Abstract
The receptor-associated prorenin system (RAPS) refers to pathogenic mechanisms whereby prorenin binding to its receptor activates both the tissue renin-angiotensin system (RAS) and RAS-independent intracellular signaling pathways. Although we found significant involvement of angiotensin II type 1 receptor (AT1-R)-mediated inflammation in choroidal neovascularization (CNV), a central abnormality of vision-threatening age-related macular degeneration, the association of receptor-associated prorenin system with CNV has not been defined. Here, (pro)renin receptor blockade in a murine model of laser-induced CNV led to the significant suppression of CNV together with macrophage infiltration and the up-regulation of intercellular adhesion molecule-1, (ICAM-1) monocyte chemotactic protein-1, (MCP-1) vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR)-1, and VEGFR-2. To clarify the role of signal transduction via the (pro)renin receptor in CNV, we used mice in which renin-angiotensin system was deactivated by either the pharmacological blockade of AT1-R with losartan or the genetic ablation of AT1-R or angiotensinogen. Compared with wild-type controls, these mice exhibited significant reduction of CNV and macrophage infiltration, both of which were further suppressed by (pro)renin receptor blockade. The (pro)renin receptor and phosphorylated extracellular signal-regulated kinases (ERK) were co-localized in vascular endothelial cells and macrophages in CNV. (Pro)renin receptor blockade suppressed ERK activation and the production of MCP-1 and VEGF, but not ICAM-1, VEGFR-1, or VEGFR-2, in AT1-R-deficient mice with CNV and in losartan-treated microvascular endothelial cells and macrophages. These results indicate the significant contribution of RAPS to CNV pathogenesis.
Although several types of organ damage are known to result from the activation of tissue renin-angiotensin system (RAS), the precise mechanism for activating tissue RAS is not fully understood. (Pro)renin receptor, a recently identified transmembrane protein consisting of 350 amino acids, interacts with prorenin to exert renin activity through the conformational change of the prorenin molecule instead of the conventional proteolysis of the prorenin prosegment achieved by processing enzymes such as cathepsin B. Since the membrane-bound (pro)renin receptor is reported to exist in the major organs but not in the circulation,1 the nonproteolytic activation of prorenin is hypothesized to play a critical role in the activation of tissue, but not circulatory, RAS. In addition, prorenin binding to its receptor is shown to cause RAS-independent signal transduction via phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 in cells bearing (pro)renin receptor.1,2,3,4 Thus, we proposed the nomenclature “receptor-associated prorenin system (RAPS)” for the dual activation of tissue RAS and RAS-independent signaling pathway. In streptozotocin-induced diabetes, blockade of prorenin interaction with its receptor led to complete suppression of proteinuria, glomerulosclerosis and renal production of angiotensin I and II without affecting circulatory RAS, indicating a critical contribution of RAPS to the pathogenesis of diabetic nephropathy.3,5,6,7
Age-related macular degeneration (AMD) is the most common cause of blindness in developed countries. AMD is complicated by choroidal neovascularization (CNV), leading to severe vision loss due to hemorrhage and exudation from the immature new vessels.8,9 Epidemiological risk factors for AMD were reported to include hypertension,10 dyslipidemia,10 and atherosclerosis,11 all of which are related to the metabolic syndrome. Recently, angiotensin II type 1 receptor (AT1-R) signaling has been shown to play a significant role in various pathological processes complicating the metabolic syndrome such as angiogenesis and inflammation.12,13,14,15 CNV has proven to be an inflammatory disorder depending on intercellular adhesion molecule (ICAM)-1,16 monocyte chemotactic protein (MCP)-117 and vascular endothelial growth factor (VEGF).18 We have recently shown that AT1-R-mediated up-regulation of these inflammatory and angiogenic molecules is required for the development of CNV19; however, the role of (pro)renin receptor as a trigger to activate tissue RAS in CNV has not been defined. Although we have further shown that tissue RAS promoting retinal inflammation20 and neovascularization21 is activated by nonproteolytic activation of prorenin, it has not been determined whether (pro)renin receptor-mediated intracellular signaling, the other pathway of RAPS, is pathogenic in the eye.
We therefore hypothesize that prorenin binding to its receptor promotes CNV by dually activating tissue RAS and RAS-independent ERK pathway via the receptor. In the present paper, we report the first evidence of significant relationship between RAPS and CNV together with underlying molecular and cellular mechanisms related to inflammation.
Materials and Methods
Animals
Male C57BL/6J mice (CLEA, Tokyo, Japan) at the age of 6 to 9 weeks, age- and sex-matched AT1-R-deficient mice22 (based on the C57BL/6J strain and donated by Tanabe Seiyaku Co., Ltd., Osaka, Japan), angiotensinogen (AGT)-deficient mice23 (based on the C57BL/6J strain and purchased from YS Institute Inc, Tochigi, Japan) and Long-Evans rats (SLC, Shizuoka, Japan) were used. All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of (Pro)renin Receptor Blocker
To cover the handle region (positions 11–15) of the prorenin molecule, which is the binding site of (pro)renin receptor,24 we designed a decoy peptide, NH2-IPLKKMPS-COOH (positions 11 to 18), as murine (pro)renin receptor blocker (PRRB) and purified it by high performance liquid chromatography, as described previously.3 The specific inhibitory action of PRRB against RAPS was confirmed in our recent in vivo data.3,5 As a negative control for PRRB, we also prepared a control peptide (CP), NH2-MTRLSAE-COOH (positions 30 to 36) with an amino acid sequence outside the handle region.
Induction of Laser-Induced CNV
Laser-induced CNV is widely used as an animal model for neovascular AMD and reflects the pathogenesis of CNV-related inflammation seen in AMD. In this model, new vessels from the choroid invade the subretinal space after photocoagulation. Laser photocoagulation was performed around the optic nerve with the wavelength of 532 nm, the power of 200 mW, the duration of 100 ms and the spot size of 75 μm for mice or 100 μm for rats using a slit lamp delivery system (Novus Spectra; Lumenis, Tokyo, Japan), as described previously.17
Treatment with PRRB, CP, and Losartan
Mice received intraperitoneal injections of vehicle (PBS), CP (1.0 mg/kg), PRRB (1.0 mg/kg), or losartan (2, 20, or 50 mg/kg; Cayman Chemical, Ann Arbor, MI) 1 day before photocoagulation and the treatments were continued daily until the end of the study. The present dose of PRRB is equivalent to that applied to significantly reduce retinal neovascularization in mice.21 As for losartan, the dose of 20 mg/kg was the most potent in inhibiting CNV (data not shown) and used as the maximal-effect dose in the present data.
Quantification of Laser-Induced CNV
One week after laser injury, eyes were enucleated and fixed with 4% paraformaldehyde. Eye cups obtained by removing anterior segments were incubated with 0.5% fluorescein-isothiocyanate-isolectin B4 (Vector Laboratories, Burlingame, CA). CNV was visualized with blue argon laser on a confocal microscope (FV1000; Olympus, Tokyo, Japan). Horizontal optical sections of CNV were obtained at every 1-μm step from the surface to the deepest focal plane. The area of CNV-related fluorescence was measured by National Institutes of Health ImageJ (Bethesda, MD). The summation of the whole fluorescent area was used as the volume of CNV, as described previously.17
Quantification of Infiltrating Macrophages
Three days after laser injury, whole-mount retinal pigment epithelium (RPE)-choroid complex was incubated with a goat polyclonal antibody against platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) and a rat polyclonal antibody against F4/80 (Serotec, Oxford, UK). Alexa 488- and Alexa 546-tagged secondary antibodies (Molecular Probes, Eugene, OR) were then applied. PECAM-1-stained area of CNV and F4/80-positive macrophages were evaluated, and the volume-adjusted number of macrophages was calculated.
Quantitative Reverse Transcription-Polymerase Chain Reaction Analyses
We isolated total RNA from the RPE-choroid and performed quantitative reverse transcription (RT)-PCR with an ABI Prism 7700 HT Detection System (Applied Biosystems, Foster City, CA); and probes and primers for the rat genes that encode prorenin, (pro)renin receptor, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as described previously.5,7,25
Western Blot Analyses
Three days after laser injury, the RPE-choroid complex was carefully isolated and placed into the lysis buffer. After blocking nonspecific binding with 5% skim milk, polyvinylidene fluoride membranes were incubated with a goat polyclonal antibody against angiotensin II (Santa Cruz Biotechnology, Santa Cruz, CA) or a mouse monoclonal antibody against phosphorylated ERK1/2 (Cell Signaling Technology, Beverly, MA), total ERK1/2 (Cell Signaling Technology) or α-tubulin (Sigma, St. Louis, MO). Membranes were then incubated with biotin-conjugated secondary antibody (Jackson Immuno Research Laboratories, West Grove, PA) followed by avidin-biotin complex (Vectastain ABC Elite Kit; Vector Laboratories). Finally the signals were detected through enhanced chemiluminescence (ECL Blotting Analysis System; GE Health Care).
Enzyme-Linked Immunosorbent Assay
Protein extracts were obtained from the RPE-choroid complex 3 days after photocoagulation. The protein levels of ICAM-1, MCP-1, VEGF, VEGF receptor (VEGFR)-1 and VEGFR-2 were determined with the enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Immunohistochemistry
Three days after photocoagulation, rat eye cups were incubated with a goat anti-rat (pro)renin receptor antibody together with fluorescein-isothiocyanate-isolectin B4 (Vector Laboratories) or a rabbit anti-EMR 1 (corresponding to murine F4/80) antibody (Santa Cruz Biotechnology). The anti-(pro)renin receptor antibody was raised by using the previously established COS-7 cells producing rat (pro)renin receptor protein.5 Alexa 488- and Alexa 546-tagged secondary antibodies (Molecular Probes) were then applied. For immunohistochemical staining of phosphorylated ERK1/2, a goat polyclonal antibody against rat phosphorylated ERK1/2 (Santa Cruz Biotechnology) was applied as the primary antibody.
In Vitro Assays
Murine brain-derived capillary endothelial cells (b-End3) were cultured with Dulbecco’s modified Eagle’s medium (Sigma). After 6-hour incubation with tumor necrosis factor(TNF)-α (Sigma, 1 ng/ml) plus losartan (10 μmol/L), or TNF-α plus PRRB (100 μmol/L) and losartan (10 μmol/L), the supernatant and cell lysate were collected for protein analyses, and then the concentration of MCP-1 in the supernatant and ICAM-1, VEGFR-1 and VEGFR-2 in the cell lysate were measured by the ELISA kits (R&D Systems). Murine macrophages (RAW264.7) were treated with Dulbecco’s modified Eagle’s medium containing lipopolysaccharide (100 ng/ml) plus losartan (10 μmol/L) or lipopolysaccharide plus PRRB (100 μmol/L) and losartan (10 μmol/L). After 6-hour incubation, supernatant was processed for ELISA analyses for VEGF (R&D Systems).
Statistical Analyses
All results were expressed as mean ± SEM. The values were processed for statistical analyses (Mann-Whitney test). Differences were considered statistically significant when the P values were <0.05.
Results
Prorenin Expression Is Up-Regulated in CNV and Blockade of Prorenin Binding to Its Receptor Inhibits CNV
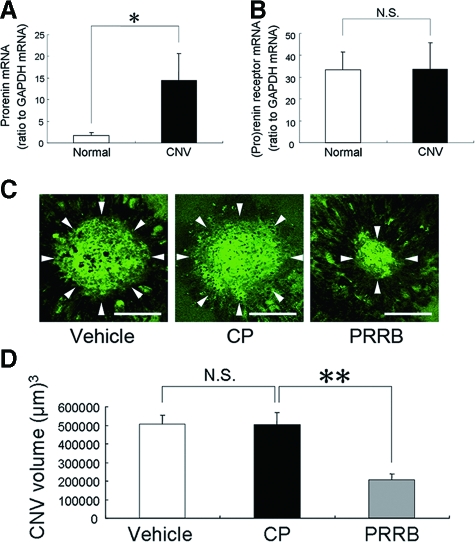
To elucidate the involvement of prorenin and (pro)renin receptor in the pathogenesis of CNV, we first performed quantitative RT-PCR analyses for prorenin and (pro)renin receptor in the RPE-choroid complex. Prorenin mRNA levels (ratio to GAPDH mRNA) were up-regulated (P < 0.05) in the RPE-choroid of laser-treated rats, compared with age-matched normal controls (Figure 1A). In contrast, mRNA levels of (pro)renin receptor showed no significant difference (P > 0.05) between laser-treated rats and normal controls (Figure 1B). The CNV volume was measured to evaluate the effects of PRRB on the development of CNV. CP treatment did not significantly (P > 0.05) change the CNV volume (501,810 ± 66,820 μm3), compared with vehicle-treated animals (504,411 ± 49,791 μm3) (Figure 1, C and D). However, PRRB-treated mice showed a significant (P < 0.01) decrease in the CNV volume (208,355 ± 29,388 μm3), compared with vehicle-treated mice (504,411 ± 49,791 μm3) or CP-treated mice (501,810 ± 66,820 μm3) (Figure 1, C and D).
Figure 1.
Prorenin expression is up-regulated in CNV and PRRB inhibits CNV. A: Up-regulation of prorenin mRNA levels in the RPE-choroid complex by inducing CNV (n = 6 to 7). B: (Pro)renin receptor mRNA levels were unchanged following CNV induction (n = 6 to 7). C: Flatmounted choroids from vehicle-, CP-, and PRRB-treated mice. D: The graph shows the CNV volume. PRRB application led to significant suppression of CNV, compared with vehicle or CP treatment. Arrowheads in (C) indicate lectin-stained CNV tissues (n = 33 to 37. **P < 0.01, *P < 0.05). Scale bars = 100 μm.
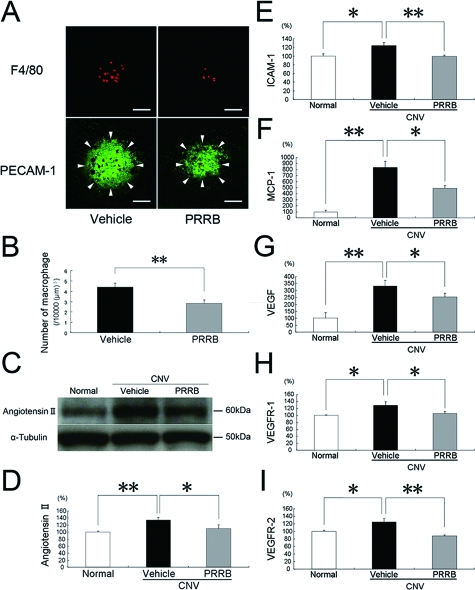
PRRB Inhibits CNV-Associated Macrophage Infiltration, Angiotensin II Generation and the Expression of Angiogenic and Inflammatory Molecules
As the cellular mechanism in the pathogenesis of CNV, infiltration of inflammatory cells including macrophages plays a critical role. We compared the number of macrophages, which was adjusted by the area of CNV, between mice treated with PRRB versus vehicle. PRRB-treated mice showed a significant decrease in the number of F4/80-positive macrophages, compared with vehicle-treated animals (P < 0.01, 2.84 ± 0.31/10,000 μm3 versus 4.39 ± 0.41/10,000 μm3) (Figure 2, A and B). To investigate the effect of PRRB on angiotensin II generation during CNV, we analyzed angiotensin II levels in the RPE-choroid complex. RPE-choroidal levels of angiotensin II were higher (P < 0.01) in animals with CNV than in age-matched normal controls (Figure 2, C and D). Application of PRRB significantly suppressed protein levels of angiotensin II in the RPE-choroid (P < 0.05) (Figure 2, C and D). To determine whether PRRB affects angiogenic and inflammatory molecules related to the pathogenesis of CNV, protein levels of ICAM-1, MCP-1, VEGF, VEGFR-1, and VEGFR-2 in the RPE-choroid complex were analyzed by ELISA. RPE-choroidal protein levels of ICAM-1, MCP-1, VEGF, VEGFR-1, and VEGFR-2 were up-regulated by inducing CNV. Systemic administration of PRRB significantly suppressed protein levels of ICAM-1 (P < 0.01), MCP-1 (P < 0.05), VEGF (P < 0.05), VEGFR-1 (P < 0.05), and VEGFR-2 (P < 0.01) (Figure 2, E–I).
Figure 2.
PRRB inhibits macrophage infiltration and RPE-choroidal production of angiotensin II and CNV-related inflammatory molecules. F4/80-positive macrophages (A, top) and PECAM-1-stained CNV (arrowheads in A, bottom) were evaluated and the volume-adjusted number of macrophages is shown in the graph (B). PRRB led to significant suppression of macrophage infiltration into CNV (n = 20). Scale bars = 50 μm. C–D: RPE-choroidal generation of angiotensin II was significantly reduced by treatment with PRRB. PRRB significantly suppressed the protein levels of ICAM-1 (E), MCP-1 (F), VEGF (G), VEGFR-1 (H), and VEGFR-2 (I) in the RPE-choroid (n = 4 to 9. **P < 0.01, *P < 0.05).
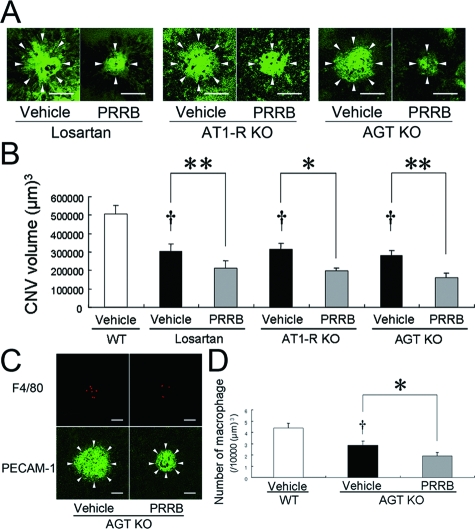
RAS-Independent (Pro)renin Receptor-Mediated Intracellular Signaling Contributes to CNV Development and Macrophage Infiltration
To clarify the role of RAS-independent intracellular signaling via (pro)renin receptor, we used mice in which RAS was deactivated by pharmacological blockade of AT1-R with losartan or genetic ablation of AT1-R or AGT. Compared with vehicle-treated wild-type animals (504,411 ± 49,791 μm3), these mice exhibited a significant (P < 0.01) reduction of CNV (305,244 ± 37,883 μm3 for losartan treatment, 314,120 ± 34,023 μm3 for AT1-R deficiency, 278,811 ± 30,462 μm3 for AGT deficiency), which was further suppressed by additional PRRB application (212,643 ± 38,779 μm3 for losartan treatment [P < 0.01], 198,206 ± 15,536 μm3 for AT1-R deficiency [P < 0.05], 163,457 ± 23,767 μm3 for AGT deficiency [P < 0.01]) (Figure 3, A and B). We further examined the role of RAS-independent intracellular signaling via (pro)renin receptor in macrophage infiltration into CNV. Compared with wild-type animals (4.39 ± 0.41/10,000 μm3), AGT-deficient mice exhibited a significant (P < 0.05) decrease in the number of F4/80-positive macrophages (2.85 ± 0.38/10,000 μm3), which was further attenuated by PRRB treatment (1.89 ± 0.31/10,000 μm3, P < 0.05) (Figure 3, C and D).
Figure 3.
RAS-independent (pro)renin receptor-mediated intracellular signaling contributes to CNV development and macrophage infiltration. The graph shows the choroidal flatmounts (A) and the CNV volume (B). PRRB treatment further induced a significant decrease in the CNV volume in losartan-treated, AT1-R-deficient and AGT-deficient mice. Arrowheads in (A) indicate lectin-stained CNV tissues (n = 23 to 40). Scale bars = 100 μm. F4/80-positive macrophages (C, top) and PECAM-1-stained CNV (arrowheads in C, bottom) were evaluated in AGT-deficient mice, and the volume-adjusted number of macrophages is shown in the graph (D). PRRB further caused significant suppression of macrophage infiltration (n = 14 to 17. †P < 0.01, **P < 0.01, *P < 0.05). Scale bars = 50 μm.
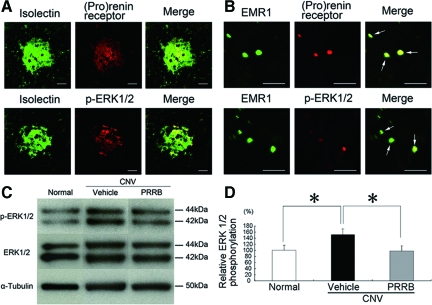
(Pro)renin Receptor and Phosphorylated ERK1/2 Are Present in CNV-Associated Macrophages and Vascular Endothelial Cells and PRRB Inhibits ERK1/2 Activation Following CNV Induction
To examine the expression and tissue localization of (pro)renin receptor and phosphorylated ERK1/2, a known downstream pathway via (pro)renin receptor, rat CNV tissues were immunostained with antibodies against (pro)renin receptor and phosphorylated ERK1/2 together with isolectin B4 or an anti-EMR 1 antibody, markers for vascular endothelial cells or macrophages, respectively. The immunohistochemical analyses of rat CNV tissues showed (pro)renin receptor immunoreactivity in isolectin B4-positive endothelial cells (Figure 4A) and EMR1-positive macrophages (Figure 4B), both of which were positive for phosphorylated ERK1/2. We examined the effect of PRRB on the activation of ERK1/2 in the RPE-choroid excised from mice with CNV. The relative ratio of phosphorylated to total ERK1/2 in the RPE-choroid, increased by inducing CNV, was significantly (P < 0.01) suppressed by PRRB treatment (Figure 4, C and D), while no significant (P > 0.05) difference was detected in total ERK1/2 protein levels.
Figure 4.
(Pro)renin receptor and phosphorylated ERK1/2 are present in CNV-associated macrophages and vascular endothelial cells, and PRRB inhibits ERK1/2 activation following CNV induction. The immunohistochemical analyses show (pro)renin receptor and phosphorylated ERK1/2 in isolectin B4-positive endothelial cells (A) and EMR1-positive macrophages (B). A: Green fluorescence from isolectin B4 (left) and red fluorescence from an anti-(pro)renin receptor (middle, top) or an anti-phosphorylated ERK1/2 antibody (middle, bottom) identified the isolectin B4-positive endothelial cells as having (pro)renin receptor or phosphorylated ERK1/2, respectively, when the images were superimposed (right). Scale bars = 50 μm. B: Green fluorescence from an anti-EMR1 antibody (left) and red fluorescence from an anti-(pro)renin receptor (middle, top) or an anti-phosphorylated ERK1/2 antibody (middle, bottom) identified the EMR1-positive macrophages as having (pro)renin receptor or phosphorylated ERK1/2, respectively, when the images were superimposed (arrows, right). Scale bars = 50 μm. C-D: Western blotting for phosphorylated and total levels of ERK1/2 in the RPE-choroid after photocoagulation. Relative phosphorylation of ERK1/2 in the RPE-choroid complex, increased by inducing CNV, was suppressed by PRRB treatment (n = 11 to 13. *P < 0.05).
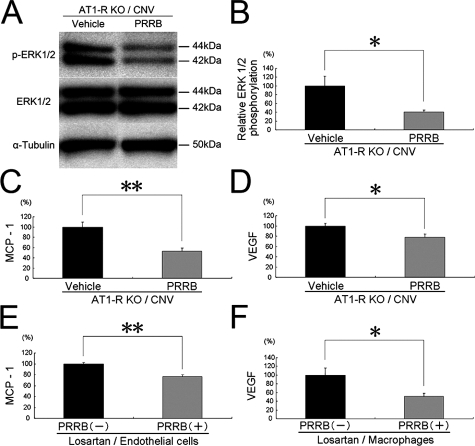
RAS-Independent (Pro)renin Receptor-Mediated Intracellular Signaling Contributes to CNV-Related ERK1/2 Activation and the Expression of Inflammatory and Angiogenic Molecules In Vivo and In Vitro
To further determine whether RAS-independent intracellular signaling via (pro)renin receptor contributes to the activation of ERK1/2 in CNV, phosphorylated ERK1/2 was examined in the RPE-choroid from AT1-R-deficient mice with CNV. PRRB treatment significantly (P < 0.05) suppressed phosphorylated but not total ERK1/2 (Figure 5A). Relative phosphorylation of ERK1/2 was significantly (P < 0.05) suppressed by PRRB application, compared with vehicle-treated AT1-R-deficient mice (Figure 5B). To examine whether RAS-independent intracellular signaling via (pro)renin receptor contributes to the up-regulation of the inflammatory and angiogenic molecules responsible for CNV (Figure 2, E–I), CNV was induced in AT1-R-deficient mice to measure protein levels of ICAM-1, MCP-1, VEGF, VEGFR-1, and VEGFR-2 in the RPE-choroid. PRRB application to AT1-R-deficient mice with CNV led to significant suppression of MCP-1 (P < 0.01) (Figure 5C) and VEGF (P < 0.05) (Figure 5D), but not ICAM-1, VEGFR-1, or VEGFR-2 (P > 0.05, data not shown), compared with vehicle treatment to AT1-R-deficient mice with CNV. To confirm the in vivo molecular mechanisms mediated by RAS-independent pathway via (pro)renin receptor (Figure 5, C and D), we further performed in vitro analyses, using murine cell lines including b-End3 microvascular endothelial cells (Figure 5E) and RAW264.7 macrophages (Figure 5F), both of which were treated by losartan. We analyzed protein levels of ICAM-1, MCP-1, VEGFR-1, and VEGFR-2 inTNF-α-stimulated endothelial cells and of VEGF in lipopolysaccharide-stimulated macrophages. In losartan-treated endothelial cells, protein levels of MCP-1 were significantly (P < 0.01) suppressed by the treatment with PRRB (Figure 5E). In contrast, no significant difference was detected in protein levels of ICAM-1, VEGFR-1, or VEGFR-2 following PRRB treatment (data not shown). In losartan-treated macrophages, VEGF protein levels were significantly (P < 0.05) suppressed by the treatment with PRRB (Figure 5F).
Figure 5.
RAS-independent (pro)renin receptor-mediated intracellular signaling contributes to CNV-related activation of ERK1/2 and expression of inflammatory molecules in vivo and in vitro. A,B: Western blotting for phosphorylated and total levels of ERK1/2 in AT1-R-deficient mice with CNV. PRRB suppressed relative phosphorylation of ERK1/2. C–F: In vivo (C,D) and in vitro (E,F) effects by blocking intracellular signaling via (pro)renin receptor on protein levels of CNV-related molecules. MCP-1 (C,E), and VEGF (D,F) levels were significantly suppressed with PRRB (n = 9 to 11). **P < 0.01, *P < 0.05.
Discussion
The present study reveals, for the first time to our knowledge, several important findings concerning the role of (pro)renin receptor in CNV generation. First, CNV development was associated with up-regulation of prorenin expression in the RPE-choroid complex and PRRB treatment showed a significant decrease in the CNV volume, indicating that prorenin binding with its receptor contributes to CNV (Figure 1). Second, the cellular and molecular mechanisms in the PRRB-induced suppression of CNV included the inhibitory effects on macrophage infiltration into CNV, angiotensin II generation and the up-regulated expression of inflammatory and angiogenic molecules such as ICAM-1, MCP-1, VEGF, VEGFR-1, and VEGFR-2, all of which were downstream molecules of angiotensin II19 (Figure 2). Although the detailed molecular and cellular mechanisms underlying CNV are not fully clarified, ICAM-1 expression16,26 and macrophage infiltration18,19 were observed in CNV tissues from human eyes with AMD and the laser-induced murine model, suggesting the close association of inflammation with the progression of CNV. Pharmacological depletion of macrophages17,27 or genetic ablation of CCR2,28 a receptor for MCP-1, was shown to result in the reduction of CNV, suggesting that macrophages, recruited by MCP-1 released from RPE or vascular endothelial cells, facilitate the development of CNV by producing VEGF. In concert with our previous data,19 the currently observed PRRB-induced suppression of CNV indicates that tissue RAS is activated during CNV by (pro)renin receptor-mediated nonproteolytic activation of prorenin, leading to AT1-R signaling-mediated up-regulation of CNV-related inflammatory molecules.
The present study further revealed the role of RAS-independent (pro)renin receptor signaling in CNV generation (Figure 3). This study is the first to show the involvement of RAPS, (pro)renin receptor-mediated signal transduction and tissue RAS activation, in in vivo angiogenesis as well as in ocular pathogenesis. We recently showed the contribution of (pro)renin receptor signaling to diabetic nephropathy using AT1-R-deficient mice.3 AT1-R-deficient mice with streptozotocin-induced diabetes exhibited reduced proteinuria and glomerulosclerosis in the early phase as compared to wild-type diabetes, indicating a significant role of tissue RAS in diabetic nephropathy. Surprisingly, these renal events in AT1-R-deficient diabetes later progressed to the equivalent levels seen in wild-type diabetic mice. The glomerulosclerosis observed in AT1-R-deficient diabetic mice was associated with ERK activation, which was completely blocked together with the phenotype by sustained application of PRRB, suggesting that the redundant pathways of RAPS were involved in the pathogenesis of diabetic nephropathy. In the present study, we administered PRRB to CNV mice receiving the AT1-R blocker losartan or genetically deficient in AT1-R or AGT, and these three different methods for deactivating RAS confirmed the significant role of intracellular signaling via (pro)renin receptor in the development of CNV (Figure 3, A and B). The data are compatible with the result of parallel experiments showing that macrophage infiltration into CNV was also suppressed by PRRB in AGT-deficient mice (Figure 3, C and D). Importantly, (pro)renin receptor was present in macrophages and vascular endothelial cells, the major cellular components in CNV tissues, together with the activation of ERK 1/2, a known intracellular signaling via (pro)renin receptor (Figure 4, A and B). Indeed, in vivo quantitative analyses for ERK 1/2 revealed PRRB-induced suppression of the phosphorylation of ERK1/2, which was enhanced following CNV induction (Figure 4, C and D). However, because AT1-R has been shown to mediate CNV generation,19 the data (Figure 4) could not exclude the possibility of ERK activation via AT1-R as well as (pro)renin receptor, leading us to further perform the following in vivo and in vitro experiments (Figure 5) to confirm that (pro)renin receptor signaling per se caused the activation of ERK and up-regulation of inflammatory molecules responsible for CNV formation. Importantly, PRRB application to AT1-R-deficient mice with CNV led to significant suppression of ERK activation (Figure 5, A and B). Out of the CNV-related molecules, the expression of which was inhibited by PRRB (Figure 2, E–I), our in vivo (Figure 5, C and D) and in vitro (Figure 5, E and F) data showed that MCP-1 and VEGF were also regulated by (pro)renin receptor signaling per se. These new findings (Figures 3–5) clarified molecular and cellular mechanisms mediated by RAS-independent intracellular signaling via (pro)renin receptor in CNV generation. In addition to our recent reports3,5,6,25 showing that RAPS contributes to glomerulosclerosis in the kidney and fibrosis in the heart, the present data are the first to show the association of RAPS with inflammation and angiogenesis in the eye.
Although hypertension is a known risk factor predisposing to AMD, there are indeed a large number of normotensive patients with CNV who have the potential risk of hypotension caused by the use of antihypertensive agents including AT1-R blockers and angiotensin-converting enzyme inhibitors. In contrast, since (pro)renin receptor is present in the major organs but not in the circulation, PRRB does not affect circulatory RAS or systemic blood pressure.5,7 Interestingly, PRRB treatment to CNV was shown to cause not only tissue RAS deactivation but also additional suppression of (pro)renin receptor signaling-mediated expression of MCP-1 and VEGF, the major pathogenic factors responsible for CNV formation. Collectively, inhibition of RAPS with PRRB may prove more useful as a novel therapeutic strategy for CNV than RAS suppression with conventional AT1-R blockers or angiotensin-converting enzyme inhibitors.
Footnotes
Address reprint requests to Susumu Ishida, M.D., Ph.D., Laboratory of Retinal Cell Biology, Inaida Endowed Department of Anti-Aging Ophthalmology, Keio University School of Medicine; 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan. E-mail: ishidasu@sc.itc.keio.ac.jp.
See related Commentary on page 1591
Supported by Grant-in-Aid for Scientific Research of Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 18791296 to S.S.).
References
- Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Nakagawa T, Nishiyama A, Kawachi H, Shimizu F, Inagami T. Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J Am Soc Nephrol. 2006;17:2495–2503. doi: 10.1681/ASN.2005121278. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H. Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J Am Soc Nephrol. 2007;18:2054–2061. doi: 10.1681/ASN.2006080820. [DOI] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2003;110:1273–1280. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3771–3777. doi: 10.1167/iovs.03-0121. [DOI] [PubMed] [Google Scholar]
- Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the irbesartan and lipoic acid in endothelial dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep. 2006;8:191–198. doi: 10.1007/s11906-006-0050-7. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodriguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1. IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int. 2005;68:2787–2793. doi: 10.1111/j.1523-1755.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Taguchi H, Anand A, Ambati BK, Gragoudas ES, Miller JW, Adamis AP, Ambati J. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Hata Y, Yoshikawa H, Nakagawa K, Sueishi K, Inomata H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1997;235:159–167. doi: 10.1007/BF00941723. [DOI] [PubMed] [Google Scholar]
- Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, Ozawa Y, Inoue M, Tsubota K, Suda T, Ishida S. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2006;26:2252–2259. doi: 10.1161/01.ATV.0000240050.15321.fe. [DOI] [PubMed] [Google Scholar]
- Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Suzuki F, Oike Y, Ishida S. Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci. 2006;47:2686–2692. doi: 10.1167/iovs.05-1458. [DOI] [PubMed] [Google Scholar]
- Satofuka S, Ichihara A, Nagai N, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Itoh H, Oike Y, Ishida S. Role of nonproteolytically activated prorenin in pathologic, but not physiologic, retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:422–429. doi: 10.1167/iovs.06-0534. [DOI] [PubMed] [Google Scholar]
- Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, Uchida H, Sugiura M, Fukuta K, Fukamizu A, Murakami K. Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- Suzuki F, Hayakawa M, Nakagawa T, Nasir UM, Ebihara A, Iwasawa A, Ishida Y, Nakamura Y, Murakami K. Human prorenin has “gate and handle” regions for its non-proteolytic activation. J Biol Chem. 2003;278:22217–22222. doi: 10.1074/jbc.M302579200. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006;47:894–900. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- Yeh DC, Bula DV, Miller JW, Gragoudas ES, Arroyo JG. Expression of leukocyte adhesion molecules in human subfoveal choroidal neovascular membranes treated with and without photodynamic therapy. Invest Ophthalmol Vis Sci. 2004;45:2368–2373. doi: 10.1167/iovs.03-0981. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]