Abstract
Context: Obesity attenuates spontaneous GH secretion and the GH response to exercise. Obese individuals often have low fitness levels, limiting their ability to complete a typical 30-min bout of continuous exercise. An alternative regimen in obese subjects may be shorter bouts of exercise interspersed throughout the day.
Objective: The objective of the study was to examine whether intermittent and continuous exercise interventions evoke similar patterns of 24-h GH secretion and whether responses are attenuated in obese subjects or affected by gender.
Design: This was a repeated-measures design in which each subject served as their own control.
Setting: This study was conducted at the University of Virginia General Clinical Research Center.
Subjects: Subjects were healthy nonobese (n = 15) and obese (n = 14) young adults.
Interventions: Subjects were studied over 24 h at the General Clinical Research Center on three occasions: control, one 30-min bout of exercise, and three 10-min bouts of exercise.
Main Outcome Measures: Twenty-four hour GH secretion was measured.
Results: Compared with unstimulated 24-h GH secretion, both intermittent and continuous exercise, at constant exercise intensity, resulted in severalfold elevation of 24-h integrated serum GH concentrations in young adults. Basal and pulsatile modes of GH secretion were attenuated both at rest and during exercise in obese subjects.
Conclusions: The present data suggest that continuous and intermittent exercise training should be comparably effective in increasing 24-h GH secretion.
Although the response is attenuated in obese adults, one 30-min vs three 10-min bouts of exercise are equally effective for augmenting 24-h spontaneous GH secretion.
GH is secreted by the anterior pituitary gland in a pulsatile manner under the regulation of three peptides: GHRH, somatostatin, and GH-releasing peptide (ghrelin) (1). GHRH stimulates, somatostatin inhibits, and GH-releasing peptide/ghrelin acts synergistically with GHRH and stimulates GH release directly. GH release is regulated by reversible autofeedback enforced by GH and IGF-I (1).
Acute aerobic exercise is a potent stimulus to GH release (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18). In nonobese adults, intensity and duration of exercise, fitness, and gender all influence the GH response to exercise. Recent data from our laboratory affirm that GH secretion is related to exercise intensity in a graded fashion (12,16,18). However, the GH response to exercise is attenuated in older adults (18).
Obesity also attenuates spontaneous GH secretion as well as the GH response to exercise (19,20,21). Mechanistically the decrease in spontaneous 24-h GH secretion in obesity has been attributed to a decrease in pulsatile GH release and a shorter half-life of endogenous GH (22). Analogously, reduced exercise-induced GH release in obesity has been attributed to a reduction in the mass of GH secreted per burst (20). Many obesity-related physical adaptations resemble those recognized in GH-deficient adults, including reduced muscle mass and exercise capacity, increased body fat especially abdominal visceral fat (AVF), and increased cardiometabolic risk.
Exercise training increases spontaneous 24-h GH release in nonobese women (13), whereas short-term aerobic exercise training had no effect on exercise-stimulated GH release in obese women (20). One of the concerns regarding exercise prescription for obese individuals is their low fitness level, which may limit their ability to complete the prescribed amount of exercise (e.g. 30 min of continuous exercise), thus restricting training effects (23). This has led to the suggestion that multiple short bouts of exercise interspersed throughout the day may be an effective training strategy for less fit individuals and those who report an inability to devote 30–40 min to continuous exercise due to perceived lack of time.
Several reports indicate that an intermittent exercise regimen is as effective as the more traditional continuous approach, particularly in obesity (23,24,25,26). This may have implications for GH release. As few as 10 min of aerobic exercise stimulate GH release (27) and repeated bouts of exercise during the day augment 24-h GH release (28). Thus, the purpose of the present study was to examine whether intermittent and continuous exercise result in similar patterns of 24-h GH secretion and whether each response is attenuated similarly in both exercise conditions in obese subjects. We hypothesized that three bouts of intermittent exercise would augment 24-h GH secretion compared with one bout of continuous exercise of equal intensity and duration and minimize attenuation of the 24-h GH response in obese individuals.
Subjects and Methods
Subjects and preliminary screen procedures
Twenty-nine sedentary, healthy nonobese and obese men and women participated after providing voluntary written informed consent as approved by the Human Investigation Committee of the University of Virginia. Descriptive characteristics are presented in Table 1. Each subject underwent a detailed medical history and physical examination. No subject had a history of hypothalamo-pituitary, renal, hepatic, hematological, or metabolic disease. The subjects were nonsmokers, did not abuse alcohol, and were not taking any systemic medications. Screening laboratory data revealed normal hematological, renal, hepatic, metabolic, and thyroid function. Subjects refrained from exercise for 24 h before each evaluation. For the purposes of the present study, nonobese was defined as a body mass index (BMI) less than 27 kg · m−1 and overweight/obese was defined as a BMI equal to or greater than 27 kg · m−1.
Table 1.
Descriptive characteristics of nonobese and obese men and women
| Variable | Nonobese men (n = 8) | Nonobese women (n = 7) | Obese men (n = 8) | Obese women (n = 6) |
|---|---|---|---|---|
| Age (yr) | 23.3 (2.0) | 25.4 (2.2) | 30.6 (2.4) | 29.8 (4.1)a |
| Height (cm) | 179.5 (1.8) | 168.3 (2.1) | 177.3 (3.1) | 159.9 (2.0)b,c |
| Weight (kg) | 76.3 (2.6) | 66.0 (3.5) | 103.6 (4.4) | 97.6 (5.7)a,c |
| BMI (kg · m−1) | 23.7 (0.7) | 22.6 (0.8) | 33.3 (2.1) | 36.8 (2.5)a |
| Percentage fat | 15.1 (3.1) | 27.9 (2.2) | 25.6 (2.4) | 46.3 (2.3)a,d |
| VO2LT (ml/kg · min) | 18.8 (1.2) | 15.2 (1.9) | 13.2 (1.0) | 8.4 (0.7)b,c |
| VO2peak (ml/kg · min) | 38.3 (2.1) | 31.0 (3.3) | 28.5 (3.9) | 16.1 (1.3)b,c |
Data are presented as mean (se).
Obese is greater than nonobese (P < 0.05).
Obese is less than nonobese.
Men is greater than women (P < 0.05).
Men is less than women (P < 0.05).
Body composition analysis
Body density was determined by hydrostatic weighing (29). Residual lung volume was measured using an oxygen-dilution technique (30). The computational procedure of Brozek et al. (31) was used to estimate percentage body fat.
Peak oxygen uptake (VO2peak) and lactate threshold (LT) test
VO2peak and LT were evaluated via a continuous cycle ergometer protocol. The initial power output was set at 60 W, with increases in power output of 15 W every 3 min until volitional fatigue. Open-circuit spirometry was used to collect metabolic data (Viasys Vmax 229, Yorba Linda, CA). Heart rate was determined electrocardiographically. Blood samples were taken at rest and during the last 15 sec of each stage for the measurement of blood lactate concentration (YSI 2700 select biochemistry analyzer; Yellow Springs Instruments, Yellow Springs, OH). VO2 peak was chosen as the highest O2 consumption (VO2) attained.
Determination of LT and the constant load treadmill velocity
Blood lactate concentration was plotted against power output, and LT was chosen as the highest power output obtained before the curvilinear increase in blood lactate concentration (32).
Exercise admissions consisted of one 30-min session (1 × 30) or three 10-min bouts (3 × 10) of exercise with power output set midway between VO2 at LT and VO2peak.
General Clinical Research Center (GCRC) admissions
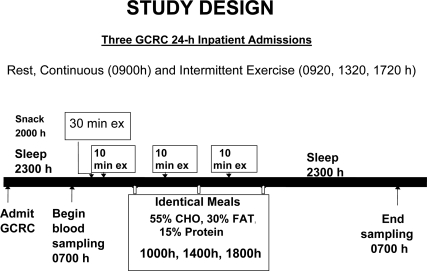
Subjects were studied at the GCRC on three randomly assigned occasions, two with exercise and one at rest. Young women were tested in the early follicular phase of the menstrual cycle. The research design is shown in Fig. 1.
Figure 1.
Design of the present study. CHO, Cholesterol.
Subjects were admitted to the GCRC the evening before the studies, consumed their evening meal by 1700 h, were allowed to consume water ad libitum, and received a standardized snack (500 kcal, 55% carbohydrate, 15% protein, and 30% fat) at 2000 h. At 2100 h an iv cannula was placed bilaterally in each forearm vein. Subjects were asked to turn lights off by 2300 h. Beginning at 0700 h, blood samples were withdrawn every 10 min until 0700 h the next day for later measurement of serum GH. To avoid the confounding effects of meals on GH secretion, subjects consumed standardized meals at 1000, 1400, and 1800 h and then remained fasting until the next morning. The caloric and macronutrient content of each meal was identical (kilocalories based on measured basal metabolic rate + an activity factor, 55% carbohydrate, 15% protein, and 30% fat). Subjects were asked to sit quietly in bed during the nonexercise portion of the day. They were allowed to read, watch television, work on their computers, and ambulate to the rest room.
Exercise admissions
The 1 × 30 min exercise bout exercise began at 0900 h and the 3 × 10 min exercise bouts were initiated at 0920, 1320, and 1720 h. During the same time frame for each condition (9000–1000, 1300–1400, 1700–1800 h), blood lactate was measured every 10 min, heart rate and electrocardiogram were monitored continuously, and metabolic data were measured minute by minute during rest or exercise and during the immediate 30 min after exercise. These assessments were made to assure achievement appropriate exercise intensity during the exercise admissions and for safety reasons.
Nonexercise admission
The above procedure except for exercise was followed on the nonexercise day.
GH assay
GH concentrations were measured in duplicate by modified ultrasensitive chemiluminescence assay (Nichols Institute Diagnostics, San Clemente, CA) using 22-kDa recombinant human GH as standard (20). Cross-reactivity with 20 kDa GH was 30%. Assay sensitivity (defined as 3 sd above the zero dose tube) was 0.005 μg/liter. Median intra- and interassay coefficients of variation were 5.2 and 6.3%, respectively. All samples from a single subject were assayed together to eliminate interassay variability.
Data reduction
Assay data were analyzed by a model-free dose-dependent extrapolation of triplicate standards (33). Mean and integrated serum GH concentrations (IGHC) were calculated (34). Hormonal secretion profiles were analyzed using a recently developed deconvolution method (35,36,37).
Statistical analysis
To produce symmetric measurement distributions and equalize measurement, variability data for the deconvolution parameters were transformed to the natural logarithmic scale and analyzed via mixed-effects ANOVA for repeated measures (38). The ANOVA model specification included three classification factors to estimate the main effect of obesity (nonobese, obese), gender (female, male), and the level of the exercise condition (control, 1 × 30 min, 3 × 10 min). One-, two-, and three-way interactions were also estimated.
Model parameters were estimated by way of residual maximum likelihood, and the variance-covariance matrix was modeled in the compound symmetry form (39). A priori comparisons were formulated by way of linear contrasts of the least-squares means. Fisher’s restricted least significant difference criterion was used to maintain an overall two-sided multiple comparisons type I error of 0.05. Confidence interval construction was based on Fisher’s least significant difference.
For the deconvolution parameters, the within- and between-group comparisons are expressed in terms of fold change in the value of the geometric mean (GM). The GM is a location parameter similar to the arithmetic mean and median. The value of the GM identifies the central location of the measurement distribution and is calculated by taking the antilogarithm of the mean of the distribution of logarithmically transformed data (40). We compared GMs instead of arithmetic means because a critical statistical assumption of ANOVA is that, to obtain valid statistical tests, residual variation should be approximately equal within treatment groups. When the magnitude of the variance in the response increases as the mean of the response increases in value, the natural logarithmic transformation is generally used to stabilize the residual variance among two or more treatment groups.
The within- and between-group comparisons for IGHC are presented as a difference between the arithmetic mean. The PROC MIXED procedure of SAS version 8.2 (SAS Institute Inc. Cary, NC) was used to carry out statistical analyses. A Bonferroni multiplier with a prespecified experimental type I error rate of 0.05 was used to maintain a type I error rate of 0.05.
The effects of obesity status and gender on the relationship between IGHC and exercise condition were examined by way of a random coefficient regression model and a random coefficient piecewise regression model. The values for IGHC were transformed to the natural logarithmic scale for these analyses.
Linear-mixed regression models were developed to examine the effects of gender and the linear and nonlinear effects of VO2 and BMI on pulsatile GH at rest and during exercise.
Data are presented as mean ± se.
Results
Nonobese individuals had lower BMI, less percentage body fat, and greater fitness than obese individuals. Men were taller, heavier, had lower BMI, and had less percentage body fat and higher LT and VO2 peak values than women. Obese subjects were older than nonobese subjects (P < 0.05, Table 1).
IGHC
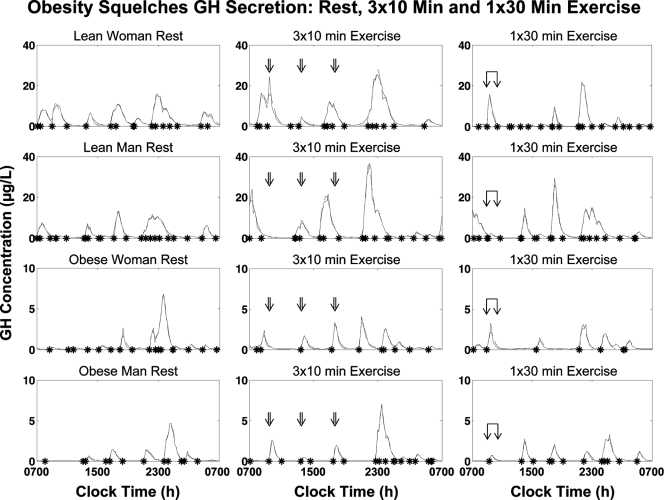
Figure 2 shows the 10-min 24-h GH profiles from representative nonobese and obese male and female subjects. Each exercise bout resulted in a GH pulse toward the end of or immediately after exercise, with the response attenuated in obese subjects.
Figure 2.
Twenty-four-hour serum GH concentration profiles sampled every 10 min in a nonobese and obese man and woman during three study sessions each (control; a 30 min continuous bout of exercise, 1 × 30 min; three 10 min intermittent bouts of exercise, 3 × 10 min). Asterisks denote significant pulse onsets. The overlying interrupted curves are predicted (fit) by the deconvolution model (methods). Sampling began at 0700 h (time 0). Exercise was performed at 0900–0930 h (single exercise bout) and at 0920–0930, 1320–1330, and 1720–1730 h (three 10 min bouts). Standardized meals were fed at 1000, 1400, and 1800 h. Lights were put out at 2300 h.
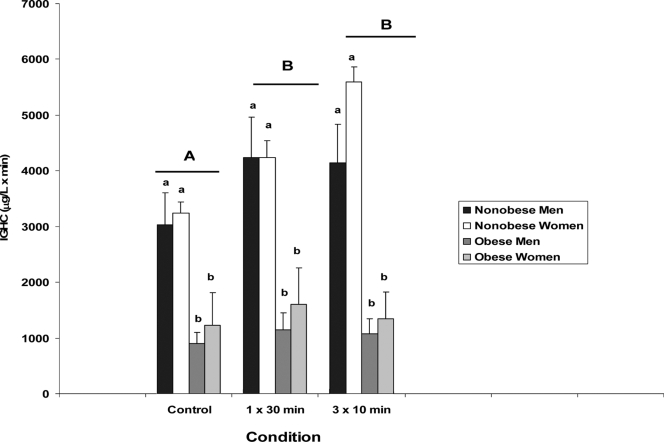
Figure 3 presents the mean and se values for 24-h integrated GH concentrations (0700 h to 0700 h) for the three study conditions in nonobese and obese males and females. Significant main effects were observed for obesity status (P < 0.001) and condition (P = 0.003). Within each condition 24-h IGHC in nonobese subjects exceeded that in obese individuals (P < 0.002 for all comparisons). Both exercise conditions resulted in augmented 24-h IGHC compared with the resting condition (P = 0.004 for 1 × 30 min vs. control and P = 0.002 for 3 × 10 min vs. control). No interactions were observed for 24-h IGHC.
Figure 3.
IGHC (0700–0700 h) across three exercise conditions (control; a 30 min continuous bout of exercise, 1 × 30 min; three 10 min intermittent bouts of exercise, 3 × 10 min) in nonobese and obese men and women. Data are means ± se. ANOVA revealed the following: significant main effects for obesity status (P < 0.001) and condition (P = 0.003). Within each condition, 24-h IGHC in nonobese subjects exceeded that of obese individuals (P < 0.002 for all comparisons). Both exercise conditions resulted in augmented 24-h IGHC compared with the control condition (P = 0.004 for 1 × 30 min vs. C and P = 0.002 for 3 × 10 min vs. C). There were no differences observed between exercise conditions (P = 0.82), no main effect for gender, and no interactions. Capital letters give differences between exercise conditions and lower-case letters differences among subjects (e.g. A and B indicate that 1 × 30 min and 3 × 10 min were different than control; a and b indicate that within each condition nonobese subjects differed from obese subjects).
Deconvolution analyses
Table 2 presents deconvolution analyses.
Table 2.
GH deconvolution parameters for the three study conditions in nonobese and obese males and females
| Nonobese men (n = 8)
|
Nonobese women (n = 7)
|
Obese men (n = 8)
|
Obese women (n = 6)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Control | 1 × 30 | 3 × 10 | Control | 1 × 30 | 3 × 10 | Control | 1 × 30 | 3 × 10 | Control | 1 × 30 | 3 × 10 |
| Basal secretion (μg · liter−1 · min) | 4.1 (2.0) | 7.2 (2.5) | 6.2 (1.7) | 10.2 (2.2) | 10.9 (2.8) | 25.2 (9.2) | 2.5 (0.9) | 1.2 (0.5) | 1.3 (0.2) | 6.1 (2.2) | 5.3 (1.4) | 9.7 (4.3) |
| Burst frequency (n per 24 h) | 18.1 (2.1) | 18.9 (1.7) | 15.6 (1.8) | 15.9 (2.1) | 16.9 (2.2) | 15.9 (2.4) | 13.1 (1.2) | 15.9 (1.0) | 12.4 (2.0) | 13.5 (2.2) | 16.7 (2.9) | 13.8 (2.2) |
| Slow half-life (min) | 13.8 (1.4) | 15.4 (1.5) | 12.7 (1.2) | 13.4 (1.1) | 16.3 (1.8) | 13.5 (1.1) | 14.9 (1.6) | 15.8 (1.9) | 13.3 (1.0) | 13.6 (1.1) | 13.0 (1.0) | 13.4 (1.2) |
| Pulsatile secretion (μg · liter−1 · min) | 145.8 (29.0) | 179.8 (31.5) | 200.9 (37.3) | 148.2 (19.1) | 166.6 (21.6) | 237.0 (48.3) | 38.3 (8.2) | 45.5 (10.4) | 58.3 (17.0) | 54.8 (29.4) | 68.0 (27.0) | 60.2 (21.6) |
| Mass/pulse (μg · liter−1) | 7.41 (1.0) | 10.1 (1.8) | 12.8 (1.2) | 10.0 (2.2) | 10.3 (0.9) | 18.9 (7.1) | 2.8 (0.5) | 2.8 (0.6) | 5.2 (1.6) | 3.9 (1.7) | 4.1 (1.4) | 4.3 (1.5) |
Data are presented as mean (se). Statistical analyses revealed: 1) basal GH secretion was greater in women than men (P < 0.001) and greater in nonobese than obese subjects (P < 0.001); 2) obese subjects had fewer detectable GH secretory pulses (P = 0.007); 3) slow GH half-life was not affected by gender, obesity, or the control or exercise conditions; 4) 24-h pulsatile GH secretion was significantly elevated in nonobese subjects (P = 0.001) and the 3 × 10 min and 1 × 30 min exercise conditions compared with control (P < 0.001 and P = 0.003, respectively); and 5) nonobese subjects had greater mass of GH secreted per pulse, P < 0.0001) and the 3 × 10 min exercise condition was associated with greater mass of GH secreted per pulse compared with control, P < 0.0001 and with the 1 × 30 min exercise condition (P = 0.016).
Basal secretion
Basal GH secretion was greater in women than men (P < 0.001) and in nonobese than obese subjects (P < 0.001). There were no differences in basal secretion among the three conditions.
GH secretory pulse frequency
There were no differences in GH secretory-pulse frequency between men and women or among the three conditions. Obese subjects had fewer detectable GH secretory pulses than nonobese subjects (P = 0.007).
Slow GH half-life
The calculated slow GH half-life (an estimate of GH clearance) was not affected by gender, obesity, or the three conditions.
Pulsatile GH secretion
Twenty-four hour pulsatile GH secretion was significantly reduced in obese subjects (P = 0.001) and was affected by condition with the 3 × 10 and 1 × 30 min conditions, resulting in greater GH pulsatile secretion than the control condition (P < 0.001 and P = 0.003, respectively).
Mass of GH secreted per pulse
Significant main effects were observed for mass of GH secreted per pulse for obesity state (nonobese had greater mass of GH secreted per pulse, P < 0.0001) and condition (the 3 × 10 min exercise condition was associated with greater mass of GH secreted per pulse compared with control, P < 0.0001 and with the 1 × 30 min exercise condition, P = 0.016).
Effects of gender, VO2, and BMI on pulsatile GH at rest and during exercise
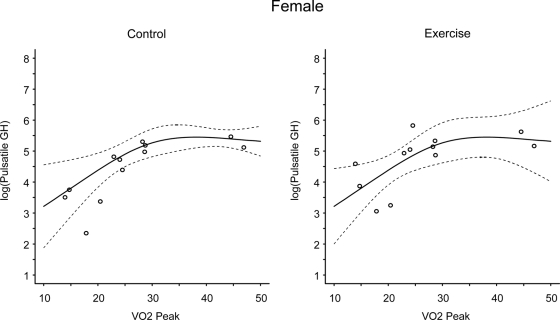
There was no effect of gender at rest or during exercise on pulsatile GH. VO2 peak (Figs. 4 and 5, P = 0.017) and BMI (Figs. 6 and 7, P = 0.044) influenced pulsatile GH. There were significant linear trends in the relationship between VO2peak and log(pulsatile GH) and between BMI and log(pulsatile GH) under both the control and exercise conditions (P values ranged from P = 0.025 to P < 0.001 for individual comparisons). The linear trend was not condition dependent. The regression model summary examining all two-way and three-way interactions indicated that at rest both VO2peak and BMI influenced pulsatile GH (with higher VO2peak and lower BMI being associated with elevated pulsatile GH, P = 0.02 and P = 0.04, respectively) and that there was a trend for a VO2peak by BMI interaction (P < 0.08). During exercise BMI (P < 0.008) but not VO2peak influenced pulsatile GH and there was trend for a VO2peak by BMI interaction (P < 0.07).
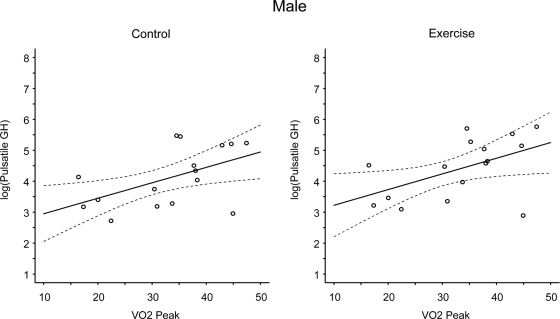
Figure 4.
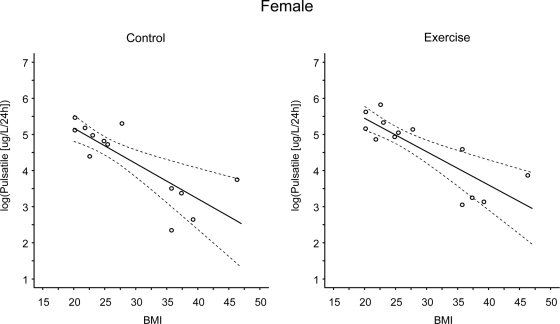
Linear-mixed regression models examining the linear and nonlinear effects of VO2 (milliliters per kilogram per minute) on pulsatile GH (micrograms per liter per 24 h) at rest and during exercise in females. The solid line represents the predicted values and the dashed lines represent the simultaneous 95% confidence intervals. There were significant linear trends in the relationship between VO2peak and log(pulsatile GH) under both the control and exercise conditions (P < 0.001 and P = 0.008 at rest and exercise, respectively). The linear trend was not condition dependent.
Figure 5.
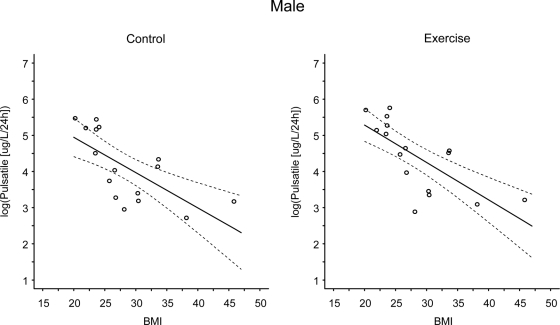
Linear-mixed regression models examining the linear and nonlinear effects of VO2 (milliliters per kilogram per minute) on pulsatile GH (micrograms per liter per 24 h) at rest and during exercise in males. The solid line represents the predicted values and the dashed lines represent the simultaneous 95% confidence intervals. There were significant linear trends in the relationship between VO2peak and log(pulsatile GH) under both the control and exercise conditions (P = 0.016 and P = 0.025 at rest and exercise, respectively). The linear trend was not condition dependent.
Figure 6.
Linear-mixed regression models examining the linear and nonlinear effects of BMI on pulsatile GH (micrograms per liter per 24 h) at rest and during exercise in females. The solid line represents the predicted values and the dashed lines represent the simultaneous 95% confidence intervals. There were significant linear trends in the relationship between BMI and log(pulsatile GH) under both the control and exercise conditions (P < 0.001 at rest and exercise). The linear trend was not condition dependent.
Figure 7.
Linear-mixed regression models examining the linear and nonlinear effects of BMI on pulsatile GH (micrograms per liter per 24 h) at rest and during exercise in males. The solid line represents the predicted values and the dashed lines represent the simultaneous 95% confidence intervals. There were significant linear trends in the relationship between BMI and log(pulsatile GH) under both the control and exercise conditions (P < 0.001 at rest and exercise). The linear trend was not condition dependent.
Discussion
Comparison of GH secretion by obesity status, gender, and continuous or intermittent exercise revealed several important findings: 1) continuous and intermittent exercise were equally effective in augmenting 24-h IGHC and GH secretory profiles, 2) obese subjects manifested marked attenuation of resting and exercise-stimulated 24-h IGHC, and 3) no gender differences were observed for 24-h IGHC.
The present data only partially support our original hypotheses. As hypothesized, both continuous and intermittent exercise increased 24-h IGHC. However, whereas we hypothesized that repeated intermittent exercise would augment GH secretion, there was no difference in 24-h IGHC values between the two exercise conditions. We based our original hypothesis on the facts that the GH response to aerobic exercise is related to exercise intensity (12,16,18); repeated bouts of aerobic exercise augment 24 h GH release (28); and as little as 10 min of aerobic exercise will stimulate GH secretion (27). In earlier studies, an acute bout of aerobic exercise stimulates a large GH pulse that typically returns to baseline with about 2 h (11,12,14,16,18,28). Thus, we speculated that when holding total exercise duration constant, stimulating the GH axis with acute exercise three times during the day would yield pulses that were similar to those observed during a single bout of acute exercise, resulting in additional augmentation of 24-h IGHC. Subsequent to data collection in the present study, we recently reported that if exercise intensity is constant, GH release can be augmented by longer exercise duration (41). This and diminished GH responses in obesity may explain why intermittent exercise was not associated with additional augmentation in 24-h IGHC.
Previous findings indicate obesity attenuates both spontaneous and exercise-stimulated GH release (19,20,21). We observed similar attenuation in 24-h IGHC both at rest and in response to continuous and intermittent exercise in obese subjects that was not affected by gender (Fig. 3). Although we categorized obesity in the present study based on BMI, it is likely that greater AVF per se is more strongly related to attenuation of spontaneous and exercise-stimulated GH release than overall obesity. Although we did not measure AVF, we previously reported AVF is a primary determinant of 24-h GH release (42). In nonobese older adults stratified based on AVF levels, elevated AVF was associated with a marked reduction in 24-h IGHC (by 40–50%), unfavorable lipid and lipoprotein profiles, and an elevation in nontraditional risk factors for coronary artery disease (43). Abdominal adiposity rather than age and sex predicts the mass and pattern of GH secretion in middle-aged adults (44) and administration of GH to obese adults results in a greater reduction in AVF than in sc fat (45,46). Thus, exercise training at an appropriate intensity should elevate GH release (13,16,18) and lower AVF.
Deconvolution analysis was used to examine GH secretion profiles (36). Exercise (of increasing intensity or duration) is associated with an increase in the amount of GH secreted per burst rather than in the number of bursts (12,16,18,41). This likely explains the increase in 24-h IGHC in the exercise conditions because mechanistically pulsatile secretion constitutes most (>85%) of the total daily GH output (47). Importantly, there were similar increases in 24-h IGHC within both the 1 × 30 min and 3 × 10 min exercise conditions (Fig. 3). Similar to the report of Kanaley et al. (20), our results indicate attenuated 24-h IGHC in obese subjects was related to decreased basal and pulsatile secretion because no differences were observed in the slow GH half-life, which is reflective of GH clearance. Our findings also indicate that increased fitness (as measured by VO2peak) elevates 24-h resting GH secretion in proportion to the elevation of exercise-stimulated GH secretion (Figs. 4 and 5). Thus, a key clinical goal is to enhance physical conditioning, thereby jointly increasing resting and exercise-associated GH-secretion. However, the fact that increased fitness cannot overcome the inhibitory effect of a high BMI (and presumably elevated AVF; Figs. 6 and 7) suggests that a combination of increased fitness through an appropriate exercise program combined with a modest weight loss program should reduce AVF [5–10% weight loss is associated with ∼30% reduction in AVF (48)] and increase GH secretion.
The fact that both exercise conditions similarly increased 24-h IGHC may have utility in designing exercise-training programs. We have shown that traditional exercise training with appropriate intensity can increase 24-h IGHC (13). Training with multiple short exercise bouts distributed throughout the day appears as effective as a single continuous exercise bout for improving maximum VO2 and weight reduction (23,24,25,26). To our knowledge, this is the first study to examine the effects of continuous vs. intermittent exercise on 24-h GH secretion. We were able to control for confounding effects of caloric intake and expenditure by serving identical meals and ensuring equal total duration and intensity of the exercise regimens. The finding that the 1 × 30 min and 3 × 10 min exercise conditions were comparably effective in increasing 24-h GH secretion in both nonobese and obese subjects (albeit the response was attenuated in obesity) indicates that either mode of exercise should allow for developing training programs that increase GH release. Because intensity of exercise is directly related to GH release (12,16,18), one possible advantage of multiple short bouts of exercise is the potential to exercise at higher intensities for shorter durations. For example, splitting a typical 30-min bout of exercise into two 15- or three 10-min bouts should allow subjects to maintain a higher intensity of exercise over the shorter duration. This concept may be particularly important for obese subjects who find it difficult to exercise for 30 min or longer continuously. Indeed, Jakicic et al. (23) reported that in overweight women, short bouts of exercise enhanced exercise adherence compared with a single bout of longer exercise.
As mentioned previously, GH production is strongly and inversely related to AVF (42,43,44). Elevated AVF is associated with a constellation of unfavorable cardiometabolic risk factors, which may be ameliorated by reducing visceral fat (49,50). Exogenous administration of GH to obese men and women has been shown to reduce AVF and improve cardiometabolic risk factors (45,46). Endogenous elevation of GH, as a result of exercise training, could result in analogous reduction in AVF and improvement in the cardiometabolic risk profile without the potential for adverse events associated with GH administration. Interestingly, the 3 × 10 min exercise condition was associated with an elevated mass of GH secreted per pulse compared with both the control condition and the 1 × 30 min exercise condition. This distribution may be relevant, because an iv bolus of GH is more effective than the same dose given continuously in eliciting acute lipolysis (51,52). Because both exercise conditions were associated with elevated 24-h GH, exercise training of this type should be associated with increased fat oxidation and glucose and protein sparing. This in turn should be related to favorable clinical outcomes.
We did not examine several parameters that may have influenced the GH response to exercise (e.g. free fatty acids, core temperature, IGF-I, GH binding proteins, ghrelin, etc.; for a more complete review see Ref.53). It could be suggested that the difference in absolute exercise intensity may have contributed to elevated core temperature and GH secretion in nonobese subjects. However, the short duration of the exercise bouts, the fact that all subjects exercised at the same relative intensity, and elevated fat content in obese subjects suggests that the physiological stress related to exercise was similar among subjects.
In summary, compared with unstimulated 24-h GH secretion, both a single 30-min and three 10-min bouts of exercise, at constant exercise intensity, resulted in severalfold elevation in 24-h IGHC in both nonobese and obese young adults. In both paradigms, basal and pulsatile GH secretion was attenuated both at rest and during exercise in obese subjects. The present data suggest that either continuous or intermittent exercise training should be effective for increasing 24-h GH secretion.
Acknowledgments
We acknowledge the invaluable contributions of the nurses of the General Clinical Research Center (GCRC) for drawing blood and caring for patients and the members of the Core Laboratory for performing the assays.
Footnotes
This work was supported by National Center for Research Resources Grant RR-00847
Disclosure Statement: J.Y.W., D.D.W.W., K.F., J.P., P.K., and D.M.K. have nothing to declare. A.W. is a member of the Scientific Advisory Boards of Kronos, and Redcord AS. G.A.G. is a consultant for the Grains Foundation. J.D.V. is a consultant for NorvoNordisk, Organon, and Eli Lilly Inc.
First Published Online September 9, 2008
Abbreviations: AVF, Abdominal visceral fat; BMI, body mass index; GCRC, General Clinical Research Center; GM, geometric mean; IGHC, integrated serum GH concentrations; LT, lactate threshold; VO2, O2 consumption; VO2peak, peak oxygen uptake.
References
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY 2006 Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- Lassarre C, Girard F, Durand J, Raynaud J 1974 Kinetics of human growth hormone during submaximal exercise. J Appl Physiol 37:826–830 [DOI] [PubMed] [Google Scholar]
- Raynaud J, Capderou A, Martineaud JP, Bordachar B, Durand J 1983 Intersubject variability of growth hormone time course during different types of work. J Appl Physiol 55:1682–1687 [DOI] [PubMed] [Google Scholar]
- Sutton J, Lazarus L 1976 Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J Appl Physiol 41:523–527 [DOI] [PubMed] [Google Scholar]
- Bunt JC, Boileau RA, Bahr JM, Nelson RJ 1986 Sex and training differences in human growth hormone during prolonged exercise. J Appl Physiol 61:1796–1801 [DOI] [PubMed] [Google Scholar]
- Chang FE, Dodds WG, Sullivan M, Kim MH, Malarkey WB 1986 The acute effects of exercise on prolactin and growth hormone secretion: comparison between sedentary women and women runners with normal and abnormal menstrual cycles. J Clin Endocrinol Metab 62:551–556 [DOI] [PubMed] [Google Scholar]
- Chwalbinska-Moneta J, Krysztofiak H, Ziemba A, Nazar K, Kaciuba-Uscilko H 1996 Threshold increases in plasma growth hormone in relation to plasma catecholamine and blood lactate concentrations during progressive exercise in endurance-trained athletes. Eur J Appl Physiol 73:117–120 [DOI] [PubMed] [Google Scholar]
- Felsing NE, Brasel JA, Cooper DM 1992 Effect of low and high intensity exercise on circulating growth hormone in men. J Clin Endocrinol Metab 75:157–162 [DOI] [PubMed] [Google Scholar]
- Kozlowski S, Chwalbinska-Moneta J, Vigas M, Uscilko H, Nazar K 1983 Greater serum GH response to arm than to leg exercise performed at equivalent oxygen uptake. Eur J Appl Physiol 52:11–135 [DOI] [PubMed] [Google Scholar]
- Lugar A, Watschinger B, Duester P, Svoboda T, Clodi M, Chrousos GP 1992 Plasma growth hormone and prolactin responses to graded levels of acute exercise and to a lactate infusion. Neuroendocrinology 56:112–117 [DOI] [PubMed] [Google Scholar]
- Pritzlaff CJ, Wideman L, Blumer J, Jensen M, Abbott RD, Gaesser GA, Veldhuis JD, Weltman A 2000 Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. J Appl Physiol 89:937–946 [DOI] [PubMed] [Google Scholar]
- Pritzlaff CJ, Wideman L, Weltman JY, Abbott RD, Gutgesell ME, Hartman ML, Veldhuis JD, Weltman A 1999 Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol 87:498–504 [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Schurrer R, Evans WS, Veldhuis JD, Rogol AD 1992 Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. J Appl Physiol 72:2188–2196 [DOI] [PubMed] [Google Scholar]
- Wideman L, Weltman JY, Shah N, Story S, Veldhuis JD, Weltman A 1999 The effects of gender on exercise-induced growth hormone release. J Appl Physiol 87:1154–1162 [DOI] [PubMed] [Google Scholar]
- Wideman L, Weltman JY, Patrie JT, Bowers CY, Shah N, Story S, Veldhuis JD, Weltman A 2000 Synergy of l-arginine and growth hormone (GH)-releasing peptide-2 (GHRP-2) stimulation of GH in men and women: modulation by exercise. Am J Physiol Reg Integ Comp Physiol 279:R1467–R1477 [DOI] [PubMed] [Google Scholar]
- Pritzlaff-Roy CJ, Wideman L, Weltman JY, Abbott R, Gutgesell M, Hartman ML, Veldhuis JD, Weltman A 2002 Gender governs the relationship between exercise intensity and growth hormone release in young adults. J Appl Physiol 92:2053–2060 [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD 1994 Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab 78:543–548 [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Pritzlaff Roy C, Wideman L, Patrie J, Evans WS, Veldhuis JD 2006 The growth hormone (GH) response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol 100:1623–1629 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Muligan T, Johnson ML, Pincus SM, Straume M, Iranmanesh A 1995 Differential impact of age, sex-steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80:3209–3222 [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Weatherup-Dentes MM, Jaynes EB, Hartman ML 1999 Obesity attenuates the growth hormone response to exercise. J Clin Endocrinol Metab 84:3156–3161 [DOI] [PubMed] [Google Scholar]
- Hansen P 1973 Serum growth hormone response to exercise in non-obese and obese normal subjects. Scan J Clin Lab Invest 31:175–178 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarradle G 1991 Dual defects in pulsatile growth hormone release and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab 72:51–59 [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Wing RR, Butler BA, Robertson RJ 1995 Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obesity 19:893–901 [PubMed] [Google Scholar]
- Murphy MH, Hardman AE 1998 Training effects of short and long bouts of brisk walking in sedentary women. Med Sci Sports Exerc 30:152–157 [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Winters C, Lang W, Wing RR 1999 Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized trial. JAMA 282:1554–1560 [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Jacobsen DJ, Snyder Heelan K, Seip R, Smith S 2000 The effects of 18 months of intermittent vs continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females. Int J Obes 24:566–572 [DOI] [PubMed] [Google Scholar]
- Cappon J, Brasel JA, Mohan S, Cooper DM 1994 Effect of brief exercise on circulating insulin-like growth factor I. J Appl Physiol 76:2490–2496 [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Weltman JY, Veldhuis JD, Rogal AD, Hartman ML, Weltman A 1997 Human growth hormone response to repeated bouts of aerobic exercise. J Appl Physiol 79:1756–1761 [DOI] [PubMed] [Google Scholar]
- Katch FI, Michael ED, Horvath SM 1967 Estimation of body volume by underwater weighing: description of a simple method. J Appl Physiol 23:811–813 [DOI] [PubMed] [Google Scholar]
- Wilmore JH 1969 A simplified method for the determination of residual lung volume. J Appl Physiol 27:96–100 [DOI] [PubMed] [Google Scholar]
- Brozek J, Grande F, Anderson JT, Keys A 1963 Densitometric analysis of body composition of men from girth measurements: revision of some quantitative assumptions. Ann NY Acad Sci 110:113–140 [DOI] [PubMed] [Google Scholar]
- Weltman A, Snead D, Stein P, Seip R, Schurrer R, Rutt R, Weltman JY 1990 Reliability and validity of a continuous incremental treadmill protocol for the determination of lactate threshold, fixed blood lactate concentrations, and VO2max. Int J Sports Med 11:26–32 [DOI] [PubMed] [Google Scholar]
- Straume M, Johnson ML, Veldhuis JD 1998 Statistically accurate estimation of hormone concentrations and associated uncertainties: methodology, validation, and applications. Clin Chem 44:116–123 [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD 2003 Physiological control of pituitary hormone secretory-burst mass, frequency, and waveform: a statistical formulation and analysis. Am J Physiol 285:R664–R673 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD 2005 Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236:242–255 [DOI] [PubMed] [Google Scholar]
- Faria ACS, Veldhuis JD, Thorner MO, Vance ML 1989 Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab 68:535–541 [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang K-Y, Zerger SL 2003 Analysis of longitudinal data. 2nd ed. New York: Oxford University Press [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD 2000 SAS system for mixed models. Cary, NC: SAS Institute Inc [Google Scholar]
- Zar JH 1996 Biostatistical analysis. 3rd ed. Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- Wideman L, Consitt L, Patrie J, Swearingin B, Bloomer R, Davis P, Weltman A 2006 The impact of sex and exercise duration on growth hormone secretion. J Appl Physiol 101:1641–1647 [DOI] [PubMed] [Google Scholar]
- Clasey JL, Weltman A, Patrie J, Weltman JY, Pezzoli S, Bouchard C, Thorner MO, Hartman ML 2001 Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab 86:3845–3852 [DOI] [PubMed] [Google Scholar]
- Weltman A, Despres JP, Clasey JL, Weltman JY, Wideman L, Kanaley J, Patrie J, Bergeron J, Thorner MO, Bouchard C, Hartman M 2003 Impact of abdominal visceral fat, growth hormone, fitness, and insulin on lipids and lipoproteins in older adults. Metabolism 52:73–80 [DOI] [PubMed] [Google Scholar]
- Vahl N, Jorgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J 1997 Abdominal obesity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. Am J Physiol 272:E1108–E1116 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G 2005 Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90:1466–1474 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Weltman JY, Weltman AL, Iranmanesh A, Muller EE, Bowers CY 2004 Age and secretagogue type jointly determine dynamic growth hormone responses to exogenous insulin-like growth factor-negative feedback in healthy men. J Clin Endocrinol Metab 89:5542–5548 [DOI] [PubMed] [Google Scholar]
- Lemieux I, Poirier P, Bergeron J, Almeras N, Lamarche B, Cantin B, Dagenais GR, Despres JP 2007 Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology. Can J Cardiol 23(Suppl B):23B–31B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J 2006 Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23:469–480 [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE 2004 Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53:2087–2094 [DOI] [PubMed] [Google Scholar]
- Johansson JO, Oscarsson J, Bjarnason R, Bengtsson BA 1996 Two weeks of daily injections and continuous infusion of recombinant human growth hormone (GH) in GH-deficient adults: I. Effects on insulin-like growth factor-I (IGF-I), GH, and IGF binding proteins, and glucose homeostasis. Metabolism 45:362–369 [DOI] [PubMed] [Google Scholar]
- Gravhelt CH, Schmitz O, Simonsen L, Bulow J, Christiansen JS, Moller N 1999 Effects of physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. Am J Physiol 277:E848–E854 [DOI] [PubMed] [Google Scholar]
- Guistina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]