Abstract
Insulin-stimulated glucose uptake requires the activation of several signaling pathways to mediate the translocation and fusion of GLUT4 vesicles to the plasma membrane. Our previous studies demonstrated that GLUT4-mediated glucose uptake is a myosin II-dependent process in adipocytes. The experiments described in this report are the first to show a dual role for the myosin IIA isoform specifically in regulating insulin-stimulated glucose uptake in adipocytes. We demonstrate that inhibition of MLCK but not RhoK results in impaired insulin-stimulated glucose uptake. Furthermore, our studies show that insulin specifically stimulates the phosphorylation of the RLC associated with the myosin IIA isoform via MLCK. In time course experiments, we determined that GLUT4 translocates to the plasma membrane prior to myosin IIA recruitment. We further show that recruitment of myosin IIA to the plasma membrane requires that myosin IIA be activated via phosphorylation of the RLC by MLCK. Our findings also reveal that myosin II is required for proper GLUT-vesicle fusion at the plasma membrane. We show that once at the plasma membrane, myosin II is involved in regulating the intrinsic activity of GLUT4 after insulin stimulation. Collectively, our results are the first to reveal that myosin IIA plays a critical role in mediating insulin-stimulated glucose uptake in 3T3-LI adipocytes, via both GLUT4 vesicle fusion at the plasma membrane and GLUT4 activity.
Keywords: myosin II, insulin-responsive glucose transporter (GLUT4), insulin, glucose transport
Introduction
Insulin-stimulated glucose uptake into adipose tissue and skeletal muscle play a critical role in glucose homeostasis. Insulin mediates glucose uptake via the insulin responsive glucose transporter, (GLUT4). In adipocytes, insulin stimulates (i) the translocation of GLUT4- containing vesicles from a perinuclear region to the plasma membrane; (ii) the docking and fusion of GLUT4 vesicles at the plasma membrane and (iii) the activation of the intrinsic activity of the GLUT4 transporter itself. Several signaling pathways including the phosphoinositol-3 kinase (PI3 kinase) pathway, the mitogen activated protein kinase (MAPK) pathway and the Cbl pathway [1–3] are activated by insulin to coordinate GLUT4-mediated glucose uptake by signaling GLUT4 vesicle translocation, actin reorganization, and stimulating the intrinsic activity of GLUT4 at the plasma membrane.
Previous studies have demonstrated that GLUT4 translocation and membrane fusion require reorganization of the cytoskeleton [4–6]. Specifically, actin, microtubules and myosin have been shown to participate in GLUT4-mediated glucose uptake [4–11]. Unlike most cells that have extensive arrays of stress fibers, adipocytes have a cortical layer of actin filaments adjacent to the cytoplasmic surface of the plasma membrane. In adipocytes, the actin cytoskeleton plays two critical roles in vesicle trafficking: i) as “tracks” on which GLUT4- containing vesicles are translocated from intracellular pools to the plasma membrane and ii) as a barrier between vesicles and the plasma membrane [5, 12]. Actin filaments must be reorganized for vesicle translocation and to “loosen” the actin barrier at the plasma membrane to facilitate localized remodeling of the cell cortex to facilitate vesicle fusion [13–15]. Upon cortical actin reorganization, GLUT4 vesicles are able to gain access to their proper docking and fusion sites at the plasma membrane [14]. Reorganization of cortical actin in adipocytes is insulin-dependent and thus is a critical component of GLUT4-mediated glucose uptake [5]. While previous studies have elucidated a role for actin reorganization in GLUT4-mediated glucose uptake, the role of the contractile proteins regulating actin reorganization and the subsequent remodeling of the cell cortex has only recently been examined. We have demonstrated that the contractile motor protein myosin II plays a critical role in GLUT4-mediated glucose uptake. While myosin II facilitates actin filament contraction, an activity most commonly associated with muscle contraction, it is also involved in nonmuscle cell processes such as cytokinesis, cell locomotion, maintenance of cell cortical tension, and cell surface receptor capping [16, 17]. Previous studies in mammalian nonmuscle cell lines revealed that myosin II is critical in regulating the cytoskeletal reorganization required for vesicle fusion with the plasma membrane [18, 19]. Thus, myosin II may be responsible for the cortical F-actin reorganization required for GLUT4 vesicle fusion in adipocytes.
An additional aspect of GLUT4-mediated glucose uptake that may involve the reorganization of the cytoskeleton is the regulation of GLUT4 activity. After vesicle fusion, GLUT4 must be activated before glucose uptake can occur [20]. In muscle cells, studies have shown that p38 mitogen-activated protein kinase (p38 MAPK) is necessary for complete GLUT4 activity [20–22] whereas in adipocytes, p44/p42 mitogen-activated protein kinase (p44/p42 MAPK) was found to increase GLUT4 activity [23]. The precise interaction between p38 MAPK or p44/p42 MAPK and GLUT4 is still unknown, but studies have suggested that the increased GLUT4 activity is due to either the interaction of an activator ligand with GLUT4 or the removal of an inhibitory ligand from GLUT4 [21]. It is possible that myosin II-mediated actin reorganization plays a role in the up-regulation of GLUT4 activity by allowing p38 MAPK, p44/p42 MAPK or some other protein access to membrane-bound GLUT4.
Myosin II is a hexameric protein consisting of two heavy chains, two essential light chains (ELCs) and two regulatory light chains (RLCs) [16]. The heavy chains are structurally oriented to form a globular head domain that possesses binding sites for ATP and actin. Myosin II motor activity and parallel filament assembly are regulated by the phosphorylation of residues localized on the RLCs [8, 16]. Three kinases have thus far been identified that phosphorylate the RLC of myosin II: the Ca2+ / calmodulin dependent myosin light chain kinase (MLCK), Rho kinase (RHOK) and p21 activated kinase [8, 16]. Phosphorylation of the RLC via MLCK initiates the binding of myosin II to filamentous actin [24–26]. Interestingly, the RLC of myosin II is the only known substrate for MLCK [16]. Several heavy chain isoforms of myosin II have been identified. Most vertebrate species express varying levels of the myosin II isoforms, myosin IIA and IIB. The expression and localization of each isoform is cell type-dependant [16]. In osteoclasts myosin IIA is localized at dynamic regions of cytoskeletal reorganization whereas myosin IIB remains somewhat constant throughout different osteoclast activation cycles [27]. Since two myosin isoforms have been identified in 3T3-L1 adipocytes it is possible that the two have distinct intracellular localization patterns during the dynamic process of insulin stimulated glucose uptake [10]. It is also of interest to determine whether the myosin II isoforms are recruited to facilitate glucose uptake and which of the isoforms is being activated during insulin stimulated glucose uptake. While previous studies have shown specific localization patterns of the myosin IIA and IIB isoforms in various cell types, [16, 27–31] the specific functions of each isoform are not yet clear due to their diverse distribution and dynamic localization.
Our previous studies were the first to demonstrate a fundamental role for myosin II in regulating the dynamic cellular processes driving GLUT4-mediated glucose uptake in adipocytes, but not GLUT4 translocation to the plasma membrane [10]. Our study also showed that adipocytes express both myosin IIA and IIB isoforms, and that in unstimulated adipocytes myosin IIA was localized primarily in the perinuclear region of the cell while myosin IIB was highly enriched at the cell cortex. Upon insulin stimulation myosin IIA relocalized to the cell cortex while the localization of myosin IIB was unchanged. These findings suggest that the two isoforms have different roles in adipocytes. Collectively, our results demonstrated that myosin II plays a critical role in mediating insulin-stimulated glucose uptake in 3T3-LI adipocytes, possibly via a mechanism that facilitates GLUT4 vesicle fusion at the plasma membrane. In the present study we examined myosin II isoform activation and the role the isoforms play in GLUT4-mediated glucose uptake. Our results reveal a novel role for myosin IIA in regulating GLUT4 vesicle fusion and GLUT4 activity. Thus our studies are the first to demonstrate a dual role for the myosin IIA isoform in regulating insulin-stimulated glucose uptake in adipocytes.
Materials and methods
Materials
Tissue culture reagents were obtained from Gibco (Grand Island, NY). Insulin was purchased from Roche Diagnostics Corporation (Indianapolis, IN). Dexamethasone, 3-isobytyl-1-methylxanthine, ML-7 and myosin IIA antibody were from Sigma (St. Louis, MO). Blebbistatin was purchased from Calbiochem (San Diego, CA). Myosin light chain (phospho S20) antibody was purchased from Abcam (Cambridge, MA). GLUT4 antibodies (C-20 and N-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). The myosin IIB antibody and Texas Red Phalloidin were obtained from Covance (Berkeley, CA). Alexa Fluor® 594 donkey anti-goat IgG and goat anti-rabbit IgG were from Molecular Probes, Inc. (Eugene, OR). The enhanced chemiluminescence (ECL) detection kit and horseradish peroxidase conjugated secondary antibodies were from Amersham Bioscience (Piscataway, NJ).
Glucose uptake assay
3T3-L1 preadipocytes were induced to differentiate as described previously [32]. Glucose uptake assays were performed on fully differentiated 3T3-L1 adipocytes. Adipocytes were serum-starved for 4 h in the presence of 0.1% DMSO (Basal) or inhibitor as indicated. Adipocytes were then washed twice with 37 °C Krebs Ringer Phosphate (KRP) buffer (pH 7.4) containing 128 mM NaCl, 4.7 mM KCl, 1.65 mM CaCl2, 2.5 mM MgSO4, 5 mM Na2HPO4 and then placed in KRP buffer containing vehicle, 10 µM ML-7, 10 µM ML-9 or 10 µM Y-27632 as indicated. Adipocytes were either untreated (basal) or treated with insulin (100 nM) for 10 min, followed by the addition of [1-14C]-2-deoxy-D-glucose (0.1µCi/well) (NEN) and 5 mM glucose for an additional 10 min at 37 °C. Cells were then washed three times with phosphate buffered saline (PBS) and solubilized in 0.5 M NaOH and 0.1% SDS. Samples were assayed for 14C-2-deoxy-D-glucose uptake as disintegrations per min per mg protein. Data are expressed as means ± SEM.
Immunoblot analysis
3T3-L1 adipocytes were lysed in a buffer containing 25 mM HEPES pH 7.4, 1% Nonidet P-40, 100 mM NaCl, 2% glycerol, 5 mM NaF, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaPPi, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mg/ml aprotinin, 5 mg/ml leupeptin, and 5 mg/ml pepstatin [5]. Lysates were incubated at 4°C for 20 min and then centrifuged at 6,000 × g for 20 min at 4°C. The supernatants were incubated for 5 minutes at 95°C in Laemmli sample buffer [33], and then subjected to SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore), and analyzed by immunoblotting as previously described [32]. Protein bands were quantified by densitometry using ImageQuant (version 5.2 for Windows) software.
Immunoprecipitation
Antibodies (3 µg/ml) were added to whole cell lysates (1 mg) and incubated overnight at 4 C. Agarose beads (protein A/G PLUS–agarose beads) were added to the immunoprecipitates and agitated for 1 h at 4 C. Immunoprecipitates were recovered by centrifugation at 2500 × g and washed three times with ice cold lysis buffer. Immunoprecipitated proteins were dissolved in 5 × Laemmli buffer, heated for 5 min at 95 C, and then subjected to SDS-PAGE and immunoblot analysis.
Immunofluorescence
Differentiated adipocytes grown on coverslips were serum starved for 4 h and treated according to the glucose uptake protocol (omitting 14C-2-DOG) for the time indicated. Cells were then fixed with 2% buffered paraformaldehyde, permeablized in 0.25% triton X-100 for 5 min at 4°C, and incubated with anti-GLUT4 antibody or anti-myosin IIA antibody. The slides were then incubated with the appropriate secondary labeled antibodies (Molecular Probes, Eugene, OR). Slides were viewed using an Olympus IX81 Motorized Inverted Confocal Microscope and FLUOVIEW FV5OO software. The relative intensity of immunofluroescence was quantified using Image-Pro Plus software (Silver Spring, MD).
Determination of cell surface GLUT4
Fully differentiated 3T3-L1 adipocytes were serum starved in the presence or absence of inhibitor as indicated. Cells were then either treated with 0.1% DMSO (Basal), 100 nM insulin (Insulin) or with 100 nM insulin and either 100 µM blebbistatin or 10 µM ML-7 for 30 min. Adipocytes were fixed with 3.7% formaldehyde, incubated for an hour with a GLUT4 antibody (1:100) targeted against an epitope in the extracellular domain and then incubated with anti-goat Alexaflour 594 for 45 minutes. Slides and viewed using an Olympus IX81 Motorized Inverted Confocal Microscope and FLUOVIEW FV500 software.
Analysis of embedded GLUT4
Adipocytes were incubated at 37°C in KRP buffer and then treated with 0.1% DMSO (Basal) or stimulated with 100 nM insulin alone or 100 nM insulin in the presence of either 100 µM blebbistatin or 10 µM ML-7. Immediately following, [14C]2-deoxy-D-glucose (0.1 µCi/well) and 5 mM glucose were added to each well. Cells were then washed three times with PBS at 4°C and lysed with 1 mL of a 0.5M NaOH and 0.1% SDS solution. Samples were assayed for 14C-2-deoxy-D-glucose uptake as described previously [32].
Statistical analysis
Data are expressed as means ± SEM. The significance of differences between means, set at P < 0.05, was assessed by Student’s t-test (Microsoft Excel).
Results
Myosin light chain kinase activity is required for insulin-stimulated glucose uptake
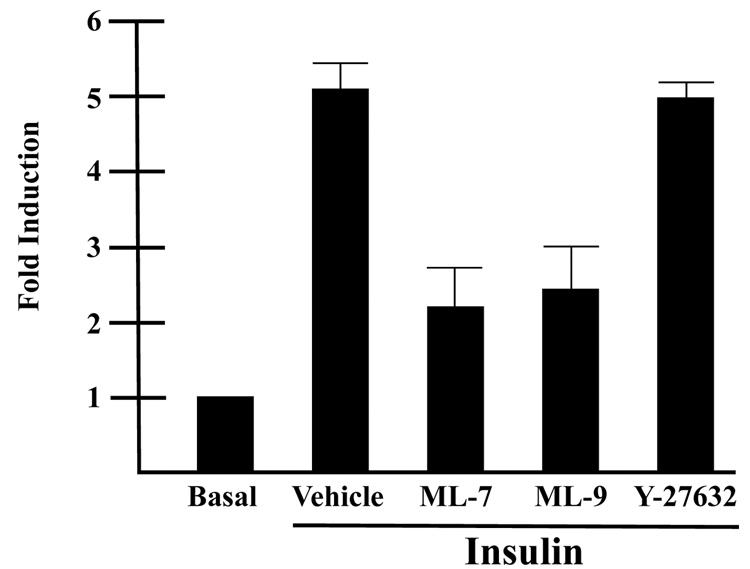
Our previous studies revealed that myosin II plays a necessary role in GLUT4-mediated glucose uptake [10]. Myosin II activity is primarily regulated by the phosphorylation status of the RLC subunit of myosin II. In order to elucidate the signaling mechanism by which insulin regulates myosin II activity in GLUT4-mediated glucose uptake, we investigated two upstream regulators of myosin II activity, myosin light chain kinase, (MLCK) and Rho kinase, (RhoK). Both of these kinases are known to phosphorylate the RLC and consequently stimulate myosin II activity (reviewed in [16]. Fully differentiated adipocytes were serum starved for 4 h in the presence or absence of two MLCK inhibitors, ML-7 or ML-9, or the RhoK inhibitor, Y27632 and then glucose uptake assays were performed in the presence or absence of the appropriate inhibitors and 14C 2-deoxy-D-glucose. As shown previously, insulin stimulated a dramatic increase in glucose uptake over basal levels (Fig. 1). Insulin typically stimulated approximately a five-fold increase in glucose uptake over basal levels. Both MLCK inhibitors, ML-7 and ML-9 impaired insulin-stimulated glucose uptake by approximately 50% compared with insulin alone. In contrast, treatment of adipocytes with the RhoK inhibitor, Y27632 had no effect on insulin-stimulated glucose uptake. These finding not only support previous studies that myosin II activity is required for insulin-stimulated glucose uptake, but also indicate that insulin stimulates myosin II activity via MLCK [8, 10].
Fig. 1. Inhibition of MLCK impairs insulin-stimulated glucose uptake in 3T3-L1 adipocytes.
3T3-L1 adipocytes were serum starved in the presence of 10 µM ML-7, 10 µM ML-9, 10 µM Y-27632 or vehicle (0.1% DMSO). Cells were then either unstimulated (Basal) or stimulated with 100 nM insulin in the presence of 10 µM ML-7, 10 µM ML-9, 10 µM Y-27632 or vehicle (0.1% DMSO) as indicated and assayed for 14C 2-deoxy-D-glucose uptake, which was calculated as disintegrations per mg protein and expressed as percent of the vehicle control. Results are the means ± SEM of three independent experiments.
ML-7 blocks the insulin-stimulated phosphorylation of the RLC associated with myosin IIA
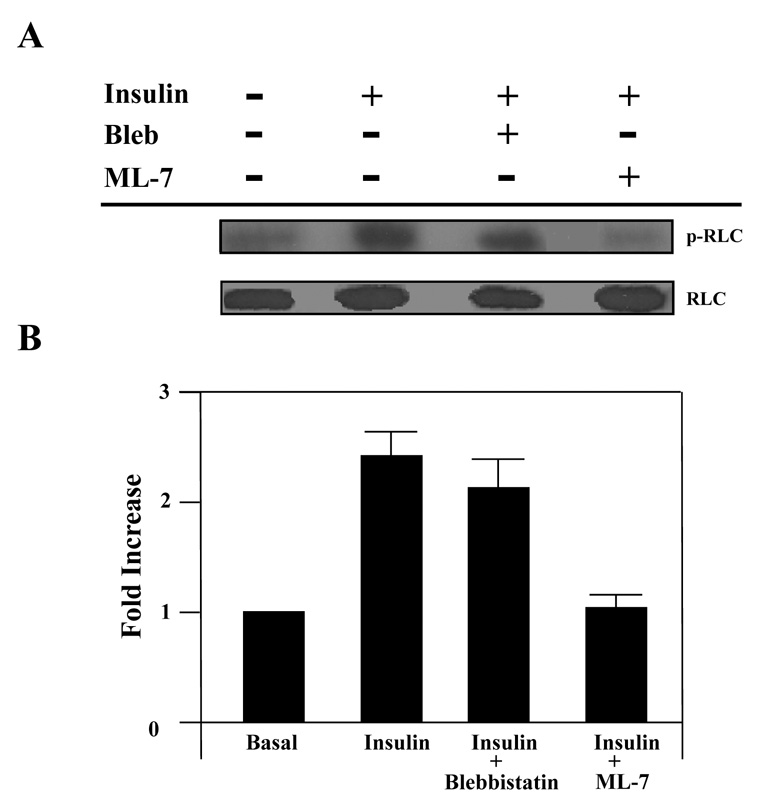
In order to determine the mechanism by which the insulin signaling pathway regulates myosin II activity, we examined the phosphorylation status of the RLC of myosin II prior to and after insulin stimulation in the presence and absence of the MLKC inhibitor ML-7. Phosphorylation of the RLC on serine 19 by MLCK regulates myosin II activity. Using a RLC-phospho serine 19 specific antibody we observed that in the basal state only a low level of phospho-RLC (p-RLC) was detected in whole cell lysates (Fig. 2A). Upon insulin stimulation, there was a dramatic increase in the level of phospho-RLC (Fig. 2A). In order to determine whether the insulin-induced phosphorylation of the RLC was due to MLCK activity, we inhibited MLCK activity with ML-7. Adipocytes treated with insulin in the presence of ML-7 had levels of p-RLC similar to unstimulated adipocytes (Fig. 2A). Since our previous studies showed that inhibition of the myosin II-actin interaction impaired insulin-stimulated glucose uptake [10], we wanted to determine whether the phosphorylation status of the RLC was dependent on a myosin II-actin association. In order to inhibit the myosin II-actin interaction, we used the myosin II specific inhibitor blebbistatin. Blebbistatin impairs the physical interaction between myosin II and actin [34–38]. Blebbistatin treatment did not affect the phosphorylation status of the RLC. Our results further showed that the differences in the levels of phosphorylated RLC were due to the degree of phosphorylation and not the levels of total RLC since the absolute levels of RLC were unchanged under all conditions (Fig. 2A). The relative levels of p-RLC to RLC were quantified by densitometry (Fig. 2B).
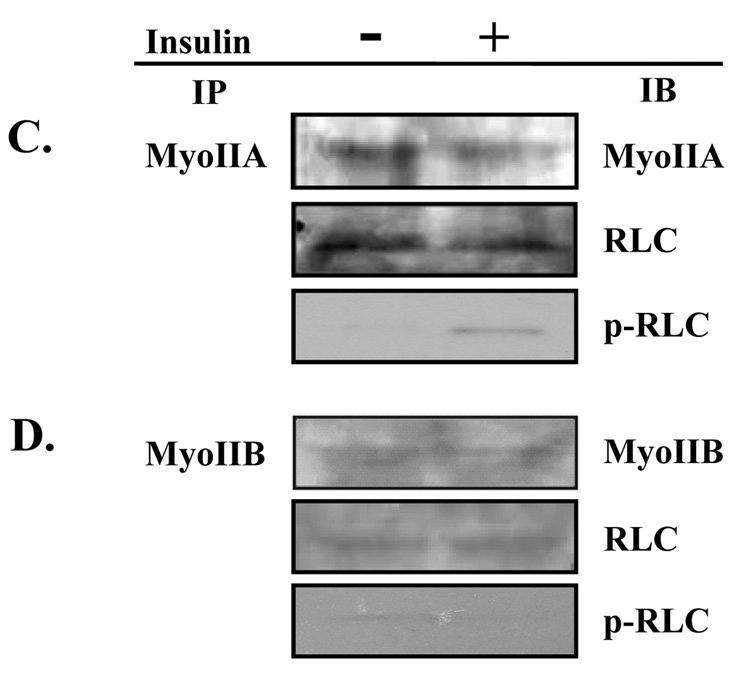
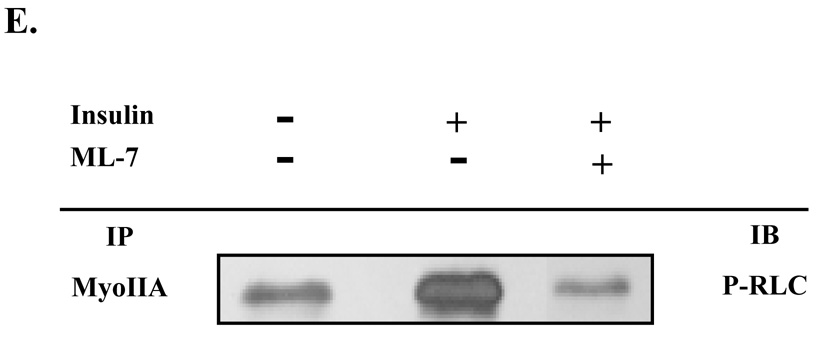
Fig. 2. Insulin stimulates phosphorylation of the regulatory light chain associated with myosin IIA.
3T3-L1 adipocytes were serum starved for 4 hours in the presence of vehicle (0.1% DMSO), 100 µM blebbistatin or 10 µM ML-7. Cells were then either left untreated (basal) or stimulated with 100 nM insulin. (A) Whole cell lysates were prepared, subjected to 10% SDS-PAGE and then immunoblotted using antibodies to either a phosphorylated myosin II RLC (p-RLC) or myosin II RLC (RLC). (B) Immunoblots were quantified by densitometry. Values are expressed as a fold of the basal value. Results are the means ± SEM of three independent experiments. Whole cell lysates from untreated and insulin-stimulated adipocytes were subjected to the immunoprecipitation (IP) protocol using either a (C) myosin IIA or (D) myosin IIB antibody. Immunoprecipitates were then subjected to 10% SDS-PAGE and immunoblotted (IB) with antibodies directed against myosin IIA, myosin IIB, RLC or p-RLC. (E) Whole cell lysates were prepared from untreated adipocytes and adipocytes stimulated with 100 nM insulin in the presence or absence of 10 µM ML-7. Lysates were then subjected to the immunoprecipitation protocol using a myosin IIA antibody. Immunoprecipitates were analyzed by 10% SDS-PAGE and immunoblotted with a p-RLC antibody. Results are representative of three independent experiments.
Our previous studies revealed that 3T3-L1 adipocytes express both myosin IIA and IIB [10]. In order to determine whether one or both isoforms are activated by insulin signaling we immunoprecipitated either myosin IIA (Fig. 2C) or IIB (Fig. 2D) using myosin II-isoform specific antibodies from unstimulated and insulin-stimulated 3T3-L1 adipocytes. As shown in Fig 2C and 2D, insulin induced a dramatic increase in the levels of phosphorylated RLC that immunoprecipitated with the myosin IIA antibody when compared to levels in unstimulated cells. In contrast, there was no alteration in the phosphorylation status of the RLC associated with myosin IIB (Fig. 2D). In order to ensure that the differences detected in the levels of phosphorylated RLC were due to phosphorylation of the RLC and not to differences in the total levels of the RLC, we also determined the levels of RLC by immunoblot analysis. As seen in Fig 2C and 2D there were no differences in the levels of total RLC. We also performed immunoblot analysis for the specific myosin isoforms to ensure the specificity of our immunoprecipitation assays (Fig. 2C and D).
Next we were interested in determining if MLCK mediated the insulin-stimulated phosphorylation of the RLC associated with myosin IIA. 3T3-L1 adipocytes were stimulated with insulin in the presence or absence of 10 µM ML-7. Myosin IIA was immunoprecipitated and then immunoprecipitates were subjected to immunoblot analysis with a phospho-RLC antibody (Fig. 2E). Inhibition of MLCK activity via ML-7 treatment during insulin stimulation blocked phosphorylation of the RLC associated with myosin IIA. Taken together these results indicate that only the myosin IIA isoform is regulated by insulin and that insulin activates myosin IIA activity via phosphorylation of the RLC by MLCK.
Myosin IIA and GLUT4 vesicles interact at the plasma membrane
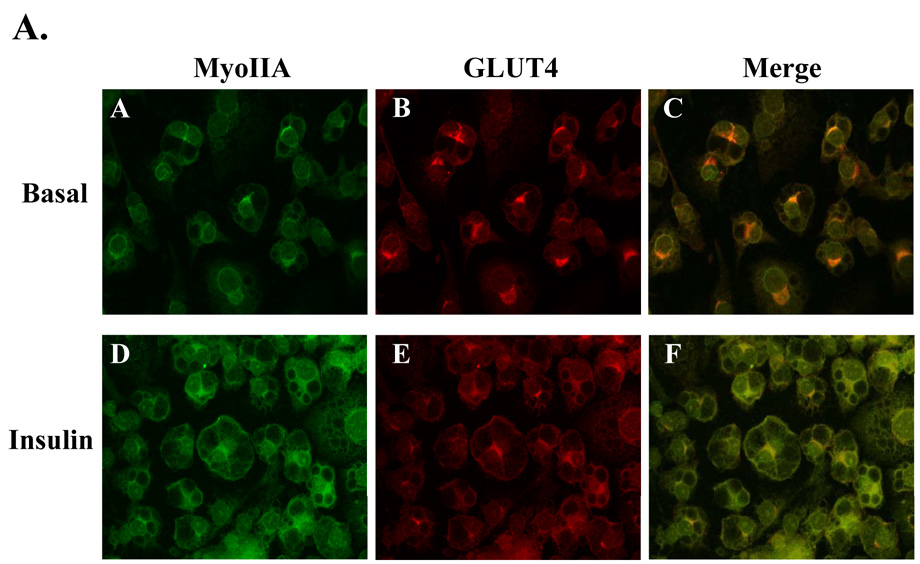
Upon insulin stimulation, GLUT4 translocates from a perinuclear region to the plasma membrane in adipocytes. Similarly, our previous studies have shown that myosin IIA is recruited from intracellular pools to the plasma membrane upon insulin stimulation [10]. We have also shown that inhibition of myosin II impairs insulin-stimulated glucose uptake but not GLUT4 translocation to the plasma membrane [10]. Collectively, these studies suggest that GLUT4 is either unable to fuse to the plasma membrane or is not embedded properly without myosin II activity. If myosin II is involved in the cytoskeletal reorganization required for GLUT4 fusion with the plasma membrane then GLUT4 and myosin IIA may colocalize at the plasma membrane upon insulin stimulation. In order to investigate this question we examined the localization patterns of GLUT4 and myosin IIA by confocal microscopy in unstimulated (Basal) and insulin-stimulated 3T3-L1 adipocytes. In the basal state, both GLUT4 and myosin IIA colocalized primarily to the perinuclear region (Fig. 3A, panels A–C). Upon insulin stimulation, both GLUT4 and myosin IIA translocate to the plasma membrane (Fig. 3A, panels D and E). We observed that myosin IIA and GLUT4 colocalized in both the perinuclear region prior to insulin treatment and at the plasma membrane after insulin stimulation (Fig. 3A).
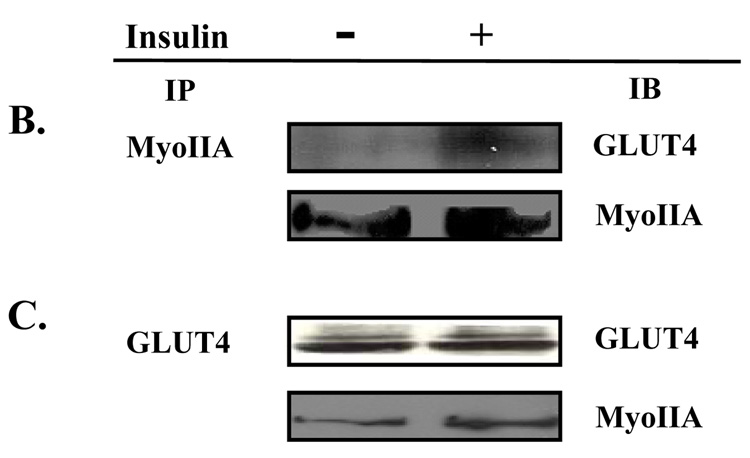
Fig. 3. Myosin IIA and GLUT4 associate in an insulin-dependent manner.
(A) 3T3-L1 adipocytes grown on coverslips were serum starved for 4 hours and then either left untreated (Basal) or stimulated with 100 nM insulin. GLUT4 and Myosin IIA localization was examined by confocal microscopy as described in Materials and Methods. Whole cell lysates from untreated adipocytes and adipocytes stimulated with 100 nM insulin in the presence or absence of 100 µM blebbistatin, or 10µM ML-7 were subjected to the immunoprecipitation protocol (IP) using a either a (B) myosin IIA antibody or a (C) GLUT4 antibody. Immunoprecipitates were then subjected to 10% SDS-PAGE and immunoblotted (IB) with either a myosin IIA antibody or a GLUT4 antibody. The results are representative of three independent experiments.
Next we wanted to determine whether there was an association between myosin IIA and GLUT4 and whether this association was insulin-dependent. Whole cell lysates from unstimulated (Basal) and insulin-stimulated 3T3-L1 adipocytes were subjected to an immunoprecipitation protocol using either a myosin IIA or a GLUT4 antibody. As shown in Fig 3B and 3C there was little association between myosin IIA and GLUT4 in the basal state. Upon insulin stimulation, there was a significant increase in the association between GLUT4 and myosin IIA. These findings indicate that while GLUT4 and myosin IIA colocalized at the perinuclear region in the basal state they did not interact. In contrast, GLUT4 and myosin IIA colocalized at the plasma membrane and co-immunoprecipitated after insulin stimulation. These findings suggest that myosin IIA may facilitate the fusion and/or functioning of GLUT4 at the plasma membrane in an insulin-dependent manner.
Myosin IIA is recruited to the plasma membrane after GLUT4 translocation
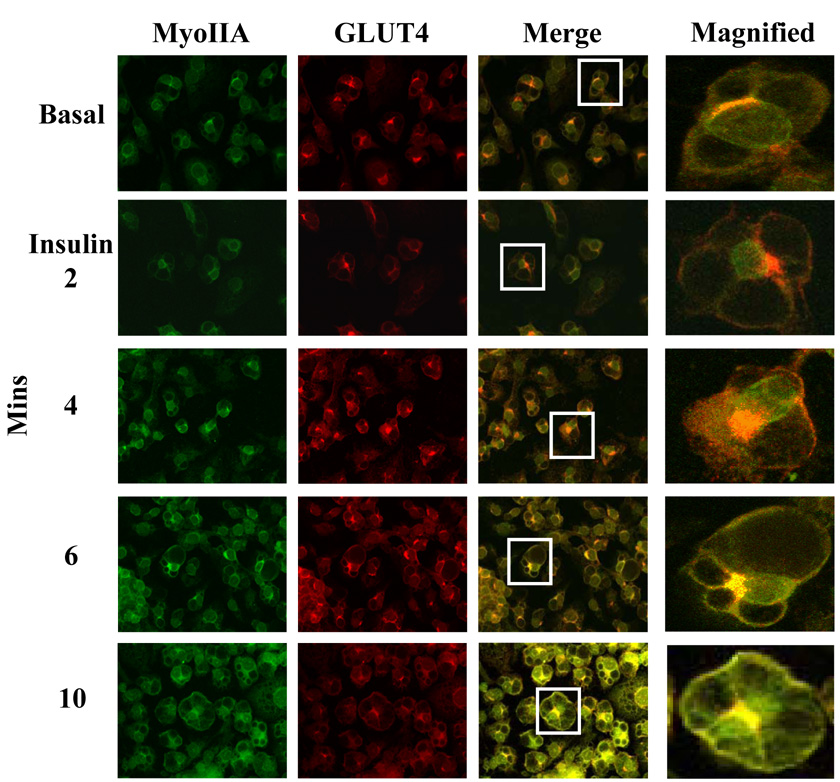
Since GLUT4 and myosin IIA colocalize at both the perinuclear region prior to insulin stimulation and at the plasma membrane after insulin treatment, we wanted to investigate whether myosin IIA is recruited prior to, with, or after GLUT4 vesicles to the plasma membrane. Our previous studies demonstrated that GLUT4 can translocate to the plasma membrane independently of myosin IIA [10]. We stimulated 3T3-L1 adipocytes with insulin for various time points and then used confocal microscopy to determine the localization of myosin IIA and GLUT4. As shown in Fig 4, in the unstimulated state (Basal) myosin IIA and GLUT4 are both colocalized to the perinuclear region. GLUT4 vesicles are detected at the plasma membrane after four minutes of insulin stimulation (Fig. 4). The level of GLUT4 at the plasma membrane increased throughout the 10 min insulin treatment. In contrast, myosin IIA is only detected at the plasma membrane 6 min after insulin stimulation and levels continue to increase up to 10 min after insulin stimulation (Fig. 4). We further show that GLUT4 and myosin IIA colocalize at the plasma membrane as early as 6 min after insulin stimulation and that GLUT4 precedes myosin IIA translocation to the plasma membrane (Fig. 4).
Fig. 4. Insulin stimulates GLUT4 translocation prior to myosin IIA recruitment to the plasma membrane.
3T3-L1 adipocytes grown on coverslips were serum starved for 4 hours and then either left untreated (Basal) or stimulated with 100 nM insulin. GLUT4 and Myosin IIA localization was examined by confocal microscopy (as described in Materials and methods) prior to and at various time points after insulin stimulation, as indicated. The results are representative images from three independent experiments.
MLCK activation of myosin IIA is required for its recruitment to the plasma membrane
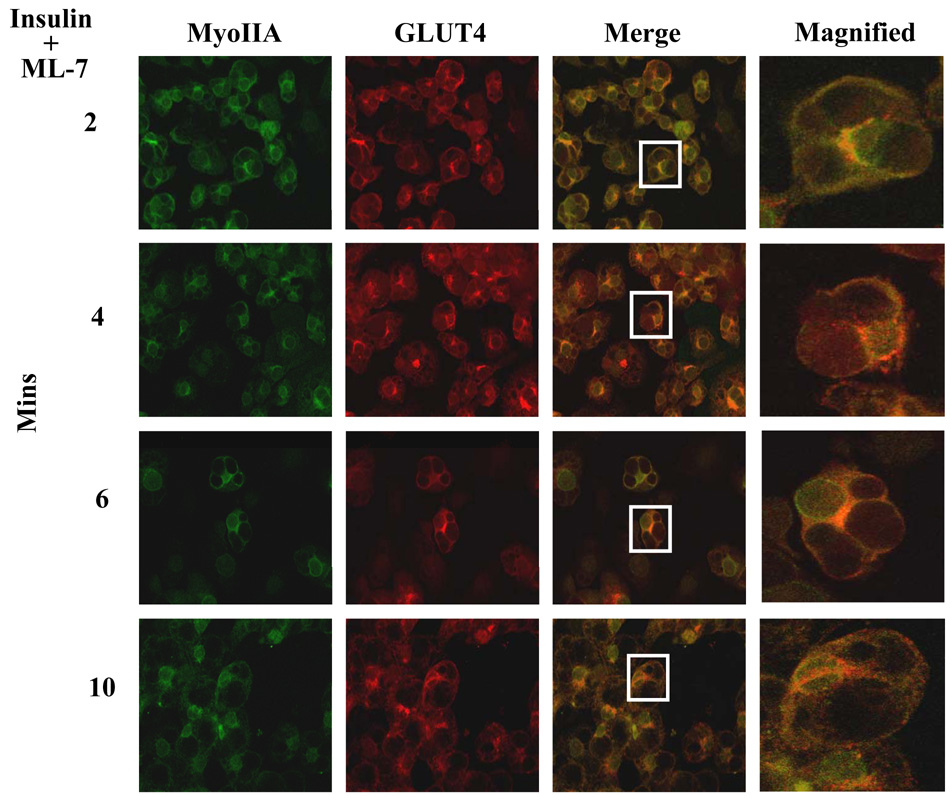
Our previous studies demonstrated that GLUT4 translocation is independent of myosin IIA recruitment but GLUT4-mediated glucose uptake is a myosin II-dependent process [10]. Since insulin signaling stimulates both the translocation of myosin IIA to the plasma membrane (Fig. 4) and the activation of myosin IIA via MLCK phosphorylation of the RLC associated with myosin IIA (Fig. 2E) we wanted to determine whether recruitment of myosin IIA to the plasma membrane required its activation via MLCK. 3T3-LI adipocytes were treated with insulin in the presence of 10 µM ML-7 for various times and the localization of GLUT4 and myosin IIA was examined by confocal microscopy. As shown in Fig. 5 myosin IIA was not recruited to the plasma membrane even after 10 minutes of insulin stimulation in the presence of ML-7 (Fig. 5). In contrast, GLUT4 vesicles translocated to the plasma membrane in the presence of ML-7 (Fig. 5, also see merged image). These findings indicate that myosin IIA activation via MLCK is required for its recruitment to the plasma membrane upon insulin stimulation of adipocytes.
Fig. 5. Myosin IIA activity is required for its recruitment to the plasma membrane.
3T3-L1 adipocytes grown on coverslips were serum starved for 4 hours and then stimulated with 100 nM insulin and 10 µM ML-7. GLUT4 and Myosin IIA localization was examined by confocal microscopy (as described in Materials and methods) at various time points after insulin stimulation, as indicated. The results are representative images from three independent experiments.
Myosin II is required for GLUT4 vesicle fusion at the plasma membrane
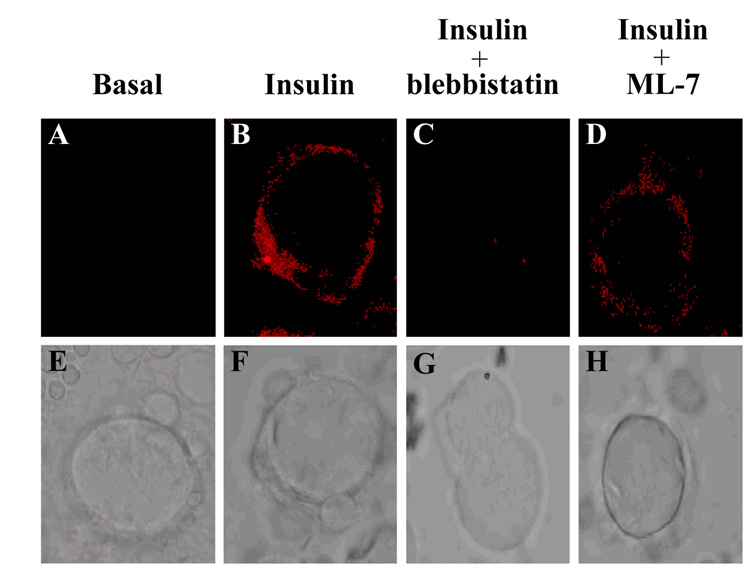
The translocation, docking and fusion of GLUT4 vesicles is required for insulin-stimulated glucose uptake in adipocytes. Our previous studies have found that myosin II is not required for GLUT4 vesicle translocation, but is necessary for proper GLUT4-mediated glucose uptake [10]. What is not known is if myosin II is required for GLUT4 vesicle fusion with the plasma membrane. To determine whether myosin II is required for GLUT4 vesicle fusion, 3T3-L1 adipocytes were serum starved for 4 hours in the presence of either 0.1% DMSO, 100 µM blebbistatin, or 10 µM ML-7 and then left untreated (Basal) or stimulated with insulin in the presence or absence of 100 µM blebbstatin, or 10 µM ML-7. Cells were fixed, incubated with a GLUT4 antibody directed against an epitope in an exofacial domain of GLUT4 and then plasma bound GLUT4 was visualized by confocal microscopy. As previously shown, insulin-stimulated a dramatic increase in plasma membrane bound GLUT4 compared to unstimulated adipocytes (Fig. 6, panel A and B). To determine whether a myosin II-cortical actin interaction was necessary for the GLUT4 vesicle fusion at the plasma membrane, adipocytes were treated with insulin and blebbistatin. Blebbistatin treatment significantly decreased the levels of plasma membrane bound GLUT4 compared to insulin-stimulated cells (Fig. 6, panel B and C). In order to determine whether myosin II activity was required for proper GLUT4 insertion into the plasma membrane, cells were treated with the MLCK inhibitor ML-7. Adipocytes treated with insulin and ML-7 showed a substantial decrease in the level of properly fused GLUT4 vesicles compared to adipocytes stimulated with insulin alone (Fig. 6, panel B and D). Differential interference contrast (DIC) images of each treatment (Fig. 6, panels E–H) were taken in addition to the confocal images. Thus these studies demonstrate that myosin IIA recruitment, activation and association with actin are necessary for proper GLUT4 insertion in the plasma membrane.
Fig. 6. Inhibition of myosin II activity inhibits GLUT4 vesicle fusion with the plasma membrane.
3T3-L1 adipocytes grown on coverslips were serum starved for 4 hours in the absence or presence of 100 µM blebbistatin or 10 µM ML-7 as indicated. Cells were then left untreated (basal) or stimulated with 100 nM insulin in the absence or presence of inhibitors and then treated according to the immunofluorescence protocol (as described in Materials and Methods). Fused GLUT4 vesicles were visualized using a GLUT4-specific antibody targeting an epitope in an exofacial domain. GLUT4 localization in adipocytes was examined in (A) unstimulated (Basal), (B) stimulated with 100 nM insulin (Insulin) or in the presence of 100 nM insulin and (C) 100 µM blebbistatin or (D) 10 µM ML-7. Differential Interference Contrast (DIC) images of each treatment were taken as well (E–H). The results are representative images from three independent experiments.
Myosin II facilitates GLUT4 intrinsic activity
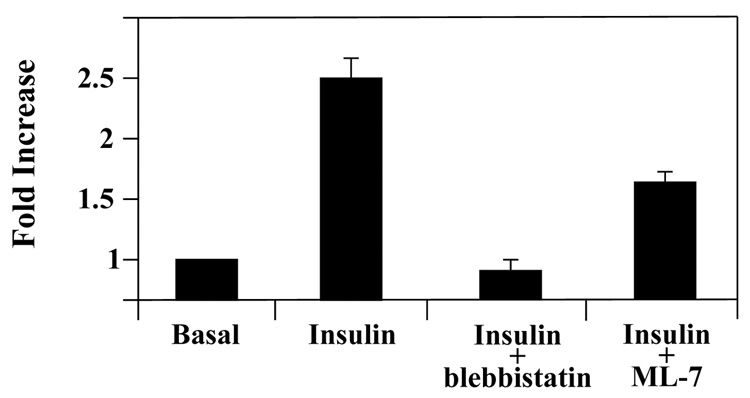
Previous studies have found that myosin II is required for GLUT4-mediated glucose uptake [8, 10]. This could reflect a requirement of myosin II for GLUT4 vesicle fusion and/or GLUT4 activity. Our findings here demonstrate that myosin IIA is required for proper GLUT4 insertion. Next we wanted to determine whether myosin II also affects GLUT4 intrinsic activity, so we examined the activity of plasma membrane embedded GLUT4 in the absence of GLUT4 translocation by performing short-term glucose uptake assays. In the basal state most but not all of the plasma membrane bound GLUT4 is internalized, resulting in a very low level of GLUT4 at the plasma membrane. Like GLUT4, myosin IIA is also present at low levels at the plasma membrane in the basal state (Fig. 3A, panels A–C). Thus using a short-term glucose uptake assay allowed us to examine the role of myosin IIA on the activity of embedded GLUT4. 3T3-L1 adipocytes were serum starved for 4 hours to internalize most, but not all the membrane bound GLUT4. After serum starvation, adipocytes were subjected to short-term glucose uptake assays using [14C]2-deoxy-D-glucose. Previous studies have shown that maximum GLUT4 translocation to the plasma membrane occurs between 10–14 minutes [12, 39]. Thus, the short time period should only examine glucose uptake from embedded GLUT4. Insulin-stimulated adipocytes showed a two-fold increase in glucose uptake compared to unstimulated (Basal) adipocytes (Fig. 7). To determine whether myosin II association with actin was necessary for GLUT4 activity, adipocytes were stimulated with 100 nM insulin in the presence of 100 µM blebbistatin. Blebbistatin treated adipocytes showed approximately a 70% inhibition of insulin-stimulated glucose uptake (Fig. 7). Next we wanted to determine whether insulin signaling via MLCK and thus myosin II activity was required for GLUT4 activity. Serum starved adipocytes were treated with 100 nM insulin and 10 µM ML-7 and then subjected to the short-term glucose uptake assay. ML-7 treated adipocytes showed approximately a 40% inhibition of insulin-stimulated glucose uptake compared to insulin-stimulated adipocytes (Fig. 7). These results show for the first time that myosin II activity via MLCK is required for GLUT4 intrinsic activity and that myosin II-interaction with cortical actin is required for GLUT4 activity.
Fig. 7. Myosin II regulates the intrinsic activity of GLUT4 during insulin-stimulated glucose uptake in 3T3-L1 adipocytes.
Adipocytes were serum starved for 4 hours and stimulated with insulin for 2 min and assayed for embedded GLUT4 (as described in Materials and Methods) in the presence of 0.1% DMSO (Basal), 100 nM insulin, 100 nM insulin and either 10 µM blebbistatin or 10 µM ML-7. Adipocytes were assayed for incorporated [14C]2-deoxy-D-glucose which was calculated as disintegrations per mg protein. The data are expressed as a percent of the vehicle control. Results are means ± SEM of three independent experiments.
Discussion
Recent studies have identified a critical role for myosin II in GLUT4-mediated glucose uptake in adipocytes [8, 10]. Since 3T3-L1 adipocytes express both myosin IIA and IIB isoforms, it is important to define the role of each of the isoforms during insulin-stimulated glucose uptake. While a role for myosin II in actin contraction has been well characterized in muscle cells, the role for myosin II in nonmuscle cells is less well defined. Several studies have demonstrated a role for the members of the myosin family in a nonmuscle cell context [15, 18, 25, 26, 28]. Myosin family members have been shown to traffic vesicles along filamentous actin tracks, and to mediate localized actin reorganization during cell migration and cytokinesis [16, 17, 40]. Insight into the role for myosin II in nonmuscle cells comes from chromaffin cells where myosin II mediates contraction of the actin cytoskeleton which leads to localized remodeling at the cell cortex that may be required for vesicle fusion with the plasma membrane [18].
Our study is the first to show that insulin differentially regulates the activity of the myosin II isoforms in adipocytes. We show that insulin specifically signals phosphorylation of the RLC associated with the myosin IIA isoform while the RLC associated with the myosin IIB isoform is not affected by insulin. These studies may provide insight into the different roles of the myosin II isoforms in adipocytes. Furthermore, we show that inhibition of MLCK using two different inhibitors (ML-9 and ML-7) but not a Rho kinase inhibitor impaired insulin-stimulated glucose uptake and that ML-7 treatment prevented the insulin mediated phosphorylation of myosin IIA. These findings demonstrate that insulin regulates the activity of the myosin IIA isoform specifically via MLCK to promote glucose uptake in adipocytes. These results are similar to those reported in a recent study showing that repulsive guidance molecule regulated the myosin IIA isoform specifically to inhibit neurite outgrowth [41]. Additionally, our studies demonstrated that phosphorylation of the RLC is required for the translocation of myosin IIA from the perinuclear region to the plasma membrane. Similarly histamine has been shown to induce the redistribution of only the myosin IIA isoform to lamellipodial extensions at the cell periphery of gastric parietal cells [42]. Our previous studies demonstrated that in the basal state myosin IIA is present in the perinuclear region while myosin IIB is localized at the cell cortex [10]. In other cell types where both isoforms are expressed, distinct localization patterns have been identified where only one isoform may occupy a specific intracellular space at a given time [27, 31]. While in MDA-MB-231 breast cancer cells, increased levels of RLC phosphorylation coincided with recruitment of both myosin IIA and IIB to the lamellar margin during active spreading [43]. The novel findings in this study demonstrate that insulin differentially regulates the myosin II isoforms and provides further insight into the differing role of the myosin isoforms in nonmuscle cells. These studies also identify another specific downstream target (myosin IIA) of insulin signaling and also another insulin-stimulated pathway required for GLUT4-mediated glucose uptake in adipocytes.
Furthermore, our studies demonstrate that while myosin IIA and GLUT4 colocalize at both the perinuclear region (prior to insulin stimulation) as well as at the plasma membrane (after insulin stimulation) there is only a physical association between myosin II and GLUT4- containing vesicles at the plasma membrane after insulin stimulation. We also show that insulin stimulates GLUT4 translocation to the plasma membrane prior to myosin IIA recruitment. Furthermore we demonstrate that myosin IIA must be phosphorylated on its RLC by MLCK to translocate to the plasma membrane. Inhibition of myosin IIA recruitment to the plasma membrane does not inhibit GLUT4 translocation but does result in impaired glucose uptake. These studies demonstrate that insulin activation of myosin IIA is required for its recruitment to the plasma membrane and GLUT4-mediated glucose uptake. Myosin IIA may be involved in the actin reorganization required at the plasma membrane for GLUT4 vesicle membrane fusion. [4– 6, 44]. This would explain the recruitment of active myosin IIA to sites where GLUT4 is present to aide in the coordinated insertion of GLUT4 into the plasma membrane.
The cortical actin cytoskeleton acts as a barrier to either endocytosis or exocytosis at the plasma membrane. When myosin II activity is inhibited, insulin stimulated glucose uptake is impaired yet GLUT4 vesicles still translocated to the plasma membrane. This suggests that myosin II is involved in some downstream event in GLUT4 mediated glucose uptake such as GLUT4 vesicle fusion with the plasma membrane. Our studies demonstrate that inhibition of myosin activity with either blebbistatin or ML-7 significantly reduced the level of GLUT4 embedded in the plasma membrane after insulin stimulation. These findings suggest that although GLUT4 translocated to the plasma membrane it is unable to embed into the membrane without active myosin IIA at the plasma membrane. Additionally we show that myosin must interact with actin to allow GLUT4 to embed into the membrane. Previous studies have shown that contraction of the actin cytoskeleton in rat chromaffin cells and human MRC-5 fibroblasts resulted in localized remodeling at the cellular cortex [14, 31]. The dynamic process of actin remodeling may be responsible for the docking and fusion of GLUT4 vesicles with the plasma membrane.
In the present study we not only demonstrate that myosin II is required for GLUT4 vesicle fusion with the plasma membrane but that it is also involved in GLUT4 activity. In the basal state, adipocytes are serum starved to allow GLUT4 internalization. We found that like GLUT4, myosin IIA is also present at low levels at the plasma membrane in the basal state. Inhibition of myosin II activity or association with actin impaired glucose uptake from embedded GLUT4. Previous studies have shown that after vesicle fusion, GLUT4 has to be activated before glucose uptake can occur [20]. While both p38 MAPK in muscle cells and p44/p42 MAPK in adipocytes were found to increase GLUT4 activity, the precise role of these factors is not well understood [1, 20–22]. It has been suggested that GLUT4 activity is regulated by its interaction with either an activator ligand or the removal of an inhibitory ligand [21]. It is tempting to speculate that myosin II-mediated actin reorganization is involved in increasing GLUT4 activity by allowing p38 MAPK, p44/p42 MAPK or some other protein access to membrane-bound GLUT4.
In summary, our studies show that insulin specifically stimulates the myosin IIA isoform via MLCK. We also show that inhibition of myosin IIA activity prevented its translocation to the plasma membrane and subsequently impaired GLUT4-mediated glucose uptake by preventing GLUT4 from embedding into the plasma membrane. Our results also demonstrate that myosin IIA activity is required for the intrinsic activity of embedded GLUT4. Collectively, our studies demonstrate that insulin stimulates myosin IIA activity and promotes the embedding of GLUT4 into the plasma membrane and the activation of GLUT4. Our findings further delineate the role of cytoskeletal factors involved in insulin-stimulated glucose uptake and thus provide further insight on the basis of impaired insulin sensitivity.
Acknowledgements
This work was supported by an NIH grant to Y.M.P. (1R15DK073180-01). Microscopy studies were supported in part by grants from the NSF (DBI-0319021) and the North Carolina Biotechnology Center (003-IDG-1011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harmon AW, Paul DS, Patel YM. MEK inhibitors impair insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2004;287:E758–E766. doi: 10.1152/ajpendo.00581.2003. [DOI] [PubMed] [Google Scholar]
- 2.Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem. 1999;274:33085–33091. doi: 10.1074/jbc.274.46.33085. [DOI] [PubMed] [Google Scholar]
- 3.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme A, Emoto M, Buxton JM, Bose S, Sabini R, Theurkauf WE, Leszyk J, Czech MP. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3-L1 adipocytes. J Biol Chem. 2000;275:38151–38159. doi: 10.1074/jbc.M003432200. [DOI] [PubMed] [Google Scholar]
- 5.Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- 6.Tsakiridis T, Tong P, Matthews B, Tsiani E, Bilan PJ, Klip A, Downey GP. Role of the actin cytoskeleton in insulin action. Microsc Res Tech. 1999;47:79–92. doi: 10.1002/(SICI)1097-0029(19991015)47:2<79::AID-JEMT1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Choi YO, Ryu HJ, Kim HR, Song YS, Kim C, Lee W, Choe H, Leem CH, Jang YJ. Implication of phosphorylation of the myosin II regulatory light chain in insulin-stimulated GLUT4 translocation in 3T3-F442A adipocytes. Exp Mol Med. 2006;38:180–189. doi: 10.1038/emm.2006.22. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Imamura T, Babendure JL, Lu JC, Olefsky JM. Disruption of microtubules ablates the specificity of insulin signaling to GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 2005;280:42300–42306. doi: 10.1074/jbc.M510920200. [DOI] [PubMed] [Google Scholar]
- 10.Steimle PA, Fulcher FK, Patel YM. A novel role for myosin II in insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;331:1560–1565. doi: 10.1016/j.bbrc.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patki V, Buxton J, Chawla A, Lifshitz L, Fogarty K, Carrington W, Tuft R, Corvera S. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol Biol Cell. 2001;12:129–141. doi: 10.1091/mbc.12.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- 14.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 15.Laevsky G, Knecht DA. Cross-linking of actin filaments by myosin II is a major contributor to cortical integrity and cell motility in restrictive environments. J Cell Sci. 2003;116:3761–3770. doi: 10.1242/jcs.00684. [DOI] [PubMed] [Google Scholar]
- 16.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 17.de la Roche MA, Cote GP. Regulation of Dictyostelium myosin I and II. Biochim Biophys Acta. 2001;1525:245–261. doi: 10.1016/s0304-4165(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 18.Neco P, Giner D, Viniegra S, Borges R, Villarroel A, Gutierrez LM. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 19.Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell. 2004;15:688–695. doi: 10.1091/mbc.E03-06-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somwar R, Kim DY, Sweeney G, Huang C, Niu W, Lador C, Ramlal T, Klip A. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J. 2001;359:639–649. doi: 10.1042/0264-6021:3590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelle Furtado L, Poon V, Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand. 2003;178:287–296. doi: 10.1046/j.1365-201X.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 22.Somwar R, Koterski S, Sweeney G, Sciotti R, Djuric S, Berg C, Trevillyan J, Scherer PE, Rondinone CM, Klip A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–50395. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- 23.Harmon AW, Patel YM. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: a mechanism for impaired cellular proliferation. Breast Cancer Res Treat. 2004;85:103–110. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 24.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 25.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 26.Xia D, Stull JT, Kamm KE. Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly. Exp Cell Res. 2005;304:506–517. doi: 10.1016/j.yexcr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Krits I, Wysolmerski RB, Holliday LS, Lee BS. Differential localization of myosin II isoforms in resting and activated osteoclasts. Calcif Tissue Int. 2002;71:530–538. doi: 10.1007/s00223-001-1112-0. [DOI] [PubMed] [Google Scholar]
- 28.Bose A, Guilherme A, Robida SI, Nicoloro SM, Zhou QL, Jiang ZY, Pomerleau DP, Czech MP. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 2002;420:821–824. doi: 10.1038/nature01246. [DOI] [PubMed] [Google Scholar]
- 29.Kolega J. Fluorescent analogues of myosin II for tracking the behavior of different myosin isoforms in living cells. J Cell Biochem. 1998;68:389–401. doi: 10.1002/(sici)1097-4644(19980301)68:3<389::aid-jcb9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial 'sorting ' of isoforms in locomoting cells. J Cell Sci. 1998;111(Pt 15):2085–2095. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh T, Takemura S, Ueda K, Hosoya H, Nagayama M, Haga H, Kawabata K, Yamagishi A, Takahashi M. Differential localization of non-muscle myosin II isoforms and phosphorylated regulatory light chains in human MRC-5 fibroblasts. FEBS Lett. 2001;509:365–369. doi: 10.1016/s0014-5793(01)03186-6. [DOI] [PubMed] [Google Scholar]
- 32.Paul DS, Harmon AW, Winston CP, Patel YM. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem J. 2003;376:625–632. doi: 10.1042/BJ20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 36.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 37.Shu S, Liu X, Korn ED. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci U S A. 2005;102:1472–1477. doi: 10.1073/pnas.0409528102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 39.Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- 40.Robinson DN, Girard KD, Octtaviani E, Reichl EM. Dictyostelium cytokinesis: from molecules to mechanics. J Muscle Res Cell Motil. 2002;23:719–727. doi: 10.1023/a:1024419510314. [DOI] [PubMed] [Google Scholar]
- 41.Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem. 2008;105:113–126. doi: 10.1111/j.1471-4159.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou R, Watson C, Fu C, Yao X, Forte JG. Myosin II is present in gastric parietal cells and required for lamellipodial dynamics associated with cell activation. Am J Physiol Cell Physiol. 2003;285:C662–C673. doi: 10.1152/ajpcell.00085.2003. [DOI] [PubMed] [Google Scholar]
- 43.Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy AM, Spisak KO, Brozinick JT, Elmendorf JS. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol. 2006;291:C860–C868. doi: 10.1152/ajpcell.00107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]