Abstract
Inflammatory markers serum amyloid A (SAA) and C-reactive protein (CRP) are predictive of cardiac disease and are proposed to play causal roles in the development of atherosclerosis, in which the retention of lipoproteins by vascular wall proteoglycans is critical. The purpose of this study was to determine whether SAA and/or CRP alters vascular proteoglycan synthesis and lipoprotein retention in a pro-atherogenic manner. Vascular smooth muscle cells were stimulated with either SAA or CRP (1 to 100 mg/L) and proteoglycans were then isolated and characterized. SAA, but not CRP, increased proteoglycan sulfate incorporation by 50 to 100% in a dose-dependent manner (P < 0.0001), increased glycosaminoglycan chain length, and increased low-density lipoprotein (LDL) binding affinity (Kd, 29 μg/ml LDL versus 90 μg/ml LDL for SAA versus control proteoglycans; P < 0.005). Furthermore, SAA up-regulated biglycan via the induction of endogenous transforming growth factor (TGF)-β. To determine whether SAA stimulated proteoglycan synthesis in vivo, ApoE−/− mice were injected with an adenovirus expressing human SAA-1, a null virus, or saline. Mice that received adenovirus expressing SAA had increased TGF-β concentrations in plasma and increased aortic biglycan content compared with mice that received either null virus or saline. Thus, SAA alters vascular proteoglycans in a pro-atherogenic manner via the stimulation of TGF-β and may play a causal role in the development of atherosclerosis.
Atherosclerosis is characterized by vascular inflammation and vascular inflammation is thought to be directly pro-atherogenic. Elevated levels of the inflammatory markers serum amyloid A (SAA) and C-reactive protein (CRP) have both been found to be predictive of cardiovascular disease risk, albeit there are more extensive data available for CRP. Both SAA and CRP are modestly increased with obesity, insulin resistance, and high cholesterol diets,1,2,3,4,5,6 which are known to increase the risk of cardiovascular disease events. There is a robust literature supporting the use of CRP in clinical evaluation of patients. A clinical trial to determine whether lowering CRP improves cardiovascular disease event rates has been performed,7 and data are expected within the next year. Thus, there is considerable interest as to whether elevated SAA and/or CRP are directly pro-atherogenic, and play a causal role in atherosclerosis development, or if they are merely elevated in reflection of the underlying atherosclerosis burden.
The response to retention hypothesis of atherosclerosis development proposes that one of the earliest steps in the formation of an atherosclerotic plaque is the retention of atherogenic lipoproteins within the subendothelial space by their interactions with vascular proteoglycans.8,9,10,11 Proteoglycans are a heterogeneous group of molecules composed of a core protein to which one or more glycosaminoglycan side chains are attached. The major vascular proteoglycans are the large chondroitin sulfate proteoglycan versican, the small dermatan sulfate proteoglycans biglycan and decorin, and the heparan sulfate proteoglycan perlecan.12 Proteoglycans bind lipoproteins through ionic interactions between the negatively charged sulfate and carboxyl groups on the glycosaminoglycan chains and the positively charged amino acid residues on apolipoproteins (apo) B and E. The critical role of lipoprotein retention by artery wall proteoglycans in the initiation of atherosclerosis was shown in a series of elegant experiments in which mice expressing proteoglycan binding-defective lipoproteins developed significantly less atherosclerosis than their littermates expressing either control lipoproteins or low-density lipoprotein (LDL) receptor-binding-defective lipoproteins, despite similar levels of cholesterol.13 Recently, we demonstrated that increases in vascular proteoglycan content precede and contribute to atherosclerosis development.14 Thus, lipoprotein retention by vascular proteoglycans is a fundamental step in the initiation of atherosclerosis.
Several recent animal studies have proposed that SAA promotes increased atherosclerosis development,15,16,17 although the mechanism is unknown. To date there are no data suggesting a pro-atherogenic role for CRP, but CRP is not an acute phase reactant in mice, which limits the interpretation of this data. Both CRP and SAA have been found in atherosclerotic lesions,18,19,20,21 but only SAA is seen co-localized with proteoglycans15 and lipoproteins.22 Vascular proteoglycans are thought to be predominantly synthesized by vascular smooth muscle cells, although endothelial cells and macrophages are also known to synthesize proteoglycans.12,23,24 This study was performed to test the hypothesis that SAA and/or CRP could modify vascular proteoglycan synthesis in a manner that increases LDL binding affinity, as a mechanism contributing to increased atherosclerosis development. We report that SAA increased vascular proteoglycan synthesis both in vitro and in vivo, but CRP did not alter vascular proteoglycan synthesis.
Materials and Methods
Chemicals and Reagents
Cell culture media and additives were obtained from Invitrogen (Carlsbad, CA). SAA was obtained from BioVision Inc. (Mountain View, CA) and CRP was obtained from Calbiochem (San Diego, CA). All other reagents were from Sigma (St. Louis, MO) unless otherwise specified. Human LDL and high-density lipoprotein (HDL) were isolated from pooled plasma from normal, healthy patients by sequential ultracentrifugation as previously described.25 HDL was incubated with SAA in vitro to form HDL-SAA complex, as previously described.26 These studies were approved by the University of Kentucky Institutional Review Board.
Cell Culture
Monkey vascular smooth muscle cells (generously provided by T.N. Wight, Seattle, WA) were maintained in commercially available Dulbecco’s modified Eagle’s medium with 5.6 mmol/L glucose as previously described.27,28 Cells were grown to confluence then made quiescent by reducing the serum concentration to 0.1% for 48 hours. Quiescent cells were stimulated with SAA (1 to 100 mg/L) or CRP (1 to 100 mg/L) for 0 to 72 hours. In some experiments cells were stimulated with SAA complexed to HDL or to HDL without SAA. Because transforming growth factor (TGF)-β is known to stimulate vascular proteoglycan synthesis,29,30 parallel wells were stimulated with TGF-β (2 ng/ml) as a positive control. To investigate the signaling pathways involved in some experiments cells were co-incubated with SAA (100 mg/L) in the presence of TGF-β neutralizing antibody 1D11 (10 μg/ml), control antibody 13C4 (10 μg/ml; both antibodies from R&D Systems, Minneapolis MN), Lipoxin A4 (LXA4, 5 μmol/L), or pertussis toxin (PTX, 0.5 μg/ml). Cells were metabolically labeled with 35S04 (50 to 100 μCi/ml), which labels glycosaminoglycan side chains. To study glycosaminoglycan chain synthesis, cells were supplied with 0.5 mmol/L methyl-β-d-xylopyranoside (xyloside), which serves as an artificial glycosaminoglycan acceptor.31
Proteoglycan and Glycosaminoglycan Purification and Characterization
Sulfate incorporation into secreted proteoglycans and glycosaminoglycans was quantified using cetyl pyridinium chloride precipitation, as previously described.29,32 After removal of the radiolabeled culture media, the cell layer was washed with phosphate-buffered saline, then cell protein was quantified using a bicinchroninic acid protein assay kit (Bio-Rad, Hercules, CA). The radiolabeled culture media from each condition was combined immediately with protease inhibitors, then proteoglycans or glycosaminoglycans were purified and concentrated by ion-exchange chromatography.29,32 The hydrodynamic size of glycosaminoglycans was determined by molecular sieve chromatography using Sepharose CL-6B columns.27,28 To estimate the size of proteoglycans, equal counts (20,000 dpm) of purified 35S04-labeled proteoglycans, were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis 4 to 12% gradient gels with a 3.5% stacking gel, as previously described.29,32 Dried gels were exposed to Fuji Imaging Plates for 48 to 72 hours and then visualized using FLA-5000 (Fujifilm, Tokyo, Japan) and analyzed using Multi Gauge software (Fujifilm). To evaluate proteoglycan synthesis total proteins were precipitated from the culture media of each condition by trichloroacetic acid, and biglycan, versican, and decorin were evaluated by Western blots as previously described33 using antibodies to biglycan (R&D Systems); decorin (generously provided by Dr. Larry Fisher, National Institutes of Health, Bethesda, MD), and versican (Chemicon, Temecula, CA). β-Actin was used as the loading control (Abcam, Cambridge, MA).
LDL Binding Assays
LDL binding to proteoglycans and glycosaminoglycans synthesized under the various experimental conditions was assessed by a modified gel mobility shift assay as previously described.27,28,34 The amount of proteoglycan bound by LDL in each lane is expressed as the proportion of radioactivity retained at the lane origin relative to the total radioactivity per lane.
TGF-β Quantification
Aliquots of conditioned media and plasma were assayed for content of total and bioactive TGF-β using the TGF-β1 Emax ImmunoAssay System (Promega, Madison, WI) according to the manufacturer’s directions. Bioactive samples were measured directly, whereas total TGF-β was determined on samples that were acid-activated before quantification.35,36
Murine Studies
Male apoE−/− mice 10 times backcrossed to C57BL6 (generously provided by Dr. Alan Daugherty, University of Kentucky) were housed in temperature-controlled vivarium facilities with 12-hour light/dark cycles. Mice were fed normal rodent chow ad libitum, and had free access to water. Animal care and experimental procedures were performed in accordance with University of Kentucky Animal Care and Use Committee guidelines. At age 6 to 8 weeks mice were injected intravenously with a replication-defective adenovirus expressing human SAA-1 (ad-SAA), a null adenovirus (ad-null), or saline. The construction and use of these viral constructs has been previously described.26,37 Mice were bled on days 1, 3, 7, 10, 14, 21, and at study end (28 days), and plasma human and murine SAA concentrations were quantified by species-specific enzyme-linked immunosorbent assay kits (Anogen, Mississauga, Canada, for human SAA; and Invitrogen for mouse SAA). Plasma lipoproteins from individual mice were analyzed by fast performance liquid chromatography as previously described.38 Fractions from the peaks of VLDL, LDL, and HDL were analyzed by Western blot for SAA content (antibody from Santa Cruz Biotechnology, Santa Cruz, CA). During injection and bleedings mice were anesthetized by inhaled isoflurane. Livers were collected from all mice 3 or 28 days after injections, and hepatic TGF-β1 expression was evaluated by real-time reverse transcriptase-polymerase chain reaction (iCycler, Bio-Rad), using primers forward 5′-TGGAGCAACATGTGGAACTC-3′ and backward 5′-GTCAGCAGCCGGTTACCA-3′ (provided by Dr. Jianhua Shao, University of Kentucky), and corrected for 18S expression. Twenty-eight days after injections, aortas were collected, stripped of adventitia, and total protein was extracted and used in Western blot analyses to quantify proteoglycan content as previously described.33 β-Actin was used as the loading control. Atherosclerosis was quantified in the aortic root, en face aorta, and brachiocephalic artery as previously described.14,39,40 Immunohistochemical staining was performed on adjacent aortic root sections as previously described.33
Statistical Analyses
Results are expressed as mean ± SEM. Data were analyzed by one-way analysis of variance for SAA effects, with pair-wise comparisons using Dunnett’s multiple comparison test. The effect of TGF-β was evaluated by t-test compared to control (unstimulated cells). A P value <0.05 was considered statistically significant. Binding curves were evaluated using GraphPad Prism software (GraphPad Software, San Diego, CA) to determine best fit, and binding constants were compared by t-test.
Results
SAA Stimulates Vascular Proteoglycan Synthesis
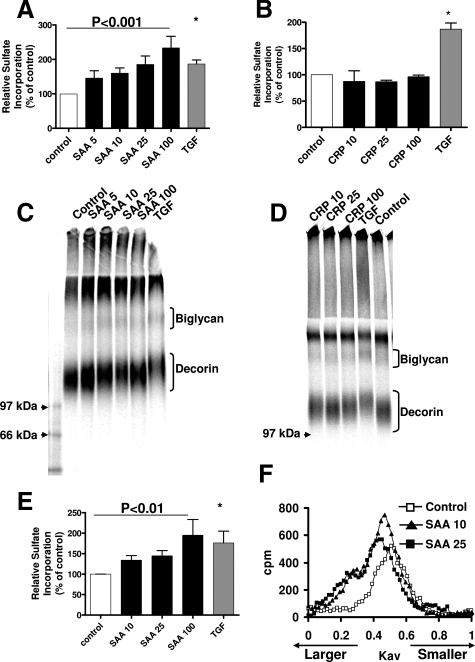
Incubation of vascular smooth muscle cells with SAA led to a dose-dependent increase in sulfate incorporation (50 to 100%, P < 0.0001; Figure 1A). The increase in sulfate incorporation was seen even at very modest, physiologically relevant SAA concentrations (5 mg/L), and SAA at 25 to 100 mg/L induced a similar stimulation of sulfate incorporation as TGF-β. Time course studies demonstrated that the effect of SAA to stimulate proteoglycan sulfate incorporation was maximal at 24 hours (data not shown). There was no effect of CRP (1 to 100 mg/L for 0 to 72 hours) on sulfate incorporation (Figure 1B). The size of the proteoglycans synthesized by vascular smooth muscle cells stimulated with SAA was estimated by electrophoresis. Proteoglycans synthesized by cells stimulated with SAA had decreased electrophoretic mobility indicating increased apparent size of both biglycan and decorin (Figure 1C); however, there was no effect of CRP on proteoglycan electrophoretic mobility (Figure 1D). An effect on the larger versican and heparan sulfate proteoglycans cannot be determined because they do not enter the resolving gel. Because the size of the proteoglycan core proteins does not change, this indicates an increase in glycosaminoglycan chain length. To confirm the effect of SAA was to increase glycosaminoglycan chain length, cells were supplied with xyloside, which acts as an artificial acceptor of glycosaminoglycan synthesis.31 Incubation with SAA stimulated a dose-dependent increase in glycosaminoglycan sulfate incorporation (30 to 100%, P < 0.01; Figure 1E). Glycosaminoglycan chains synthesized by cells incubated with SAA were larger compared to glycosaminoglycans synthesized by unstimulated cells (Figure 1F). The stimulation of proteoglycan or glycosaminoglycan sulfate incorporation and size was observed only with lipid-free SAA, but not with SAA-HDL complex or HDL alone (data not shown). Stimulation of vascular smooth muscle cells with CRP had no effect on glycosaminoglycan sulfate incorporation or length (data not shown).
Figure 1.
SAA, but not CRP, increases vascular proteoglycan and glycosaminoglycan synthesis. A and B: Vascular smooth muscle cells were stimulated with SAA (A) or CRP (B) at the indicated concentrations (mg/L) or with TGF-β (gray bars, 2 ng/ml) for 24 hours. Sulfate incorporation was determined by cetyl pyridinium chloride precipitation as described in the Materials and Methods. Data shown are mean ± SEM from eight separate experiments (A) and three separate experiments (B). C and D: Proteoglycan size was estimated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (3.5% stacking gel with 4 to 12% gradient resolving gel). Lanes were loaded with equal counts; gels shown are representative of eight independent experiments (C) or three separate experiments (D). E and F: Vascular smooth muscle cells were stimulated with SAA at the indicated concentrations (mg/L) or with TGF-β (gray bars, 2 ng/ml) for 24 hours in the presence of 0.5 mmol/L xyloside. E: Sulfate incorporation was determined by cetyl pyridinium chloride precipitation as described in the Materials and Methods. Data shown are mean ± SEM from five separate experiments. F: Glycosaminoglycans were applied to Sepharose CL-6B columns as described in the Materials and Methods for analysis of hydrodynamic size. The curves shown are representative of five separate experiments. *P < 0.01 versus control.
SAA Stimulates the Production of Proteoglycans with Increased LDL Binding Affinity
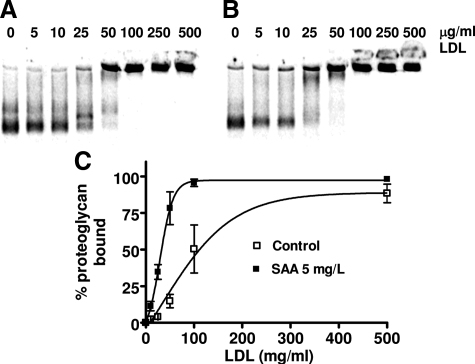
Proteoglycans bind LDL via ionic interactions between the sulfate groups on the glycosaminoglycan chains with positively charged residues on apolipoproteins, and increased binding affinity is seen when there is increased size and sulfation of the glycosaminoglycan chains.41 The LDL binding affinity of proteoglycans synthesized by vascular smooth muscle cells stimulated with SAA was determined using a modified gel mobility shift assay. Proteoglycans synthesized by vascular cells stimulated with as little as 5 mg/L SAA had increased LDL binding affinity compared to proteoglycans synthesized by unstimulated cells (Kd, 29 μg/ml LDL versus 90 μg/ml LDL, respectively; P < 0.0005) (Figure 2). However, no further increase in LDL binding affinity was observed with proteoglycans synthesized by cells stimulated with higher concentrations of SAA (data not shown).
Figure 2.
Proteoglycans secreted by cells stimulated with SAA have higher LDL binding affinity than proteoglycans secreted by unstimulated cells. Shown are representative gels showing the binding of proteoglycans synthesized by unstimulated cells (A) and proteoglycans synthesized by cells stimulated with 5 mg/L SAA (B) to native human LDL. C: Binding curves show mean ± SEM from four separate experiments.
SAA Up-Regulates Biglycan Expression via TGF-β
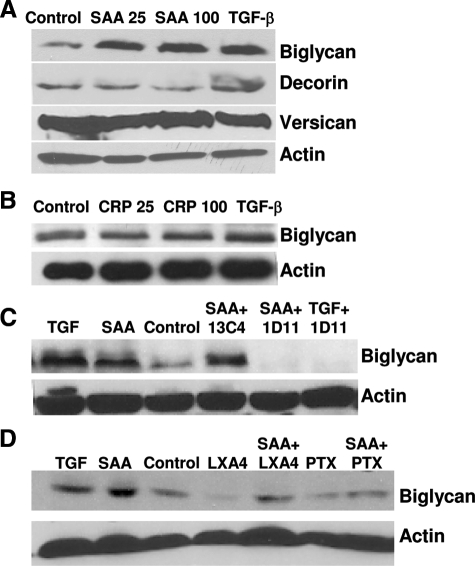
The effect of SAA and CRP on the expression of proteoglycan core proteins was determined using Western blot analyses. SAA stimulation resulted in increased biglycan core protein synthesis (Figure 3A), similar to the known effect of TGF-β to stimulate biglycan synthesis (Figure 3A, lane 4).29 No changes in the core proteins for decorin or versican were observed (Figure 3A). CRP had no effect on the core proteins of biglycan (Figure 3B) or the other proteoglycans (data not shown). The mechanisms by which SAA exerts its effects are not clear; however, there is striking similarity between observed effects of SAA on biglycan with that previously observed with TGF-β.29,30,33,42,43,44 Thus, TGF-β levels were quantified to determine whether SAA stimulated biglycan expression via stimulation of endogenous TGF-β. Conditioned media from vascular smooth muscle cells exposed to SAA had increased amounts of both total TGF-β (not shown) and endogenously active TGF-β compared to media from unexposed cells (165 ± 11 pg/ml versus 68 ± 24 pg/ml, respectively; P = 0.04). To determine whether TGF-β was required for SAA to stimulate biglycan expression, cells were exposed to SAA (100 mg/L) in the presence of the TGF-β neutralizing antibody 1D1145 or irrelevant control antibody 13C4. The presence of 1D11 completely eliminated detection of TGF-β, whereas there was no inhibition of TGF-β concentrations when cells were exposed to the irrelevant control antibody with SAA (TGF-β, 184 ± 1 pg/ml). Western blot analyses showed that biglycan was increased in media from cells exposed to SAA alone, TGF-β alone, and SAA with the 13C4 antibody, but was not increased in media from cells exposed to no additives, or when SAA or TGF-β were provided with the 1D11 TGF-β neutralizing antibody (Figure 3C).
Figure 3.
SAA stimulates biglycan via TGF-β and the FPRL1 receptor. A: Cells were stimulated with SAA at 25 or 100 mg/L, or with TGF-β (2 ng/ml). B: Cells were stimulated with CRP at 25 or 100 mg/L, or with TGF-β (2 ng/ml). C: Cells were stimulated with TGF-β (2 ng/ml), SAA 100 mg/L alone or in combination with TGF-β neutralizing antibody 1D11 (10 μg/ml), or with irrelevant control antibody 13C4 (10 μg/ml). D: Cells were stimulated with TGF-β (2 ng/ml), SAA 100 mg/L alone or in combination with Lipoxin A4 (LXA4, 5μmol/L), or PTX (0.5 μg/ml) for 24 hours. Proteoglycan core protein synthesis was analyzed by Western blot. Lanes were loaded with an equal amount of protein, and blotted for biglycan, versican, decorin, and actin. Blots shown are representative of three independent experiments (A) or two independent experiments (B--D).
SAA Stimulation of Biglycan Involves the FPRL1 Receptor
Several studies have demonstrated that SAA is a ligand for the formyl peptide receptor-like 1 (FPRL1)/Lipoxin A4 (LXA4) receptor,46,47,48 which is a G-protein-coupled receptor. FPRL1 mRNA is expressed by these vascular smooth muscle cells (data not shown). To investigate the mechanisms by which SAA affects proteoglycan synthesis cells were co-incubated with SAA (100 mg/L) and agents that inhibit either SAA binding to FPRL1 or G-protein-coupled signaling pathways. Co-incubation of SAA with LXA4, which competes for FPRL1 binding, attenuated the SAA-induced up-regulation of biglycan, whereas co-incubation with PTX, which inhibits G-protein-coupled receptor signaling, inhibited the effect of SAA to stimulate biglycan expression (Figure 3D). Furthermore, the binding of SAA to the FPRL1 receptor and stimulation of G-protein-coupled receptor signaling is involved in SAA’s stimulation of TGF-β because both LXA4 and PTX attenuated the SAA stimulation of TGF-β in the media (TGF-β concentrations 20% of those seen when cells stimulated with SAA alone, P < 0.01 for each).
SAA Stimulates Proteoglycan Synthesis in Vivo
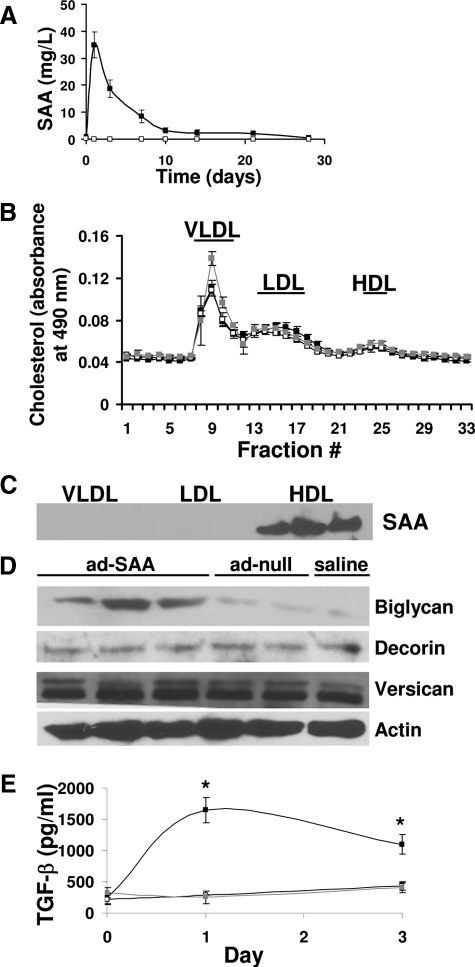
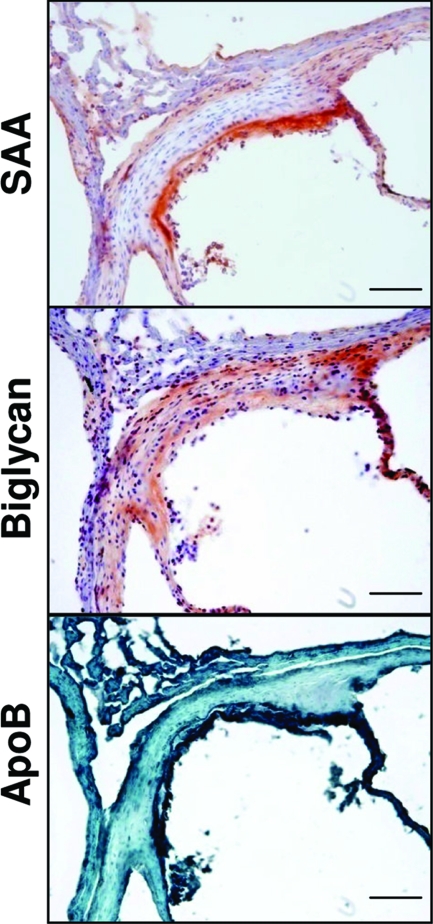
To determine whether SAA stimulates vascular proteoglycan synthesis in vivo, ApoE−/− mice were injected with ad-SAA, ad-null, or saline. Time-course studies demonstrated peak human SAA levels on day 1 (35 ± 5 mg/L), with levels still detectable through 21 days (Figure 4A). Murine SAA was equally elevated in all groups on day 1 (10 ± 1 mg/L), then rapidly returned to baseline at all other time points and did not differ between groups at any time (data not shown). Plasma aliquots drawn 1 and 3 days after adenovirus or saline injections were separated using fast performance liquid chromatography then lipoprotein fractions were blotted for SAA. There was no effect of ad-SAA on plasma lipoprotein distribution (Figure 4B) and SAA was found predominantly associated with HDL (Figure 4C), as previously described in both mice26 and in humans.49 Mice that received ad-SAA had increased aortic biglycan content (determined by Western blot, Figure 4D). No differences in vascular decorin or versican content were seen (Figure 4D). Plasma total and bioactive TGF-β levels were quantified in mice that received ad-SAA, ad-null, or saline. Only mice that received ad-SAA had increased TGF-β levels (Figure 4E). The TGF-β is likely hepatic in origin because mice that received ad-SAA had twofold increased hepatic TGF-β expression compared to mice that received ad-null or saline (data not shown). Although mice had minimal atherosclerosis that did not differ between groups at this time (data not shown), immunohistochemical analyses demonstrated co-localization between biglycan, SAA, and apoB in the aortic root of mice injected with ad-SAA (Figure 5).
Figure 4.
Elevated human SAA stimulates proteoglycan synthesis in vivo. A: Mice were injected with an adenovirus expressing SAA (ad-SAA, black squares), a null virus (ad-null, open squares), or saline (gray squares, not visible because of overlap with open squares) on day 0, then samples were collected on the indicated days and human SAA quantified by enzyme-linked immunosorbent assay. Shown are mean ± SEM for n = 22 (ad-SAA), n = 11 (ad-null), and n = 9 (saline). B: Plasma aliquots from individual mice drawn 1 day after adenovirus or saline injection were analyzed by fast performance liquid chromatography for lipoprotein cholesterol distribution. Shown are mean ± SEM for n = 5 (ad-SAA and ad-null) and n = 4 (saline). C: Fractions from the VLDL, LDL, and HDL peaks (indicated by the horizontal bars) were collected and SAA content analyzed by Western blot. Blot shown is from a mouse that received ad-SAA, representative of five. D: Aortic biglycan content was determined by Western blot analysis of total aortic protein. Each lane represents individual mice, representative of n = 22 (ad-SAA), n = 11 (ad-null), and n = 9 (saline). E: Endogenously active TGF-β was measured in six mice per group from samples collected before, and 1 and 3 days after injections. Data shown are mean ± SEM. *P < 0.01 versus saline group.
Figure 5.
SAA increased aortic biglycan content, which co-localizes with SAA and apoB. Mice were injected with ad-SAA, ad-null, or saline, and 28 days later vascular tissues were collected. Shown are representative photos from a mouse that received ad-SAA. In each photo the lumen is shown to the bottom right. Scale bars = 100 μm. Original magnifications, ×200.
Discussion
Elevated SAA and CRP levels are predictive of cardiovascular disease events, and have been proposed to play a direct role in the development of atherosclerosis. However, the mechanisms by which these inflammatory markers may affect atherogenesis are unclear. Our data demonstrate that SAA, but not CRP, increased vascular smooth muscle cell proteoglycan synthesis in a pro-atherogenic manner compared to unstimulated cells. SAA stimulation induced an increase in sulfate incorporation because of both an up-regulation of biglycan synthesis as well as elongation of glycosaminoglycan chains. The net result was that proteoglycans synthesized by vascular smooth muscle cells stimulated with SAA, but not CRP, have increased LDL binding affinity compared to proteoglycans synthesized by unstimulated cells. Furthermore, the effect of SAA to stimulate proteoglycan synthesis also occurred in vivo, in that mice with elevated levels of SAA had increased aortic biglycan content. The short duration of study (SAA elevation persisted <28 days after adenoviral administration) was not sufficient to affect atherosclerosis development; however, we found increased biglycan content in mice that had received ad-SAA, and immunohistochemistry demonstrated co-localization between biglycan, SAA, and apoB.
Our finding that SAA specifically up-regulated biglycan synthesis is of particular relevance to atherosclerosis because proteoglycan-mediated LDL retention is thought to play a key role in the development of atherosclerosis.8,9,13 All proteoglycans can bind LDL, thus, which proteoglycan is the most atherogenic is not clear. However, biglycan is the proteoglycan that is consistently reported to co-localize with apoB in both mice and humans.14,50,51,52 ApoB has also been reported to co-localize with perlecan in mice,33,50 and decorin in humans.51 However, decorin overexpression in mice was associated with decreased atherosclerosis development,53 and studies examining atherosclerosis in mice heterozygous for perlecan (perlecan deficiency is lethal) are complex, with only male apoE−/− mice showing decreased atherosclerosis, and no differences in atherosclerosis extent in female apoE−/− or either gender of LDLR−/− mice.54 A recent study using apoE−/− mice that express perlecan lacking heparan sulfate glycosaminoglycan chains demonstrated decreased atherosclerosis.55 Using in vitro analyses the authors demonstrated decreased lipoprotein binding to both complex extracellular matrix and isolated proteoglycans from the mice with glycosaminoglycan-less perlecan compared to wild-type, but in vivo analyses did not demonstrate differences in lipoprotein binding, thus the mechanisms by which glycosaminoglycan-less perlecan is atheroprotective are not clear.55 The role of proteoglycans in atherosclerosis is complex because they play many roles in addition to lipoprotein binding, and the relative importance of proteoglycan-mediated lipoprotein retention may alter with progression of the atherosclerotic lesion development.10 However, given the co-localization observed between apoB and biglycan in both mouse and human atherosclerosis, we propose that biglycan is a key proteoglycan of interest. There are no data on the effect of biglycan deficiency or overexpression on atherosclerosis development, but overexpression of biglycan in vitro led to increased retention of LDL on complex extracellular matrices.56 We recently demonstrated that increased vascular biglycan content predisposed to accelerated atherosclerosis development,14 further supporting a key role for biglycan in mediating LDL retention.
Thus, the increased vascular biglycan content induced by SAA could lead to increased LDL retention and initiation of atherosclerosis. Although we did not find any difference in atherosclerosis between groups in this study, the mice were young at the time of atherosclerosis analysis (10 to 12 weeks old) and the duration of elevation of SAA was short (<28 days), whereas most studies evaluating atherosclerosis in this murine model require consumption of cholesterol-enriched diets for 12 weeks, or examine chow-fed mice at much older ages (6 to 12 months old). Further studies are required to determine whether prolonged duration of elevated SAA indeed increases atherosclerosis development. However, two previous studies that used dietary cholesterol feeding to induce elevated plasma SAA concentrations have shown increased atherosclerosis development in LDL receptor-deficient mice that correlated with increased SAA concentrations.15,17 Our data combined with the literature suggest that elevated SAA concentrations contribute causally to the development of atherosclerosis.
The mechanisms by which SAA exerts its effects are not yet understood. However, as previously suggested,46,47,48 we demonstrate that SAA exerts at least some of its effects via binding to FPRL1 and G-protein-coupled receptor signaling. Furthermore, we now demonstrate a novel effect of SAA: the stimulation of TGF-β. We and others have previously shown that TGF-β up-regulates proteoglycan synthesis, especially biglycan synthesis, and increases the LDL binding affinity of all proteoglycans.29,30,32,33,44 Inhibition of FPRL1 using LXA4 or PTX or inhibition of TGF-β using a neutralizing antibody inhibited SAA stimulation of biglycan expression and administration of adenoviral SAA in mice led to increased plasma TGF-β concentrations, increased hepatic TGF-β expression, and increased vascular biglycan content. Thus, we suggest that SAA stimulates biglycan synthesis via its effect to increase TGF-β concentrations. The role of TGF-β in atherosclerosis development is complex with decreased atherosclerotic fibrosis but increased inflammation observed with the inhibition of TGF-β signaling.57,58 However, both SAA and TGF-β may play a pro-atherogenic role in the development of atherosclerosis because of their induction of vascular biglycan content and increase in proteoglycan-LDL binding affinity.
SAA is usually seen in association with HDL, although some studies have shown SAA in association with apoB-containing lipoproteins.15,17,59,60 SAA itself can bind to proteoglycans, and SAA-containing lipoproteins have been shown to bind proteoglycans in proportion to their SAA content.15,61 Additionally, SAA has been shown to be synthesized by all cell types pertinent to atherosclerosis, including vascular smooth muscle cells, endothelial cells, and macrophages.18 The co-localization of SAA with biglycan observed in our studies could be attributable to production or deposition of SAA in the vascular wall where it directly stimulates vascular smooth muscle cell biglycan synthesis, or could be attributable to biglycan-mediated retention of SAA-containing lipoproteins. Although our tissue culture studies only found stimulation of biglycan with lipid-free SAA, it is plausible that lipid-free SAA exists within the milieu of the vascular wall. Furthermore, in our mouse study we used adenoviral expression of SAA, in which the vast majority of the SAA is associated with HDL. We confirm the relevance of our in vitro findings by demonstrating increased plasma TGF-β concentrations and increased vascular biglycan content in mice that had received ad-SAA compared to ad-null or saline-treated mice. Thus, SAA appears to act via induction of TGF-β to stimulate vascular smooth muscle cell biglycan synthesis, elongate glycosaminoglycan chains, increase LDL retention, and thus could stimulate the initiation and/or progression of an atherosclerotic lesion.
Importantly, these effects of SAA to stimulate proteoglycan synthesis were observed with only modestly and/or transiently elevated SAA concentrations. In a previous study we found mean plasma SAA concentrations of 3.5 mg/L in lean, insulin-sensitive, healthy, community-dwelling individuals, and mean SAA concentrations of 7.1 mg/L in obese, insulin-resistant individuals.62 In the present study, stimulation with SAA concentrations as low as 5 mg/L was sufficient to increase vascular smooth muscle cell proteoglycan synthesis and LDL binding affinity compared to unstimulated cells. Furthermore, after adenoviral SAA expression, the plasma level of SAA declined to levels <5 mg/L by 10 days, yet increased vascular biglycan content was seen 28 days after viral administration, indicating a prolonged or durable effect of SAA on proteoglycan synthesis. In humans, although acute inflammatory events can lead to 100- to 1000-fold elevations of SAA, insulin resistance, obesity, or cholesterol feeding are all associated with modest but chronic elevations of SAA.62,63 Modest elevations of SAA are associated with increased cardiovascular risk64,65; thus, the concentrations used in this study are physiologically relevant.
The relationship between CRP and cardiovascular disease has been well studied, and the linkage is established. It is not clear, however, if elevated CRP levels are contributory toward the development of vascular pathology, or merely reflective. We did not find any effect of CRP to stimulate vascular proteoglycan synthesis, LDL binding, or biglycan expression, which is in agreement with a recent report that mice transgenic for human CRP did not have any increase in atherosclerosis development.66 It is not yet known whether lowering CRP and/or SAA will be protective against cardiovascular disease events. However, our data suggest a possible mechanism by which elevated SAA is detrimental, and thus one can extrapolate that lowering SAA may be a therapeutic step in limiting atherosclerosis progression. Many current medical therapies that have been shown to reduce cardiovascular disease risk, such as statins, also lower CRP and SAA, and thus it is conceivable that part of the mechanism of benefit of these agents may be through pleiotropic effects on SAA and the vessel wall. In summary, our data demonstrate that modestly elevated SAA concentrations stimulate proteoglycan synthesis and increase proteoglycan LDL binding affinity, suggesting a causal role for SAA in atherosclerosis development.
Footnotes
Address reprint requests to Lisa R. Tannock, Room 567 Wethington Building, 900 S. Limestone, University of Kentucky, Lexington, KY, 40536-0200. E-mail: lisa.tannock@uky.edu.
Supported in part by the National Institutes of Health (grants HL082772 to L.R.T. and HL086670 to N.R.W. and F.C.D.), the American Heart Association (award to L.R.T.), and the University of Kentucky (the physician scientist program to L.R.T.).
This work has previously presented in part at the American Heart Association Scientific Sessions, Chicago, IL, November 2006.
References
- McLaughlin T, Abbasi F, Lamendola C, Liang L, Reaven G, Schaaf P, Reaven P. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908–2912. doi: 10.1161/01.cir.0000041046.32962.86. [DOI] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millan JL. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46:625–633. doi: 10.1007/s00125-003-1090-z. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Gustafsson M, Levin M, Skalen K, Perman J, Friden V, Jirholt P, Olofsson SO, Fazio S, Linton MF, Semenkovich CF, Olivecrona G, Boren J. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ Res. 2007;101:777–783. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Wight TN. The Vascular Extracellular Matrix. Fuster V, Ross R, Topol EJ, editors. Philadelphia: Lippincott-Raven,; 1996:pp 421–440. [Google Scholar]
- Skålén K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–530. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O'Brien KD, Chait A. Increase in serum amyloid A evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007;48:2344–2353. doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, III, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci USA. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao JH, Xie PZ, Fishbein MC, Kreuzer J, Drake TA, Demer LL, Lusis AJ. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994;14:1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- Jabs WJ, Theissing E, Nitschke M, Bechtel JF, Duchrow M, Mohamed S, Jahrbeck B, Sievers HH, Steinhoff J, Bartels C. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. 2003;108:1428–1431. doi: 10.1161/01.CIR.0000092184.43176.91. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–379. doi: 10.1016/s0021-9150(99)00105-7. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kakihara T, Kamishima T, Fukuda T, Kawai T. Both acute phase and constitutive serum amyloid A are present in atherosclerotic lesions. Pathol Int. 1996;46:797–800. doi: 10.1111/j.1440-1827.1996.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Chang MY, Olin KL, Tsoi C, Wight TN, Chait A. Human monocyte-derived macrophages secrete two forms of proteoglycan-macrophage colony-stimulating factor that differ in their ability to bind low density lipoproteins. J Biol Chem. 1998;273:15985–15992. doi: 10.1074/jbc.273.26.15985. [DOI] [PubMed] [Google Scholar]
- Halvorsen B, Aas UK, Kulseth MA, Drevon CA, Christiansen EN, Kolset SO. Proteoglycans in macrophages: characterization and possible role in the cellular uptake of lipoproteins. Biochem J. 1998;331:743–752. doi: 10.1042/bj3310743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- Cabana VG, Feng N, Reardon CA, Lukens J, Webb NR, de Beer FC, Getz GS. Influence of apoA-I and apoE on the formation of serum amyloid A-containing lipoproteins in vivo and in vitro. J Lipid Res. 2004;45:317–325. doi: 10.1194/jlr.M300414-JLR200. [DOI] [PubMed] [Google Scholar]
- Tannock LR, Little PJ, Tsoi C, Barrett PH, Wight TN, Chait A. Thiazolidinediones reduce the LDL binding affinity of non-human primate vascular cell proteoglycans. Diabetologia. 2004;47:837–843. doi: 10.1007/s00125-004-1358-y. [DOI] [PubMed] [Google Scholar]
- Tannock LR, Little PJ, Wight TN, Chait A. Arterial smooth muscle cell proteoglycans synthesized in the presence of glucosamine demonstrate reduced binding to LDL. J Lipid Res. 2002;43:149–157. [PubMed] [Google Scholar]
- Schönherr E, Jarvelainen HT, Kinsella MG, Sandell LJ, Wight TN. Platelet derived growth factor and transforming growth factor-beta1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler Thromb. 1993;13:1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- Little PJ, Tannock L, Olin KL, Chait A, Wight TN. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol. 2002;22:55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- Potter-Perigo S, Braun KR, Schonherr E, Wight TN. Altered proteoglycan synthesis via the false acceptor pathway can be dissociated from beta-D-xyloside inhibition of proliferation. Arch Biochem Biophys. 1992;297:101–109. doi: 10.1016/0003-9861(92)90646-e. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor, beta1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–530. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- Hurt-Camejo E, Camejo G, Sartipy P. Measurements of proteoglycan-lipoprotein interaction by gel mobility shift assay. Methods Mol Biol. 1998;110:267–279. doi: 10.1385/1-59259-582-0:267. [DOI] [PubMed] [Google Scholar]
- Nomura K, Tada H, Kuboki K, Inokuchi T. Transforming growth factor-beta-1 latency-associated peptide and soluble betaglycan prevent a glucose-induced increase in fibronectin production in cultured human mesangial cells. Nephron. 2002;91:606–611. doi: 10.1159/000065020. [DOI] [PubMed] [Google Scholar]
- Wilson P, Drennon K, Tannock LR. Regulation of vascular proteoglycan synthesis by metabolic factors associated with diabetes. J Invest Med. 2007;55:18–25. doi: 10.2310/6650.2007.05067. [DOI] [PubMed] [Google Scholar]
- Hosoai H, Webb NR, Glick JM, Tietge UJ, Purdom MS, de Beer FC, Rader DJ. Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoA-I levels in human apoA-I transgenic mice. J Lipid Res. 1999;40:648–653. [PubMed] [Google Scholar]
- Cole TG, Kitchens R, Daugherty A, Schonfeld G. An improved method for separation of triglyceride-rich lipoproteins by FPLC. Pharmacia Biocommunique. 1990;4:4–6. [Google Scholar]
- Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- Tannock LR, Chait A. Lipoprotein-matrix interactions in macrovascular disease in diabetes. Front Biosci. 2004;9:1728–1742. doi: 10.2741/1248. [DOI] [PubMed] [Google Scholar]
- Chang MY, Potter-Perigo S, Tsoi C, Chait A, Wight TN. Oxidized low density lipoproteins regulate synthesis of monkey aortic smooth muscle cell proteoglycans that have enhanced native low density lipoprotein binding properties. J Biol Chem. 2000;275:4766–4773. doi: 10.1074/jbc.275.7.4766. [DOI] [PubMed] [Google Scholar]
- Chen WB, Lenschow W, Tiede K, Fischer JW, Kalthoff H, Ungefroren H. Smad4/DPC4-dependent regulation of biglycan gene expression by transforming growth factor-beta in pancreatic tumor cells. J Biol Chem. 2002;277:36118–36128. doi: 10.1074/jbc.M203709200. [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Lenschow W, Chen WB, Faendrich F, Kalthoff H. Regulation of biglycan gene expression by transforming growth factor-beta requires MKK6–p38 mitogen-activated protein kinase signaling downstream of Smad signaling. J Biol Chem. 2003;278:11041–11049. doi: 10.1074/jbc.M300035200. [DOI] [PubMed] [Google Scholar]
- Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- Kumon Y, Hosokawa T, Suehiro T, Ikeda Y, Sipe JD, Hashimoto K. Acute-phase, but not constitutive serum amyloid A (SAA) is chemotactic for cultured human aortic smooth muscle cells. Amyloid. 2002;9:237–241. doi: 10.3109/13506120209114099. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim MK, Park KS, Bae YH, Yun J, Park JI, Kwak JY, Bae YS. Serum amyloid A stimulates matrix-metalloproteinase-9 up-regulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem Biophys Res Commun. 2005;330:989–998. doi: 10.1016/j.bbrc.2005.03.069. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci USA. 1977;74:4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjathoor VV, Chiu DS, O'Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:462–468. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, Olin KL, Alpers CE, Chiu W, Hudkins K, Wight TN, Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: co-localization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- Al Haj Zen A, Caligiuri G, Sainz J, Lemitre M, Demerens C, Lafont A. Decorin overexpression reduces atherosclerosis development in apolipoprotein E-deficient mice. Atherosclerosis. 2006;187:31–39. doi: 10.1016/j.atherosclerosis.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Vikramadithyan RK, Kako Y, Chen G, Hu Y, Arikawa-Hirasawa E, Yamada Y, Goldberg IJ. Atherosclerosis in perlecan heterozygous mice. J Lipid Res. 2004;45:1806–1812. doi: 10.1194/jlr.M400019-JLR200. [DOI] [PubMed] [Google Scholar]
- Tran-Lundmark K, Tran PK, Paulsson-Berne G, Friden V, Soininen R, Tryggvason K, Wight TN, Kinsella MG, Boren J, Hedin U. Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res. 2008;103:43–52. doi: 10.1161/CIRCRESAHA.108.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KD, Lewis K, Fischer JW, Johnson P, Hwang JY, Knopp EA, Kinsella MG, Barrett PH, Chait A, Wight TN. Smooth muscle cell biglycan overexpression results in increased lipoprotein retention on extracellular matrix: implications for the retention of lipoproteins in atherosclerosis. Atherosclerosis. 2004;177:29–35. doi: 10.1016/j.atherosclerosis.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Marhaug G, Sletten K, Husby G. Characterization of amyloid related protein SAA complexed with serum lipoproteins (apoSAA). Clin Exp Immunol. 1982;50:382–389. [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Mashiba S, Wada Y, Sahara M, Uchida K, Aizawa T, Kodama T. A serum amyloid A and LDL complex as a new prognostic marker in stable coronary artery disease. Atherosclerosis. 2004;174:349–356. doi: 10.1016/j.atherosclerosis.2004.01.030. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- Tannock LR, O'Brien KD, Knopp RH, Retzlaff B, Fish B, Wener MH, Kahn SE, Chait A. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005;111:3058–3062. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. The association of C-reactive protein, serum amyloid A and fibrinogen with prevalent coronary heart disease—baseline findings of the PAIS project. Atherosclerosis. 2001;156:451–456. doi: 10.1016/s0021-9150(00)00681-x. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Tennent GA, Hutchinson WL, Kahan MC, Hirschfield GM, Gallimore JR, Lewin J, Sabin CA, Dhillon AP, Pepys MB. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE(−/−) mice. Atherosclerosis. 2008;196:248–255. doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]