Abstract
Improved hygiene has been suggested to influence certain autoimmune disorders, such as multiple sclerosis. In this study, we addressed whether altering the composition of gut flora may affect susceptibility to experimental autoimmune encephalomyelitis (EAE), an animal model of MS. We administered a mixture of non-absorbing antibiotics, kanamycin, colistin, and vancomycin (KCV), orally to mice induced to develop EAE. The antibiotic treatment, beginning 1 week prior to sensitization, altered the composition of gut flora and, intriguingly, also ameliorated the development of EAE. While this result was associated with a reduced production of pro-inflammatory cytokines from the draining lymph node cells, a reduction of mesenteric Th17 cells was found to correlate with disease suppression. In addition, we found that Vα14 invariant NKT (iNKT) cells were necessary for maintaining the mesenteric Th17 cells. The homologous effects of KCV treatment and iNKT cell depletion led us to speculate that KCV treatment may suppress EAE by altering the function of iNKT cells. Consistent with this hypothesis, KCV treatment did not suppress EAE that was induced in iNKT cell-deficient mice, although it was efficacious in mice that lacked Vα19 mucosal-associated invariant T cells. Thus, gut flora may influence the development of EAE in a way that is dependent on iNKT cells, which has significant implications for the prevention and treatment of autoimmune diseases.
The immunopathology of autoimmune diseases is still poorly understood, although comprehensive and multidisciplinary approaches continue to give us new insight into the mechanisms of disease. Previous studies have generally supported a pathogenic role of interferon (IFN)γ-producing Th1 cells in autoimmune diseases such as multiple sclerosis (MS) that affect the central nervous system (CNS).1 As Th1 cells are cross-regulated by Th2 cells producing interleukin (IL)-4, IL-5, and IL-13, the counterbalance between Th1 and Th2 cells has been posed as a key issue in understanding the pathogenesis of MS.2 However, the traditional “Th1/Th2” paradigm is now facing a fundamental challenge since a third class of helper CD4+ T cells, named Th17 cells, have been found to cause autoimmune inflammation.3,4,5 Th17 cells are IL-23-dependent cells that are distinct from Th1 and Th2 cells in their ability to produce IL-176,7,8 and their use of the RORγt transcription factor.9 Although the relationship between Th17 cells and Th1 or Th2 cells remains to be fully characterized, Th17 cells are likely to exert a predominant pathogenic activity in various inflammatory conditions associated with autoimmunity or allergy either independently or collaboratively with Th1 cells.10
It is widely accepted that development of autoimmune disease is under control of both genetic and environmental factors. For example, recent whole genome analysis has revealed that several genes including human leukocyte antigen-DR are positively linked with the susceptibility to MS.11 In contrast, most of our knowledge about environmental factors relies on epidemiological data. Results of migration studies, as well as the reported presence of clusters or outbreaks of MS, have illustrated potential environmental influences on MS, including infection, stress, sunlight exposure, and sex hormone.12,13,14 While an altered intestinal microflora has been suggested to be an environmental risk factor for rheumatoid arthritis,15 inflammatory bowel disease,16 and human allergy and asthma,17 the status of gut flora has rarely been evaluated as a potential risk factor for MS.
Recent studies have shown that animals bred in a germfree environment are characterized by having low densities of lymphoid cells in the gut mucosa, a reduced size of specialized follicle structures, and low concentrations of immunoglobulins in the peripheral blood.18,19,20,21 It is also of note that the intestinal lamina propria (LP) has been identified as a site that is constitutively inhabited by Th17 cells.9 Thus the dialogue between host and bacteria at the mucosal interface seems to be critical in the development of the competent immune system.
To explore a possible role of intestinal microflora in the development of autoimmune disease, we tested if oral administration of the mixture of non-absorbing antibiotics kanamycin, colistin, and vancomycin (KCV) could modify the development of experimental autoimmune encephalomyelitis (EAE) induced in C57BL/6 (B6) mice sensitized against a myelin oligodendrocyte glycoprotein (MOG) peptide of amino acids 35 to 55 [MOG (35–55)]. Here we report that continuous oral KCV treatment, starting one week before immunization, significantly suppressed the development of EAE along with altering gut flora. Suppression of EAE was accompanied by a reduced production of pro-inflammatory cytokines from the draining lymph nodes (dLNs) in response to MOG (35–55). While the antibiotic treatment suppressed MOG (35–55) reactive Th17 cells within the mesenteric lymph nodes (MLNs), it also reduced the total number of mesenteric Th17 cells in naïve mice. Furthermore, unexpectedly we found that the Th17 cells in the MLNs are greatly reduced in Cd1−/− mice or Jα281−/− mice, which lack invariant Vα14 natural killer T (iNKT) cells,22 and that the KCV-induced reduction of the mesenteric Th17 cells was only marginal in the iNKT cell-deficient mice. As such, KCV treatment and iNKT cell deletion showed homologous effects on the mesenteric Th17 cells, which led us to speculate that gut flora may influence the development of CNS autoimmune disease in a way dependent of iNKT cells. Consistently, oral KCV treatment did not alter the development of EAE in iNKT cell-deficient mice. These results indicate that iNKT cells play a critical role in the dialogue between host and commensal flora.
Materials and Methods
Mice and Induction of EAE
Six-week-old female B6 mice were purchased from CLEA Laboratory Animal Corporation (Tokyo, Japan). Mr1−/− mice were provided by Dr. Susan Gilfillan, (Washington University School of Medicine, St. Louis)23 and were backcrossed to B6 mice for ten generations. β2-microglobulin−/− mice were purchased from Jackson Laboratories. Cd1 −/−24 and Jα281−/−25 mice were provided by Dr. Steve B. Balk (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA) and Dr. Masaru Taniguchi (Riken Research Center for allergy and Immunology, Yokohama, Japan) respectively. These mice were also back-crossed to B6 mice for ten generations. Animals were maintained in specific pathogen-free conditions in accordance with the institutional guidelines. For induction of EAE, B6 mice were injected subcutaneously with 100 μg MOG (35–55) (MEVGWYRSPFSRVVHLYRNGK) (TORAY Laboratory, Tokyo, Japan) and 1 mg heat-killed Mycobacterium tuberculosis H37RA (Difco) emulsified in incomplete Freund’s adjuvant. 200 ng of pertussis toxin (List Biological Laboratories) in 200 μl PBS was injected i.p. on days 0 and 2 after immunization. Clinical symptoms of EAE were daily evaluated and scored as follows: 0, no clinical signs; 1, loss of tail tonicity; 2, impaired righting reflex; 3, partial hindlimb paralysis; 4, total hindlimb paralysis; 5, moribund or dead.
Antibiotic Treatment of Mice
To treat mice with a mixture of non-absorbing antibiotics, we used a previously described protocol after adding minor modifications.26 Briefly, to examine the effects of altering gut flora, a group of mice were given ad libitum access to drinking water supplemented with kanamycin (1 mg/ml), colistin (2000 U/ml), and vancomycin (0.1 mg/ml). Normal drinking water was given to another group of mice serving as control. For immunological studies of MLNs, LPLs, and splenocytes, the antibiotic-containing water was continuously given for 1 week until individual experiments were conducted. To evaluate the effect of antibiotics on EAE and recall responses, the treatment was started 1 week before immunization, and continued during the entire observation period.
Cell Proliferation and Cytokine Analysis
To measure cell proliferation and cytokine production, we stimulated lymph node cells (1 × 106/well) with anti-CD3 antibody (2C11) at 5 μg/ml for 72 hours in 96-well round-bottomed plates. For evaluating MOG (35–55)-specific recall responses, we stimulated lymph node cells (1 × 106/well) with MOG (35–55) peptide at 1 to 100 μmol/L for 72 hours. The cells were suspended in RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf serum, 2 mmol/L l-glutamine, 100 U/ml of penicillin-streptomycin, 2 mmol/L sodium pyruvate and 50 mmol/L β-mercaptoethanol. T cell proliferation to MOG (35–55) was determined by measuring the incorporation of [3H] thymidine (1 μCi/well) during the last 24 hours of culture in a β-1205 counter (Pharmacia, Uppsala, Sweden). Assays were conducted in triplicate wells and data were expressed as counts per minute (c.p.m.). Culture supernatant was collected 72 hours after stimulation, and cytokines in the supernatant were measured by using cytometric bead array kits for mouse inflammatory cytokines (BD Biosciences) and IL-17 enzyme-linked immunosorbent assay (ELISA) kit (R&D systems).
Surface Marker Analysis, Quantification of CNS Leukocytes and Histology
Cells were stained with fluorescence-labeled specific antibodies after incubation with anti-CD16/32 to avoid nonspecific staining and were analyzed with a FACSCalibur (BD). Except for Foxp3-APC from eBioscience, all of the other antibodies were obtained from BD Pharmingen. For flow cytometric analysis of CNS-infiltrated cells, spinal cords were homogenized, passed through 70-μm nylon mesh and separated by Percoll density-gradient centrifugation to obtain single-cell suspensions. In some experiments, paraffin-embedded spinal cords were stained with either luxol fast blue or H&E for conventional histological analysis.
Intracellular Staining
Cells collected from MLN were stimulated with phorbol 12-myristate 13-acetate (50 ng/ml) and Ionomycin (750 ng/ml) for 5 hours in the presence of GolgiPlug (BD Biosciences). Cells were first stained extracellularly with PerCP-conjugated anti-CD4, APC-conjugated anti-T cell receptor-β and α-GalCer-loaded Dimer X recombinant soluble dimeric mouse CD1 days (BD Pharmingen), and then stained with fluorescein isothiocyanate-conjugated mAb A85-1 specific for mouse IgG1 (BD Pharmingen), and fixed and permeabilized with Fixation/Permiabilization solution (BD Biosciences). Finally, cells were stained intracellulary with phycoerythrin-conjugated anti-IL-17 (BD Biosciences). Samples were acquired on a FACSCalibur (BD Biosciences), and data were analyzed with CELLQuest software (BD Biosciences).
Isolation of Lamina Propria Lymphocytes
Intestines were removed from euthanized mice and placed in ice-cold PBS containing 25 mmol/L HEPES. After removal of residual mesenteric fat tissue, Peyer’s patches were carefully excised, and the intestine was opened longitudinally. The intestine was then thoroughly washed in ice-cold PBS and cut into 1.5-cm pieces. The pieces were incubated four times in 5 ml of 5 mmol/L EDTA, in 10% fetal calf serum/25 mmol/L HEPES/PBS for 15 minutes at 37°C with fast rotation (200 rpm). After each round of incubation, the epithelial cell layer, containing the intraepithelial lymphocytes, was removed. After the fourth EDTA incubation, the pieces were washed in PBS, and placed in 25 ml of RPMI containing 20% fetal calf serum, 25 mmol/L HEPES, and 300 U/ml of Collagenase H (Roche). Digestion was performed three times by incubating the pieces at 37°C for 40 minutes with slow rotation (100 rpm). The solution was then vortexed intensely and passed through a 70-mm cell strainer. The pieces were collected and placed into fresh digestion solution. The procedure was repeated three times. Supernatants from all three digestions from a single small intestine were combined, washed once in cold PBS, resuspended in 5 ml of the 40% fraction of a 40:80 Percoll gradient, and overlaid on 2 ml of the 80% fraction in a 15 ml Falcon tube. Percoll gradient separation was performed by centrifugation for 20 minutes at 2800 rpm at room temperature. LPLs were collected at the interphase of the Percoll gradient, washed once, and resuspended in FACS buffer or T cell medium. The cells were used immediately for experiments.
RNA Extraction and Real-Time Reverse Transcription-PCR
The SV Total RNA isolation kit (Promega) was used for isolation of total RNA from mesenteric lymphocytes or splenocytes according to the manufacturer’s instruction. First-strand cDNA was generated with the Advantage-RT kit (Clontech). The Light Cycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics) was used for quantitative PCR analysis. Gene expression values were normalized to expression of the hypoxanthine guanine phosphoribosyl transferase (Hprt) as ‘housekeeping’ gene. QuantiTect Primer Assay (Qiagen) was used for amplification of IL-21 and IL-23. The other primers used were as follows: HPRT forward, 5′-GTTGGATACAGGCCAGACTTTGTTG-3′; HPRT reverse, 5 ′-GAGGGTAGGCTGGCCTATAGGCT-3′; RORγtforward, 5′-TGTCCTGGGCTACC CTACTG-3′; RORγt reverse, 5′-GTGCAGGAGTAGGCCACATT-3′; TGF-β1 forward, 5′-TGCGCTTGCAGAGATTAAAA-3′; TGF-β1 reverse, 5′-GCTGAATCGAAA GCCCTGTA-3′; IL-6 forward, 5′-TTCCATCCAGTTGCCTTCTT-3′; IL-6 reverse, 5′-CAGAATTGCCQATTGCCATTGCACAAC-3′.
Statistics
EAE clinical severity was daily scored as mean ± SEM for each group, and analyzed by the Mann-Whitney U nonparametric ranking test. Differences in cumulative scores of each group of mice were evaluated by Student’s t-test. Cytokine secretion data were analyzed with Student’s t-test.
Results
Oral KCV Treatment Suppressed the Development of EAE and Inhibited Pro-Inflammatory Cytokine Production from Draining Lymph Node Cells
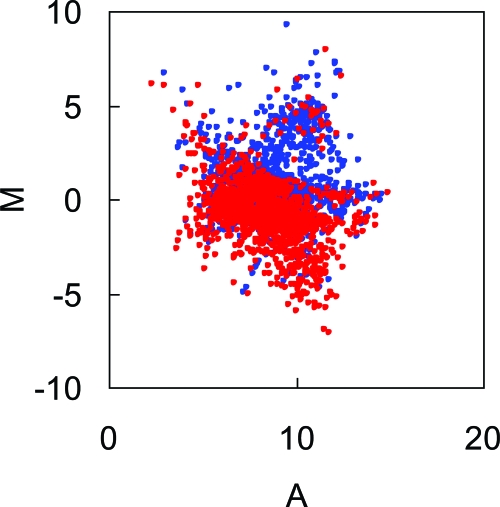
With an attempt to modulate the composition of intestinal flora, we treated wild-type B6 mice orally with a combination of antibiotics KCV as described in Materials and Methods. Because these antibiotics are not absorbed through gut mucosa,27 any effect caused by this treatment is thought to arise from within the gut lumen. To examine whether our treatment protocol would change the composition of intestinal flora, we applied the DNA microarray system referred to as ‘FloraArray’28 and made a comprehensive analysis for intestinal flora derived from KCV-treated mice and control mice. To compare the signal intensities of intestinal flora from the two groups of mice, MA plots were illustrated from the fluorescent images. Although each spot on the FloraArray is derived from a number of different strains in the commensal microflora, this analysis gives us useful information regarding the composition of gut flora. The MA-plot analysis revealed that 722 out of 1536 spots showed more than twofold increase in the fecal DNA sample from KCV-treated mice as compared with those from control mice. By contrast, 894 spots showed more than twofold increase in fecal DNA from control mice as compared with the mice treated with antibiotics (Figure 1). We additionally performed quantitative PCR analysis and revealed that the antibiotic treatment caused differential and reciprocal changes in the quantity of each bacterium species. For example, a great reduction of Lactobacillus murinus and Bacteroides fragilis was seen in the feces from KCV-treated mice, whereas Bacteroides thetaiotaomicron was significantly increased in the same samples of feces (data not shown). These results demonstrate that the protocol of the antibiotic treatment significantly affects the content of intestinal flora.
Figure 1.
Altered composition of the intestinal microflora by oral administration of antibiotics KCV. A custom DNA microarray named ‘FloraArray’27 was used for evaluating the gut flora of mice. Briefly, genomic DNA was extracted from freshly collected fecal samples and fragmented by physical force. DNA fragments of approximately 2.0 kb were inserted into the pUC vector to construct a shotgun library. Plasmid DNA was then extracted from this library. A DNA microarray was fabricated by spotting the randomly selected plasmid DNA without amplification on a glass slide. For analysis of sample DNA by the array, genomic DNA was extracted from fecal content of either control or KCV-treated mice after 1-week treatment with antibiotics KCV, and purified DNA was labeled with Cy3 or Cy5, respectively. Then fluorescent images were analyzed by scanning the array after performing competitive hybridization with mixed labeled DNA on the array. To compare the signal intensities between the two samples with or without antibiotics treatment, the data spots were displayed as MA plots. Red circles and blue circles represent data of samples from control and KCV-treated mice, respectively.
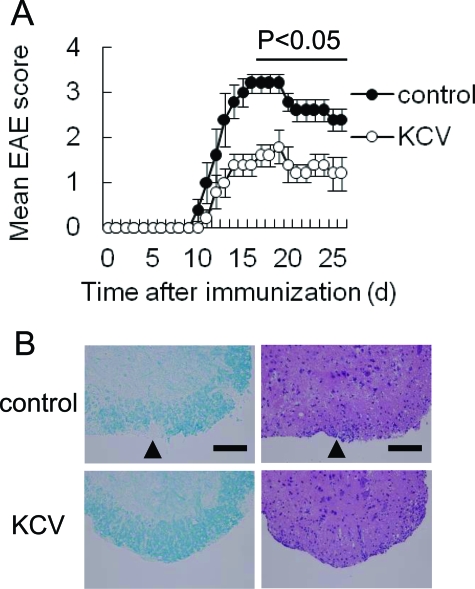
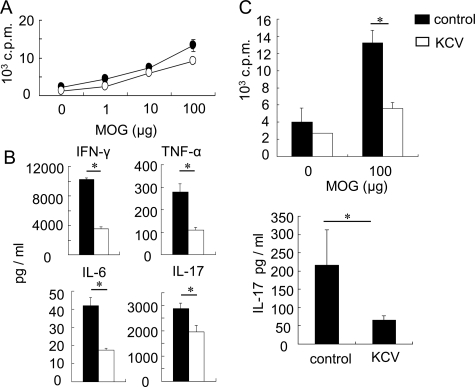
We next addressed whether the change of intestinal flora could also modulate the progression of EAE, an animal model of MS. When we continuously treated the mice with KCV-containing drinking water from 1 week before immunization, clinical signs of MOG (35–55)-induced EAE were significantly suppressed in comparison with control mice (Figure 2A). Accordingly, histological examination showed a reduced infiltration of mononuclear cells and less noticeable demyelination at the lumbar region of the spinal cord of the treated mice (Figure 2B). Moreover, we observed a lower number of total CNS infiltrating cells at an active stage of EAE (day 18) in KCV-treated mice than in control mice when we isolated mononuclear cells from CNS of those mice (data not shown). In parallel, we examined the recall responses of the dLNs to MOG (35–55) on day 11 after immunization. Although proliferation rates of the dLNs in response to MOG (35–55) were comparable between KCV-treated mice and control mice (Figure 3A), the dLN cells from the treated mice produced significantly lower amounts of pro-inflammatory cytokines IFN-γ, TNF-α, IL-6, and IL-17 in response to MOG (35–55) (Figure 3B), consistent with the suppressed signs of EAE. We also measured the recall response of the MLNs to MOG (35–55). The MLN cells from control mice immunized with MOG (35–55) showed significant responses to the MOG peptide in the proliferative responses as well as IL-17 production (Figure 3C). However, those from KCV-treated mice showed only marginal responses, indicating that induction of MOG (35–55) specific encephalitogenic Th17 cells in both dLNs and MLNs is impaired by an alteration of intestinal contents caused by the antibiotic treatment.
Figure 2.
Suppression of EAE by oral KCV treatment. A: Clinical score of EAE. After immunized with MOG (35–55) mice were treated with KCV as described in Materials and Methods. Clinical EAE scores of KCV-treated mice (KCV) and of control mice (control) are shown. Data represent mean score ± SEM from a representative of three experiments (n = 5 for each group of mice). The bar indicates the duration during which a significant difference was observed between KCV and control; *P < 0.05 (Mann-Whitney U-test). B: Histopathological assessment of the CNS region in EAE-induced mice. Shown are cellular infiltration and demyelination (arrowheads) of the lumbar spinal cord of control or KCV-treated mice on day 18. Paraffin-embedded spinal cords were stained with luxol fast blue (left panels) or H&E (right panels). Representative figures from two separate experiments are demonstrated. Scale bar = 100 μm.
Figure 3.
Reduced MOG-specific responses in dLN and MLN cells from KCV-treated mice. A: Effect of KCV on the lymphocyte proliferative responses. Draining lymph nodes (dLNs) were removed from control or KCV-treated mice 11 days after immunization with MOG (35–55) and the total lymphoid cells (1 × 106) were stimulated with varying doses of MOG (35–55) peptide for 72 hours. Proliferative responses were assessed by [3H] thymidine incorporation. Data are from one of three independent experiments, showing the mean of triplicate samples. B: Effect of KCV treatment on MOG (35–55)-reactive T cells in the dLN. Supernatants were collected after stimulating the dLN cells of day 11 with 100 μmol/L MOG (35–55) peptide in vitro for 72 hours. Cytokine concentration was measured by cytometric bead array or ELISA as described in Materials and Methods. Data represent the mean ± SEM of duplicated samples from one of three separate experiments (n = 2 mice). *P < 0.05 (two-tailed Student’s t-test.) C: Effect of KCV treatment on MOG (35–55)-reactive T cells in the MLN. Whole MLN cells were isolated from control or KCV-treated mice (n = 2) 11 days after EAE induction. The cells were stimulated with MOG (35–55) as conducted for dLN cells and proliferative responses (upper panel) and IL-17 production (lower panel) were measured. IL-17 was measured by using ELISA. Data represent the mean ± SEM of triplicate samples from one of two independent experiments (n = 2 mice). *P < 0.05 (two-tailed Student’s t-test).
Mesenteric Lymphocytes from Naive Mice Produce a Lower Amount of IL-17 after KCV Treatment
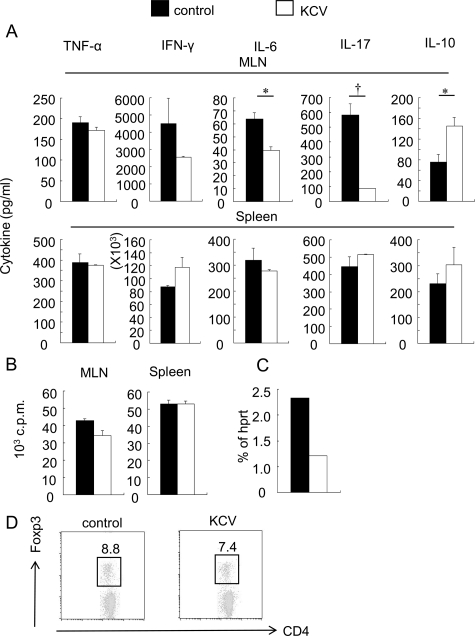
MLNs are thought to offer an important site for the functional cross talk between intestinal microflora and gut immunity.29,30 Next we investigated whether the antibiotic treatment induced an alteration of the MLN cell functions in naïve wild-type mice. First we compared the ability of the MLN cells to produce pro-inflammatory cytokines on stimulation with plate-bound anti-CD3 antibody. Proliferative responses of the MLN cells were not affected or slightly suppressed at most by KCV treatment. Interestingly, MLN cells from KCV-treated mice secreted significantly lower amounts of IL-6 and IL-17 compared with those from control mice, whereas production of TNF-α and IFN-γ was not significantly suppressed (Figure 4, A and B). In contrast, splenocytes from both groups of mice showed essentially similar result following stimulation with anti-CD3 (Figure 4, A and B). Recently, Ivanov et al showed that an orphan nuclear receptor RORγt is the key transcription factor that orchestrates the differentiation of the Th17 cell lineage.9 They also showed that Th17 cells tend to accumulate in the mucosa of the small intestine. Quantitative RT-PCR analysis revealed a lower expression of RORγt in the MLN cells from KCV-treated mice as compared with control mice (Figure 4C). We also found that the MLN cells from KCV-treated mice secreted significantly greater amounts of IL-10 than those from control mice (Figure 4A), suggesting that the mesenteric T cells would acquire less inflammatory properties after the antibiotic treatment.
Figure 4.
Decreased production of inflammatory cytokines from MLN cells after oral KCV treatment. A: Cytokine production from MLN T cells of naïve mice after KCV treatment. Mice were continuously given KCV-containing or control water for 7 days. Then MLN cells and splenocytes were isolated and stimulated by immobilized anti-CD3. MLNs (top panels) or splenocytes (bottom panels) from control or KCV-treated mice (unprimed) were stimulated with immobilized anti-CD3 antibody for 72 hours. Cytokines in the supernatants were measured by using cytometric bead array or ELISA. Data are from a representative out of three independent experiments (n = 2 mice). *P < 0.05, †P < 0.001, (Student’s t-test). B: Proliferative responses of MLN cells after anti-CD3 stimulation. MLN cells and splenocytes were prepared as described in (A). Proliferative responses were assessed by [3H] thymidine incorporation. Data represent the mean ± SEM of triplicate samples from one out of three independent experiments (n = 2 mice). *P < 0.05 (Student’s t-test). C: Reduction of RORγt expression after KCV treatment. Total RNA was isolated from pooled MLN cells prepared from control mice or from mice given oral KCV treatment for 7 days. RORγt mRNA was estimated by quantitative RT-PCR and all data were normalized to hprt (n = 2 mice). D: Intracellular expression of Foxp3 for gated CD4+ T cells derived from MLNs. Mice were given control or KCV-containing water for 1 week. Dot plots are gated on CD4+ T cells. Data are representative of three independent experiments showing similar results.
Next we examined whether this treatment may alter the composition of lymphocytes in the MLN. We found that the total number of MLN cells was almost equal in KCV-treated and control mice (data not shown). Furthermore, flow cytometric analysis demonstrated that the proportion of dendritic cells, macrophage/monocytes, B cells, conventional CD4+ and CD8+ T cells, NK cells, and NKT cells in the MLN did not change after treatment with KCV (data not shown). These data indicate that the antibiotic treatment protocol does not exhibit any cytotoxic effect on the mesenteric lymphocyte populations, although it remarkably alters the cytokine profile of T cells. We also examined the frequency of Foxp3+ regulatory CD4+ T cells in the MLN. Although recent studies have revealed the presence of reciprocal developmental pathways between Th17 cells and Foxp3+ regulatory T cells,31 we could not detect any increase of CD4+Foxp3+ T cells in the MLN cells after KCV treatment (Figure 4D).
A Role of Vα14 iNKT Cells in the Regulation of Mesenteric Th17 Cells that Are Vulnerable to KCV Treatment
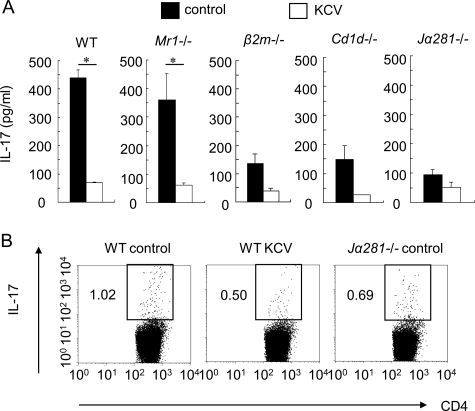
Recent studies have revealed that MR1-restricted invariant Vα19-Jα33 T cells, also referred to as mucosal associated invariant T (MAIT) cells, are preferentially distributed to gut LP and are strikingly influenced by the presence of gut flora.23,32 We have recently shown that the MAIT cells could play a regulatory role in EAE.33 Because of their dependence on commensal flora23,32 we speculated that the antibiotic treatment might suppress the Th17 cell-mediated EAE disease by using the regulatory function of MAIT cells triggered by a change of flora. To verify this idea, we treated MAIT cell-deficient Mr1−/− mice as well as wild-type B6 mice with oral KCV, and examined the ability of the MLN cells to produce IL-17 after anti-CD3 stimulation. Contrary to our speculation, the results showed that the MLN cells from Mr1−/− mice and wild-type mice produced an equivalent amount of IL-17 either before or after KCV treatment (Figure 5A), indicating that MAIT cells do not play a major role in the suppression of Th17 cells by KCV treatment. However, in additional experiments using β2-microglobulin−/− (β2m−/−) mice, we found that the baseline production of IL-17 by the MLN T cells after anti-CD3 stimulation was remarkably diminished in the mice, whereas the mesenteric T cells from β2m−/− mice and wild-type mice produced a similar amount of IL-17 after KCV treatment. Accordingly, oral KCV causes only a marginal reduction of IL-17 in β2m−/− mice, indicating that class I-restricted T cells other than MAIT cells play a critical role in the KCV-induced suppression of the Th17 cells within MLN.
Figure 5.
A role of Vα14 iNKT cells in the regulation of mesenteric Th17 cells. A: IL-17 production by the MLN T cells of mice lacking invariant iNKT or MAIT cells. After 1 week of KCV treatment, MLN cells were isolated from control or KCV-treated mice, including wild-type (WT), Mr1−/−, β2m−/−, Cd1−/−, or Jα281−/− mice. The cells were stimulated with immobilized anti-CD3 antibody for 72 hours. IL-17 in the supernatant was measured using ELISA. Data are a representative of two independent experiments (n = 2 mice). *P < 0.05 (Student’s t-test). B: Th17 cells in MLNs in KCV-treated or iNKT deficient mice. MLN cells were isolated from wild-type mice (WT control), KCV-treated wild-type mice (WT KCV), or iNKT cell-deficient Jα281−/− mice and stimulated for 5 hours with phorbol 12-myristate 13-acetate and ionomycin in the presence of GolgiPlug. We conducted surface labeling with the indicated antibody and αGalCer-loaded CD1 days dimer as well as intracellular IL-17 staining. Dot plots are gated on CD4+ T cells devoid of iNKT cells. Data are representative of two independent experiments (n = 2 mice).
Then we explored a possible role of Vα14 iNKT cells restricted by CD1 days, an MHC class 1b molecule. As is widely known, iNKT cells produce a variety of regulatory cytokines after recognizing glycolipid antigens such as α-galactocylceramide (α-GalCer) in association with CD1 days. Numerous reports have supported the role of iNKT cells in the regulation of autoimmunity.22,34,35 We, therefore, repeated our above experiments using Cd1−/− mice,24 which do not express either iNKT cells or non-invariant type II NKT cells,36, as well as with Jα281−/− mice,25 in which iNKT cells alone are specifically deleted. In these iNKT cell-deficient mice, we again found a great reduction in the baseline production of IL-17 from the MLNs after anti-CD3 stimulation. Furthermore, effects of oral KCV on the Th17 cells were only marginal, if any, in the mice (Figure 5A), raising a possibility that the host immune system may sense the change of gut flora by using iNKT cells.
It is now known that IL-17 secreting CD4+ MLN cells comprise not only Th17 cells but also CD4+ Vα14 iNKT cells.37 To evaluate the alteration of mesenteric Th17 cells with accuracy, we next evaluated the proportion of IL-17+ CD4+ T cells after excluding iNKT cells by gating. By analyzing the MLN cells from wild-type mice (WT control), KCV-treated wild-type mice (WT KCV), or iNKT cell-deficient Jα281−/− mice (Figure 5B), we have confirmed that the number of IL-17+ CD4+ T cells corresponding to Th17 cells is reduced in the KCV-treated wild-type mice and in the iNKT cell-deficient Jα281−/− mice. We also noticed that IL-17+ iNKT cells are 15 times lower than IL-17+ CD4+ T cells in wild-type mice (data not shown).
Oral KCV Treatment Inhibits Production of Th17-Promoting Cytokines in the Intestinal Lamina Propria
Next we sought to identify a primary event that would take place in the intestinal immune system following oral KCV treatment. Because the vast majority of Th17 cells in the MLNs appear to depend on iNKT cells (Figure 5A), we evaluated the number and function of iNKT cells in the MLNs. However, neither reduction nor increase of iNKT cells was found in the MLNs after the antibiotic treatment (data not shown). In addition, the MLN cells from KCV-treated mice and from control mice produced similar levels of cytokines in response to α-GalCer (data not shown). These results indicate that as seen with CD4+Fox3+ T cells (Figure 4D), iNKT cells in the MLN are not significantly influenced by the status of gut flora. Therefore, we postulate that local accumulation of regulatory cells is probably not the mechanism for the reduction of Th17 cells in the MLN of KCV-treated mice (Figures 3 and 4). By using quantitative RT-PCR, we also measured mRNA expression of TGF-β, IL-6, IL-21, and IL-23 in the MLNs, which play key roles in the development or maintenance of Th17 cells in the intestine.7,31,38,39,40 However, expression of these Th17-promoting cytokines did not change after KCV treatment (data not shown). Taking these results together, we assumed that the reduction of Th17 cells in the MLNs might result from a primary event that takes place upstream to the MLNs. Therefore, we shifted our attention from MLNs to intestinal LPLs.
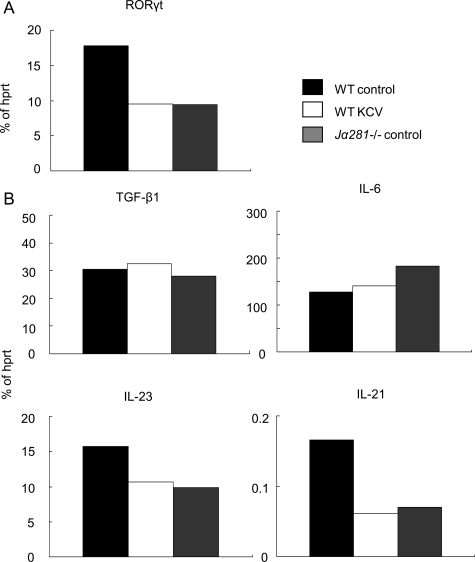
Notably, Th17 cells constitutively inhabit LP,9 and more iNKT cells are detected in LP than in MLN (our unpublished data). We first confirmed that RORγt expression was significantly reduced in the LPLs from KCV-treated wild-type mice as compared with those from control wild-type mice (Figure 6A), indicating that a reduced number of Th17 cells could be traced upstream to the LP. Moreover, the LPLs from iNKT cell deficient Jα281−/− mice showed a reduced expression of RORγt, again indicating the importance of iNKT cells for the maintenance of Th17 cells. We further quantified mRNAs of TGF-β1, IL-6, IL-23, and IL-21 expressed by LPLs by RT-PCR. Compared with the LPLs from control wild-type mice, those from KCV-treated wild-type mice and from Jα281−/− mice showed a reduced expression of IL-21 (Figure 6B). Expression of IL-23 was also reduced in KCV-treated wild-type mice as well as in Jα281−/− mice. These results support our postulation that LPLs are primarily influenced by the antibiotic treatment, resulting in a downstream decrease in the number of Th17 cells.
Figure 6.
Reduced expression of Th17-promoting cytokines in the intestinal lamina propria lymphocytes from KCV-treated mice as well as iNKT-deficient mice. A: RORãt expression in the intestinal LP after treatment with oral KCV. Total RNA was isolated from pooled LPLs prepared from wild-type control B6 mice (WT control), KCV-treated wild-type B6 mice (WT KCV), and control Jα281−/− mice (Jα281−/− control) (n = 2). Wild-type KCV were given oral KCV for 7 days before the analysis. RORγt mRNA was estimated by quantitative RT-PCR and all data were normalized to hprt. Data are representative of two independent experiments. B: Expression of Th17-promoting cytokines in the intestinal LPL. Total RNA was isolated from LPLs of the three groups of mice as described in (A). Expression of TGFβ−1, IL-6, IL-23, and IL-21 mRNA was estimated by quantitative RT-PCR and all data were normalized to hprt. Data are a representative of two independent experiments.
Suppressive Effect of KCV Treatment on EAE Is Abolished in iNKT-Cell Deficient Mice
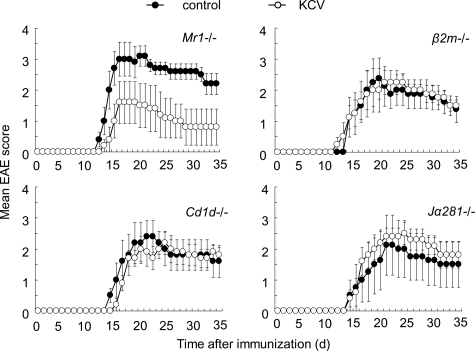
The ex vivo experiments have demonstrated that Th17 cells in the MLN and LP are affected by KCV treatment in association with suppressed signs of EAE. Moreover, we showed that the KCV effects on Th17 cells could not be seen in the absence of iNKT cells. Although the results indicate an intimate relationship between Th17 cells and iNKT cells in the intestinal immune system, it does not necessarily imply that altering gut flora would suppress the development of EAE in a way dependent of iNKT cells. To make this point clear, we examined the effects of oral KCV treatment on the development of EAE induced in iNKT cell-deficient mice (β2m−/−, Cd1−/−, Jα281−/−) as well as in MAIT cell-deficient mice (Mr1−/−) (Figure 7). First, we noted that clinical EAE induced in Mr1−/− mice was significantly suppressed by KCV treatment, which coincides with the fact that the mesenteric Th17 cells are not affected by the absence of MAIT cells (Figure 6A). In contrast, suppressive effects of oral KCV was almost completely abolished in β2m−/−, Cd1−/−, and Jα281−/− mice (Figure 7), allowing us to conclude that iNKT cells play a key role in the KCV-induced suppression of EAE.
Figure 7.
Suppressive effect of oral KCV treatment on EAE is abolished in Vα14 iNKT-deficient mice. Mr1−/−, β2m−/−, Cd1−/−, and Jα281−/− mice were treated with KCV as described in Materials and Methods. After immunization of mice with MOG (35–55) clinical EAE scores of mice were assessed. Data represent mean score ± SEM from two independent experiments (n = 4 or 5 mice).
Health Status of KCV-Treated Mice
We have observed that antibiotic treatment tended to cause loose stool in the KCV treated mice. However, this happened in both wild-type mice and iNKT cell-deficient mice, which does not validate speculation on any relation with the EAE disease suppression by KCV. Furthermore, KCV-treatment did not cause a significant change in body weight. We also examined the histology of gut lumen, and found that KCV treatment did not cause any pathological changes.
Discussion
The present study has experimentally demonstrated that altering gut flora by non-absorbing antibiotics could lead to protection against autoimmune disease EAE. Although the suppressive effect of antibiotics on EAE has been previously described,41 the prior study did not address the possible contribution of the altered gut flora and has correlated the EAE suppression with an altered Th1/Th2 balance. In contrast, the present study has linked the antibiotic effects with a reduced number of Th17 cells in the gut-associated immune system. Most notably, the immunomodulatory effects of KCV could not be seen in iNKT cell-deficient mice, as assessed by the number of mesenteric Th17 cells or by severity of EAE. Comparison of wild-type and iNKT cell-deficient mice revealed that iNKT cells in the wild-type mice are able to promote the maintenance of mesenteric Th17 cells in the steady state, whereas the disease promoting ability of iNKT cell is impaired by KCV treatment. Given that oral administration of synthetic glycolipid ligands stimulatory for iNKT cells could alter the manifestation of autoimmune diseases,22,42 one may speculate that oral KCV treatment leads to the appearance or disappearance of glycolipid ligands in the intestinal content that critically influence the function of iNKT cells.
The mucosal sites continuously sample foreign materials mainly via M cells in Peyer’s patch and dendritic cells (DCs) in the LP.43 The DCs in the LP would present orally applied antigens, migrate and enter the MLN.44,45 Therefore, we wondered if the MLN might serve as the primary site where a contraction of Th17 cells takes place via mechanisms involving regulatory cells or changes of local cytokine milieu. However, the antibiotic treatment did not influence iNKT cells or Foxp3+ regulatory T cells in the MLNs. Cytokines needed for promoting Th17 cell development and survival were not altered either, indicating that a critical event causing a reduction of Th17 cells probably takes place upstream. Consistent with this idea, we showed that expression of IL-21 and IL-23 in the LPLs was significantly suppressed in KCV-treated mice and iNKT cell-deficient mice. The role of IL-21 in the development of Th17 cells39 has been demonstrated in mice lacking IL-6, the cytokine originally identified as a crucial promoter of Th17 cells. Intriguingly, it has recently been reported that IL-21 plays a critical role in the regulation of Th17 cells involved in gut inflammation.38 Taken together, we suggest that the suppression of IL-21 and IL-23 may be a primary event after KCV treatment, which leads to the reduction of mesenteric Th17 cells. It is known that both iNKT cells and Th17 cells are able to produce IL-21.46,47 Given that iNKT cells in the MLNs were not altered after KCV treatment, we speculated that iNKT cells within LP may numerically or functionally be altered, which could account for the reduced IL-21 in the LPLs. However, because of technical limitations, we have not definitively demonstrated that this is the case. Although a recent report using IL-21 knockout mice showed that IL-21 is not essential for the development of Th17 cells in vitro and in vivo,48 it does not exclude the role of IL-21 in wild-type mice.
It is arguable that the reduced Th17 cells in the MLN cells from KCV-treated mice may result from a direct or indirect effect of KCV on DCs. However, flow cytometric analysis did not reveal any difference between KCV-treated and control mice with regard to the surface levels of MHC class II, CD80 or CD86 on the MLN-DCs (data not shown). In addition, there was no alteration of CD103 on the MLN-DCs that is described as an inducer of Foxp3+ regulatory T cells.49
Although we have so far focused on analysis of Th17 cells and iNKT cells in the gut immune system, we cannot overlook that dLN cells from KCV-treated mice produced a lower amount of IFN-γ in response to MOG (35–55) indicating that Th1 cells in the dLNs could be also affected by KCV treatment. Interestingly, a concomitant reduction of Th1 cells and Th17 cells has recently been demonstrated in EAE mice treated with anti-IL-6 receptor antibody, which was used for aiming at specific suppression of Th17 cells.50 These homologous results suggest the possible induction of regulatory T cells in the dLN that may regulate both Th1 and Th17 cells. Although Foxp3+ regulatory T cells are qualified suppressors, total number of the CD4+Foxp3+ T cells in dLN was not altered after KCV treatment. It is possible that MOG (35–55) specific regulatory T cells might be selectively induced by altering gut flora. It is obvious that further studies are needed to clarify the total picture of NKT cell-dependent suppression of EAE by altering gut flora.
There is a clear tendency for an increased incidence of immune-mediated disorders in developed countries.51 Although this increase has often been linked with improved hygiene, a number of studies have suggested a role for commensal flora affected by life style.52 This is an attractive idea, in particular for inflammatory bowel disease, where the target is the gut and is inhabited by pathogenic Th17 cells as well as regulatory cells such as MAIT cells. In contrast, much less attention has been paid on the role of commensal flora in the development of the CNS autoimmune disease MS. The present study emphasizes that the repertoire of the immune system is greatly regulated by gut flora, which has broad implications for understanding the pathogenesis of autoimmune disease and allergy, and could be applied for future studies. However, it is too early to suggest that antibiotic treatment will be beneficial for MS. Indeed, altering gut flora could trigger or prevent the development of autoimmune conditions. Future studies coping with such variables as timing, duration, choice of antibiotics used for treatment will not only give us deeper understanding on the interaction between gut flora and Th17 cells, but also provide important information related to the human health.
Acknowledgments
We thank Dr. Masanobu Nanno (Yakult Central Institute for Microbiological Research, Tokyo, Japan) for providing the protocol of LPL isolation. We also thank Miho Mizuno, Chiharu Tomi, and Tomoko Ozawa for their great technical assistance.
Footnotes
Address reprint requests to Dr. Takashi Yamamura, Department of Immunology, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1 Ogawahigashi, Kodaira, Tokyo 187-8502, Japan. E-mail: yamamura@ncnp.go.jp.
Supported by the Health and Labour Sciences Research Grants on Brain Science and on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan.
References
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Coffman RL. Origins of the TH1-TH2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Yamamura T. Interleukin 17-producing T-helper cells and autoimmune diseases: time for a paradigm shift? Curr Rheumatol Rep. 2007;9:93–95. doi: 10.1007/s11926-007-0001-6. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis EE, Visser MR, Kavelaars A, Cobelens PM, Fleer A, Harmsen W, Verhoef J, Akkermans LM, Heijnen CJ. Oral antibiotics as a novel therapy for arthritis: evidence for a beneficial effect of intestinal Escherichia coli. Arthritis Rheum. 2000;43:2583–2589. doi: 10.1002/1529-0131(200011)43:11<2583::AID-ANR28>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Sun J, Weber P, Navarro P, Francis D. Antibody repertoire development in fetal and newborn piglets. III Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology. 2000;100:119–130. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock GW. Molecular assessment of intestinal microflora. Am J Clin Nutr. 2001;73:410S–414S. doi: 10.1093/ajcn/73.2.410s. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Sakuishi K, Illes Z, Miyake S. Understanding the behavior of invariant NKT cells in autoimmune diseases. J Neuroimmunol. 2007;191:8–15. doi: 10.1016/j.jneuroim.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- Chambers HF. General Consideration of Antimicrobial Therapy. Brunton LL, Lazo JS, Parker KL, Goodman LS, Gilman AG, editors. New York: McGraw-Hill; Goodman & Gilman’s The Pharmacological Basis of Therapeutics eleventh edition. 2005:pp. 1095–1110. [Google Scholar]
- Yokoi T, Kaku Y, Suzuki H, Ohta M, Ikuta H, Isaka K, Sumino T, Wagatsuma M. ‘FloraArray’ for screening of specific DNA probes representing the characteristics of a certain microbial community. FEMS Microbiol Lett. 2007;273:166–171. doi: 10.1111/j.1574-6968.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, Maron R, Weiner HL. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol. 2006;18:519–526. doi: 10.1016/j.coi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant Vα19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- Miyake S, Yamamura T. Therapeutic potential of glycolipid ligands for natural killer (NK) T cells in the suppression of autoimmune diseases. Curr Drug Targets-Immune, Endocrine, and Metabolic Disorders. 2005;5:315–322. doi: 10.2174/1568008054863772. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Ann Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, Pallone F, Macdonald TT, Monteleone G. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13:485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Turnbull EL, Yrlid U, Jenkins CD, Macpherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–1384. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkotter HJ. Site-specific expression of CD11b and SIRPα (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur J Immunol. 2005;35:1418–1427. doi: 10.1002/eji.200425726. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T, Takahashi T, Ripley B, Kimura A, Kishimoto T, Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25 Suppl:74–80. doi: 10.1016/j.jaut.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54:317–320. doi: 10.1136/gut.2004.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]