Abstract
Context: A past history of gestational diabetes mellitus (GDM) confers a very high risk of postpartum development of diabetes, particularly type 2 diabetes.
Objective: The Diabetes Prevention Program (DPP) sought to identify individuals with impaired glucose tolerance (IGT) and intervene in an effort to prevent or delay their progression to diabetes. This analysis examined the differences between women enrolled in DPP with and without a reported history of GDM.
Design: The DPP was a randomized, controlled clinical trial.
Setting: The study was a multicenter, National Institutes of Health-sponsored trial carried out at 27 centers including academic and Indian Health Services sites.
Patients: A total of 2190 women were randomized into the DPP and provided information for past history of GDM. This analysis addressed the differences between those 350 women providing a past history of GDM and those 1416 women with a previous live birth but no history of GDM.
Interventions: Subjects were randomized to either standard lifestyle and placebo or metformin therapy or to an intensive lifestyle intervention.
Main Outcomes: The primary outcome was the time to development of diabetes ascertained by semiannual fasting plasma glucose and annual oral glucose tolerance testing. Assessments of insulin secretion and insulin sensitivity were also performed.
Results: Whereas entering the study with similar glucose levels, women with a history of GDM randomized to placebo had a crude incidence rate of diabetes 71% higher than that of women without such a history. Among women reporting a history of GDM, both intensive lifestyle and metformin therapy reduced the incidence of diabetes by approximately 50% compared with the placebo group, whereas this reduction was 49 and 14%, respectively in parous women without GDM. These data suggest that metformin may be more effective in women with a GDM history as compared with those without.
Conclusions: Progression to diabetes is more common in women with a history of GDM compared with those without GDM history despite equivalent degrees of IGT at baseline. Both intensive lifestyle and metformin are highly effective in delaying or preventing diabetes in women with IGT and a history of GDM.
Diabetes development is more common in women with gestational diabetes mellitus histories compared to those without, despite similar degrees of impaired glucose tolerance; lifestyle and metformin equivalently delay diabetes.
The original identification of gestational diabetes mellitus (GDM) by O’Sullivan and Mahan in 1964 recognized this population’s increased risk of the future development of diabetes (1). Pregnant women undergoing a 3-h, 100-g oral glucose tolerance test with glucose values exceeding 2 sd above the mean on two of the four values were defined as having GDM. In this landmark study, the investigators described a population of pregnant women with a lifetime risk of diabetes exceeding 70% (2).
In recognition of this marked risk for diabetes, the Diabetes Prevention Program (DPP) sought to identify women with a previous history of GDM for inclusion in this large prospective study of the ability of metformin therapy or intensive lifestyle (ILS) to prevent diabetes in individuals with impaired glucose tolerance (IGT) (3). The population enrolled included a multiethnic population of men and women spanning the age range of 25–89 yr (4). As previously reported for the cohort as a whole, ILS reduced the incidence of diabetes by 58%, whereas metformin reduced it by 31%, compared with the placebo control group (5). We now report a comparison of baseline characteristics and responses to intervention in subgroups of women enrolled in DPP with a history of GDM compared with parous women in the DPP without a history of GDM.
Subjects and Methods
Subjects
The DPP design, eligibility, and baseline characteristics have been reported elsewhere (3,4). The study prespecified a recruitment target and planned hypotheses regarding women with a history of GDM. Briefly, 3234 participants with IGT were identified, qualified as having IGT by a 2-h oral glucose tolerance test and randomized to three different treatment groups (placebo, metformin, and ILS) in 27 clinical centers throughout the United States. By entry criterion, all participants in DPP were required to be older than 25 yr of age. Eligibility required a fasting plasma glucose value of 95–125 mg/dl and a 2-h value, after a 75-g glucose load, between 140 and 199 mg/dl. (The minimum fasting glucose was not a requirement in the American Indian centers.) At the time of randomization, all women completed a simple questionnaire relating to their number of pregnancies and live births and whether they were complicated by gestational diabetes. No further information on the index pregnancies was possible due to the time past from the pregnancy until recruitment. Of 2190 women in DPP, 350 reported a history of GDM and 1840 did not. Of the 1840 women without history of GDM, 321 had never been pregnant. Of the 1519 with pregnancies, 1416 had at least one live birth. This report restricted analyses to the 1416 women without a history of GDM reporting at least one live birth and all 350 women with a history of GDM because all reported at least one live birth. Of the 350 women with a history of GDM, 122 were assigned to placebo, 111 to metformin, and 117 to ILS, whereas among the 1416 women without a history of GDM, 487 were assigned to placebo, 464 to metformin, and 465 to ILS.
Statistical analysis
Baseline characteristics were described using percentages for categorical variables and means ± sd for quantitative variables. For variables with highly skewed distributions a logarithmic transformation was done first, and the geometric means were reported instead. Comparisons among groups were done using the χ2 test of independence for categorical variables and the t test for quantitative variables.
Cox proportional hazards models (6) were used to assess the effect of the treatment group on the development of diabetes. Analyses stratified on reported history of GDM were performed, and a test of heterogeneity was used to determine whether the effect of the treatment varied between the subgroups based on GDM history, after adjusting for age at randomization. Because interactions were examined as tests of hypotheses, they were interpreted cautiously because of the multiplicity of such tests in DPP analyses. Treatments were compared within each GDM subgroup using the log-rank test. Similarly, analyses stratified on treatment group were performed comparing the GDM subgroups within each treatment.
The impact of the interventions on the development of cardiovascular risk factors in women with a history of GDM compared with women without a history of GDM was evaluated with mixed-effects models (7) that estimated mean differences over time in physical activity and weight, adjusted for the baseline values as well as age at randomization. Generalized estimating equations (8) were used to assess differences over time in the percentage of participants with adherence to medication, adjusted for age at randomization. P values for comparisons between any two groups were adjusted for multiple comparisons using the Holm procedure (9).
A P < 0.05 was considered to be statistically significant and all tests were two sided. The Statistical Analysis Software (SAS) version 8.2 was used for all analyses (SAS Institute, Inc., Cary, NC).
Results
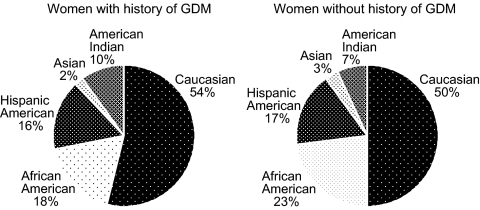
Those women with a reported history of GDM enrolled in DPP (n = 350) had a mean 12-yr interval (for n = 207 due to incomplete data) since the delivery of their first GDM pregnancy. They were significantly younger than parous women (n = 1416) without a GDM history (43.0 ± 7.6 vs. 51.5 ± 9.7 yr, P < 0.001), so baseline analyses were adjusted for age. Other characteristics were comparable, including parity, body mass index (BMI), fasting glucose, 2-h postglucose load glucose, glycosylated hemoglobin (HbA1c), insulin sensitivity, and insulin secretion (Table 1). After adjusting for age, lipid levels were similar between those with and without a history of GDM, with LDL-C levels (arithmetic means ± sd) of 121.2 ± 30.9 vs. 125.1 ± 33.6 mg/dl, HDL-C of 45.5 ± 10.3 vs. 48.7 ± 12.3 mg/dl, and triglycerides (geometric means) of 135.3 vs. 140.3 mg/dl, respectively. Systolic blood pressure was lower in those with a history of GDM (117.4 ± 13.1 vs. 124.1 ± 15.4 mm Hg, adjusted for age, P = 0.004). Ethnic distribution was similar in women with vs. without a history of GDM (Fig. 1), with ethnic minority groups representing 46% of women with a history of GDM.
Table 1.
Baseline characteristics of parous women in DPP
| History of GDM | No history of GDM | P value | |
|---|---|---|---|
| n | 350 | 1416 | |
| Age (yr) | 43.0 ± 7.6 | 51.5 ± 9.7 | <0.001 |
| Live births | 2.61 ± 1.32 | 2.63 ± 1.49 | 0.794 |
| BMI (kg/m2) | 34.2 ± 6.2 | 34.6 ± 6.8 | 0.379 |
| Fasting glucose (mg/dl) | 105.8 ± 8.4 | 105.2 ± 7.9 | 0.246 |
| Two-hour glucose (mg/dl) | 165.8 ± 18.0 | 164.2 ± 17.0 | 0.118 |
| HbA1c (%) | 5.87 ± 0.50 | 5.91 ± 0.49 | 0.105 |
| Fasting insulin (μU/ml) | 26.7 ± 14.5 | 26.3 ± 14.2 | 0.616 |
| Insulin to glucose ratio (μU/mg) | 118.3 ± 85.1 | 128.1 ± 92.4 | 0.076 |
Figure 1.
Ethnic representation of parous women with and without a history of GDM randomized into the DPP.
Randomization resulted in balance by age, BMI, HbA1c, insulin to glucose ratio, and reported history of GDM across the three treatment groups. Medication adherence was similar between women with and without a history of GDM, with placebo adherence (72.9 and 76.7%, respectively) significantly greater (P < 0.05) than metformin adherence (68.8 and 70.3%, respectively).
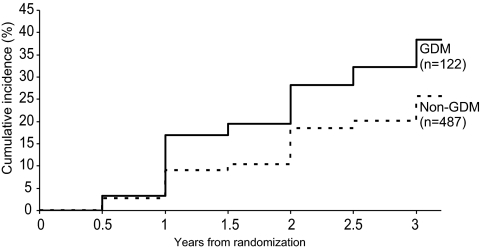
Those women randomized to placebo therapy represent the uninterrupted progression of glucose intolerance within the DPP. Figure 2 illustrates the cumulative incidence of diabetes development over time among parous women by history of GDM. Three years after randomization, the estimated cumulative incidence of diabetes was 38.4% for women with a history of GDM compared with 25.7% for women without a history of GDM. Despite both groups entering the study with similar glucose levels, women with a history of GDM had a 71% increased crude incidence rate per 100 person-years for the development of diabetes compared with women without such a history.
Figure 2.
Cumulative incidence of diabetes mellitus among the placebo group by history of GDM.
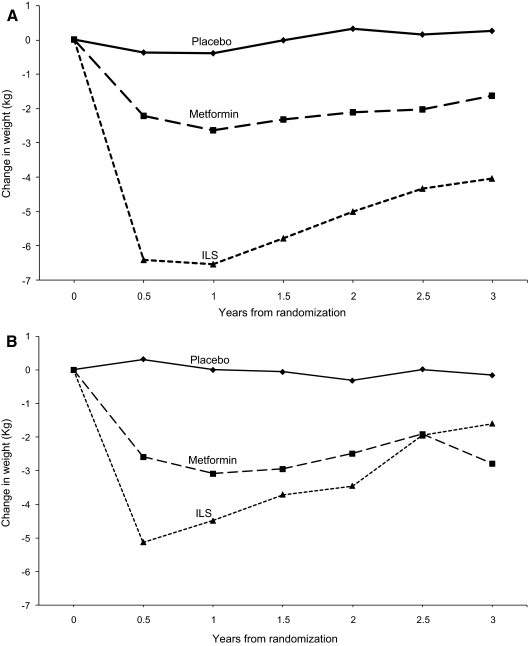
Women randomized to ILS increased their physical activity comparably during the first year of intervention (approximately 1.5 h/wk from baseline activity) in both the GDM and non-GDM groups; however, this was not sustained by the women with a history of GDM (Fig. 3B), falling to less than 30 min of increased physical activity by yr 3. Additionally, as shown in Fig. 3, women with a GDM history had a weight loss nadir of 5.13 ± 0.43 kg at 6 months with a steady weight regain to a mean weight loss of only 1.60 ± 0.80 kg at yr 3 compared with a nadir of 6.40 ± 0.20 kg and a mean weight loss of 4.03 ± 0.40 kg at yr 3 in those women without a history of GDM (Fig. 3A) (P = 0.021 for differences in weight at 3 yr).
Figure 3.
Change in weight during DPP by randomized treatment group. Panel A, Women without a history of GDM; Panel B, women with a history of GDM.
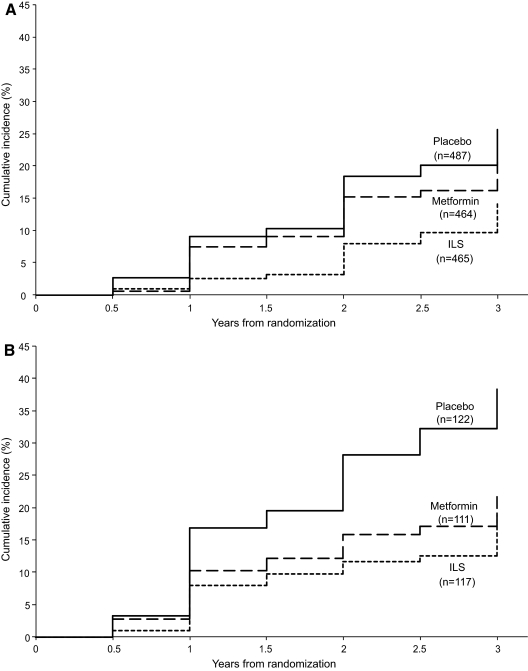
Figure 4 depicts the progression of IGT to diabetes by randomized treatment in those women with and without a history of GDM. Parous women without a history of GDM (Fig. 4A) benefited from ILS, with a 49% risk reduction compared with placebo (P < 0.001) and a 41% risk reduction compared with metformin (P = 0.001). Unlike in the original complete DPP cohort, we did not detect a significant benefit from metformin therapy for this subgroup, with only a 14% risk reduction compared with placebo, although our study was not adequately powered for this subgroup comparison. In contrast, among women with a history of GDM (Fig. 4B), metformin afforded this group a 50% risk reduction (P = 0.006) compared with placebo, whereas ILS achieved a 53% risk reduction (P = 0.002).
Figure 4.
Cumulative incidence of diabetes in DPP by randomized treatment group. Panel A, Women without a history of GDM; Panel B, women with a history of GDM.
Table 2 summarizes the observed hazard rates for the development of diabetes in women with and without a history of GDM, the impact of the therapeutic interventions, and the public health implications of implementing these treatments. In the placebo group, a history of GDM conferred a significantly greater incidence of progression from IGT to diabetes (15.2 cases per 100 person-years compared with 8.9 cases per 100 person-years, P < 0.05). Despite the difference in underlying hazard rate, ILS had a similar impact on risk reduction (compared with placebo) in the two groups (53.4 vs. 49.2%, interaction P = 0.74), whereas metformin tended to be more effective in reducing the incidence of diabetes (compared with placebo) in those women with a history of GDM (50.4 vs. 14.4%, interaction P = 0.06) vs. those without a history of GDM. Taking into account these treatment effects, we estimate that only five to six women with IGT and a history of GDM would need to be treated over 3 yr with either metformin or ILS to prevent one case of diabetes. In women without a history of GDM, the estimated numbers needed to treat to prevent a single case of diabetes over 3 yr are 24 and nine for metformin and ILS, respectively.
Table 2.
Effect of DPP treatment on incidence of diabetes
| Placebo
|
Metformin
|
ILS
|
||||
|---|---|---|---|---|---|---|
| GDM (n = 122) | No GDM (n = 487) | GDM (n = 111) | No GDM (n = 464) | GDM (n = 117) | No GDM (n = 465) | |
| Incidence of diabetes (number of cases per 100 person-years)a | 15.2b | 8.9 | 7.8 | 7.8 | 7.4 | 4.7 |
| Reduction in incidence (compared with placebo)a | 50.4c | 14.4 | 53.4c | 49.2c | ||
| Number needed to treat (to prevent one case in 3 yr compared with placebo)a | 6.1 | 24.0 | 5.3 | 9.0 | ||
Adjusted for age.
P < 0.05 compared with non-GDM group.
P < 0.05 compared with placebo.
Discussion
The historical observations of rapid and progressive development of type 2 diabetes after pregnancy complicated by GDM (2,10,11,12) encouraged DPP investigators to recruit such women into the study. Our inclusion criteria required IGT with an elevated fasting glucose and excluded those with diabetes, thus excluding women with early postpartum conversion to diabetes. Kjos et al. (13) described persistence of diabetes postpartum occurring in as many as 10% of women with a history of GDM and conversion to diabetes in those normalizing glycemia postpartum occurring quickly over the next 5–10 yr with a paucity of data beyond that time (14). The mean age of women with a history of GDM entering DPP was 43 yr with a mean interval of 12 yr from the index GDM pregnancy, suggesting that we may have excluded many women with the highest risk of diabetes conversion. Despite this and the fact that those reporting a history of GDM were younger, the DPP subgroup with a history of GDM had a crude incidence rate of diabetes that was 71% higher than that of parous women without a history of GDM. Even many years after the pregnancy complicated by GDM, it appears that women continue to be at risk for development of diabetes. Therefore, continued follow-up and testing for diabetes should be part of the lifetime assessment of women with a history of GDM.
Although tests of interaction between the effect of interventions and the history of GDM were not significant and the study was not powered for these tests, our data suggest a differential success of the interventions between those with and without history of GDM. Achieving targets for ILS was far less successful among those with a history of GDM. On average, these women were less able to sustain the prescribed level of physical activity and demonstrated a lower peak weight loss as well as a more rapid weight regain, resulting in a significantly lower weight loss over time than women in the ILS group without a history of GDM. Because weight loss was strongly associated with a reduced risk of diabetes in DPP (hazard ratio for 5 kg weight loss = 0.42; 95% confidence interval 0.35–0.51, P < 0.001), it is not surprising that, within ILS, women with a history of GDM had a higher crude incidence rate of diabetes than those without (7.4 vs. 4.7 cases per 100 person-years, P = 0.065). Nonetheless, ILS remains an effective treatment modality for diabetes prevention among women with a history of GDM, requiring therapy of only five women to prevent one case of diabetes over a 3-yr period.
We estimate that metformin therapy, on the other hand, may be as much as 3 times more effective in reducing the incidence of diabetes in those with a history of GDM compared with those without. This may in part be explained by the younger age (mean of 43 yr) of the GDM group because women between 25 and 44 yr of age within DPP as a whole had a similar risk reduction with either metformin or ILS, but metformin was no more effective than placebo in women over age 60 yr (5).
The Troglitazone in Prevention of Diabetes (TRIPOD) study data provide the closest comparison to the DPP results (15). TRIPOD enrolled an exclusively Latina population, whereas DPP was ethnically mixed with 54% Caucasian. In the DPP, the GDM population was older (43 vs. 34 yr) and considerably more distant from their index pregnancies (12 vs. < 4 yr). As a result, we lost those individuals converting to diabetes in the early postpartum years before entering DPP. Nevertheless, parous female DPP participants, both with and without history of GDM, had a marked risk of progressing to diabetes (15.2 and 8.9 cases per 100 person-years, respectively) over the subsequent 3–5 yr. TRIPOD demonstrated a 55% risk reduction with troglitazone treatment, comparable with our observed reductions of 50.4% for metformin and 53.4% for ILS among women with history of GDM.
In summary, in DPP, women with a reported history of GDM had a markedly increased risk for diabetes compared with parous women with a similar degree of glucose intolerance and no GDM history. Intervention with metformin and ILS were comparable in reducing the development of diabetes with only an estimated five to six individuals requiring treatment to prevent one case of diabetes over 3 yr. In women without a history of GDM as well as in the DPP cohort overall, ILS was found to be far more effective than metformin therapy. In those with history of GDM, the absence of a difference between ILS and metformin may be due to the minimal weight loss achieved with ILS among GDM women. We conclude that women with a history of GDM who currently have IGT remain at an increased risk of developing diabetes years after the index pregnancy and appear to benefit from either lifestyle or pharmacologic interventions.
Acknowledgments
The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the coordinating center for the design and conduct of the study and the collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the coordinating center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies. A complete list of centers, investigators, and staff can be found elsewhere (5). Members of the DPP Research Group are listed elsewhere (5).
Footnotes
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 30, 2008
For editorial see page 4646
Abbreviations: BMI, Body mass index; DPP, Diabetes Prevention Program; GDM, gestational diabetes mellitus; HbA1c, glycosylated hemoglobin; IGT, impaired glucose tolerance; ILS, intensive lifestyle; TRIPOD, Troglitazone in Prevention of Diabetes.
Medstar Research Institute (R.E.R.), Hyattsville, Maryland 20783; The Biostatistics Center (C.A.C., S.F.), Diabetes Prevention Program Coordinating Center, The George Washington University, Rockville, Maryland 20852; Northwestern University Feinberg School of Medicine (B.E.M.), Chicago, Illinois 60611; Department of Preventive Medicine and Biometrics (D.D.), University of Colorado, Denver, Colorado 80262; National Institute of Diabetes and Digestive and Kidney Diseases (P.H.B.), Phoenix, Arizona 85016; St. Luke’s-Roosevelt Hospital Center (X.P.-S.), New York, New York 10019; and Veterans Affairs Puget Sound Health Care System and University of Washington (S.E.K.), Seattle, Washington 98108
References
- O'Sullivan JB, Mahan CM 1964 Criteria for the oral glucose tolerance test in pregnancy. Diabetes 13:278–285 [PubMed] [Google Scholar]
- O'Sullivan JB 1991 Diabetes mellitus after GDM. Diabetes 40(Suppl 2):131–135 [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group 1999 The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes mellitus. Diabetes Care 22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group 2000 The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group 2002 The Diabetes Prevention Program: reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR 1972 Regression models in life-tables. J R Statistical Soc (B) 34:187–220 [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL 1994 Analysis of longitudinal data. Oxford, UK: Clarendon Press [Google Scholar]
- Liang KY, Zeger SL 1986 Longitudinal data analysis using generalized linear models. Biometrika 73:13–22 [Google Scholar]
- Holm S 1979 A simple sequentially rejective Bonferroni test procedure. Scan J Statistics 6:5–70 [Google Scholar]
- Damm P, Kuhl C, Bertelsen A, Molsted-Pedersen L 1992 Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol 167:607–616 [DOI] [PubMed] [Google Scholar]
- Metzger BE, Bybee DE, Freinkel N, Phelps RL, Radvany RM, Vaisrub N 1985 Gestational diabetes mellitus: correlations between the phenotypic and genotypic characteristics of the mother and abnormal glucose tolerance during the first year post-partum. Diabetes 34(Suppl 2):111–115 [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Knowler WC, Baird HR, Bennett PH 1980 Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care 3:458–464 [DOI] [PubMed] [Google Scholar]
- Kjos SL, Buchanan TA, Greenspoon JS, Montoro M, Bernstein GS, Mestman JH 1990 Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months postpartum. Am J Obstet Gynecol 163:93–98 [DOI] [PubMed] [Google Scholar]
- Kim C, Newton KM, Knopp RH 2002 Gestational diabetes mellitus and the incidence of type 2 diabetes. Diabetes Care 25:1862–1868 [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP 2002 Preservation of pancreatic B-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 51:2769–2803 [DOI] [PubMed] [Google Scholar]