Summary
We demonstrate here that LXR–dependent sterol homeostasis is a physiologically-regulated determinant of cell proliferation and acquired immune responses. T cell activation triggers simultaneous suppression of the LXR pathway for cholesterol transport and induction of the SREBP pathway for cholesterol synthesis. This coordinated program is engaged in part through induction of the sterol-metabolizing enzyme SULT2B1, expression of which in T cells blocks LXR signaling. Forced induction of LXR target genes during T cell activation markedly inhibits mitogen-driven expansion, whereas loss of LXRβ confers a proliferative advantage. Inactivation of the sterol transporter ABCG1 in T cells uncouples LXR signaling from proliferation, directly linking sterol homeostasis to the anti-proliferative action of LXR. Mice lacking LXRβ exhibit lymphoid hyperplasia and enhanced responses to antigenic challenge, indicating that proper regulation of LXR-dependent sterol metabolism is important for immune responses. These data implicate LXR signaling in a metabolic checkpoint that modulates cell proliferation and immunity.
Keywords: nuclear receptor, T cell, proliferation, cholesterol
Introduction
The capacity of lymphocytes to undergo rapid expansion in response to antigenic challenge is essential for adaptive immunity. Mounting evidence suggests that metabolism plays a key role in determining lymphocyte responses. For example, activated T lymphocytes are functionally glycolytic despite sufficient oxygen tension (Fox et al., 2005). As a consequence of this metabolic program, T cell activation requires high glucose availability (Roos and Loos, 1973). In the absence of sufficient glucose, T cells fail to proliferate and undergo apoptosis (Alves et al., 2006; Rathmell et al., 2000; Vander Heiden et al., 2001). The influence of cellular lipid metabolism on immune responses, however, is poorly understood.
Cholesterol is an important component of cell membranes and has long been recognized to be necessary for cell growth and proliferation (Brown and Goldstein, 1974; Chen et al., 1975; Chen et al., 1974). Early studies defined a relationship between the pathway for de novo sterol synthesis and movement through the cell cycle in lymphocytes. Blocking mevalonate production with certain cholesterol derivatives or HMG-CoA reductase inhibitors (statins) inhibits DNA synthesis and mitosis (Chakrabarti and Engleman, 1991; Chen et al., 1975). A complicating factor in these studies, however, is the requirement for the mevalonate pathway in production of non-sterol mevalonate derivatives such as geranylgeraniol and farnesol. Indeed, much of the immunomodulatory effects of statins have been attributed to the biology of these products (Kwak et al., 2000; Weitz-Schmidt et al., 2001). The question of whether intracellular cholesterol distribution or availability represents a dynamic signal for control of immune responses has not been adequately explored, and the molecular mechanisms that link lipid metabolism to cellular growth and the cell cycle machinery remain unknown.
The Liver X Receptors (LXRα and LXRβ) are members of the nuclear receptor superfamily that regulate cholesterol homeostasis. The endogenous activators of these receptors are oxysterols and intermediates in the cholesterol biosynthetic pathway (Janowski et al., 1996). The two LXR isotypes share considerable sequence homology and respond to the same ligands, but their tissue distribution differs markedly. LXRα is highly expressed in liver, adipose tissue and macrophages, and LXRβ is expressed ubiquitously (Repa and Mangelsdorf, 2000). Many LXR target genes are involved in cholesterol and fatty acid metabolism, such as ABCA1, ABCG1, SREBP-1c and fatty acid synthase (Tontonoz and Mangelsdorf, 2003). Other targets, such as AIM/SPα, are involved in the regulation of apoptosis and innate immune responses (Joseph et al., 2004; Valledor et al., 2004).
LXRs also have the capacity to negatively regulate inflammatory gene expression via transrepression (Joseph et al., 2003). The LXR pathway has been shown to impact inflammatory responses in models of atherosclerosis, contact dermatitis, sepsis and multiple sclerosis (Hindinger et al., 2006; Joseph et al., 2003; Joseph et al., 2002; Tangirala et al., 2002). Although the mechanisms underlying signal-specific transrepression remain incompletely understood, it is clear that activation of LXR inhibits inflammatory responses to LPS or cytokines in part via blockade of NF-κB signaling (Joseph et al., 2003; Ogawa et al., 2005). Recent studies have suggested that LXR-dependent repression of inflammatory target genes occurs via an N-CoR- and sumoylation-dependent process (Ghisletti et al., 2007). Interestingly, activation of TLR3 or TLR4 inhibits LXR cholesterol function via the transcription factor IRF-3, suggesting that LXR functions to regulate the crosstalk between inflammatory and metabolic pathways (Castrillo et al., 2003). Despite recent progress in understanding links between LXR and innate immunity, little is known regarding the impact of LXR signaling on T and B cell biology and acquired immunity.
We demonstrate here that transcriptional regulation of intracellular cholesterol homeostasis impacts cell proliferation acquired immune responses. We show that T cell activation is accompanied by the downregulation of LXR target genes involved in cholesterol transport and the simultaneous induction of the SREBP-2 pathway for cholesterol synthesis. Ligand activation of LXR inhibits mitogen-driven T cell expansion by altering cellular sterol content through a pathway requiring the transporter ABCG1. Conversely, loss of LXR expression confers a proliferative advantage to lymphocytes, resulting in enhanced homeostatic and antigen-driven responses. LXR-dependent coupling of cholesterol metabolism and proliferation represents a previously unrecognized mechanism for the regulation of immune responses.
Results
Age-dependent lymphoid hyperplasia in mice lacking LXRβ expression
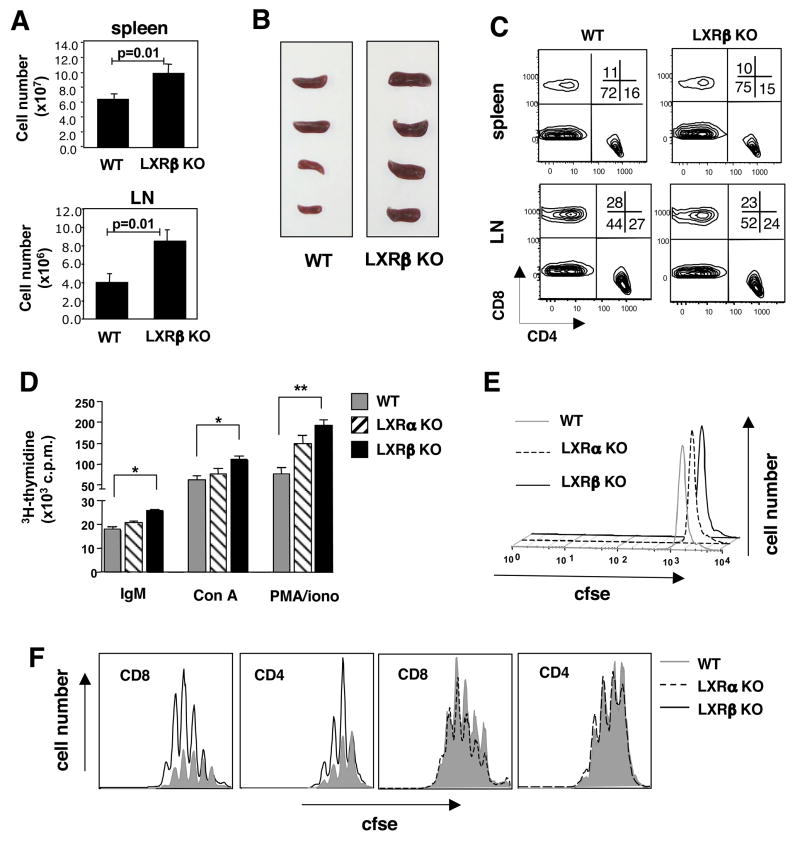
To investigate the role of LXRs in lymphoid cells, we examined the immune system of young Lxrα and Lxrβ null mice (Peet et al., 1998) on a C57BL/6 background (>10 generations backcrossed). Flow cytometric analysis of spleen, lymph node and thymus from 6–8week old Lxrα and Lxrβ null mice revealed no significant differences in the frequency of CD4+ and CD8+ T and CD19+B220+ B lymphocyte populations when compared to LXR-sufficient controls (Fig. S1A and data not shown). Thus, global loss of either LXR isotype does not grossly alter the development of T or B lymphocytes. However, analysis of hematopoetic tissues from older Lxrβ null mice (5–6 months) revealed moderate splenomegaly and an expansion in the total number of lymphocytes in spleen (p=0.01) and LN (p=0.01) (Fig. 1A,1B). No significant difference in the frequency of T and B cells was noted in the spleen, whereas LNs from Lxrβ null mice consistently showed a modest expansion in the B cell compartment (Fig. 1C).
Figure 1. Splenomegaly and lymphocyte expansion in mice lacking LXRβ.
(A) Total cellularity of spleen and LN from 6 month-old WT and LXRβ KO mice. (B) Gross morphology of spleens. (C) Frequency of CD4 and CD8 T cells from spleen and LN. (D) Mitogen-driven proliferation of spleen cells from 6–8 week old WT, LXRα KO or LXRβ KO mice. Cells were stimulated with anti-IgM(Fab′)2 (10μg/mL), Con A (10μg/mL), or PMA (0.5μM) and ionomycin (100mM). 3H-thymidine was added to cultures after 72 h for the final 16 h. *p<0.01; **p<0.001. (E,F) TCR- driven proliferation of spleen cells from WT, LXRα KO or LXRβ KO mice. Purified CFSE labeled T cells were stimulated with pbCD3 (10μg/mL). Cells were harvested at 24 h (E) and 72 h (F), stained with anti-CD4, CD8, and 7-AAD. 5×104 counting beads were added to samples to serve as an internal control.
Since Foxp3+ Regulatory T cells (Tregs) play an important role in maintaining lymphocyte homeostasis, we considered the possibility that the lymphoid hyperplasia might reflect a loss of Tregs. However, FACS analysis of Lxrβ null spleen and LN cells revealed no difference in the frequency of CD4+Foxp3+ T cells (Fig. S1B), nor were differences noted in the activation markers CD69, 25 and 44 (Fig. S1C; data not shown). Similarly, no differences were observed in the frequency of dendritic cells (DCs) from spleens of Lxrβ null mice or in MHC class II, CD86 and CD40 expression levels, suggesting that APCs are not activated and directly mediating the lymphoid hyperplasia (Fig. S2).
Next, we examined the expression pattern of Lxrα and Lxrβ in murine immune cells. Lxrα was expressed at high levels in peritoneal-derived macrophages and bone marrow-derived macrophages, whereas little or no mRNA was detected in resting purified B and T cells (Fig. S3A). Lxrβ was expressed in macrophages, T and B cells. We considered the possibility that activation of lymphocytes might alter Lxrα expression, but we failed to detect expression in splenic T cells from C57BL/6 mice activated with plate bound anti-CD3ε (pbCD3) mAb in the presence or absence of 2 μM GW3965 (synthetic LXR agonist; Fig. S3B). Human T cells were also found to express LXRβ but not LXRα protein (Fig. S3C). As expected, expression of LXRα in THP-1 macrophages was induced by GW3965 as a result of autoregulation (Laffitte et al., 2001) (Fig. S3C). Activation of LXR induced target gene expression in human T cells, but did not lead to the upregulation of LXRα mRNA (Fig. S3D; data not shown).
LXRβ is an intrinsic regulator of lymphocyte proliferation
We hypothesized that LXR may regulate proliferation and/or acquisition of effector functions in lymphocytes. To test this possibility, we examined 3H-thymidine incorporation into whole spleen cells from WT, Lxrα null, or Lxrβ null mice stimulated with a panel of mitogenic stimuli. Interestingly, anti-IgM-(Fab′)2-, concavalin A- and pma/ionomycin-stimulated spleen cells from Lxrβ null mice incorporated significantly more thymidine than WT controls (Fig. 1D; data not shown). To determine if this effect was due to an intrinsic difference cell proliferation, purified CFSE-labeled T cells were stimulated with pbCD3 and analyzed by flow cytometry for proliferative response and viability. No difference was observed in cell viability or the dilution of CFSE-labeled T cells at 24 h, indicating that Lxrβ null T cells do not have an inherent early survival advantage (Fig. 1E). However, analysis at 72 h and 96 h revealed that the absolute number of T cells which had divided in response to TCR stimulus was markedly greater in Lxrβ null T cells than Lxrα null and WT cells (Fig. 1F). Thus LXRβ expression intrinsically regulates lymphocyte expansion in response to either TCR, BCR or pharmacologic activation.
Ligand activation of LXR inhibits lymphocyte expansion
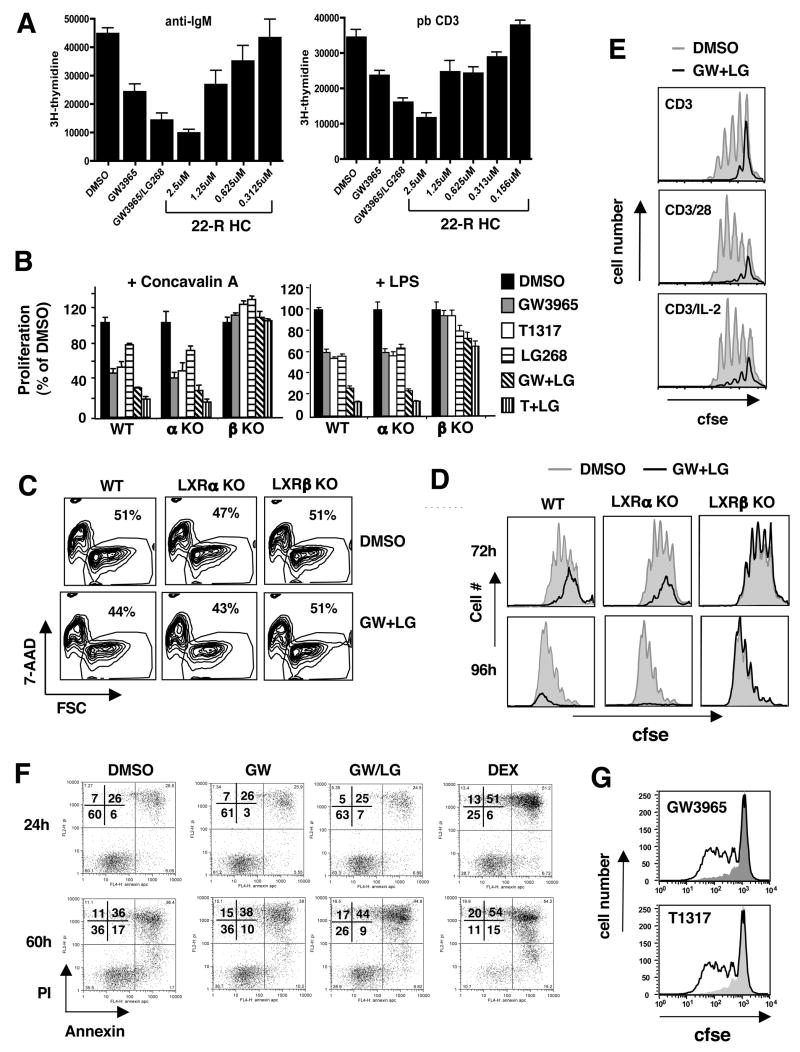
In complimentary studies, we asked whether LXR activation altered the proliferative capacity of lymphocytes in vitro. Spleen cultures from C57BL/6 mice were stimulated with anti-IgM-(Fab′)2 or pbCD3 in the presence or absence of GW3965, LG68 (RXR agonist) or a physiologic LXR ligand, 22(R)-hydroxycholesterol. After 48–96 h, cultures were pulsed with 3H-thymidine overnight to determine proliferation. Activation of LXR by LXR and/or RXR agonists markedly reduced the proliferative capacity of cultured cells (Fig. 2A). Similarly, proliferation of B and T cells was inhibited by 22(R)-hydroxycholesterol in a dose-dependent manner.
Figure 2. LXR activation inhibits lymphocyte proliferation.
(A) Decreased mitogen-driven proliferation of WT spleen cells stimulated with anti-IgM(Fab′)2 (10μg/mL) or pbCD3 (10μg/mL) and LXR ligands GW3965 (2μM), 22(R)-hydroxycholesterol (0.156 μM–2.5μM) or RXR ligand LG268 (100 nM) as indicated. 3H-thymidine was added to cultures after 72 h for the final 16 h. (B) WT, LXRα KO or LXRβ KO splenocytes were stimulated with ConA (10μg/mL) or LPS (100μg/mL) and treated with LXR ligands GW3965 (2μM), T1317 (1μM) and RXR ligand LG268 (100nM) as indicated. 3H-thymidine was added to cultures after 72 h for the final 16 h. (C,D) CFSE dilution and viability of purified WT, LXRα KO or LXRβ KO T cells stimulated with pbCD3 in the presence of GW3965 and LG268 was determined at 24–96 h. (E) CFSE dilution of purified WT T cells at 72 h stimulated with pbCD3, soluble CD28, rIL-2, LXR/RXR agonist as indicated. (F) Annexin and PI staining of pbCD3 stimulated WT T cells cultured with GW3965 and LG268. (G) CFSE dilution of human T cells stimulated with anti-CD3 crosslinked with pb goat anti-mouse and cultured with GW3965. 5×104 counting beads were added to samples (panels C,D,E,G) to serve as an internal counting control and analyzed via flow cytometry.
To determine if the inhibitory effect of LXR agonists on lymphocyte proliferation was receptor-dependent, cultures were stimulated with a panel of T and B cell mitogens in the presence or absence of receptor agonists. Activation of LXR alone or in combination with RXR markedly reduced 3H-thymidine incorporation in WT spleen cell cultures stimulated with Con A, anti-IgM, or LPS (Figs. 2B and S4). A similar reduction in 3H-thymidine incorporation was seen in spleen cells from Lxrα null mice. By contrast, LXR activation had little or no effect on 3H-thymidine incorporation into mitogen-stimulated Lxrβ null splenocytes (Fig. 2B and S4). A modest decrease in 3H-thymidine incorporation was observed in LG268-treated splenocytes stimulated with LPS and anti-IgM, indicative of an RXR-dependent, LXR-independent effect in B cells (Fig. 2B and S4). However, this decrease was not observed in Con A-treated spleen cells, suggesting that RXR activation alone does not perturb T cell expansion in this system (Fig. 2B).
We considered the possibility that activation of LXR in whole spleen cultures might indirectly impact lymphocyte expansion through actions on other cell types. To address this issue, purified T cells were CFSE labeled and stimulated with pbCD3 in the presence of either receptor agonists or vehicle. Importantly, there was no significant difference in the viability of T cells cultured with LXR/RXR ligands at 24 h, indicating that they are not inherently toxic to lymphocytes at these concentrations (Fig. 2C). FACS analysis of cultures at 48–96 h revealed that LXR activation inhibited proliferation of WT and Lxrα null T cells, but not Lxrβ null cells (Fig. 2D). Thus, LXR activation has an intrinsic effect on lymphocyte proliferation that is mediated by LXRβ. We have also observed LXR-dependent inhibition of proliferation in developing bone marrow derived monocytes cultured with M-CSF in vitro (data not shown). Interestingly, we failed to observe an effect of LXR ligands on the proliferation of a number of transformed T and B cell lines (including Jurkat and Ramos; data not shown). This observation suggests that many transformed cells have lost their sensitivity to the LXR anti-proliferative effect.
Efficient T cell activation requires two signals. The first is mediated via the TCR, while the second can be transmitted from a number of cell surface receptors (Bromley et al., 2001). To determine if costimulation could restore proliferation in the presence of ligand, we activated purified WT T cells with pbCD3 and added either soluble anti-CD28 or rIL-2. As expected, LXR activation markedly reduced proliferation in response to pbCD3 (Fig. 2E); however, the addition of anti-CD28 or IL-2 to cultures did not rescue.
Next we investigated whether the effects of LXR on lymphocyte expansion were also shared with other members of the nuclear receptor superfamily. In addition to LXR, both PPARγ and GR are known to inhibit inflammatory responses in immune cells (Ogawa et al., 2005). Treatment of T cells with dexamethasone caused cell death in 75–90% of cells after 24 h, whereas activation of LXR, RXR or PPARγ had little effect (Fig. 2F and S5C). Interestingly, in contrast to LXR activation, PPARγ signaling conferred a modest survival advantage to proliferating T cells in vitro at later time points (data not shown). Thus, LXRβ activation regulates lymphocyte expansion in a manner distinct from glucocorticoid receptor and PPARγ.
To determine if the transcriptional program initiated by LXR also regulates human lymphocytes, purified T cells were collected from peripheral blood of normal donors. Purified T cells were treated with vehicle or receptor ligands and stimulated with pbCD3 +/− soluble anti-CD28. In contrast to murine lymphocytes, LXR/RXR activation did not induce apoptosis in cultures analyzed up to 72 h (Fig. S5D). Similar to murine lymphocytes, LXR activation also decreased the proliferative capacity of human CD8+ or CD4+ T cells stimulated with pbCD3 (Fig. 2G and S5A). In contrast to murine T cells, the addition of anti-CD28 was able to overcome the LXR-mediated inhibition of proliferation (Fig. S5B), suggesting subtle differences in the biology of LXR in mouse and human lymphocytes.
LXR activation blocks cell cycle progression in lymphocytes
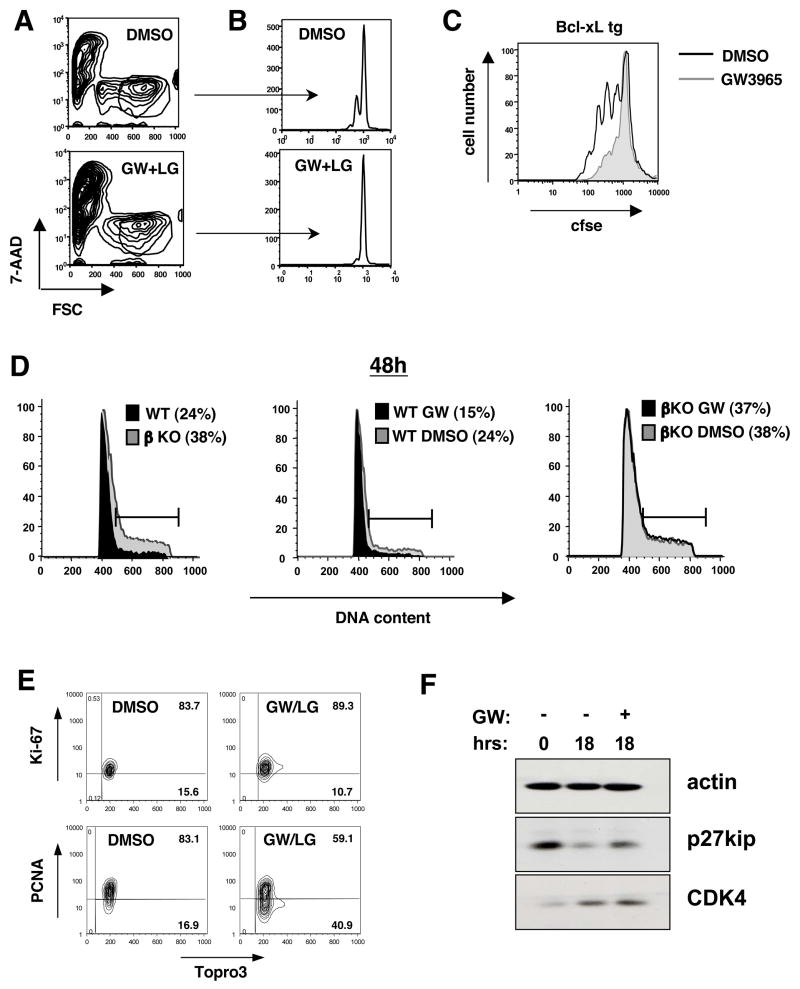
One possible explanation for the anti-proliferative effects of LXR is that the receptor is engaging an apoptotic pathway that decreases the total number of lymphocytes proliferating. Alternatively, it could be that LXR-dependent transcription is regulating cell cycle progression. Analysis of forward scatter (FSC) and side scatter (SSC) characteristics of cultured T cells demonstrated that neither the activation nor ablation of LXR altered cellular enlargement in response to pbCD3 (Fig 3A; data not shown). However, examination of T cell blasts at 36–48 h clearly established that LXR-activated cells did not divide efficiently (Fig. 3B). Transgenic overexpression of the anti-apoptotic factor Bcl-xL or addition of the caspase inhibitor ZVAD-fmk provided a modest survival advantage over their wild type counterparts, but did not restore proliferation in the presence of LXR ligand (Fig. 3C; data not shown), consistent with a primary effect of LXR on proliferation rather than cell viability.
Figure 3. LXRβ signaling regulates cell cycle progression.
(A,B,C) CFSE dilution of WT and Bcl-xL tg T cells stimulated with pbCD3 (10μg/mL) for 36–96 h in the presence of GW3965 (2μM) and LG268 (100nM). Cells were stained with 7-AAD and analyzed by flow cytometry. (D) Cell cycle analysis of WT and LXRβ KO T cells stimulated with pbCD3 and GW3965 and LG268 as indicated. Cells were stained for DNA content with propidium iodide at 48h and analyzed by flow cytometry. (E,F) Cell cycle proteins of WT T cells stimulated with pbCD3 and GW3965 and LG268 as indicated. (E) Cells were permeabilized and stained for intracellular proliferation antigens Ki-67, PCNA and topro-3 for DNA content at 36h. (F) Whole cell lysates were collected and analyzed by Western blot for p27kip and CDK4 expression at 18h.
Next we analyzed the DNA content of activated T cells in the presence or absence of LXR ligand. Interestingly, Lxrβ null T cells displayed an increased fraction of cells in S and G2/M phase and a decreased sub2N fraction at 36–48 h compared to WT cells (Fig. 3D and S6). WT but not Lxrβ null cells treated with LXR agonist showed a markedly reduced fraction of cells moving through the cell cycle and an increase in the apoptotic sub2N fraction (Fig. 3D and Fig. S6). Analysis of DNA content in pbCD3-stimulated human T cell cultures also indicated a significant reduction in cells entering S phase at 24 h with no difference in the sub2N fraction (data not shown). Thus, activation of LXRβ limits the capacity of T cells to move through the cell cycle, resulting in decreased proliferation.
We also analyzed the expression of cell cycle proteins. qPCR analysis of GW3965-treated lymphocytes showed normal upregulation of cylin D2, D3 and c-Myc suggesting movement into G1 (data not shown). Similarly, we observed normal upregulation of proliferation-associated antigen Ki-67 and CDK 4 (Fig. 3E and 3F). However, ligand-treated cells maintained increased levels of the cell cycle inhibitor p27kip, indicating a decreased ability to move through cell cycle. Accordingly, viable ligand-treated cells showed decreased PCNA expression at 48 h (Fig. 3E). We also observed decreased mRNA expression of cyclin E1 and E2 (data not shown), further supporting our assertion that enforced LXR activation decreases cell cycle progression in activated lymphocytes.
LXR signaling does not inhibit lymphocyte activation
The NF-κB signaling pathway plays an important role in lymphocyte activation (Schulze-Luehrmann and Ghosh, 2006). Since LXR is known to block NF-κB-dependent gene expression, we considered the possibility that the anti-inflammatory action of LXR might underlie its effects on lymphocyte expansion. However, LXR activation did not significantly impact the upregulation of the activation markers CD69 and CD25 at 24 h (Fig. S7A; data not shown). LXR/RXR activation also did not perturb IL-2 production (Fig. S7B). Furthermore, restimulation of resting T cells with IL-2 led to comparable induction of the IL-2 responsive genes lymphotoxin A and granzyme B (Fig. S7C) (Beadling and Smith, 2002; Kovanen et al., 2005). Coupled with the observations that Lxrβ null T cells do not express higher levels of CD25, CD44 or CD69 ex vivo (Fig. S1D and data not shown) and do not upregulate these markers to a greater extent in response to TCR crosslinking (Fig. S7D), these data strongly suggest that the regulation of proliferation by LXR is not achieved through alterations of TCR or IL-2 signaling or inhibition of proximal lymphocyte activation.
We also addressed when LXR activation must occur in relation to TCR signals in order to block proliferation. To that end, CFSE-labeled T cells were activated with pbCD3 for 24 h before LXR/RXR ligands were added to cultures. Interestingly, activation of LXR after 24 h of TCR signaling perturbed expansion only in later rounds of division (Fig. S8). If LXR ligands were washed out 2 h after stimulation, proliferation was restored (Fig. S8), indicating that sustained LXR signaling is required to inhibit proliferation. These observations suggested that the effects of LXR were likely to be mediated by activation or repression of downstream target genes (see below).
Reciprocal regulation of the SREBP-2 and LXR transcriptional programs during lymphocyte activation
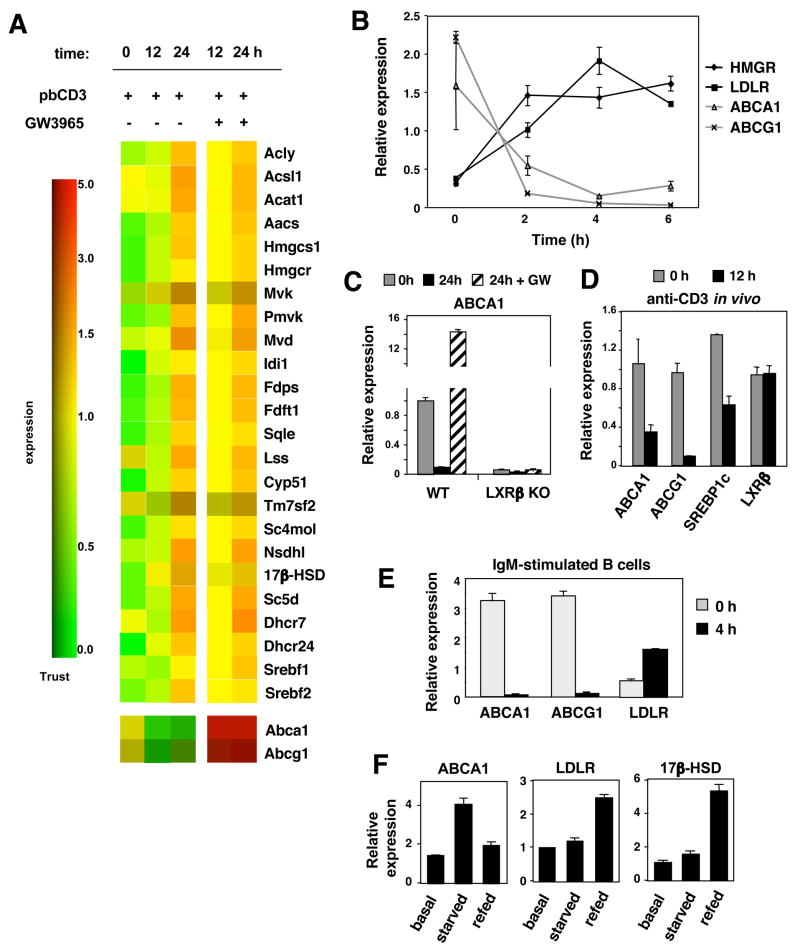
To determine the mechanism by which LXR modulates proliferation, we profiled gene expression in splenic murine T lymphocytes before or after stimulation with pbCD3 in the presence or absence of LXR agonists. In particular, we examined genes involved in de novo cholesterol biosynthesis, reverse cholesterol transport and cholesterol uptake. Consistent with previous reports showing that cholesterol synthesis is stimulated in activated T cells (Chen et al., 1975), we found that the entire pathway of SREBP-2 target genes was induced following activation (Fig. 4A). Pathways for fatty acid and phospholipid synthesis were also induced, consistent with activation of SREBP-1 (data not shown). Unexpectedly, TCR activation also resulted in the concomitant and profound downregulation of LXR target genes, including two key genes involved in cholesterol transport, Abca1 and Abcg1 (Fig. 4A). By contrast, culturing activated lymphocytes with LXR led to a robust induction of these genes. The transcriptional effects of LXR were notably more limited in T cells as compared to other cell types such as macrophages. A relatively small number of genes were altered by LXR activation and the vast majority were linked to cholesterol metabolism. In fact, many LXR target genes expressed in other cell types (e.g. apoE, PLTP, apoD, ABCG5, ABCG8) are absent in T cells (data not shown). We also noted that culturing activated lymphocytes with LXR agonist modestly promoted SREBP-2 target gene expression (Fig. 4A). This effect may be secondary to increased SREBP-1 expression driven by LXR, or may reflect alterations in cholesterol content in the ER (see below).
Figure 4. Reciprocal regulation of the LXR and SREBP-2 transcriptional programs during lymphocyte activation.
(A) DNA microarray analysis of SREBP-2 and LXR target genes of purified WT T cells stimulated with pbCD3 and GW3965 (2μM). (B) Real-time PCR analysis of mRNA from purified WT T cells stimulated with pbCD3 (10μg/mL) for the indicated time. (C) Real-time PCR analysis of mRNA from purified WT and LXRβ KO T cells stimulated for 24 h with pbCD3 and GW3965 as indicated. (D) Real-time PCR analysis of LXR and LXR target genes from mRNA of purified WT T cells of mice treated with 20 μg anti-CD3e i.p. for 12 h. (E) Real-time PCR analysis of mRNA from purified WT B cells stimulated for 6h with anti-IgM and GW3965 as indicated. (F) Real-time PCR analysis of mRNA from proliferating (basal), serum starved (starved), and refed WT mouse embryonic fibroblasts.
Analysis of LXR and SREBP1 target gene expression by realtime PCR confirmed that T cell activation is accompanied by the rapid and reciprocal regulation of these transcriptional pathways. For example, Abca1 and Abcg1 expression begins to decline whereas Ldlr and Hmcgr expression begins to increase as early as 2 hours post-activation (Fig. 4B). The regulation of Abca1 and Abcg1 during T cell activation is LXR-dependent, since deletion of LXRβ in T cells abolished both basal and ligand-inducible target gene expression (Fig. 4C). To determine if LXR target genes were also downregulated during lymphocyte activation in vivo, C57Bl/6 mice were injected i.p. with anti-CD3ε antibody. Spleens were harvested 12 h after injection and mRNA was collected from purified T cells. In line with our in vitro observations, the LXR target genes Abca1, Abcg1 and Srebp-1c were markedly suppressed upon activation, while Lxrβ expression was maintained (Fig. 4D).
Reciprocal regulation of the LXR and SREBP pathways by proliferative stimuli was also observed in primary murine B cells activated via the BCR and with PMA/ionomycin (Fig. 4E; data not shown). A similar reciprocal relationship between LXR and SREBP target genes was observed in human lymphocytes activated with PMA/ionomycin (Fig. S9). We also examined C57BL/6 mouse embryonic fibroblasts (MEFs) under basal conditions, serum starvation (G1 arrest) and refeeding (re-entry in to cell cycle). Real-time PCR confirmed that proliferative stimuli trigger suppression of LXR targets and induction of SREBP-2 targets in MEFs. Interestingly, serum deprivation for 24 h upregulated LXR target gene expression in MEFs (Fig. 4F). Release from cell cycle arrest via refeeding for 6 h with serum upregulated the SREBP-2 target genes Ldlr and 17β-Hsd and downregulated expression of LXR target genes. These data mirror the gene expression patterns observed in quiescent and activated lymphocytes, suggesting that the reciprocal regulation of LXR and SREBP-2 activity is a general feature of cells moving through early stages of the cell cycle.
Proliferative stimuli induce oxysterol sulfotransferase (SULT2B1) expression and reduce LXR ligand availability
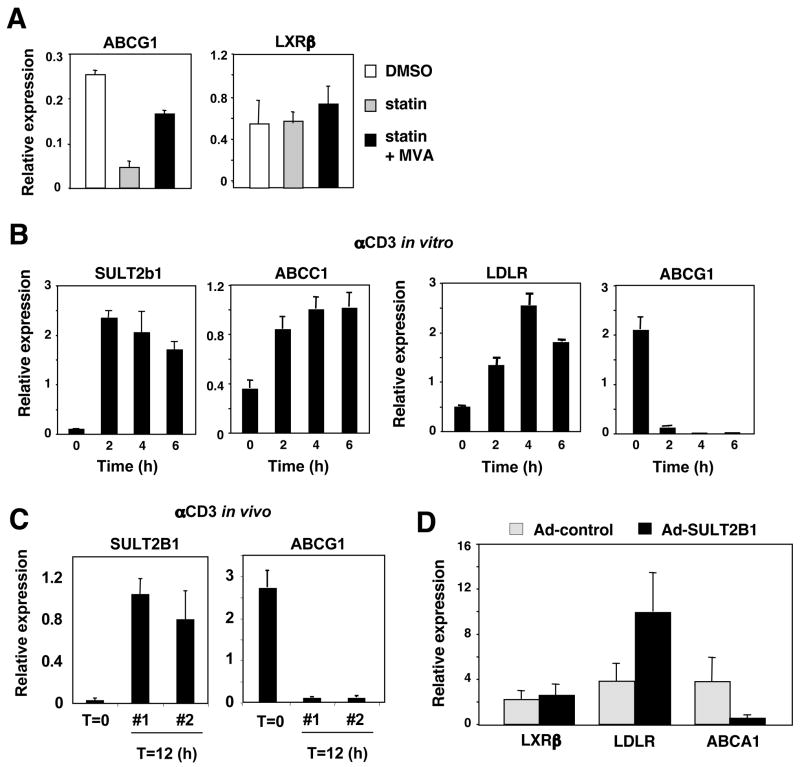
The loss of LXR target gene expression despite preserved expression of the receptor strongly suggests that the abundance of endogenous LXR ligand is reduced during T cell activation. Consistent with this hypothesis, culturing primary T cells in the presence of the HMG-CoA reductase inhibitor simvastatin, previously shown to block production of LXR agonists, led to a reduction in Abca1 and Abcg1 expression (Fig. 5A). Moreover, the addition of mevalonic acid restored their expression, indicating that oxysterol production from the mevalonate pathway is required for the maintenance of LXR activity in quiescent lymphocytes. Finally, target gene expression was also restored by the direct addition of the endogenous LXR agonist 22(R)-HC (data not shown).
Figure 5. Regulation of LXR signaling by SULT2b1 during T cell activation.
(A) Real-time PCR analysis of LXRβ and ABCG1 from quiescent WT T cells cultured with simvastatin and 5 mM mevalonic acid. (B) Real-time PCR analysis of SULT2b1, ABCC1, LDLR and ABCG1 mRNA from purified WT T cells stimulated with anti-CD3 in vitro for the indicated times. (C) Real-time PCR analysis of SULT2b1 and ABCG1 mRNA from purified WT T cells stimulated for 12 h with anti-CD3 in vivo. (D) Real-time PCR analysis of LXRβ, LDLR and ABCA1 mRNA from CAR-tg T cells transduced with SULT2b1- containing or control Adenovirus.
To investigate the potential mechanism for regulation of LXR ligand availability during T cell activation, we returned to our transcriptional profiling analysis. Remarkably, expression of the oxysterol metabolizing enzyme SULT2B1 was rapidly induced in response to T cell activation. SULT2B1 catalyzes the transfer of sulfate groups to oxysterols, inactivating them as LXR ligands and facilitating their export from the cell by ABCC1 and other membrane transporters (Chen et al., 2007; Javitt et al., 2001; Zelcer et al., 2003). Realtime PCR revealed that both SULT2B1 and ABCC1 were rapidly induced by proliferative stimuli (pbCD3 or PMA/ionomycin) in T cells (Fig. 5B; data not shown). Importantly, a pronounced upregulation of SULT2B1 was also observed in T cells activated in vivo (Fig. 5C). This observation strongly suggests that LXR target gene expression is suppressed during T cell activation through active metabolism of endogenous oxysterol LXR agonists. In support of this hypothesis, adenoviral expression of SULT2B1 in resting primary T cells from adenoviral receptor-expressing transgenic mice (Wan et al., 2000) recapitulated the effects of proliferative stimuli on both LXR and SREBP-2 target gene expression (Fig. 5D).
LXR inhibits proliferation through Abcg1-dependent alteration of cholesterol homeostasis
The observation that T cell activation is associated with a reduction in endogenous LXR activators, coupled with the prominent regulation of LXR-dependent sterol transporters, suggested LXR may modulate T cell proliferation through control of cholesterol metabolism. We ruled out the possibility that limiting extracellular cholesterol availability was involved in the effects of LXR agonist on T cell proliferation by supplementing cultures with excess LDL. Addition of as much as 100 μg protein/ml LDL had no effect on cell proliferation in the presence or absence of LXR agonist (Fig. 6A; data not shown). We next addressed the possibility that LXR-dependent alteration of intracellular cholesterol metabolism was linked to proliferation. Assessment of global membrane cholesterol content by fillipin-staining showed increased total cholesterol content in blasting T cells, but no gross difference between vehicle and LXR agonist treatment (Fig. 6B). However, intracellular cholesterol trafficking is complex and intracellular pools are not necessarily in equilibrium with the plasma membrane (Chang et al., 2006; Simons and Ikonen, 2000). Therefore, this crude analysis does not rule out more subtle or compartment-specific changes in sterol levels or changes in the abundance of rare sterol species (such as oxysterols; (Yancey et al., 2003)).
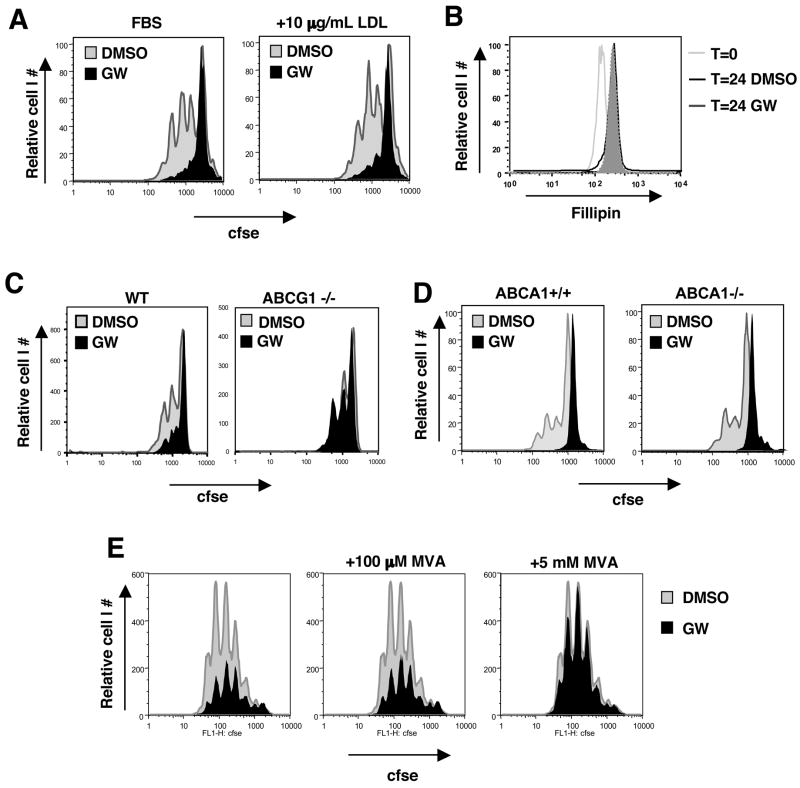
Figure 6. LXR inhibits proliferation through Abcg1-dependent alteration of cholesterol homeostasis.
(A) CFSE dilution of WT T cells stimulated with pbCD3 and GW3965 in the presence of 10μg/mL LDL for 60 h. (B) Cholesterol content of plasma membrane from WT T cells revealed by fillipin staining ex vivo or after 24 h stimulation with pbCD3 and GW3965 as indicated. (C,D) CFSE dilution of purified ABCG1−/−, ABCA1−/− and control T cells stimulated with pma and ionomycin in the presence of LXR ligands for 60 h. (e) CFSE dilution of purified WT T cells stimulated with pbCD3 for 96 h in the presence of GW3965 and mevalonic acid (MVA) as indicated.
Previous work has established that ABCG1 and ABCA1 play central roles in both LXR-dependent sterol efflux and intracellular sterol trafficking. We therefore tested the requirement for these transporters in the anti-proliferative effects of LXR agonists. Purified WT and Abcg1 null lymphocytes were activated with pma/ionomycin in the presence or absence of GW3965 and/or LG268. After 48–96 h, CFSE proliferation profiles were assessed by flow cytometry. As expected, LXR agonist reduced the proliferative capacity of WT T cells (Fig. 6C and S11). Remarkably, however, the effects of LXR activation were greatly attenuated in cells lacking ABCG1. We also analyzed effects of LXR agonists on ABCA1 null and background matched (B6/129) ABCA1+/+ controls. Unfortunately, the ABCA1 null mutation is lethal in the C57BL/6 background, precluding a direct comparison of ABCG1 and ABCA1 on the same background. Nevertheless, loss of ABCA1 expression did not alter the response to LXR agonist (Fig. 6D), suggesting this transporter is not required for the LXR effect.
The above observations indicate that transport of sterols by ABCG1 is required for LXR agonists to inhibit cell proliferation. In strong support of this mechanism, the inhibitory effect of LXR activation could be completely overcome by providing lymphocytes with an excess of mevalonate (5 mM), the precursor for cholesterol and oxysterols (Fig. 6E). Importantly, providing cells with levels of mevalonate sufficient to allow non-steroidal modifications, such as protein prenylation, but insufficient to drive sterol synthesis (100 μM), had no effect on LXR-dependent inhibition. These data identify ABCG1 as a key component of a sterol trafficking pathway that must be downregulated during T cell activation. Enforced expression of ABCG1 during T cell activation by exogenous LXR agonists engages a metabolic checkpoint that blocks proliferation through alteration of intracellular sterol metabolism.
Endogenous LXR signaling regulates acquired immune responses
To definitively determine if the intrinsic loss of LXRβ signaling in T cells would translate into a proliferative advantage in vivo, we used a competitive adoptive transfer model of homeostatic proliferation. For these studies 1×106 Lxrβ null (Thy1.2+) T cells and an equivalent number of congenic WT (Thy1.1+) T cells were co-adoptively transferred into the same B6.RAG null host and allowed to undergo homeostatic proliferation for 1 week. Strikingly, FACS analysis of spleen and blood from host animals revealed that the frequency of Lxrβ null (Thy1.1−, CD3+) T cells was much higher (p=0.003) than WT (Thy1.1+, CD3+) T cells isolated from the same host (Fig. 7A).
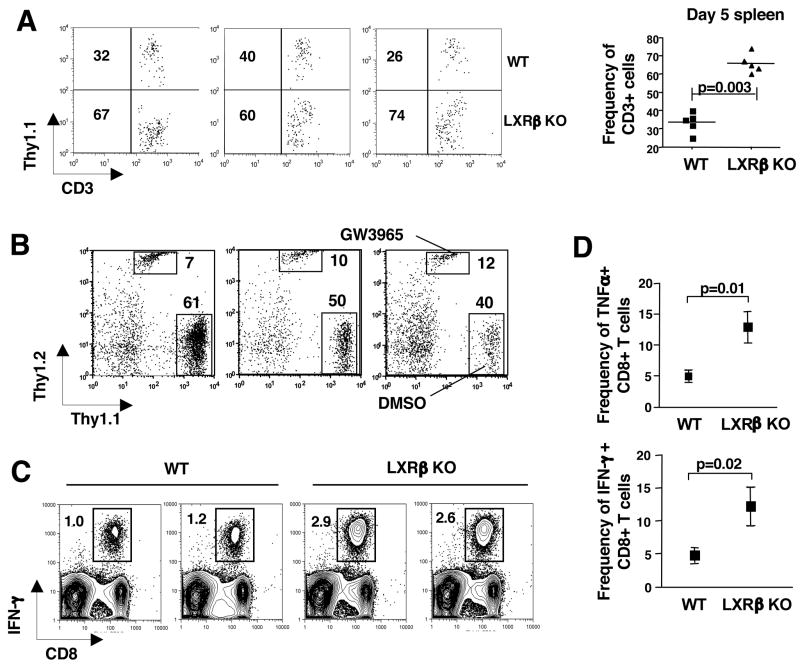
Figure 7. LXR signaling regulates T cell proliferation and immunity.
(A) Homeostatic proliferation of WT and LXRβ KO T cells. 1×106 purified WT (Thy1.1+) and LXRβ KO T cells (Thy1.2+) were co-adoptively transferred into B6Rag −/− hosts. After 5 days, spleens were harvested and cells stained with anti-CD3 and Thy1.1 to determine the frequency of recovered T cells. Each FACS plot represents an individual mouse and graph represents 5 mice from one experiment. (B) Homeostatic proliferation of WT T cells stimulated with LXR/RXR ligands. WT (Thy1.2+) T cells were pre-treated with GW3965 in vitro for 18 h. Control (Thy1.1+) T cells were pre-treated with DMSO in vitro for 18 h. 1×106 live cells per treatment group were co-adoptively transferred into B6Rag −/− hosts. After 5 days, spleens were harvested and cells stained with anti-CD3, Thy1.1 and Thy1.2. Each FACS plot represents an individual mouse of 5 mice from one experiment. (C,D) Increased antigen specific immune response in LXRβ KO mice. WT and in LXRβ KO mice were immunized with 1×107 syngeneic MECs transfected with the human adenovirus type 5 early region 1 (Ad5e1). (C) The frequency of antigen-specific CD8+ T cells in whole spleen was enumerated ex vivo one week after immunization by intracellular IFN-γ staining after a short term in vitro restimulation with the E1B192-200 (VNIRNCCYI) peptide. FACS data presented is 2 mice per genotype representative of 4 mice per group. (D) The frequency of antigen specific IFN-γ and TNFα producing CD8 T cells within the CD8 T cell compartment. Data is presented as the mean +/− SEM of 4 mice per group. Each experiment was repeated twice.
In complimentary studies, we asked if the activation of LXR would prevent the expansion of WT T cells in vivo. To that end, we pretreated- purified WT Thy1.2+ T cells and WT Thy1.1+ T cells with GW3965 or vehicle respectively for 16 h in vitro to allow for initiation of the LXR transcriptional program. Live cells were collected and 1×106 vehicle (Thy1.1+) and GW3965 (Thy1.2+) treated cells were injected into B6.RAG null mice. Peripheral blood and spleen were subsequently analyzed for Thy1.1+ and Thy1.2 + CD3+ T cells on days 5–7. Consistent with the above loss-of-function studies, pretreatment of T cells with LXR ligand markedly reduced the frequency of T cells undergoing homeostatic proliferation (Fig. 7B).
The ability of LXR to modulate lymphocyte expansion strongly suggested that endogenous LXR signaling might have a functional impact on acquired immune responses. To determine if loss of LXRβ would augment antigen driven proliferation in vivo, we immunized mice with 1×107 Tap-deficient mouse embryo cells (MECs) expressing the human adenovirus type 5 early region 1 (Ad5E1). Antigen-specific CD8+ T cells were enumerated ex vivo one week post immunization by intracellular IFN-γ and TNF-α staining after a short term in vitro restimulation with the E1B antigen E1B192-200 (VNIRNCCYI) (Toes et al., 1998). Remarkably, FACS analysis indicated that the frequency of antigen-specific Lxrβ null IFN-γ+ (p=0.02) or TNFα+ (p=0.01) CD8+ T cells was 2–3 fold higher than their WT counterparts (Fig. 7C,D). Thus, antigen-driven expansion of CD8+ T cells is negatively regulated by LXRβ in WT mice. Taken together, these data establish LXR-dependent sterol metabolism as a novel signaling pathway regulating T cell function and immune responses.
Discussion
An important characteristic of adaptive immunity is the capacity of antigen-specific lymphocytes to undergo rapid and extensive proliferation in response to antigenic challenge. Thus, understanding the signaling pathways that impact proliferation is critical to understanding how immune responses are generated and controlled. Previous work from a number of laboratories has outlined the importance of glycolytic metabolism in T cell responses (MacDonald and Cerottini, 1979; Rathmell et al., 2000; Vander Heiden et al., 2001). The influence of lipid metabolism on lymphocyte function, however, is poorly understood. We have shown here that intracellular sterol metabolism has a previously unrecognized regulatory role in the control of acquired immune responses. Cholesterol is an essential component of membranes and therefore the requirement for adequate cholesterol in cell reproduction is obvious. However, our data reveal that the intracellular availability of sterols is dynamically regulated during T cell activation, and that this is linked to transcriptional responses mediated by SREBP and LXR, as well as to cell cycle control. Moreover, we show that the ability of LXR to alter cellular sterol metabolism through regulation of the ABCG1 sterol transporter impacts both lymphocyte proliferation and antigen-stimulated immune responses.
The initial clue to a potential role for LXR signaling in innate immune cells came from analysis of LXR null mice, which exhibit age-dependent splenomegaly and lymphadenopathy. This phenotype reflects expansion in both the T and B cell compartments and is linked to the previously unrecognized ability of LXRβ to regulate mitogen- and antigen-driven lymphocyte proliferation. Analysis of purified primary lymphoid cultures established that activation of LXRβ by physiologic or pharmacologic ligands diminishes the proliferative capacity of B and T cells. Conversely, genetic loss of LXRβ rescues cells from the inhibitory effect of LXR agonist and potentiates mitogen-and antigen-driven expansion. We also demonstrated that the ability of LXR to impact cell proliferation has a functional consequence for lymphoid homeostasis and antigen-driven immune responses in vivo. Lymphocytes lacking LXRβ expression show an exaggerated response in both homeostatic and vaccine-driven proliferation models. Thus, endogenous LXR signaling is a physiologically important determinant of immune responses and pharmacological LXR activation has the potential for immune modulation.
Given the ability of LXR to inhibit NF-κB signaling in macrophages, we initially suspected that LXR might interfere with TCR signaling and proximal pathways involved in lymphocyte activation. However, this appears not to be the case, since markers of activation and IL-2 production were not affected by LXR ligands. We also excluded the possibility that LXR activation was causing apoptosis directly, as is known to occur with GR agonists. Rather, cell cycle analysis indicated that LXR signaling was regulating the G1 to S transition. Collectively, these observations suggested that LXR was controlling the expression of one or more genes whose action was inhibitory to cell cycle progression. To identify such genes we profiled gene expression during lymphocyte activation. We found that a battery of genes linked to cholesterol homeostasis is dynamically regulated during lymphocyte activation, and that exogenous LXR ligand alters this metabolic program. In particular, the genes encoding the sterol transporters ABCA1 and ABCG1–both LXR targets–are rapidly downregulated upon T cell receptor crosslinking. The addition of LXR agonist strongly stimulates their expression. These findings led to the hypothesis that ABCG1 action alters sterol metabolism in lymphocytes in a manner that is inhibitory to proliferation. Definitive support for the requirement of ABCG1 in this model is provided by the demonstration that the ability of LXR to inhibit proliferation is markedly reduced in lymphocytes from Abcg1 null mice. A definitive link to sterol metabolism is established by the observation that the inhibitory effect of LXR is completely blocked if lymphocytes are provided with an excess of mevalonate, the precursor for cholesterol and oxysterols.
Our results reveal the existence of an endogenous sterol signaling pathway that regulates lymphocyte proliferation through coordinate regulation of SREBP and LXR activity. The changes in SREBP and LXR target gene expression we observe during T cell activation are indicative of alterations in endogenous sterol regulators of both transcription factors. Brown and Goldstein have delineated an elegant mechanism for the regulation of cholesterol synthesis in which SREBP is held as an inactive precursor in the ER by the sterol-sensing protein SCAP (Goldstein et al., 2006). A logical conclusion of our data is that sterol content in the ER (cholesterol and/or oxysterols) must be decreasing rapidly upon lymphocyte activation. Furthermore, the reduction in LXR target gene expression during activation indicates that the nuclear availability of sterol LXR activators is also reduced. Indeed, basal expression of LXR target genes in lymphocytes is dependent on the production of endogenous LXR agonists by the mevalonate pathway.
Unexpectedly, the mechanism by which LXR activity is regulated during T cell activation does not involve alteration in ligand production, rather it is due to induction of enzymatic ligand metabolism. The enzyme SULT2B1 transfers sulfate groups to oxysterols, inactivates them as LXR ligands, and facilitates their export from the cell. We have shown that expression of SULT2B1 is dramatically increased in T cells by proliferative stimuli. This induction would be predicted to deplete the cell of LXR ligand and suppress expression of LXR target genes (Chen et al., 2007; Fuda et al., 2007). In fact, expression of SULT2B1 in resting lymphocytes using an adenoviral vector recapitulates the effects of proliferative stimuli on both the LXR and SREBP gene expression programs. Thus, upregulation of SULT2B1 during cell proliferation provides an elegant mechanism to affect changes in cellular cholesterol metabolism required to support new membrane synthesis and cell division. Whether additional mechanisms also contribute to regulation of LXR activity during cell proliferation remains to be addressed.
The ability of ABCG1 expression to block proliferation implicates a sterol substrate of this transporter in a metabolic checkpoint that regulates cell-cycle progression. Early studies on cholesterol synthesis in lymphocytes found that manipulation of the mevalonate pathway by the addition of sterol metabolites, such as 25-hydroxycholesterol, resulted in a G1 arrest (Chakrabarti and Engleman, 1991). Similarly, HMG-CoA inhibitors block the proliferation of cells in multiple systems. A complicating factor in these studies is that suppression of the mevalonate pathway also perturbs synthesis of non-sterol mevalonate derivatives such as geranylgeraniol and farnesol. However, attempts to uncouple the cholesterol synthetic pathway from non-steroidal protein modifications either using low dose statins or inhibitors of downstream enzymes have revealed an absolute requirement for cholesterol in cell cycle progression and mitosis (Martinez-Botas et al., 2001; Martinez-Botas et al., 1999). We have observed a similar arrest in the cell cycle; however, our gene profiling studies show that we are not blocking the SREBP-2 pathway. Rather, we are likely enforcing sterol efflux or redistribution, resulting in a localized depletion of sterols.
ABCG1 is known to play an important role in cholesterol and oxysterol efflux (Kennedy et al., 2005; Terasaka et al., 2007; Wang et al., 2004). Recent studies have also reported that ABCG1 is found in intra-cellular compartments, such as the ER and vesicles, and that ABCG1 expression stimulates SREBP-2 activity through the redistribution of sterols out of the ER (Tarr and Edwards, 2007). We hypothesize that adequate levels of one or more sterols in a particular cellular compartment, likely the ER, are read by the cell cycle machinery as an indication of appropriate metabolic conditions for cell division. Downregulation of ABCG1 during activation may be necessary to maintain compartmentalization of these sterols. Forced induction of ABCG1 by LXR activation reduces the availability of this signaling sterol. In the absence of LXR, increased sterol levels act as a stimulus to proliferation. At present, we favor the hypothesis that cholesterol itself is the sterol being sensed by the cell cycle machinery, because the ability of LXR agonists to block proliferation indicates that the signaling sterol is not an LXR agonist.
In summary, this work outlines a previously unrecognized role for LXRβ and sterol signaling in the regulation of lymphocyte function. Although our focus in this report has been on lymphocytes, the ability of the SULT2B1-LXR-ABCG1 axis to couple cellular cholesterol metabolism and proliferation is likely to be applicable to many cell types, particularly those undergoing rapid cell division. Finally, given that LXR responds to endogenous lipids whose availability may be altered in disease, our results raise the possibility that LXR signaling may impact acquired immune responses in human metabolic diseases such as dyslipidemia and atherosclerosis.
Materials and Methods
Mice and Cell lines
C57BL/6 and C57BL/6 recombination activating gene 1-deficient mice were purchased from The Jackson Laboratory. Lxrα and Lxrβ null mice were a gift from D. Mangelsdorf (University of Texas- Southwestern) and are greater than 10 generations backcrossed to C57BL/6. BCL-xL transgenic mice were a gift from D. Green (St. Jude’s). ABCG1 and ABCA1 null mice have been previously described (Kennedy et al., 2005; Timmins et al., 2005). Do11.10 CAR transgenic mice were purchased from the Taconic Laboratory. All mice were maintained under SPF conditions in the animal facilities of the University of California, Los Angeles. Tap- deficient- mouse embryo cells (MECs) expressing the human adenovirus type 5 early region 1 (Ad5E1) have been described (Schoenberger et al., 1998).
Media, Reagents, Antibodies, and Flow Cytometry
GW3965, T0901317, and GW7845 were provided by T. Willson and J. Collins (GlaxoSmithKline). Lymphocytes were grown in RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 10 mM Hepes (all from Gibco), and 50 μM 2-ME (Sigma-Aldrich). 7-AAD, Propidium iodide, annexin V, anti-mouse CD25 (PC61), CD3 (2C11), CD4 (RM4-5), CD8 (53-6.7), CD28 (37.51), CD69 (HI.2F3), Foxp3 (FJK-16s) and anti-human CD4 (RPA-T4), CD8 (RPA-T8), Ki-67 (B-56) and PCNA (PC-10) were purchased from Bd biosciences and eBioscience. Purified anti-human CD3 (OKT3) was purchased from Ortho Scientific. Murine rIL-2 was purchased from Peprotech. Flowbright counting beads (Invitrogen) were added to FACS tubes before analysis to normalize CFSE dilution profiles. Intracellular PCNA and Ki-67 staining was performed per manufactures instruction (Bdbiosciences). Cells were analyzed on FACSCalibur or LSR (Becton Dickinson) using FlowJo software (Treestar, Inc).
Cell Purification and Cell Counts
In some experiments, mouse or human T cells were enriched using negative selection. For mouse, T cell or B cell enrichment was performed using Pan T or B cell isolation kit (Miltenyi Biotec) and selection on MidiMACS columns (Miltenyi Biotec) per manufacturer instructions. For human T cells, enrichment was performed using Rosette Sep (Stem Cell Technologies) per manufacturer instructions. Purity was confirmed at >95% by flow cytometry. Cells counts were performed on an Improved Neubauer Hemacytometer using trypan blue exclusion. All LN cell counts reported are from 2 inguinal and 4 axillary LNs. For adenoviral transductions, purified CAR transgenic T cells were incubated with adenovirus at an MOI of 10:1 for 1 h in complete media. Cells were then washed twice and resuspended at 1×106 cells per mL in complete media.
Proliferation Studies
For 3H-thymidine incorporation studies, single cell suspensions of murine spleen were initially prepared using ACK lysis buffer. Whole spleen cells were plated in 96-well round bottom plates (2×105 cells/well) and stimulated with LPS (10 μg/mL), Concavalin A (10 μg/mL), anti-IgM (Fab′)2 (10 μg/mL) or PMA (0.5 μM) and ionomycin (100 mM). Cells were cultured for 24–96 h at 37°C/5% CO2 and pulsed with 3H-thymidine for the final 16 h. For some studies, T cells or B cells were purified and enriched from spleen and LNs by negative selection as indicated above. Single cell suspensions were labeled with CFSE (Molecular Probes) as described previously (Lyons and Parish, 1994). Cells were stimulated as above. Human T cells were CFSE labeled and stimulated with anti-CD3 (1 μg/mL) crosslinked with plate bound goat anti-mouse (100 μg/mL) in 96 well flat bottom plates (2×105 cells/well). Some cultures received 2μg/well of soluble anti-CD28. Cells were stained for surface expression of CD8 and CD4 on the indicated days, incubated with 7-AAD and 5 ×104 counting beads added to tubes before analysis by flow cytometry.
Immunization and Homeostatic Proliferation Assays
For immunization studies WT and Lxrβ null mice were immunized subcutaneously with 1×107 irradiated (3000 rad) TAP- deficient- Ad5E1- MEC. Mice were sacrificed on day 7 and antigen-specific CD8+ T cells were enumerated from spleen as described (Schoenberger et al., 1998). Single cell suspensions were incubated for 5 h with E1B192-200 peptide ((aa:VNIRNCCYI) A&A labs LLC)) at 2.5 μg/mL in the presence of Brefeldin A (BD biosciences) ex vivo. Cells were stained for surface expression of CD8 and CD4, fixed and permeabilized using Cytofix/Cytoperm kit (BD biosciences) and stained for intracellular IFN-γ and TNFα according to manufacturer’s protocol. For homeostatic proliferation studies 1x 106 purified LXRβ null (Thy1.2+) T cells and WT (Thy1.1+) T cells were co-adoptively transferred into the same B6.RAG null host. Peripheral blood and spleen was harvested, stained for CD3, and Thy1.1 expression and analyzed by FACS.
RNA Isolation, DNA Microarray and Real-time PCR Analysis
For DNA microarray total RNA was isolated from cells by using Trizol (Invitrogen) and further purified RNAeasy columns (Qiagen). Preparation and hybridization to Affymetrix 430 v2.0 was performed at the UCLA microarray core and data analyzed using GeneSpring GX 7.3 (Agilent Technologies). For real-time PCR, total RNA was isolated as above. 1 microgram of total RNA was reverse transcribed with random hexamers using the Taqman Reverse Transcription Reagents Kit (Applied Biosystems). Sybergreen (Diagenode) real-time quantitative PCR assays were performed using an Applied Biosystems 7900HT sequence detector. Results show averages of duplicate experiments normalized to 36B4.
Protein Isolation and Analysis
Total cell lysates were prepared in ysis buffer (10 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl, pH 7.4, 1% SDS) supplemented with protease inhibitors (Roche Molecular Biochemicals). Samples were separated on a 12% SDS-polyacrylamide gel and transferred to nitrocellulose. Membranes were probed with Anti-hLXRα and hLXRβ (Perseus Proteomics), anti-P27kip (Cell Signaling) and anti-CDK4 (Santa Cruz Antibodies) at 1:1000 overnight to detect expression. Goat anti-mouse and Donkey anti-rabbit (DAKO) were used at 1:3000 for 1 h at room temperature and visualized with chemiluminescence (ECL, Amersham Pharmacia Biotech).
Statistical Methods
Real-time PCR data, in vitro proliferation assays and cell counts are expressed as the mean ± standard deviation. The frequency of antigen specific T cells and homeostatically proliferating T cells are presented as the mean ± standard error of the mean (S.E.M). All statistical analysis was done using the student’s t test. A probability value of p<0.05 was considered statistically significant.
Supplementary Material
Figure S1. Normal distribution of T and B lymphocytes in LXR null mice. (a) Splenocytes from male, 6–8 week old WT, Lxrα−/− and Lxrβ−/− mice were stained with anti-CD4, CD8, CD19 and B220. The frequency of B cells is shown in the upper panel and the frequency of CD4 and CD8 T cells are marked in the upper right quadrant of lower panels. (b) LN and spleen cells from WT and Lxrβ−/− mice were stained for CD4 and intracellular Foxp3. (c) Splenocytes from WT, Lxrα−/− and Lxrβ−/− mice were stained with anti-CD4, CD8, CD44 and CD25. FACS plots are representative of one mouse per genotype repeated greater than 5 times.
Figure S2. Normal APC phenotype in aged LXRβ null mice. (a,b) Spleen cells from 5 month old WT and Lxrβ−/− mice were stained for CD11c, MHC class II, CD40, and CD86 expression. FACS plots are representative of one mouse per genotype repeated greater than 3 times.
Figure S3. LXRβ is expressed in quiescent and activated lymphocytes. (a) Real-time PCR analysis of C57BL/6 peritoneal macrophages, bone marrow derived macrophages, ex vivo purified splenic B and T cells. (b) Purified splenic T cells were activated for 24 h with pbCD3 and LXR ligand as indicated and Real-time PCR analysis was performed. (c,d) Purified human T cells were cultured with LXR ligand and PMA/ionomycin for 24 h as indicated. (c) Whole cell lysates were collected at the indicated time and probed for LXRα and LXRβ expression by Western. (d) mRNA was collected at the indicated time and analyzed for Lxrα, Lxrβ, expression by real-time PCR.
Figure S4. Gain of LXR function inhibits B lymphocyte proliferation. Spleen cells from 6–8 week old WT, Lxrα and Lxrβ−/− mice were stimulated with anti-IgM(Fab′)2. Cultures were treated with LXR and/RXR ligand as indicated. 3H-thymidine was added to cultures after 72 h for the final 16 h.
Figure S5. Gain of LXR function negatively regulates human CD4 T cell expansion, but can be rescued by costimulation and does not induce apoptosis. (a,b,c) Purified CFSE labeled human T cells were and stimulated with pbCD3, soluble anti-CD28 and LXR ligands as indicated. Cultures were stained for CD4, annexin and PI at the indicated times. 5×104 counting beads were added to samples to serve as an internal counting control (d) Purified WT mouse T cells were activated with pbCD3 and cultured with LXR, RXR, GR, PPARγ ligands or DMSO control. After 24h cells were stained with 7-AAD., and analyzed via flow cytometry.
Figure S6. LXRβ signaling regulates cell cycle progression. WT and Lxrβ−/− T cells were stimulated with pbCD3 and LXR/RXR ligand as indicated. Cells were stained for DNA content with propidium iodide at the indicated times and analyzed by flow cytometry.
Figure S7. Gain- and loss- of LXR function does not perturb activation or IL-2 signaling. (a) CD69 and CD25 expression on WT T cells ex vivo or after 24h activation with pbCD3 in the presence LXR/RXR ligand as indicated. (b) IL-2 production from purified WT T cells activated with pbCD3 for 24 h and then cultured with Brefeldin A and restimulated with PMA/ionomycin. After 5h stimulation, cells were stained with anti-CD4 and CD8, then fixed, permeabilized, stained for intracellular IL-2 and analyzed by flow cytometry. (c) Real-time PCR analysis of Lta and Grzmb from Purified WT T cells stimulated with pbCD3 for 5 days, rested for 18 h and then restimulated with IL-2 for 8h. (d) CD69 and CD25 expression on WT, Lxra−/− and Lxrβ−/− T cells after 24h activation with pbCD3.
Figure S8. Sustained LXR signaling is required to inhibit proliferation. (a) CFSE dilution of purified WT T cells stimulated with pbCD3 for 72h. In addition, indicated cultures received LXR/RXR agonist at 24h. (b) Cultures were pre-treated with LXR/RXR agonist for 2 h, then washed, placed in fresh media and stimulated with pbCD3. Cells were harvested at 72 h and analyzed by flow cytometry. 5×104 counting beads were added to serve as an internal counting control and samples were analyzed via flow cytometry.
Figure S9. Reciprocal regulation of SREBP-2 and LXR transcriptional programs during human lymphocyte activation. Real-time PCR analysis of Abca1, Abcg1, Ldlr, Hmgcr, Stard4 from purified human peripheral blood lymphocyes ex vivo or stimulated with pma/ionomycin for 4h.
Figure S10. IRF-3 does not influence TCR mediated downregulation of LXR target genes in lymphocytes. Realtime PCR analysis of Abca1 and Abcg1 from purified WT and Irf3−/− T cells ex vivo or after stimulation with pbCD3 for 18h.
Figure S11. LXR inhibits proliferation through Abcg1-dependent alteration of cholesterol homeostasis. CFSE dilution of purified Abcg1−/− and WT T cells stimulated with pma/ionomycin and LXR ligand for 96h. 5×104 counting beads were added to serve as an internal counting control and samples were analyzed via flow cytometry.
Acknowledgments
We are grateful to D. Mangelsdorf for the LXR null mice and D. Russell for SULT2B1 adenovirus. We thank T. Willson and J. Collins for the GW3965, T0901317, and GW7845. Flow cytometry was performed in the UCLA Flow Cytometry Core Facility that is supported by NIH awards CA-16042 and AI-28697. We thank Dr. Kye Won Park for helpful discussions. P.T. is an investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (RR021975 to S.B., HL30568 to P.T. and P.A.E., and HL049373 to J.S.P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Beadling C, Smith KA. DNA array analysis of interleukin-2-regulated immediate/early genes. Med Immunol. 2002;1:2. doi: 10.1186/1476-9433-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974;249:7306–7314. [PubMed] [Google Scholar]
- Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in T lymphocyte proliferation. J Biol Chem. 1991;266:12216–12222. [PubMed] [Google Scholar]
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Chen HW, Heiniger HJ, Kandutsch AA. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975;72:1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Kandutsch AA, Waymouth C. Inhibition of cell growth by oxygenated derivatives of cholesterol. Nature. 1974;251:419–421. doi: 10.1038/251419a0. [DOI] [PubMed] [Google Scholar]
- Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Fuda H, Javitt NB, Mitamura K, Ikegawa S, Strott CA. Oxysterols are substrates for cholesterol sulfotransferase. J Lipid Res. 2007;48:1343–1352. doi: 10.1194/jlr.M700018-JLR200. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene-and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hindinger C, Hinton DR, Kirwin SJ, Atkinson RD, Burnett ME, Bergmann CC, Stohlman SA. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J Neurosci Res. 2006;84:1225–1234. doi: 10.1002/jnr.21038. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Javitt NB, Lee YC, Shimizu C, Fuda H, Strott CA. Cholesterol and hydroxycholesterol sulfotransferases: identification, distinction from dehydroepiandrosterone sulfotransferase, and differential tissue expression. Endocrinology. 2001;142:2978–2984. doi: 10.1210/endo.142.7.8244. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kovanen PE, Young L, Al-Shami A, Rovella V, Pise-Masison CA, Radonovich MF, Powell J, Fu J, Brady JN, Munson PJ, et al. Global analysis of IL-2 target genes: identification of chromosomal clusters of expressed genes. Int Immunol. 2005;17:1009–1021. doi: 10.1093/intimm/dxh283. [DOI] [PubMed] [Google Scholar]
- Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Joseph SB, Walczak R, Pei L, Wilpitz DC, Collins JL, Tontonoz P. Autoregulation of the human liver X receptor alpha promoter. Mol Cell Biol. 2001;21:7558–7568. doi: 10.1128/MCB.21.22.7558-7568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- MacDonald HR, Cerottini JC. Inhibition of T cell-mediated cytolysis by 2-deoxy-D-glucose (2-DG): differential effect of 2-DG on effector cells isolated early or late after alloantigenic stimulation in vitro. J Immunol. 1979;122:1067–1072. [PubMed] [Google Scholar]
- Martinez-Botas J, Ferruelo AJ, Suarez Y, Fernandez C, Gomez-Coronado D, Lasuncion MA. Dose-dependent effects of lovastatin on cell cycle progression. Distinct requirement of cholesterol and non-sterol mevalonate derivatives. Biochim Biophys Acta. 2001;1532:185–194. doi: 10.1016/s1388-1981(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Botas J, Suarez Y, Ferruelo AJ, Gomez-Coronado D, Lasuncion MA. Cholesterol starvation decreases p34(cdc2) kinase activity and arrests the cell cycle at G2. Faseb J. 1999;13:1359–1370. doi: 10.1096/fasebj.13.11.1359. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- Roos D, Loos JA. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973;77:127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, van der Voort EI, Krietemeijer GM, Offringa R, Melief CJ, Toes RE. Cross-priming of CTL responses in vivo does not require antigenic peptides in the endoplasmic reticulum of immunizing cells. J Immunol. 1998;161:3808–3812. [PubMed] [Google Scholar]
- Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr PT, Edwards PA. ABCG1 and ABCG4 are co-expressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J Lipid Res. 2007 doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci U S A. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toes RE, van der Voort EI, Schoenberger SP, Drijfhout JW, van Bloois L, Storm G, Kast WM, Offringa R, Melief CJ. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160:4449–4456. [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci U S A. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc Natl Acad Sci U S A. 2000;97:13784–13789. doi: 10.1073/pnas.250356297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Normal distribution of T and B lymphocytes in LXR null mice. (a) Splenocytes from male, 6–8 week old WT, Lxrα−/− and Lxrβ−/− mice were stained with anti-CD4, CD8, CD19 and B220. The frequency of B cells is shown in the upper panel and the frequency of CD4 and CD8 T cells are marked in the upper right quadrant of lower panels. (b) LN and spleen cells from WT and Lxrβ−/− mice were stained for CD4 and intracellular Foxp3. (c) Splenocytes from WT, Lxrα−/− and Lxrβ−/− mice were stained with anti-CD4, CD8, CD44 and CD25. FACS plots are representative of one mouse per genotype repeated greater than 5 times.
Figure S2. Normal APC phenotype in aged LXRβ null mice. (a,b) Spleen cells from 5 month old WT and Lxrβ−/− mice were stained for CD11c, MHC class II, CD40, and CD86 expression. FACS plots are representative of one mouse per genotype repeated greater than 3 times.
Figure S3. LXRβ is expressed in quiescent and activated lymphocytes. (a) Real-time PCR analysis of C57BL/6 peritoneal macrophages, bone marrow derived macrophages, ex vivo purified splenic B and T cells. (b) Purified splenic T cells were activated for 24 h with pbCD3 and LXR ligand as indicated and Real-time PCR analysis was performed. (c,d) Purified human T cells were cultured with LXR ligand and PMA/ionomycin for 24 h as indicated. (c) Whole cell lysates were collected at the indicated time and probed for LXRα and LXRβ expression by Western. (d) mRNA was collected at the indicated time and analyzed for Lxrα, Lxrβ, expression by real-time PCR.
Figure S4. Gain of LXR function inhibits B lymphocyte proliferation. Spleen cells from 6–8 week old WT, Lxrα and Lxrβ−/− mice were stimulated with anti-IgM(Fab′)2. Cultures were treated with LXR and/RXR ligand as indicated. 3H-thymidine was added to cultures after 72 h for the final 16 h.
Figure S5. Gain of LXR function negatively regulates human CD4 T cell expansion, but can be rescued by costimulation and does not induce apoptosis. (a,b,c) Purified CFSE labeled human T cells were and stimulated with pbCD3, soluble anti-CD28 and LXR ligands as indicated. Cultures were stained for CD4, annexin and PI at the indicated times. 5×104 counting beads were added to samples to serve as an internal counting control (d) Purified WT mouse T cells were activated with pbCD3 and cultured with LXR, RXR, GR, PPARγ ligands or DMSO control. After 24h cells were stained with 7-AAD., and analyzed via flow cytometry.
Figure S6. LXRβ signaling regulates cell cycle progression. WT and Lxrβ−/− T cells were stimulated with pbCD3 and LXR/RXR ligand as indicated. Cells were stained for DNA content with propidium iodide at the indicated times and analyzed by flow cytometry.
Figure S7. Gain- and loss- of LXR function does not perturb activation or IL-2 signaling. (a) CD69 and CD25 expression on WT T cells ex vivo or after 24h activation with pbCD3 in the presence LXR/RXR ligand as indicated. (b) IL-2 production from purified WT T cells activated with pbCD3 for 24 h and then cultured with Brefeldin A and restimulated with PMA/ionomycin. After 5h stimulation, cells were stained with anti-CD4 and CD8, then fixed, permeabilized, stained for intracellular IL-2 and analyzed by flow cytometry. (c) Real-time PCR analysis of Lta and Grzmb from Purified WT T cells stimulated with pbCD3 for 5 days, rested for 18 h and then restimulated with IL-2 for 8h. (d) CD69 and CD25 expression on WT, Lxra−/− and Lxrβ−/− T cells after 24h activation with pbCD3.
Figure S8. Sustained LXR signaling is required to inhibit proliferation. (a) CFSE dilution of purified WT T cells stimulated with pbCD3 for 72h. In addition, indicated cultures received LXR/RXR agonist at 24h. (b) Cultures were pre-treated with LXR/RXR agonist for 2 h, then washed, placed in fresh media and stimulated with pbCD3. Cells were harvested at 72 h and analyzed by flow cytometry. 5×104 counting beads were added to serve as an internal counting control and samples were analyzed via flow cytometry.
Figure S9. Reciprocal regulation of SREBP-2 and LXR transcriptional programs during human lymphocyte activation. Real-time PCR analysis of Abca1, Abcg1, Ldlr, Hmgcr, Stard4 from purified human peripheral blood lymphocyes ex vivo or stimulated with pma/ionomycin for 4h.
Figure S10. IRF-3 does not influence TCR mediated downregulation of LXR target genes in lymphocytes. Realtime PCR analysis of Abca1 and Abcg1 from purified WT and Irf3−/− T cells ex vivo or after stimulation with pbCD3 for 18h.
Figure S11. LXR inhibits proliferation through Abcg1-dependent alteration of cholesterol homeostasis. CFSE dilution of purified Abcg1−/− and WT T cells stimulated with pma/ionomycin and LXR ligand for 96h. 5×104 counting beads were added to serve as an internal counting control and samples were analyzed via flow cytometry.