Abstract
Treatments of advanced prostate cancer with androgen deprivation therapy inevitably render the tumors to become castration resistant and incurable. Under these conditions, neuroendocrine differentiation of prostate cancer (CaP) cells is often detected and neuropeptides released by these cells may facilitate the development of androgen independence. Exemplified by GRP (gastrin-releasing peptide), these neuropeptides transmit their signals through G-protein coupled receptor (GPCRs), which are often overexpressed in prostate cancer, and aberrantly activate androgen receptor (AR) in the absence of androgen. We developed an autocrine neuropeptide model by overexpressing GRP in LNCaP cells and the resultant cell line, LNCaP-GRP, exhibited androgen-independent growth with enhanced motility in vitro. When orthotopically implanted in castrated nude mice, LNCaP-GRP produced aggressive tumors, which express GRP, prostate-specific antigen, and nuclear-localized AR. Chromatin immunoprecipitation studies of LNCaP-GRP clones suggest that GRP activates and recruits AR to the cognate promoter in the absence of androgen. A Src family kinase (SFK) inhibitor, AZD0530 inhibits androgen-independent growth and migration of the GRP-expressing cell lines, and blocks the nuclear transloation of AR, indicating the involvement of SFK in the aberrant activation of AR and demonstrating the potential use of SFK inhibitor in the treatment of castration resistant CaP. In vivo study showed AZD0530 profoundly inhibits tumor metastasis in severe combined immunodeficient (SCID) mice implanted with GRP-autocrine LNCaP cells. This xenograft model demonstrates autocrine, neuropeptide- and Src kinase-mediated progression of androgen-independent CaP post-castration, and is potentially useful for testing novel therapeutic agents.
Keywords: GPCR, neuroendocrine, neuropeptides, androgen-independence, Src kinase, prostate cancer
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer in American men and the second leading cause of cancer deaths (1). Androgen withdrawal initially induces apoptosis and cell cycle arrest in CaP; however, CaP eventually loses its dependence on androgens and progresses to an androgen-independent state. Various mechanisms have been postulated to account for the conversion of CaP into castration-resistant state, including the aberrant activation of AR by peptide growth factors and ligands for GPCRs (2–4). If true, these mediators and components of their signal pathways are potential targets for therapeutic intervention of castration-resistant CaP. It has been reported that androgen withdrawal from androgen-dependent CaP cells (5) or treatment with stimuli such as IL-6 and forskolin in vitro promotes acquisition of the neuroendocrine phenotype through transdifferentiation (6). Cumulative evidence suggests that neuroendocrine differentiation of CaP may be a cofactor involved in tumor progression and androgen independence (7).
Neuroendocrine cells are identified by their neurosecretory granules and expression of neuron specific markers including chromogranin A, neuron-specific enolase and mitogenic neuropeptides such as bombesin/GRP, somatostatin, calcitonin, and parathyroid hormone-related peptides (7). Neuropeptides have been identified as potent paracrine and autocrine growth factors in human cancers to include lung, gastrointestinal, pancreatic, brain, and prostate (8–13). In prostate cancer, previous studies by others and by us have shown that neuropeptides promote cell growth (14), migration and protease expression (15) in PC-3 cells, and androgen-independence in LNCaP cells (3, 5). Androgen independence in CaP patients is shown to correlate well with elevated serum levels of chromogranin A (16). Elevated expression of GRP receptors are often detected in CaP specimens (17, 18).
The bombesin/GRP family is among the most studied neuropeptide group in CaP. Bombesin/GRP transduces signals by engaging heterotrimeric G protein-coupled receptors located on the cell surface (19). Upon binding to its receptors, bombesin/GRP elicits calcium mobilization in PC-3 and DU 145 cells (20, 21) and promotes growth and cell invasiveness via proteolytic activities of MMP’s in LNCaP and PC-3 cells (15). We have previously shown that exogenous bombesin/GRP activates AR and supports androgen-independent growth in LNCaP through signaling mediated by non-receptor tyrosine kinases such as Src, FAK and Etk (3). In vivo androgen withdrawal following establishment of LNCaP tumors results in increased neuroendocrine cells (5). Together, these data suggest that castration induced neuroendocrine differentiation may release soluble factors, which sustain the growth and survival of androgen-deprived cells, contributing to tumor androgen-independence and metastasis.
In this paper, we describe a neuropeptide xenograft model and use it to test inhibition of the tyrosine kinase pathway implicated in the development of androgen-independence. We introduced the GRP-expressing vector into LNCaP cells to establish an autocrine neuroendocrine model. The GRP clones exhibited enhanced proliferation and migration properties under androgen-depleted conditions, and developed significant tumors in castrated nude mice, providing evidence for GRP’s role in androgen-independent growth through modulation of AR. We tested the effect of a SFK inhibitor AZD0530 on recultured xenograft cells both in vitro and in vivo. Our results showed that AZD0530 effectively blocked the androgen-independent growth and migration of LNCaP cells mediated by autocrine GRP, through inhibiting activation of the Src/FAK/Etk complex. SCID mice implanted with GRP-autocrine LNCaP cells and treated with AZD0530 showed a complete inhibition of tumor metastasis.
MATERIALS AND METHODS
Cell culture
LNCaP cells (ATCC, passages 38–43) were kept in RPMI1640 with 10% regular FBS. When stimulated, cells were switched to phenol-red free RPMI1640 with 5% charcoal-stripped androgen-free (CS) serum.
Proliferation assays
Cells were grown in CS medium alone or with supplemented with 100 nM of bombesin, with 1 μg/ml of bombesin/GRP specific monoclonal antibody 2A11 (22), 5 μM of GRP receptor antagonist RC3095 (23) or transfection of 100 μM of small inhibitory RNA (siRNA, sense sequence GGAAACAGUUCAACACUCAUU, validated by RT-PCR for inhibition, Dharmacon) for GRP receptor. Cells were tryspinized and counted by trypan blue exclusion method after 48 hours or over 6 days for siSrc transfection.
Chemotaxis migration assay
Migration assays were performed in a Boyden chamber with 8 μm Nucleopore membrane coated with human plasma fibronectin (50 μg/ml). 2 × 104 LNCaP cells were placed in the upper wells with testing agents in the lower wells, and incubated at 37 °C for 4 hours to allow cell migration. At the end of incubation, the membrane was stained by Diff-Quik Stain Kit and mounted on microscopic slides for counting. Each experiment was performed in triplicate. AZD0530 (500 nM) or siSrc transfection was used for inhibitor studies.
GRP-expressing construct and transfection
GRP cDNA was amplified from the small cell lung carcinoma DMS53 cell line (ATCC), which expresses GRP. The amplified cDNA was inserted into mammalian expression vector pcDNA3.1-Zeocin (Invitrogen). LNCaP cells were transfected with this GRP construct or the empty vector and stable transfectants were selected with Zeocin (100μg/ml) as GRP or Zeo (the mock transfectant). Presence of the GRP gene in selected clones was confirmed by Northern blotting and RT-PCR.
Quantitation of secreted GRP
CM collected from LNCaP, LNCaP-zeo, GRP1-1, GRP4-9 and DMS53 cells was concentrated by passing through PrepSep C18 reverse columns (Fisher) and the retainee was eluted with acetonitrile:water:acetic acid (59:40:1) mixture (24). The reconstituted solvent-free eluents were assayed for bombesin/GRP with a Bombesin EIA kit (Peninsula Lab).’
Soft Agar Assay
2 × 104 cells were plated in the midst of 0.3% agar in CS medium with or without 2A11 (1 μg/ml). Colony formation was examined after 4 weeks. For the recultured GRP xenograft bicalutamide (5 μM) was used additionally.
In vivo tumor biology
Animal studies were conducted in accordance with institutional ethical guidelines for the care and use of experimental animals. Surgical castration was performed and immediately following castration, 2×106 LNCaP-Zeo and GRP cells co-suspended with 30% matrigel were injected orthotopically into twenty and twelve castrated nude mice, respectively. At the end of 4 months, mice were sacrificed and their prostates were collected for pathological analyses. Tumor sections were immunohistochemically stained with antibodies for GRP (RGG7130, Peninsula Laboratories), AR (PG21, Millipore) and PSA (ER-PR8, Dako) and detected using the DAKO Envision+ Kit. PSA levels were determined by the Micro PSA ELISA kit (Fitzgerald Industries).
Tumors were dissociated into singe-cell suspensions with collagenase and plated in CS media. The derived LNCaP GRP sublines termed GRP-Pro (derived from Prostate) were pooled together.
For inhibitor study, fourteen castrated SCID mice were orthotopically implanted with 4×106 re-cultured GRP-Pro cells. SCID mice were used to better study tumor metastasis. Two weeks after surgery, mice are divided into two groups, with 7 treated with 50mg/kg/day via esophageal gavaging (AZD0530-treated) and 7 with buffer only (control). The study was terminated when one of the control mice succumbed to tumor burden. All the mice were euthanized, their primary tumors excised for weighing and IHC staining with p-Src (Cell Signaling), p-FAK (ABR) or AR antibodies and lymph nodes examined for metastasis.
Transient transfection assays
Zeo, GRP4-9 and GRP Pro cells were seeded in 24-well plates, transfected with 0.2 μg of PSA-Luc (promoter region, 630 bp) with the internal control pTK-RL using Effectene® (Qiagen). Transactivation was examined by the dual-luciferase assay (Promega). For RNA interference, standard siCONTROL (D-001210-02, SC) and on-target plus SMART pool human Src (L-003175-00, SiSrc, Dharmacon) were complexed with Lipofectamine 2000 (Invitrogen) and delivered to cells grown in CS media at a final concentration of 100 nM.
Chromatin Immunoprecipitation
LNCaP-Zeo, GRP, and GRP-Pro cells grown to sub-confluency were switched to CS media for 3 days. Treatment with R1881 was performed 6 hours before harvesting. Chromatin immunoprecipitation was performed as described (4, 25, 26) with 6 μg of anti-AR antibody (PG-21, Millipore). Standard PCR cycling protocol was performed with 58°C for annealing for 30 cycles. Primers for AR enhancer region are: 5′catgttcacattagtacaccttg3′ and 5′tctcagatccaggcttgcttac3′; for proximal ARE region: 5′tcctgagtgctggtgtcttag3′ and 5′agccctataaaaccttcattcc3′; and for intervening region: 5′tcatccactcatcatccagcatc3′ and 5′ggagagcaatagacttgggaaacc3′.
Immunofluorescent staining of AR
Cells (2,500) were plated in 4-well chamber slides in CS media a day before fixing with 2% paraformaldehyde for staining. Anti-AR (N-20, Santa Cruz) and anti-rabbit AlexaFluor 647 (Invitrogen) were used as the primary and secondary antibodies for staining, respectively. Immunofluorescent cells were visualized using an Olympus BX61 motorized reflected fluorescence microscope system with an AMCA filter for DAPI and a Cy5 filter for AlexaFluor647 using the SlideBook4.1 software (Intelligent Imaging Innovations).
Immunoprecipitation and Western blot
LNCaP-Zeo, GRP and GRP-Pro cells were subjected to androgen withdrawal for 3 days with or without exposure to AZD0530 (1 μM). Cell lysates were collected in IP buffer containing proteinase and phosphatase inhibitors, incubated with anti-FAK and subsequently protein G agarose beads for immunoprecipitation.
Phosphorylation of the respective precipitated proteins was detected by anti-p-Src family (Cell Signaling), p-FAK (Invitrogen) and p-Etk (Cell Signaling) antibodies after Western blotting analysis. Signals were detected by ECL system (Amersham) followed by exposure to X-ray film.
Statistics
All in vitro data were from at least three independent experiments and subjected to paired t-tests using Statview program.
RESULTS
It has been shown that bombesin confers androgen-independent growth of LNCaP cells (3). We validated that bombesin signals through the GRP receptor with specific inhibitors such as bombesin/GRP specific monoclonal antibody 2A11 and GRP receptor antagonist RC3940-II. Bombesin also stimulated cell migration as compared to the negative control (supplementary data #1).
Expression of GRP enhances proliferation and migration of transfected LNCaP cells
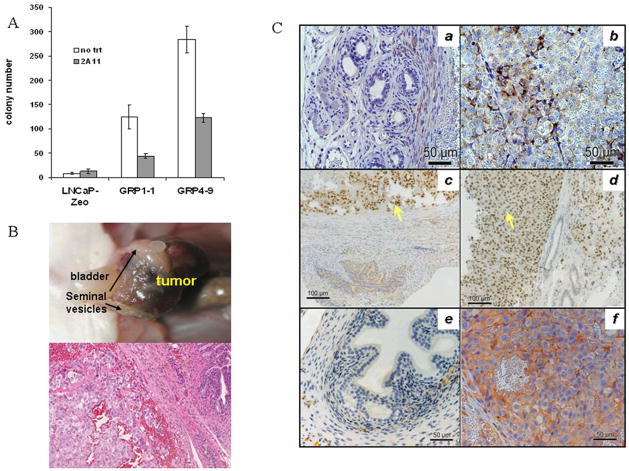
We established an autocrine model by introducing a GRP overexpressing vector to androgen-sensitive LNCaP cells to study the signaling pathways involved in androgen independence in vitro and in vivo. Stable LNCaP-GRP transfectants were established by overexpressing GRP cDNA and screened by Northern blotting and RT-PCR (Figure 1A). Positive clones (e.g. GRP1-1 and GRP4-9) were isolated and characterized. Bombesin/GRP enzyme immunoassay confirmed GRP expression in the two GRP clones (Figure 1B). GRP1-1 and 4–9 cells produce almost 5 fold more GRP than the control lines, but comparable to DMS53 cells. Antibody 2A11, GRP receptor antagonist RC3905 and siRNA for the GRP receptor effectively inhibited the androgen-independent growth of GRP1-1 and 4–9 to 20–60% of the control (Figure 1C). Negative control using siRNA targeting green fluorescence protein showed no effect on growth (data not shown). These data support the notion that GRP/bombesin is able to confer androgen-independent growth of LNCaP through binding to its membrane receptor. If the androgen independent growth is due to the autocrine release of GRP into the media, we would expect a chemotactic effect from GRP CM. As expected, LNCaP-Zeo migration was stimulated by bombesin (Figure 1D). GRP CM stimulated LNCaP-Zeo migration by more than 3-fold and this migration was significantly reduced by 2A11 (p ≤0.001), suggesting GRP’s involvement. Migration of GRP1-1 and 4–9 towards ctlCM was two-fold greater than that of LNCaP-zeo, and could be further stimulated by GRP CM, and significantly inhibited by 2A11 (p ≤0.001). These data showed that LNCaP-GRP cells release GRP, which confers androgen-independent growth and migration through autocrine loop.
Figure 1.
The model of an androgen-independent GRP expressing prostate cancer line, with evidence of enhanced proliferation and migration: A, Northern blot and RT-PCR assays verified expression of GRP gene into LNCaP GRP clones compared to the parental LNCaP/mock-transfected Zeo cells (negative controls) and DMS53 (positive control) cells. B, Quantitation of secreted GRP in CM collected from LNCaP, LNCaP-zeo, GRP1-1, GRP4-9, and small cell lung carcinoma DMS53 cells. C, Androgen-independent growth of GRP1-1 and 4–9 was targeted by inhibitors for GRP and its receptor. All inhibitors reduced the GRP cell growth in CS media with significance. D, Boyden chamber migration assay. Conditioned media (CM) were collected from cells by overlaying SF media on sub-confluent plates for 48 hr and the amount to use was normalized by the total protein concentration of each plate. CM from LNCaP-Zeo (ctlCM) or GRP (GRPCM) cells were used as the chemo-attractants. GRP-specific monoclonal antibody 2A11 (1 μg/ml) was introduced as the inhibitor. Bombesin (100 nM) was the positive control. Migration assay was conducted as described in the Materials and Methods. Means of data from at least three independent experiments were plotted and bars represent standard error of the mean.
GRP promotes in vitro and in vivo tumorigenesis in androgen-free environments
Soft agar assay was performed to assess in vitro tumorigenicity. GRP1-1 and 4–9 produced significantly more colonies than LNCaP-Zeo in CS medium, suggesting that the autocrine GRP induces both androgen- and anchorage-independent growth (Figure 2A). 2A11 significantly inhibited colony formation of both GRP1-1 and 4-9 (p≤0.05 and p≤0.0005). We then used the GRP clones for in vivo tumor study. Orthotopic prostatic implantation of GRP4-9 cells into prostates of castrated nude mice resulted in tumor growth in 8 of 12 mice. In contrast, 0 of 20 castrated mice implanted with LNCaP-zeo cells displayed any tumor growth. To generalize this finding, GRP1-1 was also orthotopically implanted and 4 of 5 mice produced tumors. H and E staining of the tumors showed characteristic human CaP tumors adjacent to normal mouse prostate tissue (Figure 2B). IHC staining (Figure 2C) showed staining of GRP was evident throughout the cytoplasm of the tumor regions, yet minimally detected in the normal mouse prostate epithelium of the tumor, despite the fact that the GRP antibody used reacts with both human and mouse GRP. Staining with anti- AR antibody demonstrated its nuclear translocalization in tumor cells, indicative of GRP ligand activation. PSA expression was extensive in the tumor specimens, again supporting GRP-mediated AR activation. Mean serum PSA level in castrated LNCaP-GRP tumor mice was 208.9±24.6 ng/ml serum, as compared to 6.13×10−5 ng/ml in castrated LNCaP-zeo mice.
Figure 2.
In vitro (soft agar assay) and in vivo (nude mice) tumorigenesis in androgen-deprived conditions: A, Soft agar assay was performed in CS medium as described in Materials and Methods. The experiment has been performed independently three times and the error bars represent standard error of the mean. B, Example of orthotopic implanted LNCaP-GRP tumor grown in a castrated nude mouse. Top: whole tumor after 4 months. Bottom: H and E staining showed LNCaP-GRP tumor on left side, mouse prostate stroma in the middle, and normal mouse prostate gland on the right. C, IHC staining of GRP (a and b), AR (c and d) and PSA (e and f) in the tumor specimens: Slides on the left (a, c and e) showed most of the normal mouse prostate region; while on the right (b, d and f) showed predominately prostate tumors.
Tumors harvested from GRP implanted mice were re-cultured in vitro to establish a xenograft cell line, labeled GRP-Pro. Expression of PSA, AR and GRP in GRP-Pro cells was analyzed by RT-PCR analysis for the authenticity of the clones (supplementary data #2). Equal endogenous level of PSA mRNA for all clones is also verified. Soft agar assay using GRP-Pro cells showed their aggressive nature as manifested by their androgen- and anchorage- independent growth in 2 weeks (Figure 3A). This growth was partially inhibited by 2A11 and the androgen inhibitor, bicalutamide, individually or in combination (with significant difference p≤0.05) suggesting that growth is dependent on both GRP and AR.
Figure 3.
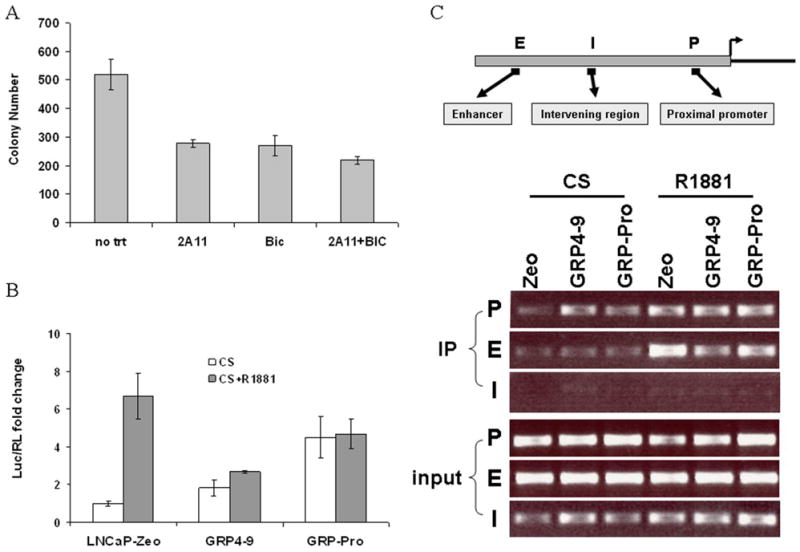
A, Soft agar assay of the re-cultured GRP-Pro xenograft: Soft agar assay was performed as described in the Materials and Methods. Treatments include monoclonal antibody to bombesin/GRP, 2A11 (1 μg/ml), anti-androgen bicalutamide (BIC, 5 μM), combination of 2A11 and BIC and synthetic androgen R1881 (1 nM). B, Transactivation assay: LNCaP-Zeo, GRP 4-9 and GRP-Pro cells were plated in CS medium and transfected with the PSA-Luc (630 bp) and pTK-RL. R1881 (1 nM) was added to some wells 24 hours post transfection and dual-luciferase assay was conducted after another 24 hours. Means of triplicate experiments were plotted and bars represent standard error of the mean. C, Chromatin immunoprecipitation assay: AR binding to both the enhancer and proximal ARE in the PSA promoter was revealed through PCR analysis using ChIP assay coupled with amplification with primers described in the Materials and Methods. “E”, “P”, and “I” designate for the upstream enhancer region, proximal ARE region, and the intervening region, respectively.
GRP modulates activation of AR
We further sought to illustrate GRP-mediated AR activation at the molecular level. Transactivation assay was performed with LNCaP-Zeo, GRP4-9 and GRP-Pro cells in CS media using promoter PSA-Luc as the reporter. Expression of PSA-Luc in GRP4-9 and GRP-Pro is 1.8 and 4.5 fold higher than in LNCaP-Zeo cells (Figure 3B). This suggests GRP secreted from GRP cells is driving the expression. Addition of synthetic androgen R1881 induced PSA-Luc expression in LNCaP-Zeo cells more than 6 fold, but much less in GRP4-9 and GRP-Pro cells probably because the GRP-activated AR, through post-translational modification, already adopted an active conformation and may not be further stimulated by R1881. If GRP activates AR in GRP-Pro cells, AR should be recruited to ARE sites in the PSA promoter. We therefore performed the ChIP assay on LNCaP-Zeo, GRP4-9 and GRP-Pro cells in CS or CS+R1881 conditions. AR binding was analyzed by PCR using respective primers against enhancer (E) and proximal (P) ARE regions, and an intervening (I) region void of any ARE sites. Figure 3C shows AR binds to PSA P region in GRP4-9 and GRP-Pro even in the absence of androgen. When treated with R1881, AR binds preferentially to the E site in LNCaP-Zeo; whereas in GRP4-9 and GRP-Pro, AR binding was evenly detected at both P and E sites.
Src and FAK tyrosine kinases play important roles in GRP-mediated androgen-independent growth and migration
Exogenous bombesin induces AR nuclear translocation, and this induction is inhibited by Src inhibitor PP2 (25). In our LNCaP GRP mouse model, AR is localized to the nuclei as shown in the tumor IHC staining (Figure 2C). We further compared the GRP cells with the mock control by immunofluorescent staining to confirm AR nuclear localization in GRP cells through autocrine GRP-mediated activation (Figure 4). Staining of AR is limited to the cytoplasm in Zeo cells grown in CS media but concentrated to the nuclei of GRP cells (counted 65% nuclei with AR). This localization was inhibited by AZD0530, a selective SFK inhibitor demonstrating significant effects on prostate cancer cells (27). Almost half of GRP cells (35% nuclei with AR remaining) lost nuclear staining of AR when Src activity is inhibited. These data confirm that GRP activates AR through Src and promotes its nuclear translocation, consistent with recent data that Src directly phosphorylates AR at Y534 resulting in nuclear translocation (28).
Figure 4.
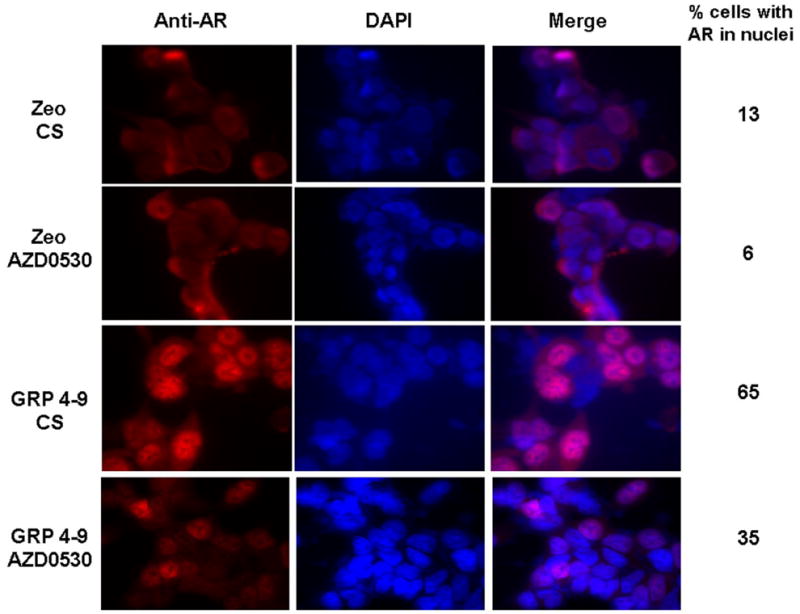
Immunofluorescent staining of AR in LNCaP-Zeo and GRP4-9 cells in response to AZD0530 treatment: AR localization in the nuclei of GRP4-9 cells under androgen-deprived conditions is inhibited by AZD0530. Numbers on the right represent the percentage of cells with AR nuclear localization.
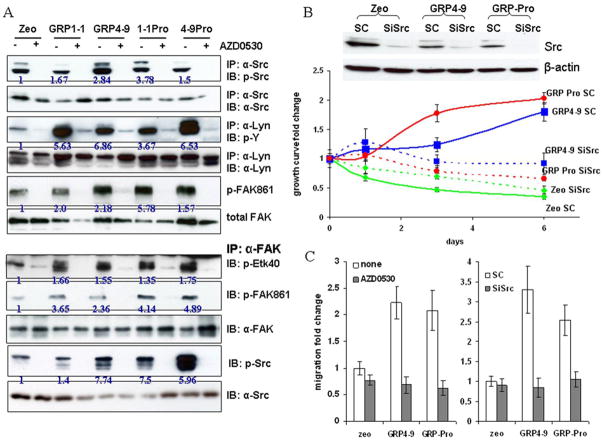
Among all the tyrosine kinases expressed in LNCaP cells, we previously showed that Src and FAK are most prominently activated by bombesin (3). Activated Src and FAK engage Etk, a tyrosine kinase shown to be involved in prostate carcinogenesis (3, 29). Src and FAK form a complex through binding between the phosphorylated Y397 in FAK and the SH2 domain in Src (30), whereas FAK associates with Etk via the FERM domain of FAK and the PH domain of Etk (31). These three kinases cross activate one another with increased tyrosine phosphorylation of the complex. Using AZD0530, we examined whether the androgen-independent growth and migration stimulation observed in our autocrine model is mediated through the Src/FAK signaling pathway. In LNCaP cells, in addition to Src, another member of SFK, Lyn, is also significantly expressed. We thus examined the phosphorylation status of Src, Lyn and FAK kinases in all cell lines grown in CS medium. We immunoprecipitated Src and Lyn proteins with their respective antibody then probed with anti-p-Src or anti-p-Y antibodies. For FAK, we used anti-p-FAKY861, residue phosphorylated by Src as another indicator of SFK activity. All the GRP and GRP-Pro lines displayed higher levels of kinase phosphorylations compared to Zeo cells after exposure to CS serum for 3 days and the phosphorylations were inhibited by AZD0530 (Figure 5A). The data showed that 1) autocrine-GRP indeed activates the SFKs; and 2) AZD0530, a pan-Src inhibitor, effectively blocks the activity of Src family members. Thus, while in the ensuing studies we will focus on the molecular characterizations of Src, the biological effects observed are likely due to the combined inhibition of all SFKs expressed in LNCaP cells. We previously reported that when activated, Src forms a complex with FAK and Etk and these kinases cross activate one another. Co-immunoprecipitation of Src, FAK and Etk kinases with the anti-FAK antibody confirms the complex formation and showed elevated activation of the three kinases in GRP and GRP-Pro cells compared to Zeo cells. Treatment with AZD0530 significantly reduced the degree of tyrosine phosphorylation of all three kinases but to a much less extent, the association between FAK and Src. (Figure 5A).
Figure 5.
A, Effect of AZD0530 on Src/FAK/Etk complex: Phosphorylation status of Src, Lyn and FAK kinases in LNCaP-zeo and GRP subclones was shown in the upper panel. Treatment of all cells with 1 μM of AZD0530 for 2 hours diminishes kinase activations in all cells without affect the total protein levels. Association of Src/FAK/Etk complex was illustrated by co-immunoprecipitation with the anti-FAK antibody. Cell lysates from Zeo and GRP cells were immunoprecipitated with anti-FAK antibody and probed for p-Etk, p-FAK, total FAK p-Src and total Src antibodies. Numbers under the untreated samples represent the densitometric quantification for phosphorylation after normalized by the total protein loading. B, Knocking down Src with siSrc transfection impaired the androgen-independent growth of GRP4-9 and GRP-Pro cells in CS media. LNCaP-Zeo cells were used as the control for GRP cells and SC (scramble control) was used as the control for siSrc. Western blots validated the siSrc transfection. C, Effect of AZD0530 and siSrc transfection on migration: AZD0530 (500 nM) or knocking down Src kinase with siSrc inhibited GRP4-9 and GRP-Pro cell migration. SC (scramble control) was used as the control for siSrc. The experiment has been performed independently at least three times and the error bars represent standard error of the mean.
Regarding proliferation, AZD0530 reduced GRP-Pro cell growth in a dose-dependent manner and inhibited the anchorage- and androgen-free growth of GRP-Pro cells (supplementary data #3). To ensure AZD0530 targets Src through which GRP mediates AR activation, RNA interference experiment for Src (siSrc) was performed. Transfection of siSrc into GRP4-9 and GRP-Pro cells greatly impaired their ability to grow in CS media compared to their respective non-target controls (SC, scramble RNA); whereas the LNCaP-Zeo cells do not grow well in the androgen-deprived condition with or without siSrc (Figure 5B). These data support that Src is a major target in neuropeptide-mediated AR activation, possibly through it downstream kinases such as FAK and Etk. Both FAK and Etk function in cell adhesion and migration, and inhibition of Src would reduce LNCaP-GRP and GRP-Pro cell migration. As a result, motility of GRP4-9 (p≤0.05) and GRP-Pro (p≤0.0005) cells was significantly inhibited by AZD0530 (500 nM) (Figure 5C). Knocking down Src with siSrc transfection into GRP4-9 and GRP-Pro cells also reduced cell migration to the comparable level as Zeo cells. These data support the notion that the GRP-mediated androgen-independent growth and migration is principally through SFK, especially Src kinase.
SFK inhibitor AZD0530 prevents tumor metastasis in SCID mice
With the encouraging results of AZD0530 inhibition in vitro, we evaluated it in our orthotopic GRP mouse model. Fourteen castrated SCID mice implanted with GRP-Pro cells; half of them were administered 50 mg/kg/day of AZD0530 (treatment) beginning two weeks after surgery (to permit tumor establishment) and half with buffer only (control) for eight weeks. All control animals grew tumor with lymph node metastasis (Figure 6A). H & E staining (insert) of the lymph node validated its human prostate cancer origin. Five of seven treated animals produced primary tumors, but none had metastasis. IHC staining using anti-p-Src and anti-p-FAK antibodies showed reduced phosphorylation levels in the treatment samples (Figure 6B) confirming the effect of AZD0530 in tumors. When probed with anti-AR antibody, the control tumor showed AR nuclear localization as in Figure 2C. AR staining became undetectable in AZD0530 treated tumor since castrated animals were used. As a result, PSA levels from sera of AZD0530 treated mice showed significant reduction (p=0.02) compared to controls (Figure 6C). Primary tumor sizes in the treated animals were smaller, although not statistically significant (p=0.104) when compared to control animals. AZD0530 however completely blocks tumor metastasis possibly through inhibiting SFK and FAK.
Figure 6.

In vivo inhibition study in SCID mice: A, The representative picture showed primary prostate tumor with lymph node metastasis in an animal from the control group. H&E staining of the lymph node sample validates its prostate cancer origin. B, IHC staining of the control and AZD0530 treated tumor samples with anti-p-Src (Y419), anti-p-FAK (Y861) and anti-AR (PG-21) antibodies. C, Means of PSA levels in sera, primary tumor weight and metastasis incidents were plotted between the control and AZD0530 treatment groups.
DISCUSSION
In this study, we report the development of a neuropeptide-autocrine model for androgen-insensitive CaP. This model was not designed to study neuroendocrine tumors of prostate, which are relatively rare, but to study the effect of neuropeptides released from neuroendocrine prostate cells on CaP progression following androgen ablation. There is abundant literature documenting the correlation of increased number of post-mitotic neuroendocrine cells with the development of castration-resistant CaP and reports showing overexpression of neuropeptides and neuropeptide receptors in advanced CaP (16–18). Yet, the biological effect of neuropeptides on CaP has not been clearly demonstrated. We present in vitro and in vivo data that the GRP autocrine loop is sufficient to establish androgen independence in LNCaP cells by inappropriate activation of AR. We also show that GRP activates Src, Lyn, FAK and Etk tyrosine kinases, which confer motility and invasiveness to CaP. Our in vivo inhibitor study demonstrates that administration of Src inhibitor AZD0530 completely blocks tumor metastasis in the androgen-independent environment.
There are numerous reports on growth factors (32), cytokines, chemokines (2, 4) and neuropeptides (3, 25) promoting androgen-independent growth of LNCaP cells. While the ligands inducing AR activation are different, many of them transmit signals through SFK (3, 4, 25). In the present model, we focused on neuropeptides which are coupled to GPCRs and as we showed before, activate the tyrosine kinase complex Src/FAK/Etk (3). We hypothesized that induced expression of GRP in LNCaP cells may facilitate a more aggressive phenotype via autocrine stimulation. Our engineered LNCaP GRP cells demonstrated androgen- and anchorage-independent growth and superior migration compared to control LNCaP-Zeo cells, and the bombesin/GRP specific antibody 2A11 partially inhibited the increased growth and migration. This incomplete inhibition by 2A11 may be due to secretion of other neuropeptides such as neurotensin from the GRP clones (data not shown). These other factors also activate GPCRs, thus there is greater inhibition with GRP receptor inhibition compared to 2A11. Consistent with the in vitro properties, autocrine GRP activity supports androgen-independent tumorigenesis of LNCaP-GRP clones in castrated mice. IHC staining demonstrated nuclear localization of AR and PSA expression in tumor cells, supporting GRP stimulation of AR in the absence of testicular androgens, which is the sole source of androgen in mice. These observations build upon those reported by Burchardt and colleagues who demonstrated that androgen withdrawal of established in vivo LNCaP tumors resulted in enrichment of neuroendocrine cells (5). Herein we demonstrate that Src mediates the nuclear-translocation and target recruitment of AR induced by GRP, based on in vitro (ChIP assay) and in vivo (tumor IHC) analyses. A related report using a neuroendocrine mouse prostate allograft also showed neuroendocrine secretions were sufficient to support androgen-independent growth of LNCaP and PSA expression in vivo (33). These data together firmly establish the potential of neuropeptides secreted by neuroendocrine differentiated cells to induce androgen independence, and this process involves Src activation.
Elevated tyrosine phosphorylations, especially Src activation were shown in hormone-refractory prostate cancer xenografts derived from castrated animals (28). In this study, we showed that Src (and likewise, Lyn) is activated both in the free form as well as in the Src/FAK/Etk complex form. As expected, FAK and Etk are also activated as indicated by their heightened phosphorylation status. Impressively, AZD0530 treatment completely blocked these activations. The exact mechanism how bombesin/GRP activates AR to induce androgen independent growth of LNCaP is not fully understood. Although GRP has been reported to mediate MAPK and Src activation through epidermal growth factor receptor (EGFR) in some human malignancies (34), we observed no increased tyrosine phosphorylation of EGFR in LNCaP cells upon bombesin stimulation (data not shown). Despite reports implicating Src kinase in the development, growth, progression and metastasis of human cancers, only one report correlates elevated Src activation and AR phosphorylation to hormone-refractory CaP (28). This report elegantly showed that tyrosine residue Y534 of AR is the direct target of Src phosphorylation, which effectively translocates AR into the nucleus for gene transcription in the absence of androgen. Another report relates expression of a truncated c-kit tyrosine kinase, which is a strong activator of Src, to advanced stages of CaP (35), suggesting the importance of Src activity in CaP progression. Here we show that reversion of androgen-independent growth of GRP lines by knocking out Src with siRNA supports a significant role for Src in GRP-mediated cell proliferation. It is speculated that modification of AR or its co-activators by phosphorylation (36) or acetylation (37) mimics the conformation change caused by androgen binding to activate AR in the absence of its cognate ligand. Src may potentially phosphorylate AR directly or through an intermediate molecule (28). Yet, since no tyrosine-phosphorylated AR was detected in bombesin-treated LNCaP cells (25), the exact mechanism how Src is involved still remains to be elucidated. In the ChIP assay, GRP mediated AR recruitment preferentially to the proximal ARE site in the PSA promoter, rather than to the enhancer ARE. This observation may reflect conformational modification of AR by Src or a downstream kinase, which facilitates AR activation by assembling a different co-activator complex to elicit gene transactivation in the absence of its natural ligand. Similarly, the reason why addition of R1881 to GRP clone did not increase the reporter activity further may be that GRP-activated AR is already in active conformation and may not be further stimulated by androgen. Our studies also revealed that post-translationally activated AR may be conformationally different from ligand bound AR, a finding supported by previous studies (4, 25). Further structural analysis will be required to substantiate this notion.
LNCaP cells are usually not very migratory, but overexpression of GRP under androgen-free conditions enhances LNCaP-GRP cell migration. Another reported mechanism is that bombesin activates RhoA and Rho-associated coiled-coil forming protein kinase to promote CaP cell migration and invasion (38). Since RhoA can be activated by Etk (39) which is activated by Src (40), our data are consistent with their findings. FAK phosphorylation in bombesin-stimulated PC-3 cells is linked to cell motility and invasion (41). In collaboration with FAK, Etk is also involved in integrin signaling and promotes PC-3M migration (31). Knocking down Etk expression with its specific siRNA inhibits LNCaP cell proliferation (29, 42), and prostates from Etk transgenic mice exhibit pathological changes resembling human prostate intraepithelial neoplasia (29). Complexing of these three kinases results in synergistic activation and may transduce GRP modulated signaling in CaP cell proliferation, migration and survival.
Targeting the bombesin/GRP receptor for cancer therapy is undergoing early clinical trials (43). Other clinical trials have reported promising results using tyrosine kinase inhibitors in cancer therapy; for instances, imatinib (Gleevec, STI571) for chronic myelogenous leukemia and gastrointestinal stromal tumors (44, 45) and trastuzumab (Herceptin, Her-2 antibody) for breast cancer (46). Our approach suggests using a SFK inhibitor to target the activation of non-receptor tyrosine kinases. Through inhibiting Src, AZD0530 prevents the Src-specific activation of FAK, AR and possibly Etk and effectively blocks tumor metastasis in our GRP autocrine model. Complex growth factors available in tumor microenvironments and the compensatory pathways involving cell proliferation downstream to Src may be factors why AZD0530 alone could not halt primary tumor growth. IC50’s for inhibiting FAK, paxillin and P130Cas responsible for migration were 4–64 fold lower than those for cyclin-D1 and c-Myc for proliferation (27). AZD0530 has been tested in tamoxifen-resistant breast cancer cells to suppress tumor cell migration through modulating FAK (47). Treating A549 lung carcinoma cells with AZD0530 results in down regulation of Id1 gene expression possibly through BMP-Smad-Id pathway involved angiogenesis and metastasis (48). The other small molecule Src inhibitor Dasatinib (49), displays similar inhibitory mechanism as AZD0530 with more inhibition on metastasis than tumor growth in vivo (50). Lyn, a member of SFK, found to play a role in PC-3 tumor progression, was also inhibited by AZD0530 (data not shown).
In addition to neuropeptides, we have previously shown Src kinase activation as central to IL-8-induced androgen-independent prostate cell growth (4). Importantly, IL-8 is also a ligand for GPCRs. As such, inhibition of signaling transduction through Src kinase as a downstream target may block the oncogenic stimulation for more than one ligand. The specific mechanisms activating AR remain to be elucidated, but the pathways identified suggest Src kinase inhibition may prove useful in the treatment of androgen-independent CaP.
Supplementary Material
Acknowledgments
We thank Dr. Cuttitta (NIH) for providing the monoclonal antibody to bombesin, 2A11, and Dr. Schally (Tulane University) for GRP receptor antagonists RC3095 and RC3940-II. The work is supported by NIH grant KO8-DK60748-01 (CPE), RO1-DK52659 (HJK), DOD PC060508 (HJK) and DOD grant PC040161 (CPE).
Footnotes
Supported by NIH Grants KO8 DK60748-01, 2RO1 DK/AG52659- 04, and Department of Defense PC10520. Mention of trade name, proprietary product or specific equipment does not constitute a guaranty of warranty by the Department of Defense, nor does it imply approval to the exclusion of other products. The views expressed herein represent those of the authors and do not necessarily represent the position of Department of Defense.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. Journal of cellular biochemistry. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 3.Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21:8385–97. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee LF, Louie MC, Desai SJ, et al. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- 5.Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–5. [PubMed] [Google Scholar]
- 6.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Research. 1999;59:3821–30. [PubMed] [Google Scholar]
- 7.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47:147–55. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Cuttitta F, Carney DN, Mulshine J, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–6. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 9.Nagata A, Ito M, Iwata N, et al. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci U S A. 1996;93:11825–30. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–19. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 11.Rozengurt E. Neuropeptides as growth factors for normal and cancerous cells. Trends Endocrinol Metab. 2002;13:128–34. doi: 10.1016/s1043-2760(01)00544-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Hu W, Kelly DR, Hellmich MR, Evers BM, Chung DH. Gastrin-releasing peptide is a growth factor for human neuroblastomas. Ann Surg. 2002;235:621–9. 9–30. doi: 10.1097/00000658-200205000-00003. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2003;63:2379–87. [PubMed] [Google Scholar]
- 14.Aprikian AG, Han K, Guy L, Landry F, Begin LR, Chevalier S. Neuroendocrine differentiation and the bombesin/gastrin-releasing peptide family of neuropeptides in the progression of human prostate cancer. Prostate Supplement. 1998;8:52–61. [PubMed] [Google Scholar]
- 15.Festuccia C, Guerra F, S DA, Giunciuglio D, Albini A, Bologna M. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. International Journal of Cancer. 1998;75:418–31. doi: 10.1002/(sici)1097-0215(19980130)75:3<418::aid-ijc16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu JTM, Astill E, Liu GH, Stephenson RA. Serum chromogranin A: early detection of hormonal resistance in prostate cancer patients. J Clin Lab Anal. 1998;12:20–5. doi: 10.1002/(SICI)1098-2825(1998)12:1<20::AID-JCLA4>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartholdi MF, Wu JM, Pu H, Troncoso P, Eden PA, Feldman RI. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int J Cancer. 1998;79:82–90. doi: 10.1002/(sici)1097-0215(19980220)79:1<82::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–9. [PubMed] [Google Scholar]
- 19.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–83. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 20.Aprikian AG, Han K, Chevalier S, Bazinet M, Viallet J. Bombesin specifically induces intracellular calcium mobilization via gastrin-releasing peptide receptors in human prostate cancer cells. Journal of Molecular Endocrinology. 1996;16:297–306. doi: 10.1677/jme.0.0160297. [DOI] [PubMed] [Google Scholar]
- 21.Han K, Viallet J, Chevalier S, Zheng W, Bazinet M, Aprikian AG. Characterization of intracellular calcium mobilization by bombesin-related neuropeptides in PC-3 human prostate cancer cells. Prostate. 1997;31:53–60. doi: 10.1002/(sici)1097-0045(19970401)31:1<53::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Siegfried JM, Guentert PJ, Gaither AL. Effects of bombesin and gastrin-releasing peptide on human bronchial epithelial cells from a series of donors: individual variation and modulation by bombesin analogs. Anat Rec. 1993;236:241–7. doi: 10.1002/ar.1092360129. [DOI] [PubMed] [Google Scholar]
- 23.Cai RZ, Reile H, Armatis P, Schally AV. Potent bombesin antagonists with C-terminal Leu-psi(CH2-N)-Tac-NH2 or its derivatives. Proc Natl Acad Sci U S A. 1994;91:12664–8. doi: 10.1073/pnas.91.26.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sausville EA, Lebacq-Verheyden AM, Spindel ER, Cuttitta F, Gazdar AF, Battey JF. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986;261:2451–7. [PubMed] [Google Scholar]
- 25.Desai SJ, Ma AH, Tepper CG, Chen HW, Kung HJ. Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 2006;66:10449–59. doi: 10.1158/0008-5472.CAN-06-2582. [DOI] [PubMed] [Google Scholar]
- 26.Louie MC, Yang HQ, Ma AH, et al. Androgen-induced recruitment of RNA polymerase II to a nuclear receptor-p160 coactivator complex. Proc Natl Acad Sci U S A. 2003;100:2226–30. doi: 10.1073/pnas.0437824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008 doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Z, Dai B, Jiang T, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Dai B, Kim O, Xie Y, et al. Tyrosine kinase Etk/BMX is up-regulated in human prostate cancer and its overexpression induces prostate intraepithelial neoplasia in mouse. Cancer Res. 2006;66:8058–64. doi: 10.1158/0008-5472.CAN-06-1364. [DOI] [PubMed] [Google Scholar]
- 30.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Kim O, Li M, et al. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol. 2001;3:439–44. doi: 10.1038/35074500. [DOI] [PubMed] [Google Scholar]
- 32.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 33.Jin RJ, Wang Y, Masumori N, et al. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64:5489–95. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SM, Grandis JR, Wentzel AL, Gooding WE, Lui VW, Siegfried JM. Gastrin-releasing peptide receptor mediates activation of the epidermal growth factor receptor in lung cancer cells. Neoplasia. 2005;7:426–31. doi: 10.1593/neo.04454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paronetto MP, Farini D, Sammarco I, et al. Expression of a truncated form of the c-Kit tyrosine kinase receptor and activation of Src kinase in human prostatic cancer. Am J Pathol. 2004;164:1243–51. doi: 10.1016/S0002-9440(10)63212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gioeli D, Ficarro SB, Kwiek JJ, et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–14. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 37.Gong J, Zhu J, Goodman OB, Jr, et al. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene. 2006;25:2011–21. doi: 10.1038/sj.onc.1209231. [DOI] [PubMed] [Google Scholar]
- 38.Zheng R, Iwase A, Shen R, et al. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25:5942–52. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- 39.Mao J, Xie W, Yuan H, Simon MI, Mano H, Wu D. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Galpha12/13. The EMBO journal. 1998;17:5638–46. doi: 10.1093/emboj/17.19.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai YT, Su YH, Fang SS, et al. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–54. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aprikian AG, Tremblay L, Han K, Chevalier S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int J Cancer. 1997;72:498–504. doi: 10.1002/(sici)1097-0215(19970729)72:3<498::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Borgesi RA, McKnight NC, Kaur R, Carpenter CL, Balk SP. Activation of Nonreceptor Tyrosine Kinase Bmx/Etk Mediated by Phosphoinositide 3-Kinase, Epidermal Growth Factor Receptor, and ErbB3 in Prostate Cancer Cells. J Biol Chem. 2007;282:32689–98. doi: 10.1074/jbc.M703412200. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Chen J, Mokotoff M, Ball ED. Targeting gastrin-releasing peptide receptors for cancer treatment. Anticancer Drugs. 2004;15:921–7. doi: 10.1097/00001813-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 45.Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006–11. [PubMed] [Google Scholar]
- 46.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 47.Hiscox S, Jordan NJ, Morgan L, Green TP, Nicholson RI. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clinical & experimental metastasis. 2007;24:157–67. doi: 10.1007/s10585-007-9065-y. [DOI] [PubMed] [Google Scholar]
- 48.Gautschi O, Tepper CG, Purnell PR, et al. Regulation of Id1 expression by SRC: implications for targeting of the bone morphogenetic protein pathway in cancer. Cancer Res. 2008;68:2250–8. doi: 10.1158/0008-5472.CAN-07-6403. [DOI] [PubMed] [Google Scholar]
- 49.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 50.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.