Abstract
Pseudoexfoliation (PEX) syndrome is a generalized disease of the extracellular matrix and the most common identifiable cause of open-angle glaucoma. Two single nucleotide polymorphisms in the lysyl oxidase-like 1 (LOXL1) gene (rs1048661 and rs3825942) have been recently identified as strong genetic risk factors for both PEX syndrome and PEX glaucoma. Here we investigated the expression and localization of LOXL1, LOXL2, and lysyl oxidase (LOX) in tissues of PEX syndrome/glaucoma patients and controls in correlation with their individual single nucleotide polymorphism genotypes and stages of disease. LOXL1 ocular expression was reduced by ∼20% per risk allele of rs1048661, whereas risk alleles of rs3825942, which were highly overrepresented in PEX cases, did not affect LOXL1 expression levels. Irrespective of the individual genotype, LOXL1 expression was significantly increased in early PEX stages but was decreased in advanced stages both with and without glaucoma compared with controls, whereas LOX and LOXL2 showed no differences between groups. LOXL1 was also found to be a major component of fibrillar PEX aggregates in both intra- and extraocular locations and to co-localize with various elastic fiber components. These findings provide evidence for LOXL1 involvement in the initial stages of abnormal fibrogenesis in PEX tissues. Alterations of LOXL1 activation, processing, and/or substrate specificity may contribute to the abnormal aggregation of elastic fiber components into characteristic PEX fibrils.
Pseudoexfoliation (PEX) syndrome represents a common, clinically significant, generalized disease of the extracellular matrix characterized by the progressive, stable deposition of abnormal fibrillar aggregates in various intra- and extraocular tissues.1 Gradual accumulation of this material in the outflow pathways may cause a common and severe type of chronic open-angle glaucoma accounting for the majority of glaucoma cases in some countries.2 In addition, increasing evidence suggests that PEX syndrome is a risk factor for cardiovascular and cerebrovascular disease.1 Although its exact etiology and pathogenesis are still unknown, recent molecular biological and biochemical data support the pathogenetic concept of PEX syndrome as a type of stress-induced elastosis, associated with the excessive production and abnormal aggregation of elastic fiber components, enzymatic cross-linking processes, increased levels of transforming growth factor-β1, a proteolytic imbalance between matrix metalloproteinases and their inhibitors, and impaired protection mechanisms against oxidative and cellular stress.1,3,4,5 Both population-based and pedigree-based studies have shown that genetic factors contribute to the pathogenesis of PEX syndrome.6,7
Recently, a strong genetic risk factor for PEX syndrome/glaucoma has been identified. Thorleifsson and associates8 established a significant association between common single nucleotide polymorphisms (SNPs) in the lysyl oxidase-like 1 (LOXL1) gene on chromosome 15q24.1 with both PEX syndrome and PEX glaucoma in Icelandic and Swedish populations. A common haplotype (G-G) formed by two nonsynonymous coding SNPs, rs1048661 (R141L) and rs3825942 (G153D), in exon 1 of LOXL1 accounted for the vast majority of PEX cases. Several replication studies in populations from the United States,9,10,11,12,13 Australia,14 Europe,15,16 Japan,17,18 and India19 confirmed genetic susceptibility of LOXL1 polymorphisms to PEX syndrome/glaucoma and indicated that the LOXL1 gene is the principal genetic risk factor for this condition.
LOXL1 is a member of the lysyl oxidase family of enzymes that catalyze the covalent cross-linking of collagen and elastin in connective tissues through oxidative deamination of lysine or hydroxylysine side chains. They comprise five characterized members: lysyl oxidase (LOX) and lysyl oxidase-like 1 to 4 (LOXL1 to LOXL4).20,21,22 LOXL1 seems to be specifically required for tropoelastin cross-linking and has been shown to be involved in elastic fiber formation, maintenance, and remodeling.23,24 Thus, it is conceivable that genetic variation in the LOXL1 gene may contribute to the formation and accumulation of the aberrant fibrillar aggregates in tissues of PEX patients representing the hallmark of PEX syndrome.
To determine the role of LOXL1 in the pathophysiology of PEX syndrome/glaucoma, we investigated the expression and localization of LOXL1 in tissues from PEX and control patients in correlation with their individual SNP genotypes and stages of disease. Expression levels of LOX and LOXL2 were also analyzed for comparison. The findings provided evidence for a selective up-regulation of LOXL1 in the initial stages of abnormal fibrogenesis in PEX tissues and for LOXL1 as a major component of PEX aggregates in clear co-localization with elastic fiber components. The risk alleles of rs1048661 were associated with reduced ocular LOXL1 expression levels. In contrast, the risk alleles of rs3825942, which were highly overrepresented in PEX cases, had no effect on expression levels, but are suggested to have functional consequences on enzyme activation and/or substrate binding and to contribute to abnormal cross-linking and aggregation of elastic fiber components into PEX fibrils.
Materials and Methods
Tissues
This study included a total of 25 eyes with PEX syndrome/glaucoma and 25 control eyes without evidence of PEX. First, ocular tissues were obtained from 18 normal-appearing donor eyes (age, 78.0 ± 6.5 years; 10 females, 8 males) and 18 donor eyes with PEX syndrome without glaucoma (age, 79.7 ± 5.0 years; 11 females, 7 males). These eyes were obtained at autopsy and were processed within 8 hours after death. The donor eyes had no documented history of any ocular disease and the presence of PEX syndrome was diagnosed by macroscopic evidence of PEX deposits on lens, iris, ciliary processes, and zonules and confirmed by electron microscopic analysis of small tissue sectors. PEX eyes were classified as early or late stage according to a semiquantitative grading score of the amount of macroscopically visible PEX material deposits on ocular structures. Because PEX deposits can be detected earliest on zonular fibers,2,25 early stages (n = 9) were defined by a frosted appearance of the zonules, whereas late stages (n = 9) revealed prominent PEX deposits on lens, iris, and ciliary processes in addition. The absence of glaucoma in these eyes was confirmed by macroscopic evaluation of the optic disk and light microscopic analysis of optic nerve cross sections.
In addition, we used four eyes of patients with a documented history of PEX-associated open-angle glaucoma (age, 78.8 ± 1.8 years; two females, two males), three eyes of patients with primary open-angle glaucoma (age, 76.7 ± 7.8 years; two females, one male), three eyes with PEX-associated angle-closure glaucoma (age, 83.3 ± 3.1 years; two females, one male), and four eyes with angle-closure glaucoma without PEX syndrome (age, 82.5 ± 5.2 years; three females, one male). These eyes had to be surgically enucleated because of painful absolute glaucoma and were processed immediately after enucleation. The demographics and relevant clinical parameters of the glaucoma patients are shown in Table 1.
Table 1.
Clinical Characteristics of Surgically Enucleated Glaucoma Eyes
| Patient | Age/Sex | Glaucoma type | IOPmax. | C/D ratio | Medication | Ocular surgery |
|---|---|---|---|---|---|---|
| 1. F.H. (L) | 81/F | SOAG, PEX | 36 | 1.0 | Travoprost | None |
| Brinzolamide | ||||||
| 2. F.S. (R) | 77/M | SOAG, PEX | 45 | 1.0 | Timolol | Filtration surgery |
| Brimonidine | ||||||
| 3. H.W. (R) | 77/M | SOAG, PEX | 56 | 1.0 | Latanoprost | Laser trabeculoplasty |
| Timolol, Dipivefrin | ||||||
| 4. E.D. (L) | 80/F | SOAG, PEX | 42 | 1.0 | Latanoprost | Filtration surgery |
| Timolol | ||||||
| 5. L.F. (R) | 87/F | POAG | 35 | 1.0 | Timolol | Filtration surgery |
| Pilocarpine | ||||||
| 6. H.G. (R) | 68/M | POAG | 48 | 1.0 | Pilocarpin | Laser trabeculoplasty |
| Carbachol | ||||||
| 7. K.H. (L) | 75/F | POAG | 26 | 1.0 | Timolol | Cataract surgery |
| 8. M.F. (R) | 86/F | ACG, PEX | 40 | 1.0 | Timolol | None |
| 9. F.S. (L) | 85/F | ACG, PEX | 42 | 0.95 | None | None |
| 10. N.P. (R) | 79/M | ACG, PEX | 40 | 0.95 | None | None |
| 11. J.D. (R) | 80/M | ACG, CRVO | 56 | 1.0 | Dorzolamide | None |
| Dipivefrine | ||||||
| 12. L.H. (L) | 84/F | ACG, CRVO | 38 | 1.0 | Latanoprost | Cataract surgery |
| Brimonidine | ||||||
| 13. B.E. (R) | 90/F | ACG, CRVO | 52 | 1.0 | None | Cataract surgery |
| 14. C.H. (L) | 76/F | ACG, CRVO | 40 | 0.95 | None | None |
F, female; M, male; R, right eye; L, left eye; ACG, angle-closure glaucoma; CRVO, central retinal vein occlusion; OAG, open-angle glaucoma; PEX, pseudoexfoliation; POAG, primary open-angle glaucoma; SOAG, secondary open-angle glaucoma.
All eyes were bisected and one half was used for RNA extraction (RNeasy kit; Qiagen, Hilden, Germany), genotyping, and real-time polymerase chain reaction (PCR), and the second half was used for immunohistochemistry. Ocular tissues were prepared under a dissecting microscope and shock-frozen in liquid nitrogen. Moreover, tissue specimens of various organ systems (skin, heart, lungs, liver, kidney, abdominal aorta) were obtained from four organ donors with PEX syndrome (age, 79.0 ± 2.0 years) and four organ donors without PEX (age, 68.4 ± 8.2 years) and processed for immunohistochemistry only. Informed consent to tissue donation was obtained from the patients or, in case of autopsy eyes, from their relatives, and the protocol of the study was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki for experiments involving human tissues and samples.
Genotyping
The two nonsynonymous coding SNPs, rs3825942 and rs1048661, of the LOXL1 gene were genotyped through direct sequencing of cDNA. A primer pair (Thermo Fisher, Dreieich, Germany) spanning the two selected SNPs in exon 1 of the LOXL1 gene were designed by means of Primer 3 software (http://fokker.wi.mit.edu/primer3/input.htm) and reverse transcriptase (RT)-PCR was performed on cDNA derived from ciliary body tissue of 25 PEX patients and 25 control individuals (Table 2). PCR products were purified using AMPure (Agencourt Bioscience, Beverly, MA) on a Biomek NX96 platform (Beckman Coulter, Fullerton, CA) and were sequenced using an ABI Prism fluorescent dye terminator system (Applied Biosystems, Foster City, CA). Purified sequence reactions using CleanSEQ (Agencourt) were resolved on an ABI 3730xl sequencer analyzer and analyzed using SeqMan software (DNASTAR, Madison, WI).
Table 2.
Primers Used for Quantitative Real-Time PCR
| Gene | Accession no. | Product | Tan | MgCl2 | Sequence |
|---|---|---|---|---|---|
| LOX | NM_002317 | 104 bp | 62°C | 3.5 mmol/L | 5′-CAGATTTCTTACCCAGCCGACC-3′ |
| 5′-GGCATCAAGCAGGTCATAGTGG-3′ | |||||
| LOX L1 | NM_005576 | 112 bp | 62°C | 3.5 mmol/L | 5′-CTACGATGTGCGGGTGCTACTG-3′ |
| 5′-GGCTGAACTCGTCCATGCTGTG-3′ | |||||
| 386 bp | 65°C | 2.5 mmol/L | 5′-CTTGCTCAACTCGGGCTCAGA-3′ | ||
| 5′-CCTGCGGGTAGGGGAACTG-3′ | |||||
| LOX L2 | NM_002318 | 73 bp | 61°C | 3.0 mmol/L | 5′-CATCCACCTCAACGAGATCCAG-3′ |
| 5′-AGACTCGGCATTGAACTTGCAG-3′ | |||||
| GAPDH | NM_002046 | 117 bp | 64°C | 3.5 mmol/L | 5′-AGCTCACTGGCATGGCCTTC-3′ |
| 5′-ACGCCTGCTTCACCACCTTC-3′ |
Tan, annealing temperature.
Real-Time RT-PCR
First-strand cDNA synthesis from 1 μg of total RNA and quantitative real-time PCR was performed using the MyIQ thermal cycler and software (Bio-Rad, Munich, Germany) as previously described.4 PCR reactions (25 μl) were run in duplicate and contained 2 μl of the 1:5 diluted first-strand cDNA, 0.4 μmol/L each of upstream and downstream primer, 3.0 to 3.5 mmol/L MgCl2, and IQ SYBR Green Supermix (Bio-Rad). Exon-spanning primers (MWG Biotech, Anzing, Germany), designed by means of Primer 3 software, (http://fokker.wi.mit.edu/primer3/input.htm) and PCR conditions are summarized in Table 2. For quantification, serially diluted standard curves of plasmid-cloned cDNA were run in parallel, and amplification specificity was checked using melting curve and sequence analyses using the Prism 3100 DNA sequencer (Applied Biosystems). For normalization of gene expression levels, mRNA ratios relative to the housekeeping gene GAPDH were calculated.
Immunohistochemistry
Light microscopic indirect immunofluorescence and electron microscopic immunogold labeling were performed on sections of ocular and extraocular tissues of PEX and control eyes, as previously described,26 using well-characterized antibodies against LOX, LOXL1, LOXL2 to -4, and against various elastic fiber components (see below). Antibody binding was detected by Alexa 488-conjugated secondary antibodies (Invitrogen-Molecular Probes, Eugene, OR) or by 10-nm gold-conjugated secondary antibodies (BioCell, Cardiff, UK). In double-labeling experiments, Alexa 555-conjugated or 18-nm gold-conjugated secondary antibodies were used in addition. Nuclear counterstaining was performed with propidium iodide or 4′,6′-diamino-2-phenylindole (Sigma-Aldrich, St. Louis, MO). For a semiquantitative evaluation of staining reactions for LOXL1, a grading scheme was used indicating the degree of cytoplasmic staining of cells in tissue sections (0 = absent, 1 = weak, 2 = moderate, 3 = intense staining). The semiquantitative findings were correlated with the patients’ individual genotypes. In negative control experiments, the primary antibodies were pre-adsorbed with the respective peptides for 2 hours before immunodetection as described.24
Antibodies
Rabbit anti-LOXL1 and anti-LOX antibodies recognizing both the proform and active forms of the enzymes were generated, affinity-purified, and tested as described24,27,28,29,30,31 and were used at a dilution of 1:2000. Moreover, an affinity-purified polyclonal goat antibody against a peptide mapping at the N-terminus of human LOXL1 (1:50) was additionally obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A polyclonal rabbit antibody to human LOX (1:2000) was also obtained from a commercial source (Novus Biologicals, Littleton, CO).
For immunolocalization of the other LOXL isoforms, affinity-purified rabbit polyclonal antibodies raised against peptides mapping near the N-terminus of mouse LOXL2, LOXL3, and LOXL4 were used at a dilution of 1:200 and confirmed to cross-react with the corresponding human LOXL isoforms. Affinity-purified polyclonal goat antibodies against peptides mapping at the N-terminus of human LOXL2 (1:50), LOXL3 (1:200), and LOXL4 (1:100) were also obtained from Santa Cruz Biotechnology.
Other antibodies used in this study were monoclonal antibodies to elastin (clone 10B8; Millipore Corp., Billerica, MA), tropoelastin (clone T11E3: Abcam, Cambridge, UK), fibrillin-1 (clone 26: Millipore Corporation), emilin (clone 2E5; G.M. Bressan, University of Padua, Padua, Italy), fibulin-5 (Abcam), LTBP-1 (D.B. Rifkin, New York University School of Medicine, New York, NY), LTBP-2 (MM425; Elastin Products Co., Owensville, MO), vitronectin (clone 8E6; Millipore Corporation), and clusterin (HS-3; BioVendor, Heidelberg, Germany) as well as polyclonal antibodies to fibulin-1, -2, -3, -4, and -5 (T. Sasaki, Shriners Hospital for Children, Portland, OR).
Statistics
Data are presented as means ± SD. Statistical evaluation of significant differences between groups of eyes was performed with the Mann-Whitney U-test for pair-wise comparison. Data were further analyzed by the Pearson correlation analysis. P < 0.05 was considered statistically significant.
Results
Expression of LOXL1, LOXL2, and LOX in Ocular Tissues
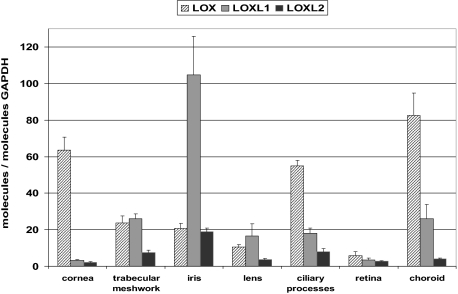
LOXL1 mRNA expression was detected at moderate levels in all ocular tissues analyzed, ie, the cornea, trabecular meshwork, iris, lens, ciliary body, choroid, and retina of normal human eyes (Figure 1). Expression levels were highest in the iris and lowest in the cornea and retina. In comparison, overall LOXL2 mRNA expression levels were markedly lower, but paralleled the LOXL1 expression pattern with highest levels in the iris and lowest levels in cornea and retina. In contrast, LOX mRNA expression was found to be highest in the cornea, ciliary body, and choroid (Figure 1).
Figure 1.
Quantitative determination of mRNA levels of LOX, LOXL1, and LOXL2 in ocular tissues of normal human donor eyes using real-time PCR technology. Data were normalized to GAPDH and are expressed as copies of gene of interest per copies of GAPDH. Values represent mean ± SD of three separate experiments.
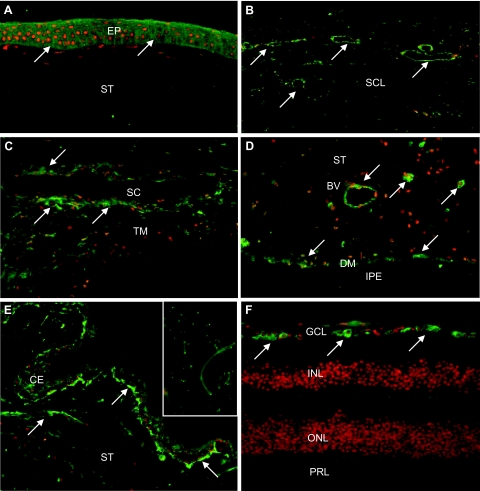
By immunohistochemistry, moderate cellular expression of LOXL1 was detected in virtually all ocular tissues of normal eyes with considerable interindividual variability in staining reactions. LOXL1 could be immunolocalized mainly to the cytoplasm of epithelial cells of the cornea (Figure 2A), conjunctiva, ciliary body (Figure 2E), and lens; to endothelial cells of the cornea, trabecular meshwork, and Schlemm canal (Figure 2C); to single stromal cells of the cornea, conjunctiva, iris, ciliary body, episclera, and choroid; to endothelial cells of conjunctival, intra- and episcleral (Figure 2B), iridal (Figure 2D), ciliary (Figure 2E), choroidal, retinal, and optic nerve blood vessels; to smooth muscle cells in the iris (Figure 2D), ciliary body, and vessel walls; and to astrocytes and lamina cribrosa cells of the optic nerve. In the retina, distinct labeling of retinal ganglion cells was present (Figure 2F). Rarely, LOXL1 was found to be expressed in nuclei of cells, particularly in those of the iris stroma. A very weak positive staining of extracellular elastic fibers, particularly at their tips, in the conjunctival stroma, choroidal stroma, ciliary stroma, the connective tissue beams of the lamina cribrosa, and in the periphery of blood vessels was occasionally observed (Figure 2E, inset), as was focal staining of basement membranes, such as the inner and outer limiting membrane of the ciliary epithelium.
Figure 2.
Immunofluorescence labeling of LOXL1 in ocular tissues of normal human donor eyes. LOXL1 immunopositivity (green fluorescence, arrows) is observed in the corneal epithelium (A); intrascleral veins and fibrocytes (B); trabecular meshwork and Schlemm canal endothelium (C); vessels, dilator muscle, and stromal cells in the iris (D); ciliary epithelium and stromal vessels (E); and retinal ganglion cells (F). The inset in E shows weak LOXL1 immunopositivity at the tips of elastic fibers in the ciliary stroma. BV, blood vessel; CE, ciliary epithelium; DM, dilator muscle; EP, corneal epithelium; GCL, retinal ganglion cell layer; INL, inner nuclear layer; IPE, iris pigment epithelium; ONL, outer nuclear layer; PRL, photoreceptor layer; SC, Schlemm canal; SCL, sclera; ST, stroma; TM, trabecular meshwork. Original magnifications: ×100; ×250 (inset).
LOX was detected in virtually all ocular tissues at a higher labeling intensity than that for LOXL1. It was also localized to the cytoplasm of various cell types, to extracellular fibers, and additionally to ocular nerves. LOXL2, LOXL3, and LOXL4 isoforms were ubiquitously present in ocular tissues, mainly within the cytoplasm of epithelial, endothelial, stromal, muscle, and vascular cells. LOXL3 and LOXL4 were additionally present in ocular nerves and in extracellular fibers in the iris stroma and ciliary muscle. LOXL4 was particularly prominent in vessel walls (data not shown).
Genotype-Correlated Expression of LOXL1
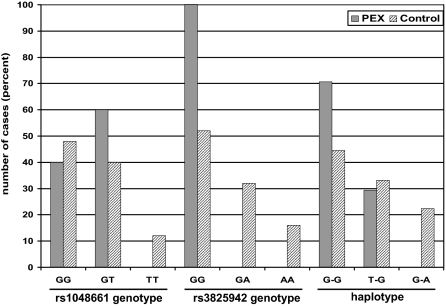
The allele frequencies, genotypes, and haplotypes of the two disease-associated coding SNPs of LOXL1 were determined in the sample groups. The frequencies of the risk genotype GG of SNPs rs1048661 and rs3825942 were 40% (10 of 25) and 100% (25 of 25) in PEX cases, and 48% (12 of 25) and 52% (13 of 25) in control cases, respectively (Figure 3). The risk alleles G of rs3825942 were highly overrepresented in PEX cases as compared to controls; in fact, all PEX cases without and with glaucoma were homozygous for the risk allele G of rs3825942. The low-risk genotypes TT of rs1048661 and AA of rs3825942 were rarely observed in control cases (3 of 25 and 4 of 25, respectively), but were lacking in PEX cases. Among the three possible haplotypes observed, 70.6% of PEX cases and 44.5% of control cases carried the high-risk haplotype (G-G), whereas the low-risk haplotype (G-A) was present in 22.4% of control cases only (Figure 3). Two copies of the high-risk haplotype were present in 41.2% of PEX and in 20.7% of control cases. Allele frequencies, genotypes, and haplotypes were not different between PEX syndrome and PEX glaucoma.
Figure 3.
Association of LOXL1 genotypes and haplotypes with PEX syndrome/glaucoma. Percentage of SNP rs1048661 and rs3825942 genotypes and SNP haplotypes in PEX (n = 25) and control (n = 25) cases.
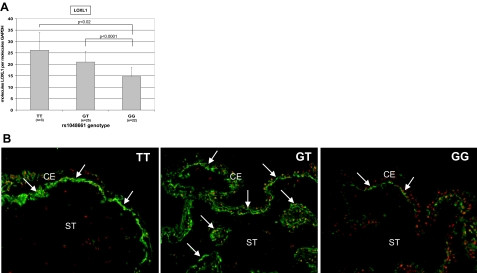
In ocular tissues, such as ciliary processes, of both PEX and control eyes, the expression of LOXL1 was reduced by ∼20% per risk G allele of rs1048661 (Figure 4A). The differences between the risk genotype GG and the genotypes GT and TT were statistically significant (P < 0.0001 and P < 0.02, respectively). In contrast, rs3825942 alleles or the haplotype formed by both SNPs did not affect LOXL1 mRNA levels in any group (data not shown). The risk allele G of SNP rs1048661, but not of rs3825942, was also associated with lower levels of LOXL1 protein in ocular tissues by immunohistochemistry (Figure 4B). Semiquantitative evaluation of LOXL1 staining reactions in ocular tissues revealed a mean score of 3.0 for the TT genotype (n = 3), of 2.3 for the GT genotype (n = 25) and of 1.1 for the GG genotype (n = 22) of SNP rs1048661.
Figure 4.
Genotype-correlated expression of LOXL1. A: LOXL1 mRNA expression in ciliary body tissue of both PEX (n = 25) and control (n = 25) eyes is significantly reduced per risk G allele of SNP rs1048661. B: LOXL1 protein expression (arrows) in the ciliary epithelium of control eyes with different genotypes is decreased per risk G allele of SNP rs1048661. CE, ciliary epithelium; ST, ciliary stroma. Original magnifications, ×100.
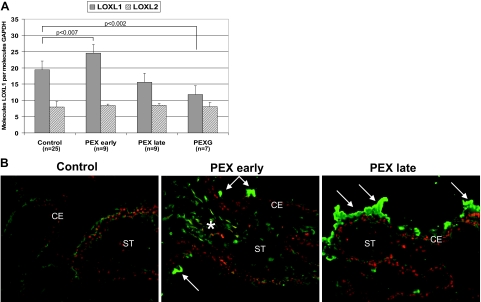
LOXL1 Expression in PEX Syndrome/Glaucoma
Comparing control and PEX eyes at various stages of disease, LOXL1 mRNA expression in ocular tissues was significantly increased by 26% in early stages of PEX syndrome (P < 0.007), but was markedly reduced by ∼20% in advanced stages of PEX syndrome and by ∼40% (P < 0.002) in PEX eyes with end-stage glaucoma as compared to controls (Figure 5A). These stage-dependent differences in expression did not correlate with the rs1048661 genotype distribution in all PEX groups. In contrast, LOXL2 (Figure 5A) and LOX (data not shown) showed no significant differences in expression levels between the various PEX and control samples. The specific stage-dependent dysregulation of LOXL1 expression was also evident on the protein level by immunohistochemistry, showing markedly increased cellular staining reactions of ocular tissues in early stages of PEX and decreased cellular labeling intensities in advanced PEX cases without and with glaucoma as compared to controls (Figure 5B).
Figure 5.
LOXL1 expression in ocular tissues of eyes with PEX syndrome/glaucoma at different stages of disease compared with control eyes. A: Quantitative determination of LOXL1 and LOXL2 mRNA expression in ciliary processes of control and PEX eyes without glaucoma at various stages of disease (PEX early, PEX late) and with glaucoma (PEXG) using real-time PCR technology. Data were normalized to GAPDH and are expressed as copies of gene of interest per copies of GAPDH. B: LOXL1 protein expression in ciliary processes of PEX eyes at early and late stages as compared with control eyes (rs1048661 genotype GG in all cases). Whereas early PEX stages disclose only minor focal PEX material deposits (arrows), advanced stages show prominent LOXL1-positive PEX material deposits on the surface of the ciliary epithelium (arrows). Cellular labeling for LOXL1 is increased in early stages of PEX (asterisk) and decreased in late stages as compared to controls. CE, ciliary epithelium; ST, ciliary stroma. Original magnifications, ×100.
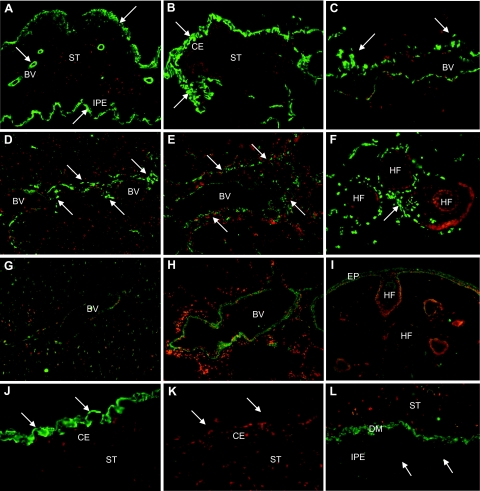
Despite decreased cellular staining levels in advanced PEX stages, LOXL1 was consistently found to be a major component of extracellular PEX material in all intra- and extraocular locations. LOXL1-positive ocular PEX deposits were observed on the surfaces of ciliary body, iris, lens, and zonules, in the iridal stroma and vessel walls, in the anterior portion of the ciliary muscle, in the trabecular meshwork, in the periphery of Schlemm canal, collector channels and intra- and episcleral aqueous veins, in the conjunctival stroma and episcleral connective tissue, in the meninges of the optic nerve, and in the walls of posterior ciliary arteries (Figure 6, A–C). In extraocular locations, LOXL1-positive clumps of PEX material were observed in the skin and connective tissue portions of all visceral organ tissues examined, such as heart and lungs, particularly in the wall of blood vessels (Figure 6, D–F). In normal control tissues, weak LOXL1 staining was associated with cells of vessel walls and connective tissues, occasionally also with extracellular fibers in the periphery of blood vessels (Figure 6, G and H). In the normal skin, LOXL1 immunoreactions could be localized to epidermal and subepithelial stromal cells, to root sheaths of hair follicles, and occasionally to extracellular fibers in the stroma and vessel walls (Figure 6I).
Figure 6.
Immunolocalization of LOXL1 in PEX material deposits in various intra- and extraocular tissues of PEX eyes. Immunopositive PEX material deposits (arrows) are present in the iris stroma, periphery of iris vessels, and on the surface of the iridal pigment epithelium (A); on the surface of the ciliary epithelium (B); in the periphery of episcleral veins (C); in myocardial tissue (D); in lung tissue, particularly in the periphery of pulmonary blood vessels (E); and in the periphery of hair follicles in the skin (F). Corresponding control tissues show weak staining for LOXL1 in vessel walls of myocardial (G) and lung tissue (H) as well as in epidermal cells and root sheaths of hair follicles in normal skin (I). J and K: Pre-adsorption of LOXL1 antibodies with the corresponding peptide completely abolished immunopositivity of PEX deposits (arrows) on the surface of the ciliary epithelium. L: PEX material deposits (arrows) in the iris were negative for LOX. BV, blood vessel; CE, ciliary epithelium; DM, dilator muscle; EP, epidermis; HF, hair follicle; IPE, iris pigment epithelium; ST, stroma. Original magnifications, ×100.
Immunopositivity of PEX aggregates could be completely abolished by pre-adsorption of antibodies with the corresponding LOXL1 peptides (Figure 6, J and K). Different antibodies or various methods of tissue processing, ie, fixation of sections in paraformaldehyde or acetone as well as paraffin-embedding, did not show any difference in staining reactions of PEX aggregates (data not shown). In contrast, PEX material was consistently negative for LOX and LOXL isoforms 2 to 4 with all antibodies used (Figure 6L).
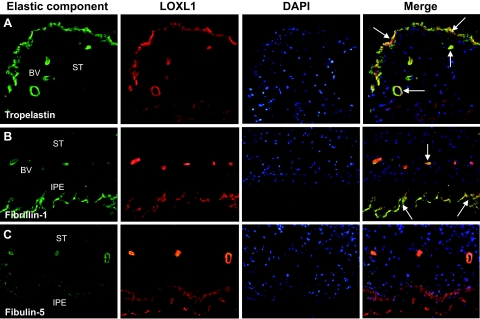
In double-labeling experiments, LOXL1 staining of intra- and extraocular PEX material deposits clearly co-localized with tropoelastin and elastin (Figure 7A), fibrillin-1 (Figure 7B), LTBP-1 and LTBP-2, emilin, vitronectin, and clusterin (not shown) on the light microscopic level. PEX material deposits were, however, immunonegative for all fibulin isoforms including fibulin-5 (Figure 7C).
Figure 7.
Double-labeling immunofluorescence of elastic fiber components (green fluorescence) and LOXL1 (red fluorescence) in ocular PEX material deposits; nuclear counterstaining with 4′,6′-diamino-2-phenylindole (blue fluorescence). A: Co-localization of tropoelastin and LOXL1 (arrows) in PEX material in the anterior border layer and periphery of vessels in the iris. B: Co-localization of fibrillin-1 and LOXL1 (arrows) in PEX material on the surface of the pigment epithelium and vessel walls of the iris. C: Lacking co-localization of fibulin-5 and LOXL1 in PEX aggregates in the wall of iridal vessels and on the surface of the iridal pigment epithelium. BV, blood vessel; IPE, iris pigment epithelium; ST, stroma. Original magnifications, ×100.
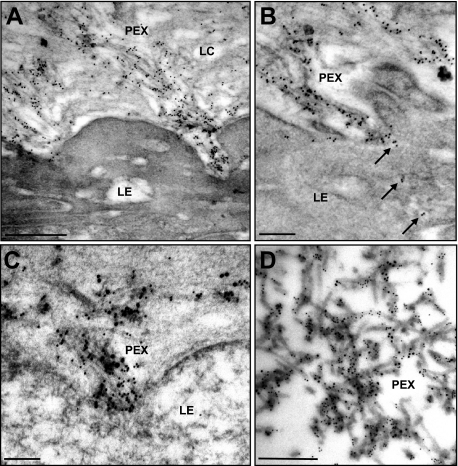
On the electron microscopic level, immunogold labeling confirmed a strong reaction of PEX fibrils and their microfibrillar subunits with antibodies to LOXL1, which was most prominent close to cellular surfaces from which the PEX fibrils appeared to emerge (Figure 8A). Occasionally, small cytoplasmic vesicles containing gold particles indicating LOXL1 could be observed in the apical cytoplasm of cells involved in PEX fiber production (Figure 8B). Double labeling of LOXL1 and elastic fiber components showed the most prominent co-localization of LOXL1 with fibrillin-1 both on emerging PEX fibrils in close association with cell surfaces (Figure 8C) and along mature PEX fibers on the surface of ocular structures (Figure 8D).
Figure 8.
Immunogold localization of LOXL1 on ocular PEX material. A: Immunopositive PEX fibers emerging from the pre-equatorial lens epithelium (LE) into the lens capsule (LC). B: Gold marker for LOXL1 is evident within cytoplasmic vesicles (arrows) of the lens epithelium (LE) and along PEX fibers on its surface. C: Co-localization of LOXL1 (10-nm gold particles) and fibrillin-1 (18-nm gold particles) along developing PEX fibers in close association to the surface of the lens epithelium (LE). D: Co-localization of LOXL1 (10-nm gold particles) and fibrillin-1 (18-nm gold particles) along mature PEX fibers on the surface of the lens capsule. Scale bars = 0.5 μm.
Discussion
Functional Significance of LOXL1
Lysyl oxidases are extracellular copper-containing enzymes that, among many other functions, catalyze cross-linking of collagen and elastin by oxidative deamination of lysine residues.20,21,22 LOX and LOXL1 have similar exon structure consisting of seven exons, five of which (exons 2 to 6) exhibit strong homology and encode the C-terminal catalytic domain. The sequence differences between the LOX and LOXL1 genes reside mainly in exon 1, which encodes the unique N-terminal domain that is required both for proper enzyme activation and for substrate recognition and binding.31,32 After attachment to the scaffolding structure, the propeptide is cleaved off by the endo-metalloproteinase procollagen-C-terminal proteinase (bone morphogenetic protein 1) for catalytic activation of the enzyme.29
The substrate preferences or specificities of the various lysyl oxidase family members are not exactly known. LOX and LOXL1 have been shown in vitro to use tropoelastin as a substrate, but their individual roles in elastogenesis remain unclear.29,31 The formation of elastic fibers has been proposed to require the deposition of tropoelastin on a pre-existing scaffold of fibrillin-containing microfibrils, cross-linking of tropoelastin monomers by LOX family enzymes, and organization of the resulting insoluble elastin matrix into mature elastic fibers.33 Several other proteins, such as members of the fibulin and emilin families, have been suggested to play a role in elastic fiber formation, but their exact function has not yet been determined. Recent studies have demonstrated that the LOXL1 propeptide is selectively targeted to elastic microfibrils at sites of elastogenesis by binding to both tropoelastin and fibulin-5.23,31,34 The ability of both LOX and LOXL1 to cross-link elastin has also been confirmed in vivo using knockout mice revealing decreased elastin content in many tissues.23 In contrast to LOX-knockout mice, which die shortly after birth,30 LOXL1-knockout mice are viable but exhibit elastic fiber defects resulting in pelvic prolapse in female animals, enlarged pulmonary air spaces, increased laxity of the skin, and vascular abnormalities.23 Thus, LOXL1 has been considered to be essential for elastic fiber assembly, maintenance, and stability in both developing and adult tissues.23,24 LOXL1 has been particularly localized to the extracellular matrix in active fibrotic processes, where its expression pattern correlated with the production of matrix components, such as type III collagen.28,35 LOXL1 was also shown to be up-regulated in cells undergoing epithelial-mesenchymal transition and in malignant lesions of the breast and lung,28,36 suggesting that it may have a particular role in connective tissue remodeling during dynamic processes such as tissue injury, fibrosis, cancer, and development.
Hence, LOXL1 has been found to be widely distributed in most tissues, such as human and murine skin, heart, placenta, lung, kidney, liver, and skeletal muscle.20,37,38,39 Although a role for LOXL1 in formation and turnover of the extracellular matrix of the eye has not been documented, LOXL1 mRNA and protein expression have been recently detected in various ocular tissues, such as cornea, iris, lens, ciliary body, lamina cribrosa, and optic nerve.14,40 The findings of the present study confirm an ubiquitous mRNA and protein expression of LOXL1 in all ocular tissues, including the retina, of normal human eyes, with moderate expression levels falling between those of LOX and LOXL2 and being influenced by the individual LOXL1 genotype. Interestingly, LOXL1 expression levels were highest in the iris, which is the ocular tissue with the earliest and heaviest involvement in the PEX process.2 Expression was mainly cellular, but faint extracellular staining was occasionally observed along elastic fibers of the conjunctival stroma, lamina cribrosa, and blood vessel walls. However, its prominent expression in elastin-poor tissues, such as the iris, its presence in nonelastogenic cell types, such as corneal epithelial and retinal ganglion cells, its occasional occurrence in cell nuclei, and its weak association with mature elastic fibers argue against a predominant role of LOXL1 in elastic fiber homeostasis in normal adult tissues of the human eye. A presumed broader functional significance of LOXL1 in ocular tissues still has to be investigated.
LOXL1 in the Pathophysiology of PEX Syndrome
Variations in the LOXL1 gene have been implicated in the pathogenesis of spontaneous cervical artery dissection and bladder cancer41,42 as well as PEX syndrome and PEX glaucoma.8,9,10,11,12,13,14,15,16,17,18,19 Two PEX-associated coding variants in exon 1 of LOXL1 (rs1048661 and rs3825942) accounted for the vast majority of PEX cases with and without glaucoma in all geographic populations studied. Whereas the risk alleles G of rs3825942 were significantly associated with PEX throughout all study populations, different alleles of rs1048661 were associated with PEX in Japanese patients17,18 or were not associated with PEX at all.12,19
The distribution of SNP genotypes and haplotypes in our study samples is consistent with previous genetic studies.8,9,10,11,12,13,14,15,16,17,18,19 Approximately 70% of PEX cases and 45% of control cases carried the high-risk haplotype (G-G), whereas the low-risk haplotype (G-A) was not present in the PEX group. The risk genotype (GG) of rs1048661 was equally distributed among PEX and control cases. Confirming previous reports on extraocular, ie, adipose tissues,8 the expression level of LOXL1 in intraocular tissues was significantly affected by rs1048661 alleles in both PEX and control samples. However, the risk alleles G of rs3825942, which were overrepresented in PEX cases and which are known to confer the greater risk for PEX,8,12,15,17,19 had no effect on LOXL1 expression levels. Although the biological effect of the rs3825942 missense change has not been determined, genetic programs (“PolyPhen,” “SIFT”) predict that the Gly153Asp amino acid exchange has functional consequences.41 This SNP leads to changes in the N-terminal propeptide of LOXL1 and might therefore affect both the catalytic activity of the protein through modifications of propeptide cleavage and substrate targeting and binding.
Available immunohistochemical, biochemical, and molecular biological data strongly support the current concept that the characteristic fibrillar PEX deposits involve components of the elastic fiber system, and that PEX syndrome is an elastic microfibrillopathy associated with an excessive production and abnormal aggregation of elastic microfibrils.1,2 Although LOXL1 was hardly detected in normal elastic fibers of human ocular tissues, the bulk of LOXL1 enzyme was bound to PEX aggregates in tissues of PEX patients. In fact, LOXL1 proved to be a major component of and an excellent marker for PEX material deposits in all intra- and extraocular locations. LOXL1 could be localized to both microfibrils and mature PEX fibers in close association with cellular surfaces. Within the PEX fiber aggregates, LOXL1 was found to co-localize with both its high-affinity protein tropoelastin and other elastic fiber constituents, such as fibrillin-1, LTBP-1/2, emilin, and vitronectin in a direct cell-associated manner suggesting known or unknown binding partners for LOXL1 at sites of pathological fibrogenesis. In contrast, interaction of LOXL1 with its normal binding partner fibulin-523,34 appears to be lacking in PEX aggregates. Therefore, sequence variations in the LOXL1 propeptide caused by rs3825942 may alter activation, processing or substrate specificity of LOXL1 and may lead to abnormal cross-linking, aggregation, and insolubilization of soluble precursors of elastic matrix proteins, eg, fibrillin-1, with accessible lysine residues into mature PEX fibrils. LOXL1 was also found to co-localize with clusterin, a highly efficient extracellular chaperone, which is known to recognize misfolded and abnormally aggregated proteins in the extracellular space, and which has been previously shown to be involved in the accumulation of PEX material in anterior segment tissues of PEX eyes.4
Expression of LOXL1 mRNA and protein in ocular tissues was obviously differentially regulated in early and late stages of the PEX process. A significant up-regulation of LOXL1 expression in early PEX stages, characterized by subtle ocular deposits of PEX material, may reflect an active involvement of LOXL1 in the initial stages of PEX fiber formation and is in line with previous studies demonstrating that LOXL1 becomes transiently activated at early stages of fibrogenesis.28 In PEX material producing cells, it is likely that LOXL1 binding occurs at the cell surface and that the enzyme participates in the early cell-associated stages of matrix polymerization. To compensate for increased LOXL1 accumulation in the extracellular space, LOXL1 expression may be successively down-regulated in later stages of the disease. The resulting inadequate levels of LOXL1 could in turn adversely affect elastin homeostasis and predispose to elastotic alterations characteristically observed in tissues, such as skin and lamina cribrosa, of PEX eyes.43
However, in view of the frequent occurrence of the high-risk haplotype and the high-risk genotypes of both SNPs in the unaffected control group, additional genetic or external co-modulating factors of yet unidentified nature are supposed to influence the abnormal matrix metabolism and the phenotypic manifestation of PEX syndrome. Potentially stimulating factors, which are known to be involved in the PEX process, include various stress factors, such as oxidative stress and hypoxia, or cytokines and growth factors, such as transforming growth factor-β1.1,5,44 In fact, environmental conditions associated with fibrotic responses, such as oxidative stress or elevated transforming growth factor-β1 concentrations, have been shown to regulate both LOXL1 expression and activity and synthesis of matrix molecules including elastin and fibrillin.45,46 Therefore, LOXL1 may become initially up-regulated in PEX tissues in parallel with elastic matrix components in reaction to specific fibrogenic stress factors.
Although the functional significance of LOXL1 in the specific PEX-associated matrix process still has to be determined, the present findings provide evidence for LOXL1 as a major component of PEX fibers in clear co-localization with various elastic fiber components, and for an involvement of LOXL1 in the initial stages of abnormal fibrogenesis in PEX tissues. The risk alleles G of rs3825942, which were overrepresented in PEX cases, had no effect on LOXL1 expression levels, but have been suggested to have functional consequences on LOXL1 activation, processing, or substrate targeting and binding. The postulated alterations of LOXL1 may lead to posttranslational modification, improper cross-linking, insolubilization, and aggregation of soluble precursors of elastic matrix proteins resulting, in cooperation with additional fibrogenic factors, in the stable accumulation of mature PEX fibrils. Exactly how the allelic variants of the LOXL1 gene lead to PEX syndrome and PEX glaucoma, the specific binding substrate for LOXL1 within PEX fibers, and the regulation of LOXL1 expression and activity in different PEX stages still remain to be determined by functional studies.
Acknowledgments
We thank Carmen Rummelt, Elke Meyer, and Katrin Bitterer for excellent technical assistance.
Footnotes
Address reprint requests to Ursula Schlötzer-Schrehardt, Ph.D., Department of Ophthalmology, University of Erlangen-Nürnberg, Schwabachanlage 6, D-91054 Erlangen, Germany. E-mail: ursula.schloetzer-schrehardt@uk-erlangen.de.
Supported by the German Research Foundation (grant SFB-539).
References
- Schlötzer-Schrehardt U, Naumann GOH. Perspective—ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Zenkel M, Pöschl E, von der Mark K, Hofmann-Rummelt C, Naumann GOH, Kruse FE, Schlötzer-Schrehardt U. Differential gene expression in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2005;46:3742–3752. doi: 10.1167/iovs.05-0249. [DOI] [PubMed] [Google Scholar]
- Zenkel M, Kruse FE, Jünemann AG, Naumann GOH, Schlötzer-Schrehardt U. Deficiency of the extracellular chaperone clusterin in eyes with pseudoexfoliation syndrome may be implicated in the aggregation and deposition of pseudoexfoliative material. Invest Ophthalmol Vis Sci. 2006;47:1982–1990. doi: 10.1167/iovs.05-1580. [DOI] [PubMed] [Google Scholar]
- Zenkel M, Kruse FE, Naumann GOH, Schlötzer-Schrehardt U. Impaired cytoprotective mechanisms in eyes with pseudoexfoliation syndrome/glaucoma. Invest Ophthalmol Vis Sci. 2007;48:5558–5566. doi: 10.1167/iovs.07-0750. [DOI] [PubMed] [Google Scholar]
- Damji KF, Bains HS, Stefansson E, Loftsdottir M, Sverrisson T, Thorgeirsson E, Jonasson F, Gottfredsdottir M, Allingham R. Is pseudoexfoliation syndrome inherited? A review of genetic and nongenetic factors and a new observation. Ophthalmic Genet. 1998;19:175–185. doi: 10.1076/opge.19.4.175.2310. [DOI] [PubMed] [Google Scholar]
- Orr AC, Robitaille JM, Price PA, Hamilton JR, Falvey DM, De Saint-Sardos AG, Pasternak S, Guernsey DL. Exfoliation syndrome: clinical and genetic features. Ophthalmic Genet. 2001;22:171–185. doi: 10.1076/opge.22.3.171.2223. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Alward WL, Kwon YH, Wang K, Streb LM, Sheffield VC, Stone EM. LOXL1 mutations are associated with exfoliation syndrome in patients from the Midwestern United States. Am J Ophthalmol. 2007;144:974–975. doi: 10.1016/j.ajo.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Aragon-Martin JA, Ritch R, Liebmann J, O'Brien C, Blaaow K, Mercieca F, Spiteri A, Cobb CJ, Damji KF, Tarkkanen A, Rezaie T, Child AH, Sarfarazi M. Evaluation of LOXL1 gene polymorphisms in exfoliation syndrome and exfoliation glaucoma. Mol Vis. 2008;14:533–541. [PMC free article] [PubMed] [Google Scholar]
- Challa P, Schmidt S, Liu Y, Qin X, Vann RR, Gonzalez P, Allingham RR, Hauser MA. Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol Vis. 2008;14:146–149. [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Pasquale L, Grosskreutz CL, Rhee D, Chen T, DeAngelis MM, Kim I, DelBono E, Miller JW, Li T, Haines JL, Wiggs JL. DNA sequence variants in the LOXL1 gene are associated with pseudoexfoliation glaucoma in a U.S. clinic-based population with broad ethnic diversity. BMC Med Genet. 2008;9:5. doi: 10.1186/1471-2350-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zabriskie NA, Hau VS, Chen H, Tong Z, Gibbs D, Farhi P, Katz BJ, Luo L, Pearson E, Goldsmith J, Ma X, Kaminoh Y, Chen Y, Yu B, Zeng J, Zhang K, Yang Z. Genetic association of LOXL1 gene variants and exfoliation glaucoma in a Utah cohort. Cell Cycle. 2008;7:521–524. doi: 10.4161/cc.7.4.5388. [DOI] [PubMed] [Google Scholar]
- Hewitt AW, Sharma S, Burdon KP, Wang JJ, Baird PN, Dimasi DP, Mackey DA, Mitchell P, Craig JE. Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet. 2008;17:710–716. doi: 10.1093/hmg/ddm342. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Krumbiegel M, Mardin CY, Paoli D, Lämmer R, Weber BHF, Kruse FE, Schlötzer-Schrehardt U, Reis A. Association of LOXL1 common sequence variants in German and Italian patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1459–1463. doi: 10.1167/iovs.07-1449. [DOI] [PubMed] [Google Scholar]
- Mossböck G, Renner W, Faschinger C, Schmut O, Wedrich A, Weger M. Lysyl oxidase-like protein 1 (LOXL1) gene polymorphisms and exfoliation glaucoma in a Central European population. Mol Vis. 2008;14:857–861. [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Gotoh N, Ueda Y, Nakanishi H, Yoshimura N. Lysyl oxidase-like 1 polymorphisms and exfoliation syndrome in the Japanese population. Am J Ophthalmol. 2008;145:582–585. doi: 10.1016/j.ajo.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Mori K, Imai K, Matsuda A, Ikeda Y, Naruse S, Hitora-Takeshita H, Nakano M, Taniguchi T, Omi N, Tashiro K, Kinoshita S. LOXL1 genetic polymorphisms are associated with exfoliation glaucoma in the Japanese population. Mol Vis. 2008;14:1037–1040. [PMC free article] [PubMed] [Google Scholar]
- Ramprasad VL, George R, Soumittra N, Sharmila F, Vijaya L, Kumaramanickavel G. Association of non-synonymous single nucleotide polymorphisms in the LOXL1 gene with pseudoexfoliation syndrome in India. Mol Vis. 2008;14:318–322. [PMC free article] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Noblesse E, Cenizo V, Bouez C, Borel A, Gleyzal C, Peyrol S, Jacob MP, Sommer P, Damour O. Lysyl oxidase-like and lysyl oxidase are present in the dermis and epidermis of a skin equivalent and in human skin and are associated to elastic fibers. J Invest Dermatol. 2004;122:621–630. doi: 10.1111/j.0022-202X.2004.22330.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Muroi S. Cycloscopy of pseudoexfoliation. Am J Ophthalmol. 1979;87:513–518. doi: 10.1016/0002-9394(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U, Zenkel M, Nüsing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci. 2002;43:1475–1487. [PubMed] [Google Scholar]
- Sommer P, Gleyzal C, Raccurt M, Delbourg M, Serrar M, Joazeiro P, Peyrol S, Kagan H, Trackman PC, Grimaud JA. Transient expression of lysyl oxidase by liver myofibroblasts in murine schistosomiasis. Lab Invest. 1993;69:460–470. [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, Sommer P. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B. Lysyl oxidase-like protein from bovine aorta. J Biol Chem. 2001;276:48944–48949. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Thomassin L, Werneck CC, Broekelmann T, Gleyzal C, Hornstra IK, Mecham R, Sommer P. The pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J Biol Chem. 2005;280:42848–42855. doi: 10.1074/jbc.M506832200. [DOI] [PubMed] [Google Scholar]
- Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647:220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Peyrol S, So C-K, Boyd CD, Csiszar K. Coexpression of the lysyl oxidase-like gene (LOXL) and the gene encoding type III procollagen in induced liver fibrosis. J Cell Biochem. 1999;72:181–188. doi: 10.1002/(sici)1097-4644(19990201)72:2<181::aid-jcb3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Takeuchi K, Quinlan MP. Identification of genes involved in epithelial-mesenchymal transition and tumor progression. Oncogene. 2001;20:6679–6688. doi: 10.1038/sj.onc.1204872. [DOI] [PubMed] [Google Scholar]
- Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270:7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- Kenyon K, Modi WS, Contente S, Friedman RM. A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25. J Biol Chem. 1993;268:18435–18437. [PubMed] [Google Scholar]
- Hayashi K, Fong KSK, Mercier F, Boyd CD, Csiszar K, Hayashi M. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004;35:845–855. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Urban Z, Agapova O, Hucthagowder V, Yang P, Starcher BC, Hernandez MR. Population differences in elastin maturation in optic nerve head tissue and astrocytes. Invest Ophthalmol Vis Sci. 2007;48:3209–3215. doi: 10.1167/iovs.07-0107. [DOI] [PubMed] [Google Scholar]
- Kuhlenbäumer G, Friedrichs F, Kis B, Berlit P, Maintz D, Nassenstein I, Nabavi D, Dittrich R, Stoll M, Ringelstein B. Association between single nucleotide polymorphisms in the lysyl oxidase-like 1 gene and spontaneous cervical artery dissection. Cerebrovasc Dis. 2007;24:343–348. doi: 10.1159/000106980. [DOI] [PubMed] [Google Scholar]
- Wu G, Guo Z, Chang X, Kim MS, Nagpal JK, Liu J, Maki JM, Kivirikko KI, Ethier SP, Trink B, Sidransky D. LOXL1 and LOXL4 are epigenetically silenced and can inhibit ras/extracellular signal-regulated kinase signalling pathway in human bladder cancer. Cancer Res. 2007;67:4123–4129. doi: 10.1158/0008-5472.CAN-07-0012. [DOI] [PubMed] [Google Scholar]
- Netland PA, Ye H, Streeten BW, Hernandez MR. Elastosis of the lamina cribrosa in pseudoexfoliation syndrome with glaucoma. Ophthalmology. 1995;102:878–886. doi: 10.1016/s0161-6420(95)30939-6. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U, Zenkel M, Küchle M, Sakai LY, Naumann GOH. Role of transforming growth factor-ß1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–780. doi: 10.1006/exer.2001.1084. [DOI] [PubMed] [Google Scholar]
- Gacheru SN, Thomas KM, Murray SA, Csiszar K, Smith-Mungo LI, Kagan HM. Transcriptional and post-transcriptional control of lysyl oxidase expression in vascular smooth muscle cells: effects of TGF-beta 1 and serum deprivation. J Cell Biochem. 1997;65:395–407. doi: 10.1002/(sici)1097-4644(19970601)65:3<395::aid-jcb9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]