Abstract
Background
Verbal self-report is the method most often used to assess sunscreen use, but the data may be confounded by recall error and social desirability. Sunscreen swabbing is a non-invasive procedure to objectively assess the presence of sunscreen on the skin. This study examined the agreement between verbal reports of sunscreen use from survey and diary measures and objectively measured sunscreen use.
Methods
Participants were 564 parents, children aged 5–10 years, and lifeguards at 16 swimming pools in four regions of the U.S. Participants completed self-reported measures, including baseline and final surveys, as well as a 4-day diary and objective swabbing measures of sunscreen presence on 2 separate days. Data were collected in 2006 and analyzed in 2006–2007.
Results
Levels of sunscreen use were relatively high based on surveys (65.7%); diary data (40.3%); and swabbing measures (59.1%). Agreement between swabbing and diary measures of sunscreen use was fair to good, with κ statistics for children at 0.40, followed by lifeguards at 0.34 and parents at 0.27. Validity coefficients across measures of sunscreen use were higher for lifeguards and parents than for children, and diary measures were higher than surveys. No systematic errors were found across groups or by gender, latitude, study arm, or risk category.
Conclusions
These findings are comparable to those in other validation studies, including studies of the validity of dietary assessments. Self-reported estimates of sunscreen use by diaries or surveys appear to be as good as objective measures.
Introduction
Skin cancer is the most commonly diagnosed cancer in the U.S.,1 and about 1 million Americans are diagnosed with it each year.2 The incidence of skin cancer has increased dramatically worldwide in the last decade,3 establishing malignant melanoma and nonmelanoma skin cancer as major public health concerns.2 Skin cancer is considered one of the most preventable types of cancer, and can be prevented by reducing ultraviolet radiation (UVR) exposure and adopting sun-protection habits (sunscreen, hats, shirts, sunglasses).4,5
Although sunscreen is the most frequent method of sun protection used across all age groups,6 most people do not use sunscreen consistently when outdoors on sunny days.7–10 Further, while sunscreen has been shown to prevent sunburn, the data are mixed with respect to its effectiveness at preventing skin cancer.11,12 Some scientists have found that sunscreen use may increase the duration of recreational sun exposure.13 However, randomized trial data have confirmed that daily sunscreen use significantly reduced the incidence of squamous cell carcinoma at both medium-term14 and long-term follow-up, and tended to decrease basal cell carcinoma rates after 8 years.15 There seems to be sufficient support to continue to recommend sunscreen to prevent the short-term (sunburn) and long-term (skin cancer) detrimental effects of excessive UVR exposure.
The available population-based data on sunscreen use are predicated on verbal reports, or self-report, mainly from surveys of sun-protection habits. Despite the well-known limitations of verbal reports of behavior, these measures are the most practical for use in both population surveillance and descriptive and intervention research, but little is known about their validity.16 Observational strategies have been used to examine the validity of the reported use of sun-protective clothing and sunglasses, but sunscreen use is not easily observed. One promising strategy to validate reports of sunscreen use objectively has been used previously in office and field studies with small samples.17–19 The current effort, a large measurement-validation study, was conducted among parents, children, and lifeguards at swimming pools to quantify the association between self-reported sunscreen use and objective measures of sunscreen presence.
This study had three main aims: (1) to describe the association between self-reported (survey and diary) and objective measures (swabbing) of sunscreen use; (2) to identify any systematic error in subgroups by gender, latitude, study group (from an intervention trial), or skin cancer risk; and (3) to explore whether specific features of sunscreen use (e.g., when applied, sun-protection factor [SPF]) relate to the validity of self-report.
MATERIALS AND METHODS
Overview
Data were collected in the summer of 2006 from the sun exposure and protection habits measurement study (SEPH), which addressed the limitations of self-report of sun exposure and sun-protection practices by testing the validity of self-reports compared to objective measures. SEPH is an ancillary study to a large ongoing study20,21 of the diffusion of an effective multicomponent skin cancer prevention program in aquatic settings known as “Pool Cool.” The Pool Cool diffusion trial is a national, three-level cluster randomized trial of basic and enhanced strategies intended to improve the implementation, maintenance, and sustainability of an effective skin cancer prevention program over four summers.21
This study was an observational, multimethod descriptive correlational study with repeated measures. Data collection took place over a 4-day period that included 2 weekdays and 2 weekend days; it involved 2 days of onsite data collection and 2 offsite days. Participants completed self-reported measures, including a baseline survey, a 4-day diary, and a final survey, as well as direct measures of sunscreen presence on 2 separate days.
Sample and Context
Sixteen pools in four metropolitan regions were selected from the 245 pools (27 metropolitan regions) that participated in the Pool Cool parent study in 2006. The four regions were stratified based on latitude (north or south= >40°N or <35°N) and study arm (basic or enhanced). The target sample was ten lifeguards and parent–child pairs from each of 16 pools (total n=480).
Recruitment and Data-Collection Procedures
At each pool, recruitment began the day before the start of data collection and continued the first morning of data collection, if necessary. Each child had to be aged 5–10 years, enrolled in swim lessons (or swim team) at the pool, and accompanied by a parent or legal guardian who was willing to participate with the child. Only one parent–child pair per family was eligible. Lifeguards were approached either as they arrived at the pool for work or during a break. Study procedures were explained to potential participants, and those who agreed to participate were asked to sign consent forms and complete a baseline survey. Verbal assent was required for children. They completed the sun-habits survey at the time of consent and were given a Pool Cool sling bag containing an instruction sheet with research staff contact information, a timeline, and a description of all study activities.
Participants were asked to arrive at the pool at 9:00 AM, or as early as possible thereafter, on 2 days of data collection: one weekday (either Thursday or Friday) and one weekend day (Saturday or Sunday). They were instructed to behave as they normally would and not to alter their behavior for participation in the study.
On the first day of data collection, skin swabs were taken of each participant’s arm and leg. These swabs were stored in alcohol and later analyzed for the presence or absence of sunscreen. Participants were given a sun-habits diary and asked to complete it daily for 4 days. On the third day, swabbing was repeated. Subjects were asked to return all study materials on the fourth day of the study, and were given a $25 gift card in appreciation of their participation.
The Self-Reported Measures: Sun-habits Survey and Diary
To compare self-reported sun exposure and sun-protection practices with objective assessments, both a survey and a 4-day diary were used. The survey included the main outcome measures used in the parent study21 and is typical of large population intervention trial measures.16 The diary provided a more precise time-matched measure of sun exposure and protection.
The sun-habits survey was completed twice: at the time of enrollment into the study and at the end of data collection. Parents answered for themselves and their child. Surveys included questions on sun-protection habits, sunscreen use, skin cancer risk factors, sunburn history, UVR exposure, and demographics. Measures were selected or adapted from previously published studies and tools used in earlier studies.20,22 The survey items were identical to those used in the parent study.21
Demographic information collected on the surveys included gender, age, race, and, for lifeguards and parents, education, income level, marital status, and number of children. Risk-factor questions from previous studies,23 with scoring adapted from the Brief skin cancer Risk Assessment Tool (BRAT),24 included untanned skin color, hair color, eye color, sunburn history, tanning propensity, and history of skin cancer, and were used to categorize participants into low-, moderate-, or high-risk groups.
Sunscreen use was assessed by asking When you are outdoors in the sun, how often do you use sunscreen? Response options were on a 4-point ordinal scale (1=rarely or never, 2=sometimes, 3=usually, 4=always). For children, the question presented to parents was How often do you have your child use sunscreen? Additional questions on sunscreen use asked when sunscreen was applied and the brand and SPF of the sunscreen used.
The sun-habits diary is a record of sun exposure and protective behavior throughout the day adapted from a diary developed previously.25 Participants were instructed to complete the diary for 4 consecutive days (including 2 weekend days), which is considered sufficient to estimate weekly sun exposure and sun protection.25 Parents were instructed to fill out separate diaries for themselves and their children (with or without input from the child, as available). Participants were asked to record their primary activity for each hour of the day between 10:00 AM and 4:00 PM and to indicate any sun-protection habits that they used (sunscreen, wearing a hat, staying in the shade, wearing a shirt with sleeves, wearing sunglasses) for each hour they were outside. At the end of the diary, participants were asked at what time(s) of the day they completed the diary (throughout the day, at the end of the day, the next day), and parents were asked if the child’s diary was filled out with the child’s input (never, sometimes, or always).
The percentage of time that sunscreen was used was calculated by dividing the amount of time each individual reported using sunscreen by the amount of time spent outdoors for that day (0%–100% use). The average of the daily percentages was used as a measure of usual sunscreen use. Another variable was created to examine sunscreen use at the time of swabbing and was scored as yes if sunscreen was reported at either the 10:00 AM or 11:00 AM hour.
Sunscreen Swabbing: Objective Measure of Sunscreen Use
Objective measures of sunscreen use were collected through participant skin swabbing, using methods developed in previous research.17,18 Swabbing was completed on one weekday morning and one weekend morning. Participants were told that a researcher would wipe their arm and leg with a towelette but were not informed that the purpose of swabbing was to check for the presence of sunscreen. Research assistants wearing disposable, non-absorbent polyurethane gloves swabbed each participant’s right forearm and right leg above the knee with medium-size disposable alcohol wipes (70% isopropyl alcohol). A 2.5 cm × 4 cm skin surface area for each body part was swabbed twice using the same wipe, reversing the side of the wipe to ensure that a thorough sample was collected. Used wipes were placed into individual, prelabeled glass vials containing 4 mL of 100% ethyl alcohol and sealed.
The swabs were analyzed for the presence of sunscreen based on ultraviolet absorption following a previously described protocol.18 After initial calibration of the spectrophotometer, samples were analyzed by group order for each pool, with a reference standard rerun after every three participants. Reference standards were made with clean wipes placed directly into 4 mL of 100% ethyl alcohol. Eluted washings were transferred to a crystal cuvette (Fisher Scientific) and analyzed in a UV-Visable spectrophotometer (Beckman DU-530). Absorbance was measured across the UV spectrum from 280 to 400 nanometers (nm) at 5 nm intervals. Between each sample the cuvette was thoroughly washed with absolute ethanol and dried.
As in previous studies,17,18 absorbance at 320 nm was used to establish the presence or absence of sunscreen. An absorbance threshold of 0.300 was selected as a positive indicator of sunscreen based on control data and the results of two controlled in-office trials.
Statistical Analysis
The objective measure of sunscreen use was compared to the two self-reported measures of sunscreen use by two approaches. The first approach involved analyzing two dichotomous measures of sunscreen use, one from the first day of swabbing and the other from the corresponding time period from the diary. For this analysis, κ statistics were used to assess agreement between the objective and self-reported diary measures. κ statistics were categorized using the cutoffs recommended by Landis and Koch26: poor (κ<0.0); slight (κ: 0.0–0.2); fair (κ: 0.2–0.4); moderate/good (κ: 0.4–0.6); substantial (κ: 0.6–0.8); and almost perfect (κ: 0.8–1.0). As the study design included respondents clustered within pools, ORs were also computed using the complex survey procedure in the Statistical Package for the Social Sciences (SPSS Version 15.0) to provide an additional measure of association. For this analysis, the outcome was a diary report of sunscreen use (1=yes, 0=no), and the predictor was the swab result for sunscreen (1=yes, 0=no). Therefore, the ORs reflect the odds of reporting sunscreen use when there was a positive swab for sunscreen versus a negative swab for sunscreen. The crosstabs procedure in the complex survey module of SPSS was used to examine the distribution of accurate and over– or under–self-reporting of sunscreen use relative to swabbing results. Statistics were computed separately for parents, children, and lifeguards, and within these groups for subgroups by gender, latitude, Pool Cool intervention arm, and skin cancer risk level.
The second approach used to assess concurrent validity involved converting each of the three measures to a value representing the percentage of time that sunscreen was used, based on data from both days of swabbing, both surveys, and the entire 4-day diary. Spearman coefficients were used to estimate validity coefficients. In addition, the method of triads as described by Ocke and Kaaks27 was used to estimate the lower and upper limits of the validity coefficients. This method has been used in a number of studies investigating the validity of dietary questionnaire measures relative to biomarkers.27–29 The method of triads is a triangular approach that uses the correlations among three methods to calculate the validity coefficients. In the case of sunscreen use, the following equation was used to estimate the correlation between true sunscreen use and sunscreen use as estimated from survey self-report:
where ρST is the validity coefficient for the survey measure, rSB the correlation between the survey measure and the swab measure, rSD the correlation between the survey and the diary measures, and rBD the correlation between the swab and diary measures. Ocke and Kaaks27 suggest that the value obtained from the above equation be used as the upper limit of the true validity coefficient and that the value rSB be used as an estimate of the lower limit.
To investigate differences in the estimates of sunscreen use based on method of assessment and participant category, means and 95% CIs were computed, accounting for people nested within pools. Comparisons were made among the three groups of participants as well as within those groups for subgroups defined by gender, latitude, Pool Cool intervention group, and skin cancer risk level. Data analysis was completed in 2006 and 2007.
RESULTS
Participation and Sample Characteristics
A total of 993 eligible participants were approached across the 16 pools; 631 (64%) consented to participate, and 564 (89%) completed the study (201 parent–child pairs and 162 lifeguards). Most people who failed to complete the study did not show up for the second day of data collection.
Most of the parent participants were female adolescents and women (95%) and the child’s mother (92.5%); most reported being white (83.5%); well-educated (65.5% college graduate or higher); and of moderate to high income (78.4% with >$50,000 household income per year). Children had a mean age of 7.2 years (SD=1.7); 52.3% were boys. Of the lifeguards, 59.3% were female adolescents and women; on average they were aged 19.4 years (SD=5.6); were mostly white (89.9%); unmarried (98.1%); and either high school students or graduates (51.3%) or college students (41%).
Sunscreen Use by Survey, Diary Reports, and Swabbing
Table 1 summarizes reported and objectively assessed sunscreen use. Swabbing detected sunscreen on at least one assessment in 61.7% of children, 59.5% of lifeguards, and 55.7% of parents. Questions about sunscreen use patterns (not shown in Table 1) revealed that 75.6% of parents said that they usually or always apply sunscreen to the child before s/he goes outside; 78.3% of parents and 82.4% of lifeguards said they put on sunscreen before going outside. Of all participants, 70.1% reported using sunscreen with an SPF of ≤30, and 29.9% reported using sunscreen with an SPF >30.
Table 1.
Sunscreen use measured by survey, diary, and swabbing methods
| Group | Survey (% usually/always) | Diary (% of time outside: median) | Swabbing (% either day) |
|---|---|---|---|
| Parents (n=201) | 53.3 | 25 | 55.7 |
| Children (n=201) | 78.9 | 42 | 61.7 |
| Lifeguards (n=162) | 64.8 | 54 | 59.5 |
Association of Sunscreen Use by Swabbing and Self-Report
The results of the analyses investigating agreement between swab results and diary self-report are reported in Tables 2, 3, and 4. The percentage of agreement between swab and diary was 64% for parents, 70% for children, and 67% for lifeguards. Based on the similarity of κ values and the 95% CIs for the ORs, there do not appear to be any significant differences in the level of agreement based on the stratification variables. Overall, agreement was fair to good across the three participant categories, with the κ value for children being 0.40, followed by lifeguards at 0.34 and parents at 0.27.
Table 2.
Day-1 swab and diary (period 10:00 AM–11:00 AM) agreement statistics for parents
| Swab and diary discrepancy
|
|||||
|---|---|---|---|---|---|
| Group | Swab and diary agreement (%) | Swab: yes/diary: no (%) | Swab: no/diary: yes (%) | κ | OR (95% CI)a |
| All parents (n=201) | 64.2 | 24.9 | 10.9 | 0.27*** | 3.5 (2.0, 6.0) |
| Latitude | |||||
| North (n=103) | 67.0 | 23.3 | 9.7 | 0.30** | 4.0 (1.7, 9.2) |
| South (n=98) | 61.2 | 26.5 | 12.2 | 0.24* | 2.9 (1.5, 5.4) |
| Study arm | |||||
| Enhanced (n=102) | 54.9 | 38.2 | 6.9 | 0.18* | 2.9 (1.3, 6.2) |
| Basic (n=99) | 73.7 | 11.1 | 15.2 | 0.41*** | 6.4 (2.4, 17.3) |
| Skin cancer risk | |||||
| Low (n=68) | 66.2 | 23.5 | 10.3 | 0.30** | 4.0 (1.7, 9.5) |
| Moderate(n=52) | 59.6 | 28.8 | 11.5 | 0.21 | 2.6 (1.0, 7.0) |
| High(n=81) | 65.4 | 23.5 | 11.1 | 0.28** | 3.6 (1.3, 9.6) |
OR and 95% CI adjusted for clustering within pool; reflects odds of reporting sunscreen use when there is a positive swab for sunscreen versus a negative swab for sunscreen
p<0.05;
p<0.01;
p<0.001
Table 3.
Day-1 swab and diary (period 10:00 AM–11:00 AM) agreement statistics for children
| Swab and diary discrepancy
|
|||||
|---|---|---|---|---|---|
| Group | Swab and diary agreement (%) | Swab: yes/diary: no (%) | Swab: no/Diary: yes (%) | κ | OR (95% CI)a |
| All children (n=200) | 70.0 | 17.5 | 12.5 | 0.40*** | 5.6 (3.2, 9.7) |
| Gender | |||||
| Male (n=102) | 67.6 | 18.6 | 13.7 | 0.34*** | 4.3 (2.0, 9.5) |
| Female (n=92) | 72.8 | 15.2 | 12.0 | 0.46*** | 7.3 (3.2,16.4) |
| Latitude | |||||
| North (n=102) | 74.5 | 14.7 | 10.8 | 0.48*** | 8.5 (4.0, 18.2) |
| South (n=98) | 65.3 | 20.4 | 14.3 | 0.31** | 3.6 (1.9, 7.0) |
| Study arm | |||||
| Enhanced (n=101) | 68.3 | 23.8 | 7.9 | 0.38*** | 6.1 (2.3,16.6) |
| Basic (n=99) | 71.7 | 11.1 | 17.2 | 0.43*** | 6.5 (3.5,12.2) |
| Skin cancer risk | |||||
| Low (n=69) | 73.9 | 14.5 | 11.6 | 0.47*** | 8.0 (3.4, 18.9) |
| Moderate (n=47) | 59.6 | 23.4 | 17.0 | 0.19 | 2.2 (0.8, 5.8) |
| High (n=83) | 72.3 | 16.9 | 10.8 | 0.45*** | 7.1 (2.9, 17.0) |
OR and 95% CI adjusted for clustering within pool; reflects odds of reporting sunscreen use when there is a positive swab for sunscreen versus a negative swab for sunscreen
p<0.01;
p<0.001
Table 4.
Day-1 swab and diary (period 10:00 AM–11:00 AM) agreement statistics for lifeguards
| Swab and diary discrepancy
|
|||||
|---|---|---|---|---|---|
| Group | Swab and diary agreement (%) | Swab: yes/diary: no (%) | Swab: no/diary: yes (%) | κ | OR (95% CI)a |
| All lifeguards (n=162) | 67.3 | 15.4 | 17.3 | 0.34*** | 4.2 (1.7, 10.7) |
| Gender | |||||
| Male (n=66) | 63.6 | 18.2 | 18.2 | 0.27* | 3.0 (0.8, 11.2) |
| Female (n=96) | 69.8 | 13.5 | 16.7 | 0.40*** | 5.4 (2.3, 12.8) |
| Latitude | |||||
| North (n=81) | 59.3 | 16.0 | 24.7 | 0.19 | 2.2 (0.6, 8.2) |
| South (n=81) | 75.3 | 14.8 | 9.9 | 0.49*** | 9.1 (3.4, 24.6) |
| Study arm | |||||
| Enhanced (n=80) | 65.0 | 17.5 | 17.5 | 0.24* | 2.8 (0.6, 12.6) |
| Basic (n=82) | 69.5 | 13.4 | 17.1 | 0.38** | 5.0 (1.6, 16.1) |
| Skin cancer risk | |||||
| Low (n=46) | 69.6 | 10.9 | 19.6 | 0.40** | 5.7 (1.5, 21.5) |
| Moderate (n=58) | 65.5 | 19.0 | 15.5 | 0.31* | 3.6 (0.7, 18.1) |
| High (n=58) | 67.3 | 15.5 | 17.2 | 0.33* | 4.0 (1.7, 9.4) |
OR and 95% CI adjusted for clustering within pool; reflects odds of reporting sunscreen use when there is a positive swab for sunscreen versus a negative swab for sunscreen
p<0.05;
p<0.01;
p<0.001
Validity coefficients for the measures of sunscreen use are reported in Table 5. The validity coefficients (and ranges) for the lifeguard and parent groups are somewhat higher than those for the children.
Table 5.
Correlation and validity coefficients among swab, diary, and survey
| Spearman coefficients | Method of triads validity coefficient range | ||||||
|---|---|---|---|---|---|---|---|
| Group | Swab anya and diaryb | Swab anya and survey | Diaryb and survey | Survey method | Diary method | ||
| Lower | Upper | Lower | Upper | ||||
| Parents (n=201) | 0.33*** | 0.23** | 0.53*** | 0.23 | 0.61 | 0.33 | 0.87 |
| Children (n=199) | 0.28*** | 0.14* | 0.30*** | 0.14 | 0.39 | 0.28 | 0.75 |
| Lifeguards (n=162) | 0.47*** | 0.36*** | 0.57*** | 0.36 | 0.66 | 0.47 | 0.86 |
Swab tested positive for presence of sunscreen on either of the 2 days of data collection
Percentage of reported time sunscreen was used
p<0.05;
p<0.01;
p<0.001
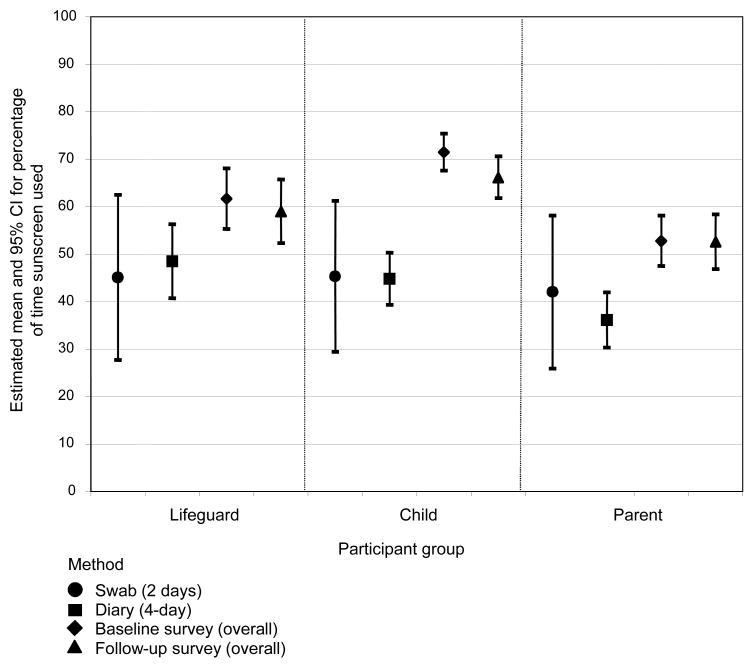
The estimated means and 95% CIs for the percentage of time sunscreen is used by method and participant category are shown in Figure 1. The CIs account for participants nested within pools. The estimated variability for sunscreen use based on swab results from 2 days is considerably larger than the variability noted for the two self-reported measures. Lifeguards and children tend to use sunscreen more often than parents.
Figure 1. Means and 95% CIs for the percentage of time sunscreen is used, by participant category and method.
BL, baseline; FU, follow-up
Systematic Errors by Subgroup
The distribution of accurate and over- or under-reporting was investigated for each participant category and for the stratification variables within each category (Tables 2, 3, and 4). The percentage of parents under-reporting (25%) sunscreen use was greater than the percentage over-reporting (11%). While parent errors in reporting were not related to latitude or skin cancer risk group, they were related to study arm (p=0.016), with more under-reporting in the enhanced group. In the child group, there was no overall difference noted for over- (13%) or under-reporting (18). Although not significant (p=0.13), those in the enhanced group tended to be more likely to under-report than over-report sunscreen use. For lifeguards, the percentage of those over-reporting (17%) did not differ from the percentage under-reporting (15%). This result was the same across all stratification variables (gender, latitude, study arm, or skin cancer risk group).
Features of Sunscreen Use and Their Association with Valid Self-Report
Exploratory analyses were conducted to examine whether any features of sunscreen use were associated with the validity of self-reported sunscreen use. There was a near-significant association between parents’ reports that they usually or always apply sunscreen on the child before going outside and a positive sunscreen swab for the child (49.3% vs 12.4%, χ2 =3.1, p=0.077). For parents, there was a significant association between reported sunscreen application before going outside and a positive swab (χ2 =6.5, p=0.01). Among lifeguards, the use of a higher SPF sunscreen was associated with a positive swab (χ2 =8.7, p=0.003).
DISCUSSION
To our knowledge, this is the first study to examine the validity of sunscreen use with validity coefficients, applying the method of triads and two different methods of self-report. The findings indicate fair to good levels of agreement between diary-reported and objectively measured sunscreen use by parents, children, and lifeguards, and across subgroups defined by gender, latitude, study arm, and skin cancer risk. Parents and their children who report putting on sunscreen before going outside are more likely to test positive for the presence of sunscreen, as are lifeguards who state that they use a higher-SPF sunscreen.
The diary method appears to be better correlated with swabbing results than the survey method. However, given the demands of a diary, the survey method validity estimates show that this method is adequate relative to the more complex method of measuring sunscreen use. Because no significant findings of systematic errors by relevant subgroups were found, there appears to be no need to calibrate survey assessments by gender, latitude, study arm, skin cancer risk, or race in an intervention trial.
For all participant categories, a method effect can be noted whereby mean sunscreen use estimated from the survey method is substantially higher than the estimates based on the diary. Although means for the survey method tend to be higher than those for the objective measure, the CIs overlap. In addition, the CIs suggest that the magnitude of the difference between the survey method and the diary method is most notable for the child group, which is not surprising because parents completed surveys about their child’s practices, whereas some children completed the daily diaries themselves or with their parents.
A major issue in comparing survey responses, diaries, and sunscreen swabbing is the time periods assessed. It is noteworthy that the 2-day (swab) and 4-day (diary) estimates are very similar. It is not at all surprising that the survey estimate is higher, as it covers a theoretically longer time period. When considering how sun-protection data are typically used in survey research and intervention trials, survey measures of usual behavior are more closely aligned with the research questions.
Given the difference in time periods measured, the agreement estimates and the validity coefficients are actually very respectable and suggest that the rank ordering of individuals is consistent across the methods. They are comparable to or better than the coefficients obtained in studies of dietary assessment compared to biochemical indicators of food intake.29,30 The CIs in Figure 1 support the notion that self-reported estimates are probably as good as an objective measure.
Although errors in reporting are noted for parents and, to some degree, the children, especially with respect to the intervention group, this does not appear to have major implications for analyses of the Pool Cool diffusion trial.21 It could make it more difficult to detect a difference between study arms if under-reporting on the diary is predictive of under-reporting on the survey questions.
Strengths of the study include the large sample across four geographic locations and three distinct types of participants (parents, children, and lifeguards) as well as high completion rates. Study limitations include the focus on a single type of outdoor recreation environment (swimming pools); the limits of swabbing only the forearm and leg; and the possible reactivity of completing multiple diaries and surveys on both participants’ responses and their decisions about using sunscreen after the beginning of the study.
While it is not practical to incorporate objective assessments of health-related behavior into large population-based studies, these findings make it possible to interpret survey data from the larger trial with greater confidence. Future studies should consider incorporating this type of validation study with a subsample of participants.16 The science of skin cancer prevention will also be advanced by the adoption of standard measures that can be compared with national survey data and across studies.31
Acknowledgments
The research reported here was supported by a grant (CA 92505-S1) from the National Cancer Institute. The authors wish to thank Tom Elliott, Kristen Burgess, Diana Chan, Erica Davis, Nicole Dubruiel, Maria Fawzy, and Nancy Marencin for their assistance with data collection and processing. The Winship Cancer Institute provided the spectrophotometer for laboratory analyses. Dr. Karen Glanz’s effort was supported in part by a Distinguished Scholar Award from the Georgia Cancer Coalition.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Devesa S, Hartge P, Tucker M. Recent trends in cutaneous malignant melanoma incidence among whites in the U. S J Natl Cancer Inst. 2001;93:678–83. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures 2007. Atlanta GA: American Cancer Society; 2007. [Google Scholar]
- 3.National Cancer Institute. SEER cancer statistics review, 1975–2002. Bethesda MD: NIH; [Google Scholar]
- 4.Armstrong BK. How sun exposure causes skin cancer: an epidemiological perspective. In: Hill D, Elwood JM, English DR, editors. Prevention of skin cancer. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2004. [Google Scholar]
- 5.USDHHS. Healthy People 2010. 2. Washington DC: U.S. Government Printing Office; 2000. www.healthypeople.gov/publications/ [Google Scholar]
- 6.Stanton WR, Janda M, Baade PD, Anderson P. Primary prevention of skin cancer: a review of sun protection in Australia and internationally. Health Promot Int. 2004;19:369–78. doi: 10.1093/heapro/dah310. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Cancer trends progress report—2007 update.progressreport.cancer.gov.
- 8.Jones SE, Saraiya M. Sunscreen use among U.S. high school students, 1999–2003. J Sch Health. 2006;76:150–3. doi: 10.1111/j.1746-1561.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 9.Cokkinides V, Weinstock M, Glanz K, et al. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among U.S. adolescents, 1998–2004. Pediatrics. 2006;118:853–64. doi: 10.1542/peds.2005-3109. [DOI] [PubMed] [Google Scholar]
- 10.Hall HI, Jorgensen CM, McDavid K, Kraft JM, Breslow R. Protection from sun exposure in U.S. white children ages 6 months to 11 years. Public Health Rep. 2001;116:353–61. doi: 10.1093/phr/116.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vainio H, Bianchini F, editors. IARC Working Group on the Evaluation of Cancer Preventive Agents. IARC handbooks of cancer prevention. Vol. 5: sunscreens. 1. Lyon, France: International Agency for Research on Cancer; 2001. [Google Scholar]
- 12.Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med. 2003;139:966–78. doi: 10.7326/0003-4819-139-12-200312160-00006. [DOI] [PubMed] [Google Scholar]
- 13.Autier P, Boniol M, Dore JF. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int J Cancer. 2007;121:1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- 14.Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–9. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 15.Van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epid Biomarkers Prev. 2006;25:2546–8. doi: 10.1158/1055-9965.EPI-06-0352. [DOI] [PubMed] [Google Scholar]
- 16.Glanz K, Mayer JA. Reducing ultraviolet radiation exposure to prevent skin cancer: methodology and measurement. Am J Prev Med. 2005;29:131–42. doi: 10.1016/j.amepre.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman DC, Brown RM, Xu C, et al. A rapid method for determining recent sunscreen use in field studies. J Photochem Photobiol B. 2003:59–63. doi: 10.1016/s1011-1344(02)00409-8. [DOI] [PubMed] [Google Scholar]
- 18.O’Riordan DL, Lunde KB, Urschitz J, Glanz K. A non-invasive objective measure of sunscreen use and reapplication. Cancer Epid Biomarkers Prev. 2005;14:722–6. doi: 10.1158/1055-9965.EPI-04-0636. [DOI] [PubMed] [Google Scholar]
- 19.O’Riordan DL, Lunde KB, Steffen AD, Maddock JE. Validity of beachgoers’ self-report of their sun habits. Arch Dermatol. 2006;142:1304–11. doi: 10.1001/archderm.142.10.1304. [DOI] [PubMed] [Google Scholar]
- 20.Glanz K, Geller AC, Shigaki D, Maddock JE, Isnec MR. A randomized trial of skin cancer prevention in aquatic settings: the pool cool program. Health Psychol. 2002;21:576–87. [PubMed] [Google Scholar]
- 21.Glanz K, Steffen AD, Elliott T, O’Riordan DL. Diffusion of an effective skin cancer prevention program: design, theoretical foundations, and first-year implementation. Health Psychol. 2005;24:477–87. doi: 10.1037/0278-6133.24.5.477. [DOI] [PubMed] [Google Scholar]
- 22.Glanz K, Lew RA, Song V, Cook VA. Factors associated with skin cancer prevention practices in a multiethnic population. Health Educ Behav. 1999;26:344–59. doi: 10.1177/109019819902600305. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock MA. Assessment of sun sensitivity by questionnaire: validity of items and formulation of a prediction rule. J Clin Epidemiol. 1992;45:547–52. doi: 10.1016/0895-4356(92)90104-u. [DOI] [PubMed] [Google Scholar]
- 24.Glanz K, Schoenfeld E, Weinstock MA, et al. Development and reliability of a brief skin cancer risk assessment tool. Cancer Detect Prev. 2003;27:311–5. doi: 10.1016/s0361-090x(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 25.Glanz K, Silverio R, Farmer A. Diary reveals sun protection practices. The Skin Cancer Foundation Journal. 1996;14:27–8,86. [Google Scholar]
- 26.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;3:363–74. [PubMed] [Google Scholar]
- 27.Ocke MC, Kaaks RJ. Biochemical markers as additional measurements in dietary validity studies: application of the method of triads with examples from the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 1997;65(4S):1240S–1245S. doi: 10.1093/ajcn/65.4.1240S. [DOI] [PubMed] [Google Scholar]
- 28.Fowke JH, Hebert JR, Fahey JW. Urinary excretion of dithiocarbamates and self-reported cruciferous vegetable intake: application of the ‘method of triads’ to a food-specific biomarker. Public Health Nutr. 2002;5:791–9. doi: 10.1079/PHN2002345. [DOI] [PubMed] [Google Scholar]
- 29.McNaughton SA, Marks GC, Gaffney P, Williams G, Green A. Validation of a food-frequency questionnaire assessment of carotenoid and vitamin E intake using weighed food records and plasma biomarkers: the method of triads model. Eur J Clin Nutr. 2005;59:211–8. doi: 10.1038/sj.ejcn.1602060. [DOI] [PubMed] [Google Scholar]
- 30.Kaaks RJ, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. Int J Epidemiol. 1992;26(1S):S26–S36. doi: 10.1093/ije/26.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
- 31.Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144:217–22. doi: 10.1001/archdermatol.2007.46. [DOI] [PubMed] [Google Scholar]