26:5850–5860. doi: 10.1128/MCB.01854-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J Cell Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982;39:315–322. doi: 10.1017/s001667230002098x. [DOI] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood. 1996;88:75–81. [PubMed] [Google Scholar]
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci USA. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garruti G, Giusti V, Nussberger J, Darimont C, Verdumo C, Amstutz C, Puglisi F, Giorgino F, Giorgino R, Cotecchia S. Expression and secretion of the atrial natriuretic peptide in human adipose tissue and preadipocytes. Obesity (Silver Spring) 2007;15:2181–2189. doi: 10.1038/oby.2007.259. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- Mano H, Mano K, Tang B, Koehler M, Yi T, Gilbert DJ, Jenkins NA, Copeland NG, Ihle JN. Expression of a novel form of Tec kinase in hematopoietic cells and mapping of the gene to chromosome 5 near Kit. Oncogene. 1993;8:417–424. [PubMed] [Google Scholar]
- Kluppel M, Donoviel DB, Brunkow ME, Motro B, Bernstein A. Embryonic and adult expression patterns of the Tec tyrosine kinase gene suggest a role in megakaryocytopoiesis, blood vessel development, and melanogenesis. Cell Growth Differ. 1997;8:1249–1256. [PubMed] [Google Scholar]
- Tang B, Mano H, Yi T, Ihle JN. Tec kinase associates with c-kit and is tyrosine phosphorylated and activated following stem cell factor binding. Mol Cell Biol. 1994;14:8432–8437. doi: 10.1128/mcb.14.12.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Miyazato A, Shimizu R, Komatsu N, Miura Y, Ozawa K, Mano H. Tec protein-tyrosine kinase is involved in the thrombopoietin/c-Mpl signaling pathway. Exp Hematol. 1997;25:211–216. [PubMed] [Google Scholar]
- Flamant S, Pescher P, Lemercier B, Clement-Ziza M, Kepes F, Fellous M, Milon G, Marchal G, Besmond C. Characterization of a putative type IV aminophospholipid transporter P-type ATPase. Mamm Genome. 2003;14:21–30. doi: 10.1007/s00335-002-3032-3. [DOI] [PubMed] [Google Scholar]