Abstract
Context: IGF-I and its binding proteins influence growth, development, and disease risk. Studies have revealed ethnic variations in the IGF system.
Objective: This longitudinal study was undertaken to test the hypothesis that the ethnic differences in the IGF system exist throughout the pubertal transition, and these differences are mediated at least in part by inherent differences in insulin dynamics.
Design: This was a longitudinal study. Annual evaluations were conducted for pubertal maturation, body composition, acute insulin response to glucose (AIRg), and reproductive-endocrine profile. Hormones and binding proteins were determined using standard assays, the AIRg during a frequently sampled iv glucose tolerance test, and body composition by dual-energy x-ray absorptiometry. Mixed model analyses were used to identify and characterize ethnic differences in the IGF system across the pubertal transition after adjusting for ethnicity, sex, age, maturation status, body composition, and reproductive hormones, and to identify the contribution of insulin to IGF binding protein (IGFBP)-1.
Participants: Subjects included African-American (AA) and European American children (n = 162 at baseline) aged 7–16 yr, evaluated across the pubertal transition.
Main Outcome Measures: Annual data on IGF-I, IGFBP-1, and IGFBP-3 were examined.
Results: IGF-I was higher in AA children at pubertal stage 1 only (P < 0.001). However, IGFBP-3 and IGFBP-1 concentrations were lower in AAs through much of puberty (P < 0.05). The lower IGFBP-1 of AAs was in part explained by greater AIRg.
Conclusions: Our data suggest that the higher IGF-I and lower IGFBP-1 and IGFBP-3 levels in AAs as compared with European Americans during puberty suggest potential ethnic differences in circulating bioavailable IGF-I. In addition, higher AIRg in AAs may lead to greater bioavailable IGF-I. Whether these differences in the IGF system account for disparities in disease risk warrants further investigation.
African Americans have higher IGF-I concentrations than European Americans only in pre-puberty and lower concentrations of insulin-like growth factor binding protein 3 (IGFBP-3) and IGFBP-1 throughout the pubertal transition; the lower IGFBP-1 of African Americans is due in part to the higher acute insulin response to glucose (AIGg), suggesting physiological relevance for the frequently reported higher AIRg among African Americans.
IGF-I has been identified as a key component in cell survival, proliferation, and differentiation throughout life. During puberty, circulating IGF-I concentrations are approximately two to three times greater than during adulthood and increase with pubertal status, peaking around midpuberty. Indeed, puberty has been suggested as a sensitive period for the programming of adult IGF-I levels (1). Production of IGF-I is stimulated by GH. Puberty is also associated with increased secretion of GH and reproductive hormones, and a transient reduction in insulin sensitivity, with an accompanying elevation in circulating insulin. All of these factors may contribute to the pubertal increase in IGF-I.
Ethnic differences in the IGF system have been identified in cross-sectional studies. Higher circulating IGF-I in African-Americans (AAs) compared with European Americans (EAs) has been observed before puberty, but not in the later part of the second decade of life (i.e. after reproductive maturity) (2,3,4,5). AAs mature earlier than EAs (6). Therefore, higher IGF-I in AA may result from greater relative proximity to puberty. This hypothesis is supported by the inconsistent observation of higher IGF-I among AA vs. EA adults (7,8,9,10,11,12,13), suggesting that this difference may not persist beyond childhood.
IGF-I bioactivity is regulated by a family of IGF binding proteins (IGFBPs) (14). Although evidence suggests that IGFBP-3 and IGFBP-1 are among the most important regulators of circulating IGF-I activity, most of the evidence for in vivo regulation of IGF-I activity by the binding proteins is inferred from in vitro data and/or indirectly hypothesized from in vivo interrelationships of these proteins. Nevertheless, these studies indicate that IGFBP-3 binds the majority of circulating IGF-I and is a significant long-term regulator of IGF-I action (14). In contrast, IGFBP-1 is considered the most important short-term regulator of IGF-I action because its production is acutely suppressed by insulin (15,16). Lower circulating concentrations of IGFBP-3 and IGFBP-1 in AAs (3,5,17) relative to EAs have been demonstrated. Lower concentrations of these binding proteins could plausibly lead to significant increases in IGF-I activity in AAs. Because insulin is known to suppress IGFBP-1 production, it has been speculated that lower IGFBP-1 among AAs is due to hyperinsulinemia (17,18). In general, AAs have a greater acute insulin response to glucose (AIRg) than EAs. We have demonstrated 42% lower insulin sensitivity and 135% higher AIRg in AAs compared with EAs, even after adjusting for body composition. However, the greater AIRg observed among AAs vs. EAs is independent of insulin sensitivity and body composition differences, i.e. is greater than that that would be predicted for compensation to insulin resistance (19). The physiological relevance of this relative hyperinsulinemia has not yet been elucidated.
This longitudinal study was conducted to determine whether there is ethnic variation in the IGF system throughout puberty. We hypothesized that: 1) AAs would have higher total and bioavailable IGF-I than EAs, 2) AAs would have lower IGFBP-3 and IGFBP-1 concentrations than EAs, and 3) the greater IGF-I to IGFBP-1 ratio would be accounted for in part by the greater AIRg in AAs.
Subjects and Methods
Study design
Subjects were recruited as part of a longitudinal observational study on disease risk factors in children and adolescents conducted from 1994–2004. Details on subject recruitment and study design have been published (19). Briefly, AA and EA children were recruited by advertisement, flyer, and word of mouth from the Birmingham, Alabama area. Children were screened for eligibility: all children were older than 5 yr of age; free of major illness; and were not taking medications known to affect body composition, metabolism, or physical activity. If deemed eligible, children were enrolled for annual visits during which body composition was assessed, fasting blood samples were obtained, and an iv glucose tolerance test was performed after an overnight stay. A pediatrician determined pubertal stage [Tanner stage (TS)] using a combination of assessments, including breast development, genital stage, in accordance with the criteria of Marshall and Tanner (20). Ethnicity was assigned according to parental self-report of parental and grandparental ethnicity. That is, for a child to be considered AA or EA, both parents and all four grandparents had to be of the indicated ethnicity. All girls who were menstruating at their annual visit were tested within 10–12 d of the start of the follicular phase. The nature, purpose, and potential risks of the study were explained to parents and children. Annually, the children and parents provided informed assent and consent, respectively, to the protocol, which was approved by the University of Alabama at Birmingham Institutional Review Board for the use of human subjects in research.
Study sample
Data on 162 children (72 AAs, 90 EAs), with varying numbers of annual evaluations, were used in this study. The majority of children were prepubertal (TS I) upon entry into the study (n = 130). There were 32 children that entered the study at early puberty (TS II). A total of 82 children progressed from prepuberty/early puberty (TS I and II) to late puberty or full maturation (TS IV and V). There were 56 children that either dropped out of the study before progressing to puberty, or did not complete the pubertal transition (TS V) during the study duration. AA and EA children had an average of five and four annual visits, respectively, commencing with 162 children completing visit 1, 147 at visit 2, 137 at visit 3, 131 at visit 4, 120 at visit 5, 100 at visit 6, 77 at visit 7, 49 at visit 8, 35 at visit 9, and six at visit 10. There were no differences in evaluation number according to ethnicity. Given the unbalanced nature of TS progression, i.e. some children progressed through puberty at a greater rate than did others, the number of observations at each TS varied.
Protocol
Children arrived at 1600 h to the Department of Nutrition Sciences for body composition assessment, and were then admitted to the General Clinical Research Center for an overnight visit. At approximately 1800 h in the evening of the overnight visit, all children received an identical dinner meal in an effort to “standardize” dietary intake before metabolic outcomes measurements. Children were not permitted to engage in any physical activities during their stay and were encouraged to remain in their rooms for the study duration. After 2000 h on the evening of their overnight stay, ingestion of only water, and energy and caffeine-free beverages was permitted until the following morning.
Body composition assessment
Body composition was assessed by dual-energy x-ray absorptiometry using a Lunar DPX-L densitometer (Lunar Corp., Madison, WI). Subjects were scanned in light clothing, while lying flat on their backs with arms at their sides. Dual-energy x-ray absorptiometry scans were performed and analyzed using pediatric software version 1.5e. On the day of each test, the DPX-L was automatically self-calibrated according to the manufacturer’s guidelines. In our laboratory the coefficient of variation (CV) for repeated measures of total body fat mass on the Lunar DPX-L is 6.55%. Height was measured to the nearest centimeter using a wall-mounted stadiometer, and weight was measured on an electronic scale while children wore light clothing.
Frequently sampled iv glucose tolerance test (FSIGT)
On the morning after the overnight fast, a topical anesthetic was applied to the antecubital space of both arms, and flexible iv catheters were placed in both arms. Three blood samples were obtained over a 40-min period, and sera were pooled for determination of all basal hormone and metabolite concentrations (except insulin and glucose). Blood drawn at times 15, −5, and −1 were analyzed and averaged for determination of fasting glucose and insulin. At time zero, glucose (25% dextrose; 11.4 g/m2) was administered iv. Blood samples (2 ml) were collected at the following times relative to glucose administration at 0 min: −15, −5, −1, 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 25, 30, 40, 50, 70, 100, 140, and 180 min. Tolbutamide (125 mg/m2) was injected iv at 20 min. The total blood drawn for this analysis was 60 cc [pool (16 cc) and FSIGT (44 cc)]. The AIRg, an approximation of first-phase insulin secretion, was calculated as the incremental area under the curve for insulin during the first 10 min after glucose injection using the trapezoidal method (21).
Hormone and glucose measurements
All analyses were performed in the Core Laboratory of the Clinical Nutrition Research Center at University of Alabama at Birmingham. Serum samples from the FSIGT were analyzed in duplicate for insulin by RIA (Diagnostic Products Corp., Los Angeles, CA; intraassay CV 5.0%, interassay CV 6.0%, sensitivity 2.5 μIU/ml), and for glucose using an Ektachem DT II System (Johnson and Johnson, Rochester, NY; a mean intraassay CV of 0.61% and a mean interassay CV of 1.45%).
Pooled sera were analyzed in duplicate for IGF-I using a standard immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX). In our laboratory the intraassay CV was 3.7%, interassay CV was 7.3%, and sensitivity was 2.6 ng/ml. Immunoradiometric assays were used to analyze samples in duplicate for IGFBP-3 (intraassay CV was 4.0%, interassay CV was 7.7%, and sensitivity was 0.5 ng/ml) and IGFBP-1 (intraassay CV was 9.4%, interassay CV was 17.9%, and sensitivity was 0.18 ng/ml). Sera were analyzed for estradiol (E2) using a double-antibody RIA (Diagnostic Products), and for total testosterone using a solid-phase immunoassay (Coat-A-Count Total Testosterone; Diagnostic Products). In our laboratory, assay sensitivity for E2 is 15.42 pmol/liter, mean intraassay CV is 4.69%, and interassay CV is 6.0%. For testosterone the sensitivity is 11.8 ng/dl, and the intraassay and interassay CVs are 2.7 and 11.4%, respectively.
Statistics
Because of the unbalanced, repeated-measures study design, the general linear mixed model procedure was used to determine the relation of ethnicity to changes in the outcome variables. This model accounts for intraperson correlations due to repeated measures, an unbalanced number of repeated observations, and for missing data (22). Initial models were constructed for the dependent variables IGF-I, IGFBP-1, and IGFBP-3. Independent variables in all models were ethnicity, sex, age, fat mass, pubertal stage, and age nested in pubertal stage. The age nested in pubertal stage variable was used to account for changes in age within each pubertal stage. Reproductive hormone levels (testosterone and E2) were added to each model to identify potential independent contributions of these hormones to each of the dependent variables, and to determine whether differences in the concentration of these hormones accounted for ethnic differences in the IGF system. Fasting insulin concentration and AIRg were added sequentially to the base IGFBP-1 model. All independent variables were modeled as fixed effects. Because of the small number of children at TS V, children at TSs IV and V were combined into one group (TS IV/V). To assess ethnic differences in the dependent variables before, and during pubertal development, models were analyzed by pubertal stage. Least squares means were determined for all outcome variables of interest after adjusting for body composition, age, reproductive hormones, and sex. To conform to the assumptions of linear regression, all statistical models were evaluated for residual normality, and logarithmic transformations were performed when appropriate. Analyses were undertaken using SAS (PROC MIXED), version 9.1 (SAS Institute Inc., Cary, NC), with statistical significance set at P < 0.05.
Results
Subject characteristics
Descriptive data are given in Table 1. AA children were younger and had higher AIRg compared with their EA counterparts at all pubertal stages (P < 0.05). EAs were taller than AAs at TSs I and II (P < 0.05). Ethnic differences in body fat mass and body mass index (BMI) did not reach statistical significance.
Table 1.
Characteristics of subjects studied at each TS
| Tanner I
|
Tanner II
|
Tanner III
|
Tanner IV and V
|
|||||
|---|---|---|---|---|---|---|---|---|
| AAs (59) | EAs (71) | AAs (43) | EAs (63) | AAs (35) | EAs (42) | AAs (45) | EAs (37) | |
| Age (yr) | 8.2 ± 0.2 | 9.3 ± 0.1a | 10.4 ± 0.2 | 11.4 ± 0.2a | 11.9 ± 0.2 | 12.8 ± 0.1a | 14.1 ± 0.2 | 14.7 ± 0.2b |
| No. of males (%) | 27 (46) | 29 (41) | 20 (47) | 19 (30) | 11 (31) | 12 (29) | 14 (31) | 18 (49) |
| Wt (kg) | 35.6 ± 1.2 | 35.7 ± 1.4 | 48.6 ± 2.1 | 47.3 ± 1.9 | 60.1 ± 2.5 | 55.9 ± 2.3 | 69.3 ± 2.7 | 65.7 ± 2.8 |
| Wt z score | −0.004 + 0.1 | −0.004 + 0.1 | 0.002 + 0.1 | −0.003 + 0.1 | 0.003 + 0.1 | 0.001 + 0.1 | 0.001 + 0.1 | 0.003 + 0.1 |
| Ht (cm) | 132.6 ± 1.2 | 135.8 ± 1.1b | 146.4 ± 1.2 | 149.7 ± 1.0b | 157.9 ± 1.2 | 159.0 ± 1.1 | 163.9 ± 1.7 | 166.5 ± 2.0 |
| Ht z score | −0.001 + 0.1 | −0.002 + 0.1 | 0.002 + 0.1 | 0.001 + 0.1 | −0.003 + 0.1 | −0.004 + 0.1 | 0.003 + 0.1 | 0.001 + 0.1 |
| BMI | 19.9 ± 0.6 | 18.9 ± 0.5 | 22.2 ± 0.8 | 20.9 ± 0.7 | 23.9 ± 0.8 | 22.0 ± 0.8 | 25.4 ± 0.9 | 23.8 ± 0.9 |
| BMI z score | −0.007 + 0.1 | −0.01 + 0.1 | 0.006 + 0.1 | 0.004 + 0.1 | 0.003 + 0.1 | −0.009 + 0.1 | −0.006 + 0.1 | 0.002 + 0.1 |
| Fat mass (kg) | 10.8 ± 0.9 | 9.7 ± 0.8 | 15.1 ± 6.7 | 14.8 ± 5.9 | 19.6 ± 1.9 | 16.9 ± 1.6 | 22.2 ± 2.1 | 20.7 ± 2.2 |
| Lean mass (kg) | 23.7 + 0.3 | 22.3 + 0.3a | 31.2 + 0.6 | 28.6 + 0.5a | 38.1 + 0.8 | 35.0 + 0.7a | 44.1 + 0.6 | 42.1 + 0.7b |
| Insulin (μU/ml) | 13.54 + 0.86 | 10.46 + 0.76a | 16.51 + 1.01 | 13.09 + 0.89b | 18.04 + 1.57 | 14.63 + 1.39 | 13.22 + 2.07 | 13.52 + 2.18 |
| AIRg (μlU/ml× 10 min) | 1530 ± 155 | 608 ± 35a | 1794 ± 141 | 685 ± 57a | 2317 ± 372 | 592 ± 54a | 1414 ± 140 | 545 ± 47a |
| IGF-I | ||||||||
| Males | 228.6 + 9.4 | 194.5 + 9.2a | 298.6 + 14.1 | 351.8 + 27.7b | 474.9 + 38.3 | 548.6 + 61.6 | 590.8 + 27.5 | 555.6 + 31.1 |
| Females | 266.2 + 12.8 | 216.7 + 8.0a | 407.4 + 27.8 | 403.0 + 17.6 | 580.4 + 25.5 | 577.4 + 16.8 | 551.4 + 17.9 | 571.4 + 21.3 |
| IGFBP-1 | ||||||||
| Males | 93.6 + 9.0 | 124.6 + 12.0b | 34.0 + 4.1 | 49.6 + 5.9b | 22.2 + 5.6 | 41.2 + 4.6b | 18.8 + 2.5 | 25.4 + 5.1 |
| Females | 84.6 + 7.4 | 100.7 + 10.7 | 33.4 + 5.1 | 45.9 + 3.8b | 26.7 + 3.3 | 42.4 + 3.4a | 21.4 + 2.1 | 30.2 + 5.1 |
| IGFBP-3 | ||||||||
| Males | 3584 + 117 | 3827 + 107 | 4100 + 104 | 4509 + 208b | 4384 + 184 | 4958 + 266 | 4685 + 192 | 5130 + 214 |
| Females | 3764 + 112 | 4152 + 106a | 4397 + 135 | 4780 + 128b | 4475 + 157 | 5179 + 139a | 4525 + 134 | 5270 + 273 |
| E2 (pg/ml) | ||||||||
| Males | 4.2 + 0 | 4.3 + 0.1 | 4.2 + 0 | 4.2 + 0 | 4.3 + 42.4 | 6.0 + 1.0 | 8.6 + 1.9 | 8.3 + 2.2 |
| Females | 4.5 + 0.3 | 4.4 + 0.2 | 9.7 + 1.7 | 12.0 + 1.4 | 41.6 + 7.5 | 35.4 + 5.2 | 49.92 + 3.0 | 29.9 + 5.2b |
| T (pg/ml) | ||||||||
| Males | 12.3 + 0.5 | 13.5 + 0.9 | 43.4 + 9.6 | 95.2 + 26.0 | 233.3 + 42.4 | 299.7 + 58.4 | 469.0 + 39.5 | 465.1 + 46.1 |
| Females | 12.4 + 0.3 | 15.8 + 4.0 | 17.1 + 1.6 | 14.4 + 0.9 | 26.9 + 2.9 | 19.0 + 1.2 | 38.2 + 3.01 | 29.0 + 3.0 |
Data are least squares means ± se. Sample size at each TS is in parentheses. Not all children entered the study at TS I (see Subjects and Methods). Data are presented as least squares means because children may have more than one measure at any given TS. Ht, Height; T, total testosterone; Wt, weight.
Significantly different by ethnicity, P < 0.01.
Significantly different by ethnicity, P < 0.05.
IGF-I
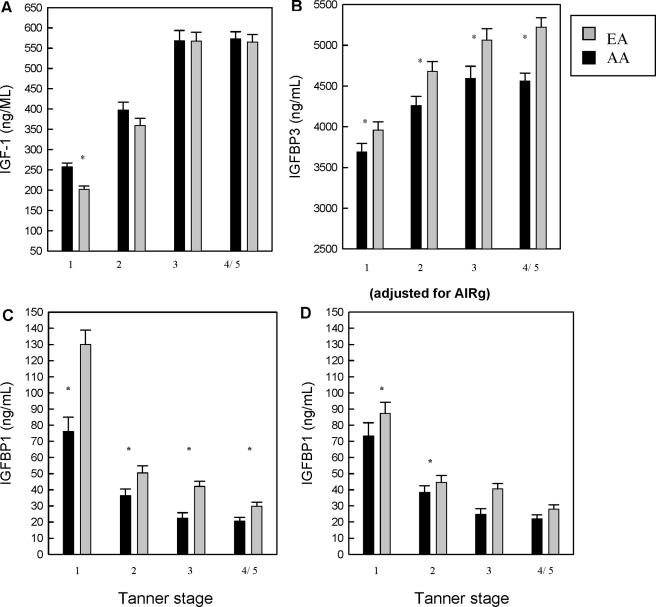
Longitudinal statistical analyses revealed a significant independent effect of ethnicity on IGF-I concentrations, such that AAs had higher IGF-I concentrations (P < 0.01) (Table 2). Significant associations between pubertal stage, age nested in pubertal stage (TS I–III), sex, reproductive hormones, and fat mass with IGF-I concentrations also were found (Table 3). E2 and testosterone were associated with IGF-I levels in the overall models and when analyzed according to sex. As indicated by the significance of ethnicity in the model, reproductive hormones did not mediate racial/ethnic differences in IGF-I. The analysis of pubertal change in IGF-I by ethnicity is illustrated in Fig. 1A. IGF-I concentrations increased throughout puberty in both ethnic groups, and tended to plateau after TS III. AAs had higher IGF-I concentrations than EAs only at TS I (P < 0.001) (Fig. 1A). The relationship between E2 and IGF-I was present in girls at TSs I and II. The relationship between testosterone and IGF-I was present in boys at TSs II and III. After adjustments for fat mass, age, hormone level, and sex, AAs had higher IGF-I concentrations at TS I (Fig. 1A).
Table 2.
Results of the mixed linear model for the dependent variable total IGF-I
| Effect | Parameter estimate | se | P value |
|---|---|---|---|
| Intercept | −0.899 | 1.223 | <0.0001 |
| Tanner I | −1.402 | 0.622 | 0.0254 |
| Tanner II | −2.001 | 0.729 | 0.0066 |
| Tanner III | −1.830 | 0.912 | 0.0466 |
| Age (Tanner I) | 0.156 | 0.128 | 0.0224 |
| Age (Tanner II) | 0.464 | 0.220 | 0.0349 |
| Age (Tanner III) | 0.425 | 0.319 | 0.0183 |
| Age (Tanner IV and V) | −0.330 | 0.231 | 0.9540 |
| Fat mass | 0.019 | 0.029 | 0.5026 |
| Lean mass | 0.664 | 0.120 | <0.0001 |
| Sex | 0.278 | 0.049 | <0.0001 |
| E2 | 0.094 | 0.019 | <0.0001 |
| Testosterone | 0.073 | 0.017 | <0.0001 |
| Ethnicity | 0.083 | 0.019 | <0.0001 |
Significant effects are in bold type. All continuously distributed variables were log transformed to correct for skewed distributions. Ethnicity and sex were coded in the models as follows: EA = 0; AA = 1; male = 0; female = 1. Age (Tanner), The age nested in the TS variable was used to account for changes in age within each TS.
Table 3.
Results of the mixed linear model for the dependent variable IGFBP-3
| Effect | Parameter estimate | se | P value |
|---|---|---|---|
| Intercept | 7.132 | 1.040 | <0.0001 |
| Tanner I | −0.362 | 0.444 | 0.0026 |
| Tanner II | −1.268 | 0.520 | 0.0158 |
| Tanner III | −1.457 | 0.644 | 0.0052 |
| Age (Tanner I) | 0.319 | 0.110 | 0.0282 |
| Age (Tanner II) | 0.088 | 0.173 | 0.0609 |
| Age (Tanner III) | 0.156 | 0.234 | 0.0505 |
| Age (Tanner IV and V) | −0.413 | 0.171 | 0.1065 |
| Fat mass | −0.012 | 0.026 | 0.6347 |
| Lean mass | 0.226 | 0.102 | 0.0277 |
| Sex | 0.121 | 0.091 | 0.1901 |
| E2 | 0.007 | 0.013 | 0.5960 |
| Testosterone | 0.018 | 0.012 | 0.1258 |
| Ethnicity | −0.228 | 0.090 | 0.0127 |
Significant effects are in bold type. All continuously distributed variables were log transformed to correct for skewed distributions. Ethnicity and sex were coded in the models as follows: EA = 0; AA = 1; male = 0; female = 1. Age (Tanner), The age nested in the TS variable was used to account for changes in age within each TS.
Figure 1.
A, Least squares mean (± se) for total IGF-I by ethnicity at each pubertal stage. Means are adjusted for sex, age, reproductive hormones, and body fat mass. Shaded bars represent EA, and dark bars represent AA children. *, AAs had significantly higher IGF-I only at TS I (P < 0.0001). To convert metric units (ng/ml) to International System of Units (nmol/liter) for IGF-I, multiply by 0.131. B, Least squares mean (± se) for IGFBP-3 by ethnicity at each TS. Means are adjusted for sex, age, reproductive hormones, and body fat mass. Shaded bars represent EA, and dark bars represent AA children. *, AAs had significantly lower IGFBP-3 at all TSs (P < 0.05). To convert metric units (ng/ml) to International System of Units (nmol/liter) for IGFBP-3, multiply by 0.035. C, Least squares mean (± se) for IGFBP-1 by ethnicity at each TS. Means are adjusted for sex, age, reproductive hormones, and body fat mass. Shaded bars represent EA, and dark bars represent AA children. *, AAs had significantly lower IGFBP-1 at TSs I–III and V (P < 0.05). D, Least squares mean (± se) for IGFBP-1 by ethnicity at each TS. Means are adjusted for sex, age, reproductive hormones, and body fat mass, and the AIRg, an approximation of first-phase insulin secretion. Shaded bars represent EA, and dark bars represent AA children. *, AAs had significantly lower IGFBP-1 at TSs I–III. Therefore, inclusion of AIRg in the model explained the ethnic differences in IGFBP-1 in childhood and early puberty. Sample size at each TS: for AAs, I (n = 59), II (n = 43), III (n = 35), and IV/V (n = 45); and for EAs, I (n = 71), II (n = 63), III (n = 42), and IV/V (n = 37).
IGFBP-3
Longitudinal analyses showed an effect of ethnicity on IGFBP-3 concentrations (P < 0.05), such that EAs had greater concentrations of IGFBP-3 (Table 3). Pubertal stage and age nested in pubertal stage also were significantly associated with IGFBP-3. Testosterone, but not E2, was associated with IGFBP-3 concentration; however, the relationship did not explain racial/ethnic differences across the pubertal transition. Furthermore, analysis by pubertal stage revealed that IGFBP-3 concentrations increased from TSs I–III in a similar manner in both ethnic groups, reaching a plateau in AAs, whereas continuing to increase in EAs (Fig. 1B). After accounting for fat mass, age, reproductive hormone concentration, and sex, AAs had lower IGFBP-3 compared with EAs at each pubertal stage assessed (P < 0.05).
IGFBP-1
Longitudinal models revealed a significant association of ethnicity with IGFBP-1 (P < 0.0001) (Table 4). Associations between pubertal stage, age nested in pubertal stage, and fat mass and IGFBP-1 were also found. Both E2 and testosterone were associated with IGFBP-1 concentration; however, the relationship did not mediate racial/ethnic differences in IGFBP-1 concentration. When analyzed according to pubertal stage, lower concentrations of IGFBP-1 were found in AAs relative to EAs at all pubertal stages (Fig. 1C). Fasting insulin concentrations and AIRg were significantly and negatively associated with IGFBP-1 at all TSs (P < 0.05; data not shown). Inclusion of fasting insulin weakened but did not remove ethnicity as a significant predictor of IGFBP-1 at TSs I–V (data not shown). However, inclusion of AIRg in the model removed ethnicity as a significant predictor of IGFBP-1 at TSs III and IV/V, and weakened ethnicity as a predictor of IGFBP-1 at TS II (P < 0.0001 to P = 0.03) (Fig. 1D).
Table 4.
Results of the mixed linear model for the dependent variable IGFBP-1
| Effect | Parameter estimate | se | P value |
|---|---|---|---|
| Intercept | 8.890 | 0.057 | <0.0001 |
| Tanner I | 2.250 | 1.360 | 0.0099 |
| Tanner II | 2.098 | 0.269 | 0.0190 |
| Tanner III | 1.458 | 0.399 | 0.4639 |
| Age (Tanner I) | 1.550 | 0.026 | 0.0034 |
| Age (Tanner II) | 1.512 | 0.046 | 0.0269 |
| Age (Tanner III) | 0.971 | 0.067 | 0.1504 |
| Age (Tanner IV and V) | 0.397 | 0.050 | 0.4286 |
| Fat mass | −0.580 | 0.057 | <0.0001 |
| Lean mass | −1.111 | 0.240 | <0.0001 |
| Sex | −0.063 | 0.092 | 0.4963 |
| E2 | −0.079 | 0.041 | 0.0564 |
| Testosterone | −0.033 | 0.036 | 0.3518 |
| Ethnicity | 0.441 | 0.081 | <0.0001 |
Significant effects are in bold type. All continuously distributed variables were log transformed to correct for skewed distributions. Ethnicity and sex were coded in the models as follows: EA = 0; AA = 1; male = 0; female = 1. Age (Tanner), The age nested in the TS variable was used to account for changes in age within each TS.
Discussion
With longitudinal data, we demonstrate for the first time that AAs had higher IGF-I concentrations than EAs only in prepuberty and lower concentrations of IGFBP-3 and IGFBP-1 throughout the pubertal transition. Furthermore, the lower IGFBP-1 of AAs was due in part to the higher AIRg, suggesting physiological relevance for the frequently reported higher AIRg among AAs. Findings were independent of fat mass, sex, reproductive hormones, and age. Overall, our analyses suggested that, relative to EAs, AAs may have greater IGF-I activity during childhood and adolescence. The biological consequences of greater IGF-I and lower IGFBP-1 and -3 among AAs on disease risk, body composition, and maturation differences between EAs and AAs have not been thoroughly studied and deserve further attention.
Ethnic differences in IGF-I were present only at TS I, suggesting that differences in pubertal maturation rate underlie this difference. Our findings are consistent with studies demonstrating similar IGF-I concentrations in AA and EA adults (7,10,12,13). AAs typically progress to puberty 1 yr earlier than their EA counterparts (6). GH and IGF-I concentrations are known to increase steadily from early prepuberty to up to 30 d before puberty onset (3,23), and, therefore, higher IGF-I in AA children may simply reflect their greater relative proximity to puberty. However, inclusion of reproductive hormones in the statistical models did not account for ethnic differences in the IGF-I concentration. We previously have demonstrated an association between IGF-I and African ancestry in prepubertal children (4), suggesting a biological basis for this difference. It is plausible that GH may mediate differences in the IGF axis, but studies regarding ethnic differences in GH are lacking. The one study that evaluated ethnic differences in GH secretion observed higher GH in AAs compared with EAs, albeit the difference was not statistically significant (24). Therefore, the biological mechanisms underlying greater IGF-I in AAs remain to be determined.
In agreement with our hypothesis, AAs had significantly lower IGFBP-1 concentrations compared with EAs throughout childhood and adolescence. Importantly, we found that adjustment for AIRg considerably weakened the association of ethnicity with IGFBP-1 at all TSs. Thus, we provide the first evidence to indicate that lower IGFBP-1 concentrations in AAs compared with EAs are likely attributable in large part to greater relative insulin responses. Our data also suggest that among AAs, greater AIRg has physiological relevance and may have implications for disease progression. In our study and in a study by Wong et al. (17), lower IGFBP-1 in AAs persisted after adjusting for fasting insulin concentrations. AIRg is likely to be more reflective of the typical AA-EA differences in insulinemia occurring after meals throughout the day. Insulin can act as both an acute and chronic suppressor of hepatic IGFBP-1 production (14,17,24). Therefore, it is likely that the relative hyperinsulinemia of AAs exerts a chronic suppressive effect on hepatic IGFBP-1 production that persists in the fasted state. Lower IGFBP-1 in AAs after accounting for AIRg was observed at TSs I–III. This observation might reflect the reduced effect of insulin on IGFBP-1 in puberty (17), likely attributable to the insulin resistance of puberty, which typically peaks at midpuberty but may not be evident prepubertally.
We also found evidence for lower IGFBP-3 concentrations in AAs than EAs throughout puberty, and these differences were independent of age, sex, reproductive hormone concentration, and fat mass. Systemic IGF-I circulates bound to IGFBP-3 and to another fraction known as the acid-labile subunit, forming a ternary complex, which binds the majority of the circulating IGF-I (14). IGFBP-3 concentrations increase with pubertal development in a pattern similar to IGF-I (14,25,26). Our findings agree with those of previous cross-sectional studies in which lower IGFBP-3 concentrations were found in adolescent AAs relative to their EA counterparts (3,17,24,25). Synthesis of IGFBP-3 is considered GH dependent but may also be regulated by other factors (14,27). Factors underlying the ethnic difference in IGFBP-3 are not readily apparent. Recent data indicate that variation of nutrient intake may have an influence on the IGF system (28).
The incidence of cardiovascular disease (CVD) risk factors, type 2 diabetes, and some cancers (29,30,31) is higher among AAs, and evidence exists linking the IGF system with each of these conditions (32,33,34,35). Numerous observational studies have examined the association between the IGF system and the presence of CVD, with most indicating that levels of IGF-I are associated with an adverse risk profile and increased incidence of CVD (36). The IGF system has been demonstrated to promote pancreatic islet cell survival and growth in vitro, and both IGF-I and its binding proteins have been associated with insulin sensitivity, fasting insulin, and apoptosis of insulin-secreting cells (25,33,37). In addition, IGF-I and IGFBP levels may be altered in type 2 diabetes. Furthermore, recent studies have linked IGF system variants to the risk of certain cancers, and some of these polymorphisms display unequal frequency between EAs and AAs (38). IGF-I levels in puberty may serve as a “biological switch” affecting gene expression that permanently alters the physiology of the individual and their response to various stimuli later in life. It is plausible that childhood IGF-I levels and greater pubertal IGF-I levels may be one of the pathophysiological pathways that increase the risk of chronic disease later in life. It has been proposed that physiological and metabolic programming that occurs during puberty may initiate events that lead to the development of these diseases later in life. Long-term studies investigating ethnic differences in the relationship between the IGF system during puberty and future disease risks are warranted.
The strengths of this study were its longitudinal design, robust measures of insulin dynamics and body composition, and the inclusion of maturation and age in all statistical models. This is the first longitudinal study to demonstrate significantly lower concentrations of IGFBP-1 and IGFBP-3 in AA compared with EA children and adolescents. However, because direct measures of free IGF-I, or measures of other IGF-I binding proteins were not obtained, our suggestion that AAs have greater IGF-I activity than EAs should be verified. Furthermore, although some of the conclusions are based on inverse changes in IGF-I and IGFBP-1 and -3, this ratio was not included in the analysis, limiting the assumption that the same individual had both a relatively lower IGFBP and relatively higher IGF-I. In addition, because dietary intake and physical activity were not available on all of our subjects, we cannot disregard ethnic differences in these and other environmental factors as the underlying cause of the observed differences in IGF-I and its binding proteins.
In conclusion, our data suggest that compared with EAs, AAs have greater IGF-I prepubertally and lower concentrations of IGFBP-3 and IGFBP-1 throughout adolescence. The lower IGFBP-1 may be due, in part, to greater AIRg in AAs. Consequently, circulating IGF-I bioactivity is likely to be greater in AAs compared with EAs during the important developmental years. These findings may have implications for ethnic differences in maturation rate, body composition, and disease risk.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 9, 2008
Abbreviations: AA, African-American; AIRg, acute insulin response to glucose; BMI, body mass index; CV, coefficient of variation; CVD, cardiovascular disease; E2, estradiol; EA, European American; FSIGT, frequently sampled iv glucose tolerance test; IGFBP, IGF binding protein; TS, Tanner stage.
References
- Sandhu J, Davey SG, Holly J, Cole TJ, Ben-Shlomo Y 2006 Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab 91:3150–3157 [DOI] [PubMed] [Google Scholar]
- Arslanian S, Suprasongsin C, Janosky JE 1997 Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab 82:1923–1927 [DOI] [PubMed] [Google Scholar]
- Girgis R, Abrams SA, Castracane VD, Gunn SK, Ellis KJ, Copeland KC 2000 Ethnic differences in androgens, IGF-I and body fat in healthy prepubertal girls. J Pediatr Endocrinol Metab 13:497–503 [DOI] [PubMed] [Google Scholar]
- Higgins PB, Fernandez JR, Goran MI, Gower BA 2005 Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res 58:850–854 [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Sovik KN, Nguyen TT, Sebring NG 2000 Insulin-like growth factors and bone mineral density in African American and White girls. J Pediatr 137:826–832 [DOI] [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS 2002 National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 110:911–919 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Mikhail M, Pagan CD, Arunachalam A, Yeh JK, Flaster E 1998 Biochemical and hormonal variables in black and white women matched for age and weight. J Lab Clin Med 132:383–389 [DOI] [PubMed] [Google Scholar]
- DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L 2004 Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev [Erratum (2004) 13(11 Pt 1):1825] 13:1444–1451 [PubMed] [Google Scholar]
- Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le Marchand L 2006 Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15:2298–2302 [DOI] [PubMed] [Google Scholar]
- McGreevy K, Hoel B, Lipsitz S, Bissada N, Hoel D 2005 Racial and anthropometric differences in plasma levels of insulin-like growth factor I and insulin-like growth factor binding protein-3. Urology 66:587–592 [DOI] [PubMed] [Google Scholar]
- Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE 2005 Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev 14:2147–2153 [DOI] [PubMed] [Google Scholar]
- Platz EA, Pollak MN, Rimm EB, Majeed N, Tao Y, Willett WC, Giovannucci E 1999 Racial variation in insulin-like growth factor-1 and binding protein-3 concentrations in middle-aged men. Cancer Epidemiol Biomarkers Prev 8:1107–1110 [PubMed] [Google Scholar]
- Tricoli JV, Winter DL, Hanlon AL, Raysor SL, Watkins-Bruner D, Pinover WH, Hanks GE 1999 Racial differences in insulin-like growth factor binding protein-3 in men at increased risk of prostate cancer. Urology 54:178–182 [DOI] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S 1997 Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18:801–831 [DOI] [PubMed] [Google Scholar]
- Liew CF, Wise SD, Yeo KP, Lee KO 2005 Insulin-like growth factor binding protein-1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy, glucose-tolerant young men. J Clin Endocrinol Metab 90:1483–1488 [DOI] [PubMed] [Google Scholar]
- Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD 1991 Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem 266:18868–18876 [PubMed] [Google Scholar]
- Wong WW, Copeland KC, Hergenroeder AC, Hill RB, Stuff JE, Ellis KJ 1999 Serum concentrations of insulin, insulin-like growth factor-I and insulin-like growth factor binding proteins are different between white and African American girls. J Pediatr 135:296–300 [DOI] [PubMed] [Google Scholar]
- Caprio S 1999 Differences between African American and white girls in the insulin-like growth factor-I and the binding proteins: importance of insulin resistance and hyperinsulinemia. J Pediatr 135:270–271 [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI 1999 Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1968 Growth and physiological development during adolescence. Annu Rev Med 19:283–300 [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P 1990 Analysis of serial measurements in medical research. BMJ 300:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P 1997 Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16:2349–2380 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Pohl CR, Wilson ME 2000 Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab 85:808–814 [DOI] [PubMed] [Google Scholar]
- Wright NM, Papadea N, Veldhuis JD, Bell NH 2002 Growth hormone secretion and bone mineral density in prepubertal black and white boys. Calcif Tissue Int 70:146–152 [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs Jr DR, Steinberger J, Cohen P, Hong CP, Prineas R, Sinaiko AR 2002 Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 87:4817–4820 [DOI] [PubMed] [Google Scholar]
- Wilson DM, Stene MA, Killen JD, Hammer LD, Litt IF, Hayward C, Taylor CB 1992 Insulin-like growth factor binding protein-3 in normal pubertal girls. Acta Endocrinol (Copenh) 126:381–386 [DOI] [PubMed] [Google Scholar]
- Andrade Olivié MA, García-Mayor RV, González Lestón D, Rodríguez Sousa T, Segura Dominguez A, Alvarez-Novoa R, Antelo Cortizas J 1995 Serum insulin-like growth factor (IGF) binding protein-3 and IGF-I levels during childhood and adolescence. A cross-sectional study. Pediatr Res 38:149–155 [DOI] [PubMed] [Google Scholar]
- McGreevy KM, Hoel BD, Lipsitz SR, Hoel DG 2007 Impact of nutrients on insulin-like growth factor-I, insulin-like growth factor binding protein-3 and their ratio in African American and white males. Public Health Nutr 10:97–105 [DOI] [PubMed] [Google Scholar]
- Djavan B, Waldert M, Seitz C, Marberger M 2001 Insulin-like growth factors and prostate cancer. World J Urol 19:225–233 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E 2000 Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92:1592–1600 [DOI] [PubMed] [Google Scholar]
- Vadgama JV, Wu Y, Datta G, Khan H, Chillar R 1999 Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology 57:330–340 [DOI] [PubMed] [Google Scholar]
- Cohen P 2006 Overview of the IGF-I system. Horm Res 65(Suppl 1):3–8 [DOI] [PubMed] [Google Scholar]
- Saydah S, Graubard B, Ballard-Barbash R, Berrigan D 2007 Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol 166:518–526 [DOI] [PubMed] [Google Scholar]
- Shim ML, Levitt Katz LE, Davis J, Dotzler WC, Cohen P, Ferry Jr RJ 2004 Insulin-like growth factor binding protein-3 is a novel mediator of apoptosis in insulin-secreting cells. Growth Horm IGF Res 14:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden AL, Elofsson S, Brismar K 2005 Gender differences in the relation of insulin-like growth factor binding protein-1 to cardiovascular risk factors: a population-based study. Clin Endocrinol (Oxf) 63:94–102 [DOI] [PubMed] [Google Scholar]
- Ezzat VA, Duncan ER, Wheatcroft SB, Kearney MT 2008 The role of IGF-I and its binding proteins in the development of type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 10:198–211 [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T 2002 Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106:939–944 [DOI] [PubMed] [Google Scholar]
- Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA 2007 IGF-I and IGFBP-3 gene variants influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis 28:2154–2159 [DOI] [PubMed] [Google Scholar]