Abstract
Context: The relationship between calcium (Ca) intake and Ca retention in adolescent boys was recently reported.
Objective: This study evaluated the influence of Ca intake, serum hormone levels, biomarkers of bone metabolism, habitual physical activity, habitual Ca intake, and physical fitness on Ca retention in the same sample.
Design: This study was a randomized, cross-over design that consisted of two 3-wk metabolic balance periods.
Setting: The study took place on a university campus as a summer camp.
Patients or Other Participants: A total of 31 American white boys (13–15 yr) participated in the study.
Interventions: Each subject consumed a controlled diet with one of five high-low Ca intake pairs that ranged from 670-2003 mg/d, which was manipulated utilizing a fortified beverage.
Main Outcome Measures: Ca retention was determined by Ca intake minus urinary and fecal Ca excretion during each balance period.
Results: Ca intake explained 21.7% of the variability in Ca retention, and serum IGF-I concentration explained an additional 11.5%. Other serum hormone levels did not significantly add to the model. Biomarkers of bone metabolism, habitual physical activity, habitual Ca intake, and physical fitness were not significant predictors of Ca retention in adolescent boys.
Conclusions: IGF-I, a regulator of growth during puberty, is an important predictor of Ca retention in adolescent boys. However, dietary Ca intake is an even greater predictor of Ca retention during this period of growth.
A metabolic balance study in adolescent boys shows that calcium intake and serum IGF-1 predict calcium retention.
Between 25 and 50% of peak bone mass in the adult is accumulated during the adolescent growth period (1). Maximizing calcium (Ca) retention during the pubertal growth spurt is essential for optimizing peak bone mass. Identifying predictors of Ca retention during the adolescent growth period can provide a rationale for making recommendations to maximize Ca retention during this crucial time in bone development.
Dietary Ca intake is a major predictor of Ca retention. It has been demonstrated that Ca retention follows a “threshold behavior” during growth (2). The mean Ca intake required for maximal retention in girls is 1300 mg/d (3), which was the basis of the recommended Ca intake for adolescents by the Institute of Medicine (4). Boys achieve maximum skeletal Ca accretion rates between 13 and 15 yr, 1 yr later than girls (1). Boys have larger skeletons than girls of the same body size (5) but are more efficient at retaining Ca at a given Ca intake. Our previous report on maximal Ca retention in adolescent boys shows that mean Ca intake for maximal retention is 1140 mg/d, at which a threshold is reached, and no significant further gains in retention occur with increased intake (6).
Ca intake and sexual maturity, as determined by postmenarcheal age (PMA), explained 15 and 10%, respectively, of the variation in Ca retention in girls (3). The goal of the present investigation was to determine predictors of Ca retention in boys. In addition to investigating the predictors that were significant in girls (Ca intake, biomarkers of bone turnover, and sexual maturity), the influence of other factors were evaluated, including: age, height, weight, serum vitamin D metabolites, PTH and IGF-I, total bone mineral content of the skeleton, serum sex hormones, habitual dietary Ca intake, physical fitness, and habitual physical activity.
Subjects and Methods
Subjects
The study design and population are described in detail elsewhere (6). Briefly, 31 healthy male American white boys, aged 13–15 yr, were recruited from Indiana schools. Medical questionnaires, physical examinations, and blood biochemistries were used to assess the health status in all subjects. Inclusion criteria for participation included no medical problems or use of medications known to interfere with Ca metabolism. Subjects lived on the Purdue University campus in a residence hall for two 3-wk periods of the summer in a camp environment.
Each subject was tested both on a relatively low dietary Ca intake and a relatively high dietary Ca intake, differing by about 400 mg/d. The study used a randomized cross-over design with 3 wk for each dietary intake, separated by a 2-wk washout period. Subjects were randomized to one of five low-high Ca intake pairs, and group assignments were adjusted when necessary to minimize mean differences in sexual maturity among pairs. The Ca intake pairs were: 800 and 1300; 900 and 1500; 1000 and 1700; 1100 and 1900; 1200 and 2100 mg/d in which actual intakes ranged from 670–2003 mg/d. Ca intakes were manipulated by a Ca citrate malate-fortified orange beverage (6). Subjects were blinded as to their treatment allocations. The study was approved by the Purdue University and Indiana University Purdue University at Indianapolis Institutional Review Boards.
Pubertal status and anthropometrics
Self-assessed pubertal status was determined by Tanner stage (7) using a questionnaire administered by a research coordinator. Total body bone mineral density (TBBMD), total body bone mineral content (TBBMC), percent body fat, and percent lean mass were measured by dual-energy x-ray absorptiometry (DPX-IQ; Lunar Corp., Madison, WI). Total body Ca content (TBCC) was calculated as 38% total bone mineral content. Height and weight of subjects lightly clad without shoes were measured at the dual-energy x-ray absorptiometry scan using a wall stadiometer and a calibrated electronic scale, respectively. Weight was measured daily throughout the study.
Habitual dietary intake and physical activity assessment
Habitual dietary Ca was assessed by 6-d food records, which were analyzed by computer software (version 5.0–3.5; Nutrition Data System for Research, University of Minnesota). The habitual physical activity level was assessed during the camp by the Godin Leisure-Time Exercise Questionnaire (8). The 12 subjects in the upper and lower quartiles of physical activity levels from the Godin Leisure-Time Exercise Questionnaire were further evaluated. Physical fitness was assessed as an estimate of maximal aerobic capacity (VO2max) from 22 observations on these 12 subjects. VO2max was extrapolated from submaximal intensity exercise gas exchange data (TrueMax; ParvoMedics, Salt Lake City, UT) during a physical work capacity test (PWC170) on a cycle ergometer (Monark, Stockholm, Sweden). Heart rate was measured by telemetry (Polar, Oy, Finland) at the end of each minute, and the test was terminated after completion of the work rate during which the heart rate reached 170 beats per minute.
Ca retention calculation
Ca retention was determined during the final 2 wk of each balance period. Ca retention was calculated by subtracting the sum of urinary and fecal Ca excretion from dietary Ca intake. The first week of each 3-wk period was regarded as a period of adaptation to the diet. A balance period began and ended on a day with a fecal sample, and included all days in between. The sum of intake minus the sum of excretion was averaged over the time period of compliance.
Analysis of daily meal composites verified dietary Ca intake. Both fecal and urine samples were pooled as 24-h collections. Diet, fecal, and urine samples were measured for Ca content by inductively coupled plasma spectrophotometry (Optima 4300DV; PerkinElmer, Shelton, CT).
Compliance measures
Creatinine was used both to assess urine collection compliance based on a constant daily urinary creatinine excretion and to adjust for timing of 24-h collections. Urinary creatinine was measured using a colorimetric procedure on a Cobas Mira Plus (Roche Diagnostic Systems, Branchburg, NJ). Each subject ingested 3 g/d polyethylene glycol (PEG) (E3350; Dow Chemical Co., Midland, MI), and percent PEG recovery in the feces was used to assess fecal collection compliance during the balance period. PEG recovery was used to decide whether data should be included rather than used for fecal Ca adjustment. Percent PEG recovery in feces was analyzed using a turbidimetric assay (9). All samples were analyzed in duplicate.
Hormones and biomarkers of bone metabolism
Blood was drawn after an 8-h overnight fast during each balance period for biochemistries. A blood draw was collected on d 4 for measurements of hematology, Ca, and sex hormones. Blood was also drawn at the end of each balance period for analysis of serum 1,25-dihydroxyvitamin D [1,25(OH)2D], 25-hydroxyvitamin D [25(OH)D], PTH, IGF-I, and IGF binding protein (IGFBP) 3.
Sera were analyzed for IGF-I and IGFBP3, testosterone, free testosterone, estrone, and SHBG by ELISA (Diagnostic Systems Laboratories, Inc., Webster, TX), with interassay coefficients of variation (CVs) of 7.0, 11.7, 5.0, 6.2, 10.5, and 10.5%, respectively. Serum PTH 1–84 was measured by a two-site immunoassay (CV of 7.1%) (Nichols Institute Diagnostics, San Juan Capistrano, CA). 25(OH)D and 1,25(OH)2D were analyzed by protein binding assays (CV of 8.1 and 9.1%, respectively) (DiaSorin Inc., Stillwater, MN). Osteocalcin (OC) was measured by immunoradiometric assay (CV of 6.7%), and bone-specific alkaline phosphatase (BAP) was measured using ELISA (CV of 4.1%) (Quidel Corp., San Diego, CA).
Cross linked N-telopeptide (NTx) and free deoxypyridinoline (fDpd) by ELISA [CV of 7.5% (Ostex Intl., Seattle, WA) and of 7.6% (Quidel), respectively] were measured in fasting urine collected after an overnight fast at the end of each balance period.
Statistics
Ca retention was modeled as a function of Ca intake. Model building methods (10) were used to determine an appropriate functional form to describe the nonlinear relationship between Ca intake and Ca retention. A basic model, developed to describe the relationship between Ca intake and Ca retention, was previously reported (6). This process led to a model of the form: Y = β0eL /(1 + eL), where Y is the mean Ca retention for a given Ca intake: L = β1(1 + β2x), x is Ca intake, and e is the base for the natural logarithm. The parameters of the fitted model are β0, β1, and β2. Multiple observations on the same subject are assumed to be correlated. Model building techniques, including nonparametric regression and the analysis of residuals, were used to determine appropriate models for the inclusion of additional explanatory variables, including age, height, weight, sexual maturity, body fat percentage, lean body mass percentage, vitamin D metabolites, PTH, IGF-I, IGFBP3, TBBMD, TBBMC, TBCC, OC, BAP, NTx, fDpd, estrone, testosterone, and SHBG, habitual dietary Ca intake, and physical fitness and habitual physical activity.
Statistical significance was set at P < 0.05. All statistical analyses were done using the Statistical Analysis System program (SAS Institute Inc., Cary, NC).
Results
A total of 32 boys enrolled in the study, 31 completed at least one 3-wk session, and 26 completed both sessions. Table 1 summarizes physical characteristics of the subjects. Baseline characteristics of subjects were similar between Ca intake pairs. Median genitalia Tanner score was III, with a distribution of stage: I (n = 2), II (n = 4), III (n = 13), IV (n = 5), and V (n = 7). Median pubic hair Tanner score was IV, with a distribution of stage: I (n = 2), II (n = 4), III (n = 8), IV (n = 12), and V (n = 6). Mean age was 13.7 ± 0.6 yr, and mean body mass index (BMI)-for-age percentile was 72.9 ± 26.6 (11) (Table 1). Mean habitual Ca intake (952 ± 311 mg/d) fell below the recommended daily intake of 1300 mg/d (Table 2).
Table 1.
Physical characteristics of subjects
| Characteristic | n | x̄ ± SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|
| Age (yr) | 31 | 13.7 ± 0.6 | 12.6 | 13.7 | 15.0 |
| Height (cm) | 31 | 165.7 ± 7.8 | 155.0 | 165.0 | 184.0 |
| Weight (kg) | 31 | 64.9 ± 18.8 | 39.0 | 61.0 | 118.0 |
| BMI (kg/m2) | 31 | 23.4 ± 5.8 | 16.2 | 22.5 | 40.4 |
| BMI-for-age (percentile)a | 31 | 72.9 ± 26.6 | 12.0 | 87.6 | 99.6 |
| TBBMD (g/cm2) | 28 | 1.1 ± 0.1 | 0.9 | 1.1 | 1.4 |
| TBBMC (g) | 28 | 2571.2 ± 657.6 | 1675.0 | 2521.0 | 3914.0 |
| TBCC (g) | 28 | 976.6 ± 249.8 | 636.0 | 957.5 | 1487.0 |
| Lean body mass (%) | 28 | 78.9 ± 12.1 | 47.4 | 82.9 | 95.9 |
| Body fat (%) | 28 | 21.1 ± 12.1 | 4.1 | 17.1 | 52.6 |
Percentiles based on the 2000 Centers for Disease Control and Prevention growth charts (11).
Table 2.
Summary of lifestyle factors
| n | x̄ ± SD | Minimum | Median | Maximum | |
|---|---|---|---|---|---|
| Pre-study values | |||||
| Habitual Ca intake (mg/d) | 29 | 952 ± 311 | 454 | 955 | 1487 |
| Leisure activity score | 29 | 85 ± 34 | 31 | 75 | 166 |
| Study values | |||||
| Actual Ca intake (mg/d) | 28 | 1198 ± 401 | 670 | 1085 | 2003 |
| VO2max (ml/kg·min) | 12 | 36.9 ± 6.3 | 24.9 | 39.3 | 44.2 |
Biochemistries taken at baseline and those taken at the end of each balance period are shown in Table 3. Serum IGFBP3 and 1,25(OH)2D were higher during high Ca intake compared with low Ca intake (P < 0.01 and P < 0.05, respectively). Serum BAP was higher during low Ca intake compared with high Ca intake (P < 0.05) (Table 3).
Table 3.
Biochemical measures at baseline and at the end of each session on low and high Ca intake levels
| n | x̄ ± SD | Minimum | Median | Maximum | x̄ ± SD | Minimum | Median | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| Baseline measurements | |||||||||
| Testosterone (ng/ml) | 26 | 5.6 ± 2.7 | 0.8 | 5.1 | 11.3 | ||||
| Free testosterone (pg/ml) | 26 | 9.5 ± 4.8 | 1.5 | 9.3 | 17.6 | ||||
| Estrone (pg/ml) | 26 | 348.3 ± 262.7 | 106.4 | 250.2 | 1249.3 | ||||
| SHBG (pmol/ml)
|
27
|
132.0 ± 102.8
|
25.2
|
86.6
|
457.3
|
|
|
|
|
| Low Ca intake (670–1104 mg/d) | High Ca intake (1168–2003 mg/d) | ||||||||
| Serum PTH (pg/ml) | 26 | 29.0 ± 12.5 | 8.6 | 26.3 | 54.1 | 27.1 ± 7.0 | 14.6 | 25.9 | 44.2 |
| 1,25(OH)2D (pg/ml) | 26 | 38.5 ± 9.2a | 22.4 | 36.4 | 61.3 | 47.6 ± 15.0 | 24.0 | 47.5 | 86.9 |
| 25(OH)2D (ng/ml) | 26 | 28.4 ± 5.3 | 16.3 | 28.4 | 37.7 | 30.4 ± 6.1 | 19.4 | 29.9 | 40.8 |
| IGF-I (ng/ml) | 26 | 369.9 ± 113.2 | 183.4 | 386.3 | 637.8 | 338.9 ± 96.8 | 130.2 | 329.4 | 520.9 |
| IGFBP3 (ng/ml) | 26 | 2296.5 ± 901.9b | 1974.0 | 2774.5 | 5989.0 | 3610.2 ± 671.1 | 2498.0 | 3449.0 | 4931.0 |
| BAP (ng/ml) | 26 | 93.8 ± 36.1a | 29.6 | 82.5 | 165.8 | 87.1 ± 33.1 | 34.4 | 89.3 | 165.8 |
| OC (ng/ml) | 26 | 64.2 ± 31.9 | 19.1 | 62.5 | 131.4 | 68.8 ± 28.2 | 29.4 | 64.1 | 123.9 |
| Urinary free Dpd:Cr (nmol/mmol) | 26 | 32.7 ± 11.9 | 12.5 | 33.8 | 59.3 | 31.0 ± 10.5 | 12.9 | 31.9 | 52.6 |
| Urinary NTx:Cr (nmol BCE/mmol Cr) | 26 | 608.3 ± 273.9 | 180.3 | 590.8 | 1327.6 | 596.7 ± 265.7 | 169.6 | 520.2 | 1149.9 |
Assigned low Ca intake levels ranged from 800-1200 mg/d, and assigned high Ca intake levels ranged from 1300–2100 mg/d. Actual Ca intakes assessed from diet composites are in parentheses at the top of each corresponding section in the table. Paired t tests were used to compare differences between biochemistries at low and high Ca intakes. Therefore, only the subjects who were measured during both conditions were included in this analysis. BCE, Bone collagen equivalents; Cr, creatinine; Dpd, deoxypyridinoline.
P < 0.01 for paired t tests between low and high Ca intakes.
P < 0.05 for paired t tests between low and high Ca intakes.
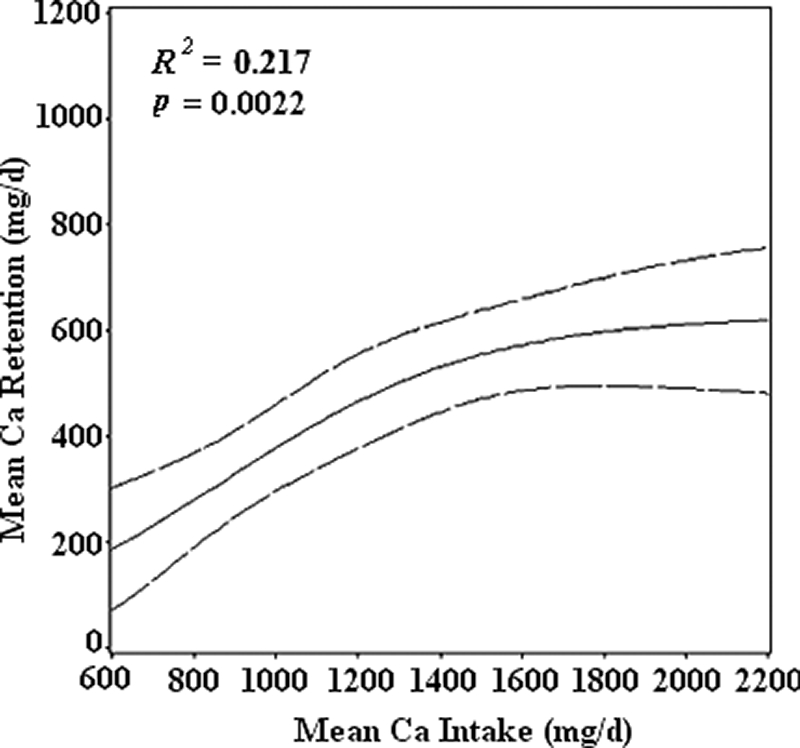
The fitted nonlinear model for predicting Ca retention from dietary intake in boys (6) is shown in Fig. 1. Ca intake explained 21.7% (P = 0.0022) of the variation in Ca retention. Based on an examination of residual plots, serum IGF-I, IGFBP3, and testosterone were identified as candidate predictors for inclusion in the model. IGF-I was the best predictor and explained an additional 11.5% (P = 0.0051) of the variation. With IGF-I in the model, neither IGFBP3 nor testosterone was statistically significant. The final nonlinear regression model is:
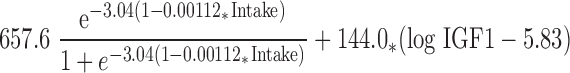 |
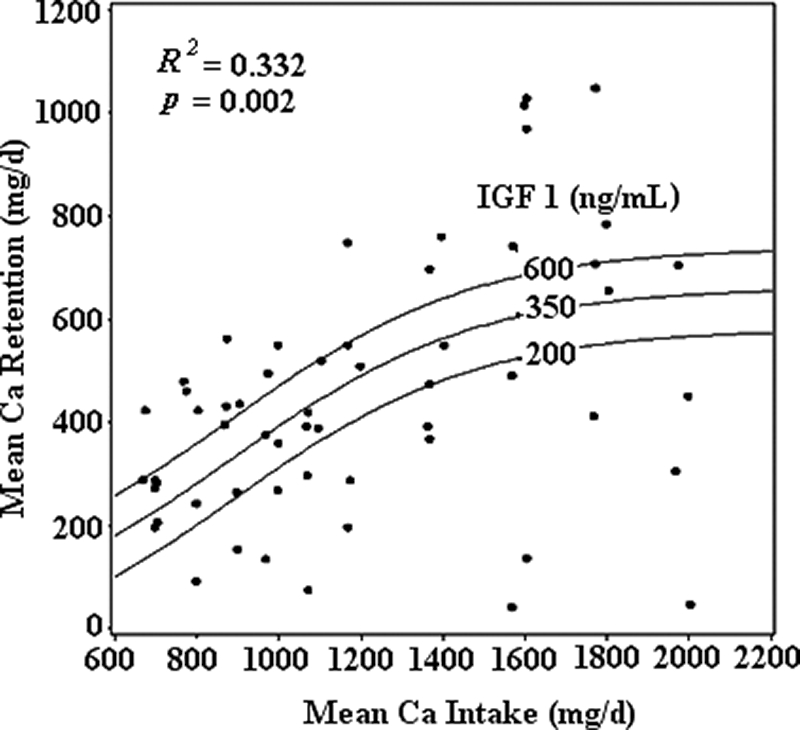
(Fig. 2). For this model, R2 = 0.332 and P = 0.0002.
Figure 1.
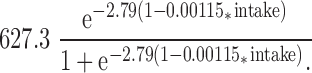 |
Figure 2.
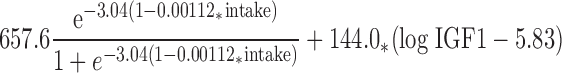 |
Serum 1,25(OH)2D, 25(OH)D, PTH, testosterone, free testosterone, estrone, SHBG, BAP, OC, and urinary NTx and fDpd were not significant predictors of Ca retention in pubertal boys.
TBBMD, TBBMC, and TBCC were not significant predictors of Ca retention. Genitalia and pubic hair Tanner scores were correlated with IGF-I (r = 0.30, P < 0.05; and r = 0.50, P < 0.001, respectively), but not with IGFBP3 (r = 0.05, P = 0.71; and r = 0.19, P = 0.18, respectively). Height, weight, BMI, percent body fat, and percent lean mass did not explain the variation Ca retention in boys. Habitual Ca intake, habitual physical activity level, and physical fitness were not significant predictors of Ca retention.
The subjects demonstrated good sample collection compliance, as indicated by creatinine and PEG analysis (85 ± 21% PEG recovery). Stable Ca to PEG ratios were observed after wk 1 in both balance periods, suggesting that subjects had adapted to Ca intake. Percent PEG recovery was consistent across Ca intakes and was not different between sessions. Neither month of balance period nor treatment order had a significant effect on Ca retention.
Discussion
In adolescent boys, 21.7% of the variation in Ca retention was explained by Ca intake, and serum IGF-I concentration explained an additional 11.5% of the variance. In contrast, Ca intake explained only 15% of the variation in Ca retention in girls (3). The ability to explain more variability in Ca retention by Ca intake in boys than girls may be attributable to lack of hormonal fluctuations associated with the menstrual cycle. In girls, PMA predicted 10% of the variation in Ca retention, in which a higher PMA predicted lower Ca retention. There is not a measurement in boys analogous to PMA in girls. Because menarche in girls is regulated by female sex hormones, the major sex hormone in boys, testosterone, could account for similar variation in Ca retention in boys.
Testosterone was a significant predictor of Ca retention in boys when added to the model that included Ca intake. However, IGF-I was a stronger predictor than testosterone. After IGF-I was included in the model of Ca retention by Ca intake, testosterone did not explain any further variation in Ca retention, which indicates that IGF-I and testosterone explain similar components of variation in Ca retention in boys but that IGF-I is a better predictor.
IGF-I is a polypeptide that is a major regulator of bone growth during childhood and adolescence, primarily through its influence on longitudinal bone growth by stimulation of proliferation and differentiation of chondrocytes at the epiphyses (12). Circulating IGF-I was associated with cross-sectional area and cortical bone area in adolescent boys and girls (13). Serum IGF-I increases and peaks during puberty closely matched with peak bone accretion rates and serum OC, a marker of bone formation (14). Serum IGF-I increases until about 6 months after menarche in girls, which corresponds to Tanner stages III–IV (15), and are maximal at 14.5 yr in girls and 15.5 yr in boys (16). We do not have IGF-I data in girls for comparison.
Matkovic and Heaney (2) demonstrated that dietary Ca intake is related to Ca retention and that Ca retention exhibits “threshold behavior.” To accomplish this, they used data from studies from different laboratories with different protocols. We tested this hypothesis under controlled conditions at the same time and in the same laboratory to determine Ca intake for maximal Ca retention in boys (6). Maximal Ca retention was observed at a mean Ca intake of 1140 mg/d and Ca retention of 442 mg/d. This threshold intake is apparent when Ca retention is plotted as maximal retention, as was done in our previous publication (6). We chose to plot Ca retention as mg/d here to illustrate quantitatively the additional effect of IGF-I. When Ca retention is plotted as mg/d, Ca retention appears to continue to increase beyond 1140 mg/d, but the further increases are not statistically significant. The mean maximal Ca retention of 442 mg/d at a Ca intake of 1140 mg/d is similar to that observed in a longitudinal study by Bailey et al. (1), which reported a mean Ca retention of 359 mg/d on a Ca intake of 1140 ± 392 mg/d estimated from diet records. Our short-term controlled-setting balance study has the advantage of precise Ca intakes compared with the large error associated with estimates of self-reported intakes and wide fluctuations in day-to-day dietary intakes in free-living subjects as in the longitudinal study by Bailey et al. (1). On the other hand, the study by Bailey et al. (1) has the advantage of more precisely measuring skeletal Ca accretion over time. It is encouraging that the results for Ca retention by these two methods are so similar. Likewise, the estimated bone Ca content that would be accrued in 1 yr for our subjects calculated from our predicted mean Ca retention (442 mg/d) and the observed Ca retention from Bailey et al. (1) (359 mg/d) are similar at 17.6 and 14.3%, respectively (6).
Bone biomarkers were not significant predictors of Ca retention in boys. This may be due to the narrow range of these biochemical markers in our study population. The boys in our study were in a narrow age range. It is likely that biomarkers of bone turnover would be significant predictors of Ca retention in boys in a cohort of wider range in age, as we reported previously in girls and young women (17).
Vitamin D metabolites and PTH were also not significant predictors of Ca retention in adolescent boys. Bone measurements and body size did not predict Ca retention in adolescent boys. Adult height has predicted Ca retention in girls (18). In addition, as shown in Table 1, the mean BMI-for-age percentile of the subjects in this study was approximately 28 percentile points over the national median according to the 2000 Centers for Disease Control and Prevention growth charts (11), which possibly reflects that Indiana was ranked eighth among the United States for the prevalence of overweight and obesity in 2001 when the study took place (19).
Habitual physical activity and physical fitness were not significant predictors of Ca retention in adolescent boys. Exercise and Ca intervention trials in children and adolescents show an overall beneficial impact of physical activity on bone acquisition during childhood and adolescence (20,21,22,23,24,25). In adolescent boys, high Ca and moderate-impact exercise interventions together resulted in increased bone mineral content at loaded bone sites compared with controls or groups that received only one of the interventions, which supports the role for both exercise and Ca in bone growth during adolescence (20). Physical fitness is associated with increased Ca absorption in men, with minimal increases in Ca excretion (26), but we only had physical fitness data on a small subset of subjects. During growth, the influence of physical fitness on Ca retention may not be detectable in the presence of stronger predictors, such as IGF-I. A limitation of the study is that it was not designed to detect differences in Ca retention at different physical fitness and activity levels.
Habitual Ca was not a significant predictor of Ca retention in our subjects. Equilibration to Ca content is seen within 1 wk on a controlled diet; therefore, it is logical that habitual Ca intake would not contribute to Ca retention during a controlled diet period. The lack of predictive ability of habitual dietary Ca intake on Ca retention, and the strong predictive ability of actual Ca intake from a controlled diet on Ca retention, indicates that the impact of Ca intake on Ca retention may be underestimated in studies in which the methods of assessing intake are those that assess habitual intake (dietary records, dietary recalls, and food frequency questionnaires) and not actual intake (controlled diets).
A general limitation of this study is that it applies to a relatively homogeneous population of 13- to 15-yr-old healthy white boys. Strengths of this study include the highly controlled diet and environment and high subject retention and the cross-over design.
In conclusion, 33.2% of the variation in Ca retention in adolescent white boys was explained by Ca intake and serum IGF-I. This compares with 25% of the variation in Ca retention that was explained in adolescent girls by Ca intake and PMA (3). Variability in Ca retention was not further explained by testosterone, other sex hormones, Tanner stage, bone biomarkers, vitamin D metabolites, PTH, body size, bone measurements, habitual physical activity, physical fitness, or habitual dietary Ca intake in adolescent boys. Although regulators of growth during puberty play an important role in Ca retention, these data indicate that dietary Ca intake plays an even greater role. Further research is needed to determine other predictors of Ca retention in adolescent boys. It is likely that genetics plays a substantial role in explaining the remaining variation.
Acknowledgments
We thank the Camp Calcium staff and the study subjects.
Footnotes
This work was supported by the National Institutes of Health Grant AR 40553.
Present address for J.W.N.: Department of Physical Education and Recreation, Western Kentucky University, 1906 College Heights Boulevard, Bowling Green, Kentucky 42101.
Disclosure Summary: C.M.W. is on the Boards of Wyeth Global Nutrition and Pharmavite. C.M.W., G.P.M., B.R.M., and M.P. were responsible for the study design. M.B., B.R.M., M.K., J.W.N., and D.A.S. were responsible for conducting the study and the data collection. K.M.H., M.B., B.R.M., G.P.M., and L.M. were responsible for the data analysis. All authors were responsible for preparing the manuscript. None of the authors had a personal or financial conflict of interest.
First Published Online October 7, 2008
Abbreviations: 1,25(OH)2D1, 25-Dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin; BAP, bone-specific alkaline phosphatase; BMI, body mass index; Ca, calcium; CV, coefficient of variation; fDpd, free deoxypyridinoline; IGFBP, IGF binding protein; NTx, cross linked N-telopeptide; OC, osteocalcin; PEG, polyethylene glycol; PMA, postmenarcheal age; TBBMC, total body bone mineral content; TBBMD, total body bone mineral density; TBCC, total body calcium content; VO2max, maximal aerobic capacity.
References
- Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R 2000 Calcium accretion in girls and body during puberty: a longitudinal analysis. J Bone Miner Res 15:2245–2250 [DOI] [PubMed] [Google Scholar]
- Matkovic V, Heaney RP 1992 Calcium balance during human growth: evidence for threshold behavior. Am J Clin Nutr 55:992–996 [DOI] [PubMed] [Google Scholar]
- Jackman LA, Millane SS, Martin BR, Wood OB, McCabe GP, Peacock M, Weaver CM 1997 Calcium retention in relation to calcium intake and postmenarcheal age in adolescent females. Am J Clin Nutr 66:327–333 [DOI] [PubMed] [Google Scholar]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine 1997 DRI dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press [Google Scholar]
- Nieves JW, Formica C, Ruffing J, Zion M, Garrett P, Lindsay R, Cosman F 2005 Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res 20:529–535 [DOI] [PubMed] [Google Scholar]
- Braun M, Martin BR, Kern M, McCabe GP, Peacock M, Jiang Z, Weaver CM 2006 Calcium retention in adolescent boys on a range of controlled calcium intakes. Am J Clin Nutr 84:414–418 [DOI] [PubMed] [Google Scholar]
- Tanner J 1962 Growth at adolescence; with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. 2nd ed. Oxford: Blackwell Scientific Publications [Google Scholar]
- Godin G, Shephard RJ 1985 A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 10:141–146 [PubMed] [Google Scholar]
- Allen LH, Raynolds WL, Margen S 1979 Polyethylene glycol as a quantitative fecal marker in human nutrition experiments. Am J Clin Nutr 32:427–440 [DOI] [PubMed] [Google Scholar]
- Kunter MHN, Christopher J, Neter J, Li W 2005 Applied linear statistical models. 5th ed. New York: McGraw-Hill/Irwin [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL 2000 CDC growth charts: United States. Advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics [PubMed] [Google Scholar]
- Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA 2004 Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol 180:247–255 [DOI] [PubMed] [Google Scholar]
- Mora S, Pitukcheewanont P, Nelson JC, Gilsanz V 1999 Serum levels of insulin-like growth factor I and the density, volume, and cross-sectional area of cortical bone in children. J Clin Endocrinol Metab 84:2780–2783 [DOI] [PubMed] [Google Scholar]
- Johansen JS, Giwercman A, Hartwell D, Nielsen CT, Price PA, Christiansen C, Skakkebaek NE 1988 Serum bone Gla-protein as a marker of bone growth in children and adolescents: correlation with age, height, serum insulin-like growth factor I, and serum testosterone. J Clin Endocrinol Metab 67:273–278 [DOI] [PubMed] [Google Scholar]
- Cadogan J, Blumsohn A, Barker ME, Eastell R 1998 A longitudinal study of bone gain in pubertal girls: anthropometric and biochemical correlates. J Bone Miner Res 13:1602–1612 [DOI] [PubMed] [Google Scholar]
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Mueller J, Hall K, Skakkebaek NE 1994 Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 78:744–752 [DOI] [PubMed] [Google Scholar]
- Weaver CM, Peacock M, Martin BR, Plaweki KL, McCabe GP 1996 Calcium retention estimated from indicators of skeletal status in adolescent girls and young women. Am J Clin Nutr 64:67–70 [DOI] [PubMed] [Google Scholar]
- Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ, Skugor M, Nagode LA, Mobley SL, Ha EJ, Hangartner TN, Clairmont A 2005 Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr 81:175–188 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2007 Behavioral risk factor surveillance system survey data. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EM, Hume C, Nowson C 2007 Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res 22:458–464 [DOI] [PubMed] [Google Scholar]
- Bradney M, Pearce G, Naughton GA, Sullivan C, Bass SL, Beck T, Carlson JS, Seeman E 1998 Moderate exercise during growth in prepubertal boys: Changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J Bone Miner Res 13:1814–1821 [DOI] [PubMed] [Google Scholar]
- Iuliano-Burns S, Saxon L, Naughton GA, Gibbons K, Bass SL 2003 Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res 18:156–162 [DOI] [PubMed] [Google Scholar]
- Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD 1997 Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res 12:1453–1462 [DOI] [PubMed] [Google Scholar]
- Specker BL, Mulligan L, Ho M 1999 Longitudinal study of calcium intake, physical activity, and bone mineral content in infants 6–18 months of age. J Bone Miner Res 14:569–576 [DOI] [PubMed] [Google Scholar]
- Stear SJ, Prentice A, Jones SC, Cole TJ 2003 Effect of a calcium and exercise intervention on the bone mineral status of 16–18-y-old adolescent girls. Am J Clin Nutr 77:985–992 [DOI] [PubMed] [Google Scholar]
- Zitterman A, Sabatschus O, Jantzen S, Platen P, Danz A, Dimitriou T, Scheld K, Stehle P 2000 Exercise-trained young men have higher calcium absorption rates and plasma calcitriol levels compared with age-matched sedentary controls. Calcif Tissue Int 67:215–219 [DOI] [PubMed] [Google Scholar]


